Abstract
In order to study the respective roles of CD4, CD8, and CD56 (NK) cells in gamma interferon (IFN-γ) production after in vitro stimulation with flu vaccine in a healthy adult human population, we depleted these cellular subtypes before stimulation with antigen (inactivated split vaccine, A/Texas H1N1, or A/Sydney H3N2). We observed that while CD4 cells were required for IFN-γ secretion in both conditions in vitro, CD56 (NK) cells and, to a lesser extent, CD8 cells had a negative effect on such synthesis upon H1N1 stimulation, as judged by an increased number of spots compared to the initial undepleted population. This regulation of IFN-γ secretion was associated with an increase in ICAM-1 expression, in particular on T and B cells. This study points out the importance of evaluating in vitro immune responses on a whole-cell population in addition to isolated subtypes if one needs to address potential cellular interactions occurring in vivo in some situations (H1N1 stimulation in the present case). Such cross-regulations occur even in vitro during the antigenic stimulation step.
Influenza is one of the most common respiratory diseases in humans and is potentially lethal in immunocompromised persons. It affects all types of people, from infants to the elderly, and is usually caused by types A and B of the influenza virus. Although efficient vaccines exist, investigations still need to be conducted to further improve such vaccines, in particular because of the high degree of virus variability, requiring annual vaccination (4). Antigenic variability is mostly due to changes in hemagglutinin (HA) and neuraminidase (NA) antigens. Among type A strains, 15 HA and 9 NA subtypes have been identified, and in humans 5 subtype combinations have been linked to flu pandemics since 1900 (10) (the recent avian H5N1 outbreak has remained localized in Hong Kong and to a limited number of persons). In the past 20 years, the predominant A strain has been of the H3N2 subtype, while H1N1 has been isolated less frequently (5, 9, 10).
Antibodies to HA and NA play a critical role in protection (3), but because of the variability of these antigens the role of cellular responses directed in particular against more conserved internal proteins has been investigated. It has been demonstrated that this response was important during the first contact, as well as during subsequent influenza infections (3). Cellular responses may be cross-protective, involving cytotoxic T lymphocytes (CTLs) that are able to recognize epitopes shared by several subtypes (13). This type of response could then be useful in vaccination for generating a pool of persistent cross-reactive T lymphocytes (8). Gamma interferon (IFN-γ)-producing CD4 T cells can also favor viral elimination from nasal tissues (16) and, more generally, IFN-γ plays a critical role in cell-mediated immunity, stimulating macrophages and NK cell activity. Apart from CD4 T cells, IFN-γ is produced by CD8 T cells and NK cells, and these cells have been demonstrated to induce and upregulate early inflammatory and cytotoxic events (11). However, their role has not been extensively studied during secondary stimulation, a situation found in the great majority of the adult and elderly populations, after these individuals receive the flu vaccine, due to prior natural infection(s).
In order to study the respective roles of CD4, CD8, and NK cells in IFN-γ production in a healthy adult human population, we depleted these cellular subtypes before in vitro stimulation with flu antigens (inactivated split virus). We observed that some depletions induced an unexpected increase in spot numbers compared to the initial undepleted population, showing that cellular cross-regulations occur even during short in vitro stimulation with antigen.
MATERIALS AND METHODS
General conditions.
Studies have been performed on fresh cells from healthy adult donors. Cell subpopulations were purified by using magnetic beads and IFN-γ secretion monitored by enzyme-linked immunosorbent assay (ELISPOT) assay after stimulation with A/Texas (H1N1) or A/Sydney (H3N2) strains. All donors have been tested at least twice for each condition in separate experiments.
Preparation of PBMC.
Peripheral blood mononuclear cells (PBMC) were obtained by centrifugation of heparinized blood on Lymphoprep gradient (Nycomed). PBMC were recovered from the gradient, washed first with saline, and twice in RPMI 1640 (Gibco-BRL) plus glutamine-streptomycin-penicillin (GSP) and 5% fetal bovine serum. Viability was assessed by trypan blue exclusion. Cells were resuspended at a concentration of 2.106/ml in AIMV (Gibco-BRL)-GSP. Overnight culture was done at a concentration of 5.106/ml in RPMI 1640-GSP-10% fetal bovine serum. Preliminary experiments demonstrated that results obtained with fresh PBMC were more consistent than those obtained with frozen PBMC in ELISPOT assays (unpublished observations). All experiments were thus carried out with fresh cells.
