Abstract
Bacterial infections are frequent complications in patients with liver cirrhosis. Cirrhotic patients present abnormalities in both innate and adaptive immune responses, including a deficient neutrophil recruitment to infected sites. The purpose of this study was to assess neutrophil-endothelium interactions in cirrhotic patients and evaluate the effects of G-CSF on this process. We studied neutrophil adhesion and transendothelial migration in 14 cirrhotic patients and 14 healthy controls. We also analyzed neutrophil expression of the adhesion molecules CD62L and CD11b in whole blood by flow cytometry. Cirrhotic patients expressed higher levels of CD11b than healthy controls, whereas CD62L expression was significantly lower, suggesting exposure of neutrophils to activating agents within the bloodstream. Neutrophils from cirrhotic patients showed increased adhesion to both resting and tumor necrosis factor alpha-stimulated microvascular endothelial cells and decreased transendothelial migration. Granulocyte colony-stimulating factor (G-CSF) (100 ng/ml) significantly enhanced neutrophil adhesion to microvascular endothelial cells in healthy controls but not in cirrhotic patients. G-CSF also significantly improved neutrophil transmigration in cirrhotic patients and healthy controls. In conclusion, cirrhotic patients exhibit increased neutrophil adhesion to microvascular endothelium and deficient transendothelial migration. G-CSF enhances neutrophil transendothelial migration in cirrhotic patients despite having no effect on neutrophil adhesion. Therefore, G-CSF may be able to increase neutrophil recruitment into infected sites in these patients.
Bacterial infections are frequent, life-threatening complications in patients with liver cirrhosis. Cirrhotic patients with severe liver failure are more likely to become infected (5, 20, 36, 56). Potential risk factors include malnutrition, possible alcohol consumption, breakdown in normal skin and mucosal barrier function, small-bowel bacterial overgrowth with increased bacterial translocation, and multiple defects in immune defense (11, 31, 39, 47). Cirrhotic patients with advanced liver disease present abnormalities in both innate and adaptive immune responses. These abnormalities include impaired neutrophil, monocyte, and T-cell function, hyperreactivity of B cells associated with immunoglobulin G (IgG) and IgA hyperproduction, depression of the reticuloendothelial system, low concentrations of complement factors C3 and C4 in serum, decreased serum hemolytic complement function, and reduced serum opsonic activity (18, 21, 26, 27, 31, 40, 43).
Neutrophils are a vital component of the host defense strategy against bacterial infections, and their ability to migrate into infected tissues is essential to their function in host protection. This process requires the initial adherence of circulating neutrophils to microvascular endothelium, neutrophil migration to the endothelial intercellular junctions, diapedesis through the intercellular junction, and subsequent migration into tissues (45). Neutrophil adhesion and migration across vascular endothelium are also essential steps in neutrophil activation that prime and modulate neutrophil activity in the inflammatory focus. Neutrophils from patients with liver cirrhosis show deficient phagocytosis, decreased intracellular bacterial killing, impaired neutrophil metabolic activity, increased apoptosis, and marked reduction in chemotaxis (29, 35, 43). Studies showing increased neutrophil adherence to nylon wool also suggest a possible defect in neutrophil-endothelial-cell interactions (2).
We recently reported that bacterial infections in cirrhotic patients are associated with decreased neutrophil influx into skin windows and impaired phagocytic activity of elicited neutrophils (19). Thus, we hypothesized that patients with advanced liver disease may experience abnormal neutrophil-endothelial-cell interactions that compromise their functional activity in tissues. In the present study, we analyzed neutrophil adhesion to microvascular endothelial cells and neutrophil migration through microvascular endothelial cell monolayers and investigated the possible influence of granulocyte colony-stimulating factor (G-CSF) on these processes. We also studied the expression of the adhesion receptors CD11b (CR3, Mac-1) and CD62L (L-selectin) in blood neutrophils. Our results suggest that the neutrophils from cirrhotic patients with advanced liver disease demonstrate increased adhesion to microvascular endothelial cells with associated decreased transendothelial migration that is improved in the presence of G-CSF. Furthermore, blood neutrophils from cirrhotic patients express higher levels of CD11b receptor and lower levels of CD62L, suggesting that neutrophil intravascular activation may be in part responsible for the previous findings.
MATERIALS AND METHODS
Reagents.
Human recombinant tumor necrosis factor (TNF) was obtained from Boehringer Mannheim (Mannheim, Germany), human recombinant G-CSF (Granulokine) was from Roche (Barcelona, Spain), BCECF-AM [2′,7′-bis-(2-carboxyethyl)-5(and -6)-carboxyfluorescein, acetoxymethyl ester] was from Molecular Probes (Leiden, The Netherlands), murine epidermal growth factor and endothelial basal medium were from Clonetics (Santa Ana, Calif.), fetal calf serum (FCS) was from Biowhittaker (Verviers, Belgium), and Ficoll-Paque was from Pharmacia (Uppsala, Sweden). CellFIX, FACS lysing solution, phycoerythrin-conjugated monoclonal antibody (MAb) to CD11b, its isotopic IgG2a control, and Calibrite beads were purchased from Becton Dickinson (San Jose, Calif.), and Quantum Simply Cellular microbeads were from Flow Cytometry Standards (Leiden, The Netherlands). Fluorescein isothiocyanate-conjugated anti-L-selectin (CD62L) MAb (DREG 56) and its isotopic IgG1a control were obtained from Serotec (Kiddington, England). All other reagents were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Study subjects and design.
The study was performed at the Surgical Infections Unit and the Division of Hepatology at the HGU Gregorio Marañon (Madrid, Spain). It was approved by the HGU Gregorio Marañon review board, and written informed consent was obtained from all patients and healthy controls. Fourteen cirrhotic patients with advanced liver disease (Child-Pugh classes B and C) and fourteen gender-matched healthy volunteers were included in the study (Table 1). Although normal controls were younger than cirrhotic patients (40.92 ± 15.27 versus 56.42 ± 11.62 years), several studies have shown that neutrophil functions of healthy elderly subjects do not significantly differ from those of healthy younger people (6, 12). The diagnosis of liver cirrhosis was based on medical records and confirmed by laboratory tests and liver biopsy. Exclusion criteria were (i) clinical or laboratory evidence of infection within the preceding week, (ii) hepatocellular carcinoma, (iii) gastrointestinal bleeding within the preceding week, or (iv) blood transfusions within 10 days. No patient or healthy control was receiving any immunosuppressive medication. No patient was a consumer of alcohol at the time of the study. Although all blood samples were obtained when cirrhotic patients were stable, total leukocyte and neutrophil counts (means ± standard deviations [SD]) were significantly (P < 0.05) lower in cirrhotic patients (leukocytes, 3,458 ± 1,976; neutrophils, 1,467 ± 806) than in healthy controls (leukocytes, 6,716 ± 1,806; neutrophils, 3,636 ± 1,022).
TABLE 1.
Demographic and clinical data of patients with liver cirrhosis and healthy controlsa
| Patient | Age and sex | Etiology | Child-Pugh class | Admission diagnosis | Healthy control | Age and sex |
|---|---|---|---|---|---|---|
| 1 | 69, M | HCV | C | Encephalopathy | 1 | 33, M |
| 2 | 58, M | HCV | C | Encephalopathy | 2 | 58, M |
| 3 | 42, F | HCV | B | Subdural hemorrhage | 3 | 46, F |
| 4 | 58, F | HCV | C | Tense ascites | 4 | 37, F |
| 5 | 54, M | Alcohol | C | OLT evaluation | 5 | 29, M |
| 6 | 70, M | HBV | B | GIB | 6 | 72, M |
| 7 | 62, M | HVC + alcohol | C | Tense ascites | 7 | 26, M |
| 8 | 50, F | HCV | B | Fever | 8 | 63, F |
| 9 | 28, M | Budd-Chiary syndrome | B | OLT evaluation | 9 | 29, M |
| 10 | 56, M | HBV | B | Encephatopathy + UTI | 10 | 54, M |
| 11 | 71, F | HCV | C | Pneumonia + SBP | 11 | 30, F |
| 12 | 56, F | HBV | B | SBP | 12 | 42, F |
| 13 | 65, M | Alcohol | C | GIB | 13 | 27, M |
| 14 | 51, M | Alcohol | C | Bacteremia | 14 | 27, M |
OLT, orthotopic liver transplantation; GIB, gastrointestinal bleeding; SBP, spontaneous bacterial peritonitis; UTI, urinary tract infection; HBV, hepatitis B virus; HCV, hepatitis C virus.
CD62L and CD11b receptor expression in blood neutrophils.