Depletion of lymphocyte subpopulations.
PBMC were washed and resuspended in 80 μl of buffer/1.107 cells (phosphate-buffered saline [PBS] without Ca2+ and Mg2+ plus 0.1% bovine serum albumin [BSA], freshly prepared). Then, 20 μl of MACS Microbeads was added per 1.107 total cells (CD4, CD8, and CD56 Microbeads; Miltenyi Biotec); this was gently mixed and incubated for 30 min at 5°C (labeling volume: buffer volume plus Microbeads volume). Cells were washed by adding 15 times the labeling volume of buffer and then centrifuged at 300 × g for 10 min. The supernatant was removed, and the pellet was resuspended in buffer (500 μl of buffer per 108 total cells). Magnetic separation was performed on a negative column (RS+) placed in the magnetic field of MACS separator (Variomacs; Miltenyi Biotec). The column was washed with 3 ml of buffer prior use. The negative fraction was obtained by elution on the column and then washed and suspended at a cell concentration of 2.106/ml in AIMV (Gibco-BRL) plus GSP. Protocols were optimized to deplete only highly positive cells: CD14 and CD56 were not lost during CD4 or CD8 depletion, respectively. Depletions were successful and specific (not more than 1% target cells remaining after depletion [not shown]), and conditions were defined in order to deplete only CD4-, CD8-, and CD56-high cells. CD4-low CD14+ cells and CD8-low CD56+ cells were then not depleted during CD4 or CD8 depletions, respectively. ELISPOT results showed that, for most donors, depletions did not induce nonspecific activation of cells as far as IFN-γ secretion was concerned.
ELISPOT assay for detection of IFN-γ-producing cells.
We coated 96-well nitrocellulose-based plates (MAHA S45; Millipore) with 5 μg of anti-human IFN-γ monoclonal antibody (MAb)/ml diluted in carbonate buffer (100 μl/well; Mabtech AB). The plates were kept overnight at 5°C. Wells were then blocked with AIMV containing GSP for 1 h at 35°C. The cells were resuspended in AIMV and added to the wells (105 cells/50 μl per well, in triplicate for each condition) and incubated for 24 h at 37°C in 5% CO2. After incubation, the plates were extensively washed first with PBS and then with PBS-0.05% Tween (PBS-Tween) to detach and remove cells. Individual wells were then treated with 100 μl of biotin-conjugated anti-human IFN-γ (1 μg/ml; Mabtech AB), and the plates were incubated for 2 h at room temperature. The plates were washed with PBS-Tween and then incubated with streptavidin-peroxidase (0.5 μg/ml; Southern Biotechnology) for 1 h at room temperature. After an extensive washing with PBS-Tween and PBS, spots corresponding to IFN-γ-producing cells were visualized with 3-amino-9-ethylcarbazole in 0.1 M sodium acetate (pH 5.0). Spots were counted with an automated ELISPOT plate counter (Microvision Instruments, Evry, France), and values corresponded to the mean number of spots (in triplicate) for 106 cells. Final values were obtained after subtraction of the control values (without stimulation). The mean value for negative controls according to the different experiments was a maximum of 1 spot/well, corresponding to 10 spots/million cells.
For the results obtained after depletion of cell subpopulations, the number of cells was normalized to the initial undepleted cell population. The mean number of spots/concentration factor (f ) was calculated as follows: f = 100/(100 − d). The depleted cell number (d) was calculated as follows: d = A − {([100 − A] × B)/[100 − B]}, where A is the percentage of cells before depletion and B is the percentage of cells after depletion.
This factor f takes in account the percentage of the considered population before and after depletion, and the resulting number of spots in the depleted population is then lowered according to it. When depletion is successful, as has always been the case (<1% cells remaining), this formula can be simplified to give: f ≈ 100/(100 − A).
Cytofluorometric analysis.