Surface expression of CD11b and CD62L receptors was measured by direct immunostaining under basal conditions and after formylmethionyl-leucyl-phenylalanine (fMLP) stimulus (10−6 M; 15 min at 37°C) in a whole-blood assay to minimize CD11b upregulation and CD62L shedding by neutrophil isolation techniques (34, 59). Aliquots of 100 μl of heparinized blood were incubated with phycoerythrin-conjugated anti-CD11b MAb, fluorescein isothiocyanate-conjugated anti-CD62L MAb, or isotype controls for 30 min at 4°C in the dark. Erythrocytes were lysed with FACSLyse solution, and all samples were washed twice with phosphate-buffered saline (PBS) and fixed with CellFix solution. Two-color flow-cytometric analysis was performed in a FACS Vantage flow cytometer (Becton Dickinson) with Lysis II analysis software. Calibration of the cytometer and two-color compensation were performed daily with Calibrite beads. Neutrophil populations were identified with forward and right angle light scatter, and the fluorescence emission of 104 events/sample was recorded as mean channel number on a logarithmic scale. To calculate the number of receptors per cell, the mean channel number of each cell population was transformed into antibody binding sites per cell with Quantum Simply Cellular microbeads as previously described (15, 49). The number of antibody binding sites corresponding to the isotopic immunoglobulin was subtracted from each cell sample.
Isolation and labeling of human neutrophils.
Blood samples from a cirrhotic patient and a match control were drawn through a 22G butterfly-type catheter into Vacutainer sterile tubes with lithium heparin (15 U/ml, final concentration) and processed immediately. Neutrophils were isolated by dextran sedimentation, gradient centrifugation, and hypotonic lysis as previously described (24). Neutrophils were washed in Hanks balanced salt solution (HBSS) without calcium and magnesium, resuspended at 107 cells/ml, and labeled with the fluorescent dye BCECF-AM (2 M; 15 min in the dark at 37°C). Neutrophils were washed twice in RPMI containing 10% FCS, counted, and resuspended at a density of 106/ml in RPMI-10% FCS without phenol red. Neutrophil suspensions were 95% pure and 98% viable by trypan blue exclusion. Where indicated, G-CSF (100 ng/ml) was added at the beginning of each assay and was present throughout.
Endothelial cell culture.
The human microvascular endothelial cell line HMEC-1 was obtained from the Biological Products Branch of the Centers for Disease Control and Prevention (Atlanta, Ga.). HMEC-1 is a transformed microvascular endothelial cell line that retains the morphological phenotype and functional characteristics of human microvascular endothelial cells (1, 7). HMEC-1 cells were grown and maintained in endothelial basal medium supplemented with 10 ng of epidermal growth factor per ml, 1 μg of hydrocortisone per ml, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 250 ng of amphotericin per ml, and 15% FCS in gelatin-coated flasks. Cell suspensions were obtained by proteolysis with 0.25% trypsin-EDTA once cell confluence was reached.
Neutrophil adhesion assay.
HMEC-1 cells were seeded into gelatin-coated 96-well microtiter plates (Costar Europe Ltd, Badhoevedorf, The Netherlands) and cultured for 48 h (37°C and 5% CO2). Confluent HMEC-1 cells were incubated with TNF-α (100 IU/ml) or control medium for 4 h. Wells were then washed twice with warm PBS, and 100 μl of the BCECF-labeled neutrophil suspension (105 neutrophils/well) with or without G-CSF (100 ng/ml) was added to each well and incubated for 1 h. Nonadherent neutrophils were removed by gentle washing and inversion (five times). The contents of each well were lysed with 10 nM Tris plus 0.1% sodium dodecyl sulfate, and fluorescence was recorded at 485/22 nm (excitation) and 530/25 nm (emission) in a microtiter plate fluorimeter (CytoFluor 2300; Millipore, Cambridge, Mass.). Final quantification (number of adherent neutrophils per well) was accomplished by linear regression analysis against wells containing serial dilutions of BCECF-labeled neutrophils (4 × 102 to 4 × 105/well). Each value is the mean for five wells.
Neutrophil transendothelial migration assay.