PBMC were analyzed on a FACScalibur (Becton Dickinson) to assess the efficacy of depletions or to measure cell surface expression of other markers. The cells were washed in PBS-0.5% BSA and saturated with human immunoglobulin (5 μg/106 cells) for 30 min. The cells were stained with the following MAbs: anti-CD3 FITC/CD19 PE (IM1293; Immunotech), anti-CD83 (IM2218; Immunotech), anti-CD4 APC (340 443; Becton Dickinson), anti-CD14 APC (340 436; Becton Dickinson), anti-CD20 PerCp (347 674; Becton Dickinson), anti-HLA-DR (347 367; Becton Dickinson), anti-CD56 FITC (340 410; Becton Dickinson), anti-CD56 PE (340 363; Becton Dickinson), anti-CD3 FITC (30114X; Pharmingen), anti-CD8 PE (30325X; Pharmingen), and anti-CD8 FITC (33294X; Pharmingen) for 30 min. The cells were finally washed twice in PBS-0.5% BSA and fixed in PBS-BSA plus Formalin overnight.
Vaccine strains.
H1N1 strain was the monovalent vaccine A/Texas from Aventis Pasteur, Marcy l'Etoile, France (batch M2ATL04; 134 μg/ml), and the H3N2 strain was the monovalent vaccine A/Sydney/5/97 from Aventis Pasteur (batch V2AYP26; 120 μg/ml). Vaccines were filtered and dialyzed against PBS (pH 7.5) and did not contain Tween or other detergents.
Cell culture stimulation.
The PBMC were cultured with flu vaccine A/Texas (H1N1) and A/Sydney (H3N2) at a final concentration of 10 μg/106 cells/ml for 24 h.
Statistical analysis.
Cell depletions were performed on the same sample (coming from one donor), and comparisons were performed with a t test for paired samples: the differences between the specific results of two populations were established for each donor, and the means of these differences were compared to “0” with the t test. Differences were considered significant when the P value was <0.05.
RESULTS
Influence of depletions on IFN-γ synthesis upon H1N1 stimulation.
Purified PBMC from healthy donors have been stimulated with H1N1 inactivated flu vaccine. In parallel, CD4, CD8, or CD56 cell subpopulations were depleted from these PBMC by using immunobeads, and the remaining cells were stimulated under the same conditions. IFN-γ-secreting cells were then quantified in an ELISPOT assay.
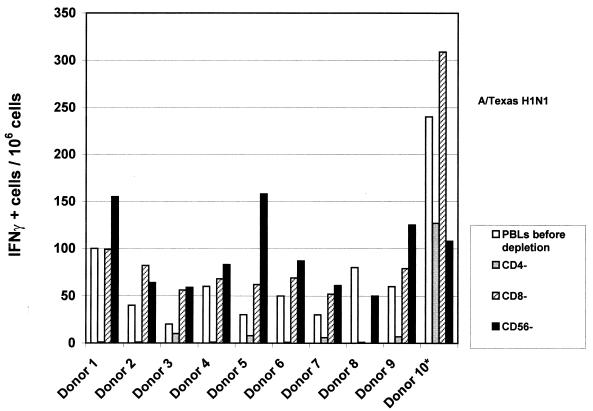
Figure 1 presents the consequences of CD4, CD8, or CD56 (NK) cells depletion after stimulation with H1N1 vaccine (24 h). CD4 depletion induced a dramatic decrease in spot numbers, which disappeared in the majority of donors. On the contrary, CD8 or CD56 depletion induced a significant increase in spot numbers in the majority of donors (P = 0.01 and P = 0.025, respectively).
FIG. 1.
Influence of cell depletion on IFN-γ synthesis after H1N1 stimulation. Depletion was performed before stimulation with flu vaccine. Spot numbers (for each donor) correspond to values obtained with PBMC before depletion or after CD4, CD8, or CD56 depletion. Stimulation was done in ELISPOT plates during 24 h by using A/Texas-H1N1 vaccine at a final concentration of 10 μg/ml; 105 cells were used per well, and experiments were performed in triplicate for each condition. The results are representative of at least two independent experiments for each donor.
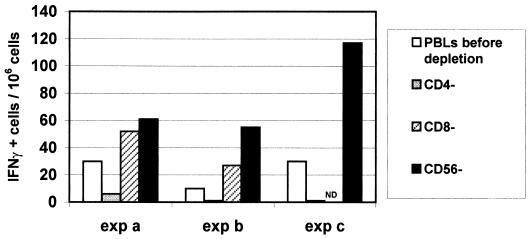
Depletions were performed at least twice for all donors on separate occasions, and an example of three experiments carried out with the same donor is presented in Fig. 2. We observed a similar pattern in all experiments after H1N1 vaccine stimulation, i.e., a decrease in spot numbers after CD4 depletion and an increase after CD8 and CD56 depletions. Moreover, double depletions (CD4-CD8, CD4-CD56, and CD8-CD56) demonstrated that each time CD4 cells were absent, a decrease in spot numbers was observed, whereas maximal increase was seen after CD8-CD56 double depletions, thus confirming our single-depletion results (Fig. 3).
FIG. 2.
Influence of cell depletion on IFN-γ synthesis after H1N1 stimulation in three different experiments on the same donor. Spot numbers correspond to values obtained with PBMC before depletion or after CD4, CD8, or CD56 depletion. Stimulation was done in ELISPOT plates during 24 h by using A/Texas-H1N1 vaccine at a final concentration of 10 μg/ml; 105 cells were used per well, and experiments were performed in triplicate for each condition.
FIG. 3.
Influence of cell double depletion on IFN-γ synthesis after H1N1 stimulation. Depletion was performed before stimulation with flu vaccine. Spot numbers (for each donor) correspond to values obtained with PBMC before depletion or after CD4-CD8, CD4-CD56, or CD8-CD56 depletion. Stimulation was done in ELISPOT plates during 24 h by using A/Texas-H1N1 vaccine at a final concentration of 10 μg/ml; 105 cells were used per well, and experiments were performed in triplicate for each condition. The results are representative of three independent experiments.
These results highlight the major role of CD4 cells in the regulation of IFN-γ secretion and/or in direct IFN-γ secretion after stimulation with killed H1N1 vaccine, and the potential inhibitory role of NK and CD8 cells in the same conditions.
Influence of depletions on IFN-γ synthesis upon H3N2 stimulation.
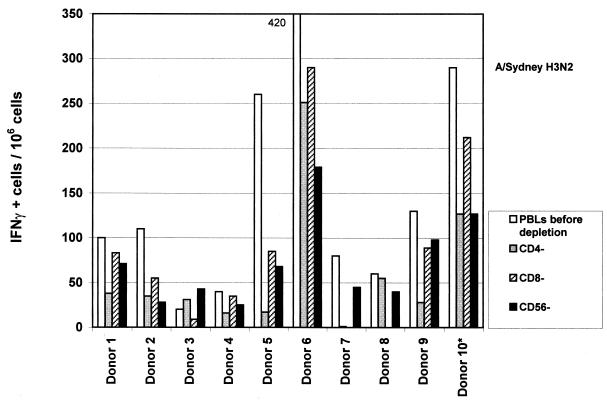
Figure 4 presents results obtained in parallel in the same donors after H3N2 inactivated vaccine stimulation. First, we observed in the majority of the donors that the number of IFN-γ-secreting cells in PBMC before depletion was irrespective of the strain used (H1N1 or H3N2). However, upon H3N2 stimulation, in contrast to what had been observed with H1N1 strain, depletions induced a decrease in spot numbers of whatever cell population was depleted (CD4, P = 0.01; CD8, P = 0.026; and CD56: P = 0.028). Although CD4 depletion resulted in almost complete disappearance of spots after H1N1 stimulation in some volunteers (donors 1, 2, 3, 4, 6, and 8), these same volunteers still presented a detectable response after H3N2 stimulation of the same CD4-depleted cell population (although presenting an average 50% decrease in spot numbers). We also observed that CD8 and CD56 cells were positively involved in IFN-γ secretion upon H3N2 stimulation, since their depletion induced a decrease in spot numbers, in contrast to the situation previously observed with the H1N1 strain.
FIG. 4.
Influence of cell depletion on IFN-γ synthesis after H3N2 stimulation. Depletion was performed before stimulation with flu vaccine. Spot numbers (for each donor) correspond to values obtained with PBMC before depletion or after CD4, CD8 or CD56 depletion. Stimulation was done in ELISPOT plates during 24 h by using A/Sydney-H3N2 vaccine at a final concentration of 10 μg/ml; 105 cells were used per well, experiments were performed in triplicate for each condition. The results are representative of at least two independent experiments for each donor.