Transwell polycarbonate inserts (6.5-mm diameter) with 3-μm-pore-size membrane (Costar, Cambridge, Mass.) were coated with 1% gelatin, seeded with HMEC-1 cells (2 × 105 cells/well), and cultured for 48 h (37°C and 5% CO2). The confluence of the endothelial cell monolayers was verified by testing their permeability to bovine serum albumin as previously described (46) with minor modifications. In brief, trypan blue (36 mg) and bovine serum albumin (800 mg) were dissolved in 100 ml of HBSS and incubated at 37°C for 10 min to yield a stable complex (trypan blue was >99.8 protein bound) with maximum absorption at 590 nm. Transwell inserts were washed with warm HBBS and transferred to 24-well plates containing 1 ml of HBSS. Trypan blue complex (0.5 ml) was added to the inserts and incubated for 5 min at 37°C in 5% CO2. Albumin diffusion was quantified by measuring the absorbance at 590 nm of the lower-well fluid. Inserts were considered confluent if the absorbance in the lower wells was less than 1% of the absorbance of the albumin-trypan blue complex. Confluent inserts were washed twice with warm medium and incubated with TNF-α (100 U/ml) or control medium for 4 h. Inserts were rinsed twice with warm PBS and placed in 24-well plates containing RPMI-10% FCS with or without the chemoattractant fMLP 10−8 M. Neutrophils (2 × 105) in RPMI-10% FCS with or without G-CSF (100 ng/ml) were added to the top chambers and allowed to migrate to the lower wells for 2 h. Migrated neutrophils were then fixed, collected from the lower wells, and counted with a hemacytometer. Results are expressed as the number of transmigrating neutrophils/well, and each data point is the mean for three wells.
Statistical analysis.
All results are given as means ± SD and were analyzed with SigmaStat 2 0.0 software (SPSS, Chicago, Ill.). The Kolmogorov-Smirnov test was used to test data for a normal distribution. Differences between normal distributions were analyzed with a t test. The nonparametric Mann-Whitney rank sum test was used for nonnormal distributions. Paired t tests were used to assess the effect of G-CSF on neutrophil adhesion and transmigration. A P value of <0.05 for two-tailed comparisons was considered significant.
RESULTS
CD11b and CD62L expression in blood neutrophils of cirrhotic patients reveals a pattern of neutrophil intravascular activation.
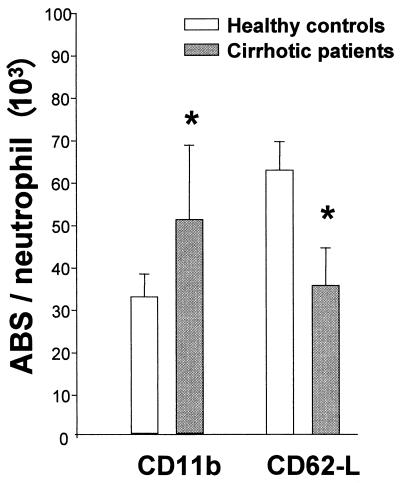
Flow cytometry analysis of the neutrophil adhesion receptors CD11b and CD62L in cirrhotic patients showed a pattern of neutrophil activation that suggests the exposure of neutrophils to activating agents within the blood. Cirrhotic patients expressed significantly higher levels of CD11b receptor on their cell membranes under basal conditions than healthy controls ([50.63 ± 17.84] × 103 versus [32.42 ± 5.34] × 103 receptors/cell; P < 0.01). In contrast, CD62L expression was significantly lower in cirrhotic patients ([35.39 ± 8.1] × 103 receptors/cell) than in healthy controls ([62.59 ± 6.65] × 103 receptors/cell; P < 0.01) (Fig. 1). CD11b shows a rapid and marked increase within minutes of neutrophil stimulation by proinflammatory mediators and is a sensitive marker of neutrophil activation (54). In contrast, activation of neutrophils with cytokines or exposure to cytokine-activated endothelium induces CD62L shedding. Whole-blood fMLP stimulation resulted in the complete loss of CD62L and upregulation of CD11b in both cirrhotic patients and healthy controls without a significant difference (data not shown).
FIG. 1.
Flow cytometry analysis of the expression of adhesion molecules CD11b and CD62L in blood neutrophils. Neutrophils from cirrhotic patients expressed significantly higher levels of CD11b receptor than neutrophils from healthy controls while expressing lower levels of CD62L (∗, P < 0.01). Data are means ± SD. ABS, antibody binding sites.
Neutrophil adhesion to microvascular endothelial cells is increased in cirrhotic patients and is not modified by G-CSF.