ICAM-1 cell surface expression after H1N1 or H3N2 vaccine stimulation.
We next tried to investigate potential mechanisms explaining such differences between H1N1 and H3N2 vaccine stimulation. Some authors have shown that influenza infection (H1N1 strain) of a tumor cell line resulted in an increased expression of ICAM-1 (CD54) and subsequent increase in NK cell activity (6). We therefore evaluated ICAM-1 expression in different cell types following stimulation of PBMC in our conditions. Figure 5 shows that, without stimulation, ICAM-1 expression was variable among cell types: ca. 99% of CD14+ cells expressed ICAM-1, whereas only 2 to 10% of the T and B cells did so. After stimulation with H1N1 vaccine, there was an increase in ICAM-1-positive cells, regardless of the subtype considered, including CD83+ cells. In contrast, H3N2 stimulation did not increase ICAM-1 expression.
FIG. 5.
Percentage of ICAM-1 (CD54)-positive cells for each considered population before and after H1N1 or H3N2 stimulation. Stimulation was done in culture plates during 24 h (10 μg of the corresponding vaccine/ml). The results are representative of three independent experiments.
Since the influence of vaccine stimulation could be an indirect phenomenon mediated by the interaction of antigen-presenting cells (APCs) and T cells, we looked at other changes in cell surface expression in the same donors. We observed that, irrespective of the antigen concentration, H1N1 stimulation increased the numbers of CD14, CD83, and CD56-high cells (data not shown). In contrast, stimulation with H3N2 vaccine, while inducing a significant increase in CD83 expression, triggered a parallel decrease in CD14 and HLA-DR and no change in CD56 expression (data not shown). Thus, the consequences on cell surface expression of different APC molecules were different for the two vaccine strains.
DISCUSSION
This study points out the importance of choosing the appropriate conditions to perform in vitro tests such as the ELISPOT according to the expected information, i.e., the number of precursors of each subpopulation or the number of secreting cells resulting of cellular cross-regulations.
The initial objective of this work was to investigate the respective roles of CD4, CD8, and CD56 (NK) cells in IFN-γ secretion upon in vitro H1N1 or H3N2 flu vaccine stimulation. We observed that, while CD4 cells were always required for IFN-γ secretion, NK cells and to a lesser extent CD8 cells had a negative effect on such synthesis, i.e., their depletion resulting in increased spot numbers, when PBMC were stimulated with H1N1 vaccine. In contrast, we observed that not only CD4 cells but also CD8 cells and NK cells were required for IFN-γ secretion upon stimulation with H3N2 vaccine.
The critical role of CD4 cells after vaccine stimulation was expected since exogenous killed antigen is likely to be presented mostly through class II molecules, resulting in CD4 activation. The mechanism by which CD8 and NK cells decrease IFN-γ secretion after H1N1 stimulation, however, remains to be elucidated. Actually, such cells have usually been involved in early inflammatory response and induction of CTL responses in flu cases (12, 14), but a negative role has not been described in cases of secondary stimulation such as we investigated here. In the case of H1N1, CD4 cell regulation could be mediated through direct lysis. In fact, some authors (7) have observed that, in vivo in mice, NK cells had a negative role on CD4 activation, presumably via the direct lysis of T cells. In addition, it has also been shown that NK cells could kill APCs such as dendritic cells or macrophages (2), and this could be the case here following H1N1, but not H3N2, vaccine stimulation that led to increased ICAM-1 expression (Fig. 5).
The different consequences of H1N1 versus H3N2 stimulation could lie in the different structures of the corresponding antigens. In fact, the major differences in H3N2 and H1N1 vaccines lie in their surface proteins HA and NA, the former being predominant in the vaccine preparations. Alternatively and/or in addition, the epidemiology of flu disease in the past 10 to 20 years could also play a role. While B and A/H1N1 strains have been detected from time to time in recent years, the most prevalent subtype has been A/H3N2. Perhaps episodic stimulation in the field with H1N1 strains versus regular contact with circulating H3N2 viruses would have different consequences on CD4, CD8, and NK cells, respectively, and in particular on CD4 and CD8 memory cells. It has been shown that, although infection of mice with a series of heterologous viruses causes a reduction of memory CD8 cells specific to viruses from earlier infections, the corresponding CD4 pool remained relatively stable (17). This could apply to H1N1-specific memory cells after multiple subsequent infections by heterologous H3N2 virus in the field. H1N1-specific CD4 response would then be predominant after infection or vaccination with such a strain, potentially requiring downregulation of pathological inflammation such as delayed-type hypersensitivity (1). On the other hand, continuous stimulation in the field with live H3N2 viruses would sustain and require other types of immune responses and regulation, involving mainly—and directly—CD8 (and NK) cells, without the need for downregulating CD4 cell activity. Supporting this hypothesis, we observed that although CD4 cells were absolutely required for IFN-γ production in the case of H1N1 stimulation, their role was less prominent after H3N2 stimulation (Fig. 1 and 4). Actually, in the case of H3N2 stimulation, the number of spots was higher in the total undepleted population for the majority of the donors, and IFN-γ was still detected after CD4 depletion; this was not the case with H1N1. In agreement, in the case of H3N2 stimulation, CD8 and NK cells were involved in cytokine production, since their depletion decreased the spot numbers. It should be noted that one donor (donor 10*) suffered from a respiratory disease 1 month before these experiments were completed. Flu was suspected, but an exact diagnosis had not been determined. This donor presented a particular profile after H1N1 stimulation, since he had a high number of spots in the undepleted population compared to most other donors, he still had spots after CD4 depletion, and he was virtually the only subject to present with a decrease in spot numbers after NK depletion. This suggests that the direct role of NK cells in IFN-γ secretion is more important shortly after infection, whereas this role may be different after some time, an idea in agreement with published data (15), if the infection in donor 10 was indeed caused by an H1N1 strain.
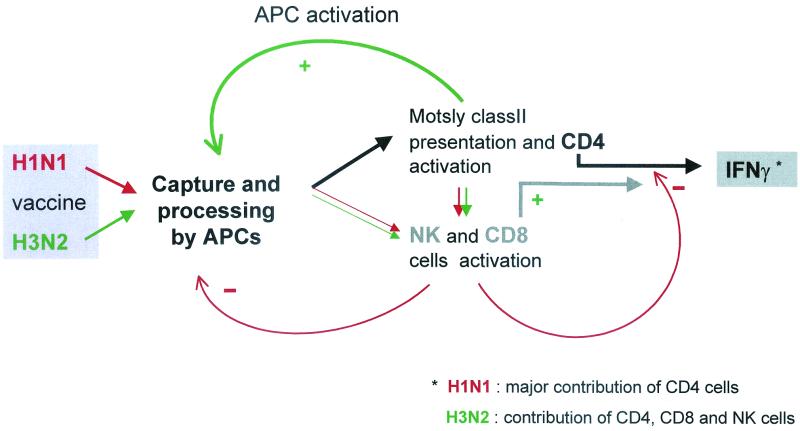
Figure 6 presents the potential mechanisms leading to IFN-γ production and its regulation by CD8 cells and NK cells in the conditions of our experiments.
FIG. 6.
Potential regulatory mechanisms involved in IFN-γ secretion after stimulation with H1N1 or H3N2 flu vaccine antigens. Both H1N1 and H3N2 antigens captured and processed by APCs stimulated a CD4 T-cell response, but only H1N1 stimulation triggered NK and CD8-negative roles, directly or indirectly, through CD4 cells. This negative regulation could result from an effect on APCs and/or IFN-γ-producing CD4 cells through direct cell lysis or inhibition of activation.
In conclusion, this study highlights the fact that different types of in vitro assays and conditions have to be considered depending on the analytical goal(s). For example, if the absolute number of CD4 or CD8 precursors producing a given cytokine is to be determined, work has to be done using isolated subtypes. Alternatively or in addition, if one needs to have in vitro a picture representing the global in vivo situation resulting from different cellular interactions, experiments have to be carried out on a total PBMC population. The role of the stimulating antigen is also critical, since two closely related stimulating agents, such as those used here, may lead to opposite results, and this has to be taken in consideration. Finally, other assays, such as intracellular cytokine staining, can also be performed, allowing the identification of each secreting subtype within the total population.
Acknowledgments
We acknowledge C. Hessler for statistical analysis, N. Fourtou and S. Gimenez for critical initial contributions to this study, and M. Bachy and N. Burdin for helpful discussions.