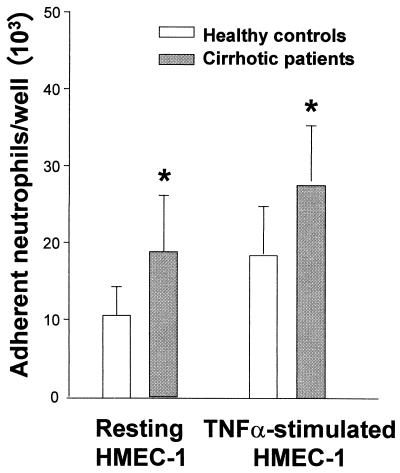
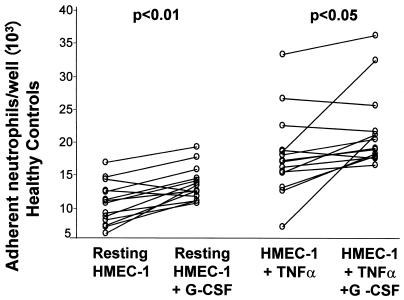
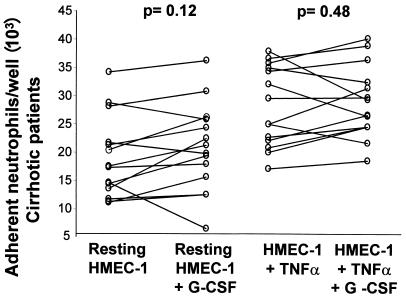
Neutrophil adhesion to monolayers of HMEC-1 was significantly higher in cirrhotic patients than in healthy controls under basal conditions ([18.97 ± 7.21] × 103 versus [10.83 ± 3.17] × 103 adherent neutrophils/well; P < 0.01) and after TNF stimulation ([27.79 ± 16.36] × 103 versus [18.66 ± 6.19] × 103 adherent neutrophils/well; P < 0.01) (Fig. 2). Addition of G-CSF to the assay medium significantly increased adhesion of neutrophils from healthy controls to both resting HMEC-1 cells ([10.83 ± 3.17] × 103 versus [13.78 ± 2.52] × 103 neutrophils/well; P < 0.01) and TNF-activated HMEC-1 cells ([18.6 ± 6.19] ×103 versus [22.25 ± 5.7] × 103 neutrophils/well; P < 0.05) (Fig. 3). In contrast, G-CSF had no significant effect on neutrophil adhesion to either resting or TNF-stimulated HMEC-1 cells in cirrhotic patients (Fig. 4).
FIG. 2.
Neutrophil adhesion to resting and TNF-α-stimulated HMEC-1 monolayers (100 IU/ml) in healthy controls and cirrhotic patients after 4 h of incubation (∗, P < 0.01). Data are means ± SD.
FIG. 3.
Effect of G-CSF on the number of neutrophils adhering to resting and TNF-α-stimulated HMEC-1 monolayers (100 IU/ml) in healthy controls. G-CSF significantly increased neutrophil adhesion to both resting (P < 0.01) and TNF-stimulated (P < 0.05) HMEC-1 monolayers after 4 h of incubation in healthy controls. Each data point is the mean ± SD for five wells, and P values represent differences between groups by paired t tests.
FIG. 4.
G-CSF failed to increase neutrophil adhesion to resting (P < 0.12) or TNF-α-stimulated (P < 0.48) HMEC-1 monolayers after 4 h of incubation in cirrhotic patients. Each data point is the mean for five wells, and P values represent differences between groups by paired t tests.
Neutrophils from cirrhotic patients exhibit decreased transendothelial migration that is improved in the presence of G-CSF.