REFERENCES
- 1.Beck, M. A., and J. F. Sheridan. 1989. Regulation of lymphokine response during reinfection by influenza virus: production of a factor that inhibits lymphokine activity. J. Immunol. 142:3560-3567. [PubMed] [Google Scholar]
- 2.Carbone, E., G. Terrazzano, G. Ruggiero, D. Zanzi, A. Ottaiano, C. Manzo, K. Karre, and S. Zappacosta. 1999. Recognition of autologous dendritic cells by human NK cells. Eur. J. Immunol. 29:4022-4029. [DOI] [PubMed] [Google Scholar]
- 3.Couch, R. B., and J. A. Kasel. 1983. Immunity to influenza in man. Annu. Rev. Microbiol. 37:529-549. [DOI] [PubMed] [Google Scholar]
- 4.Cox, N. J., and K. Subbarao. 1999. Influenza. Lancet 354:1277-1282. [DOI] [PubMed] [Google Scholar]
- 5.Dowdle, W. R. 1999. Influenza A virus recycling revisited. Bull. W. H. O. 77:820-828. [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenthal, A., O. Marder, B. Lifschitz-Mercer, Y. Skornick, R. Tirosh, Y. Irlin, R. Avtalion, and M. Deutsch. 1997. Infection of K562 cells with influenza A virus increases their susceptibility to natural killer lysis. Pathobiology 65:331-340. [DOI] [PubMed] [Google Scholar]
- 7.Fort, M. M., M. W. Leach, and D. M. Rennick. 1998. A role for NK cells as regulators of CD4+ T cells in a transfer model of colitis. J. Immunol. 161:3256-3261. [PubMed] [Google Scholar]
- 8.Jameson, J., J. Cruz, and F. A. Ennis. 1998. Human cytotoxic T-lymphocyte repertoire to influenza A viruses. J. Virol. 72:8682-8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson, B. E., and E. D. Kilbourne. 1994. Immunization with purified N1 and N2 influenza virus neuraminidases demonstrates cross-reactivity without antigenic competition. Proc. Natl. Acad. Sci. USA 91:2358-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilbourne, E. D. 1997. Perspectives on pandemics: a research agenda. J. Infect. Dis. 176(Suppl. 1):S29-S31. [DOI] [PubMed] [Google Scholar]
- 11.Kos, F. J. 1998. Regulation of adaptive immunity by natural killer cells. Immunol. Res. 17:303-312. [DOI] [PubMed] [Google Scholar]
- 12.Kos, F. J., and E. G. Engleman. 1996. Immune regulation: a critical link between NK cells and CTLs. Immunol. Today 17:174-176. [DOI] [PubMed] [Google Scholar]
- 13.Kuwano, K., M. Scott, J. F. Young, and F. A. Ennis. 1988. HA2 subunit of influenza A H1 and H2 subtype viruses induces a protective cross-reactive cytotoxic T lymphocyte response. J. Immunol. 140:1264-1268. [PubMed] [Google Scholar]
- 14.Schapiro, J. M., Y. Segev, L. Rannon, M. Alkan, B. Rager-Zisman. 1990. Natural killer (NK) cell response after vaccination of volunteers with killed influenza vaccine. J. Med. Virol. 30:196-200. [DOI] [PubMed] [Google Scholar]
- 15.Skoner, D. P., T. L. Whiteside, J. W. Wilson, W. J. Doyle, R. B. Herberman, and P. Fireman. 1996. Effect of influenza A virus infection on natural and adaptive cellular immunity. Clin. Immunol. Immunopathol. 79:294-302. [DOI] [PubMed] [Google Scholar]
- 16.Tamura, S., K. Miyata, K. Matsuo, H. Asanuma, H. Takahashi, K. Nakajima, Y. Suzuki, C. Aizawa, and T. Kurata. 1996. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant-combined recombinant nucleoprotein. J. Immunol. 156:3892-3900. [PubMed] [Google Scholar]
- 17.Varga, S. M., L. K. Selin, and R. M. Welsh. 2001. Independent regulation of lymphocytic choriomeningitis virus-specific memory pools: relative stability of CD4 memory under conditions of CD8 memory T-cell loss. J. Immunol. 166:1554-1561. [DOI] [PubMed] [Google Scholar]