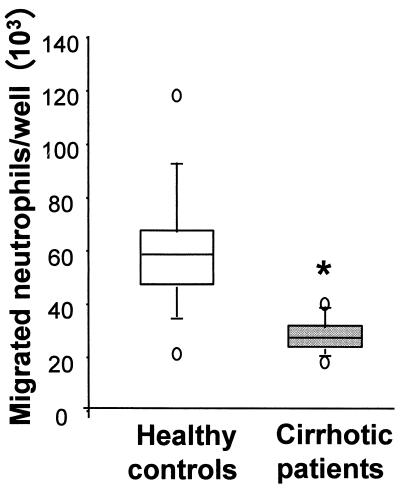
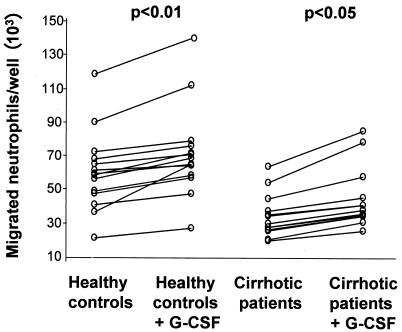
Neutrophil migration across TNF-stimulated HMEC-1 cells in response to an fMLP gradient was significantly reduced in cirrhotic patients ([36.49 ± 15.58] × 103 migrating neutrophils/well) compared with healthy controls ([60.21 ± 23.26] × 103 neutrophils/well; P < 0.01) (Fig. 5). No difference was found in neutrophil migration across resting or TNF-activated endothelium in absence of chemotactic gradients between the two groups (data not shown). G-CSF addition to the upper chamber of the Transwell system significantly enhanced neutrophil transmigration both in healthy controls (121%; P < 0.01) and in cirrhotic patients (118%; P < 0.05) (Fig. 6).
FIG. 5.
Quantification of neutrophil transendothelial migration in cirrhotic patients and healthy controls. Neutrophil migration across TNF-stimulated HMEC-1 monolayers following fMLP gradients was significantly reduced in cirrhotic patients compared to healthy controls (∗, P < 0.01 for comparison between groups by the Mann-Whitney test). Data are presented as box plots: the extents of the boxes indicate the 25th and the 75th percentiles, and the lines inside the boxes mark the 50th percentile (median) values. Capped bars indicate the 10th and 90th percentiles, and open circles mark the 5th and 95th percentiles.
FIG. 6.
Effect of G-CSF on neutrophil transendothelial migration. Addition of G-CSF (100 ng/ml) to the upper well of the transmigration system results in significantly higher migration of neutrophils across monolayers of TNF-α-stimulated HMEC-1 cells following a fMLP gradient in both healthy controls and cirrhotic patients after 2 h of incubation. Each data point is the mean of three wells, and P values represent differences between groups by paired t tests.
DISCUSSION
Various parameters of neutrophil function have been reported to be abnormal in patients with liver cirrhosis. In the present study, we utilized an in vitro model to test the hypothesis that abnormal interactions between neutrophils and microvascular endothelial cells might play a role in the pathogenesis of the deficient immune-inflammatory response that cirrhotic patients present in vivo. Our findings indicate that neutrophils from cirrhotic patients with advanced liver disease adhere significantly more to both resting and TNF-stimulated microvascular endothelial cells and migrate less across monolayers of TNF-stimulated microvascular endothelial cells following a chemotactic gradient than neutrophils from healthy controls. Flow cytometry analysis of neutrophils from whole blood showed significantly higher levels of CD11b and lower levels of CD62L in cirrhotic patients than in the control population. These findings suggest a systemic activation of neutrophils in cirrhotic patients. It is well known that the liver is a major site for the clearance of circulating cytokines and endotoxin (3, 38). Patients with liver cirrhosis present high levels of several cytokines (TNF-α, interleukin 1, interleukin 6, and gamma interferon), as well as their soluble receptors, in the blood (52). Furthermore, high levels of endotoxin without evidence of bacterial infection have been repeatedly found in the blood and ascitic fluid of cirrhotic patients (30). This most likely occurs as a consequence of increased endotoxin absorption from the intestinal lumen and impaired hepatic clearance as liver function deteriorates (33). Exposure of neutrophils to activating agents within the bloodstream might activate them in the vascular compartment and preclude further physiological functions. The higher expression of CD11b in neutrophils from cirrhotic patients could possibly account for the increased adherence to microvascular endothelial cells observed in these patients. CD11b receptor, a β2-integrin, mediates neutrophil firm adhesion to cytokine-activated endothelium and is also involved in neutrophil aggregation, cytotoxicity, and neutrophil-mediated tissue injury (17, 54).
We also observed in cirrhotic patients a significant depression of migration across TNF-stimulated microvascular endothelial cell monolayers in response to an fMLP chemotactic gradient. Interestingly, in vitro studies showed that aged neutrophils expressing low levels of CD62L have decreased ability to migrate towards an fMLP gradient and impaired deformability after fMLP stimulation (51). Thus, decreased levels of L-selectin in neutrophils of cirrhotic patients could be associated with an impaired ability to migrate across endothelial cell monolayers as we found in our model.
Our data demonstrate, in contrast to earlier adhesion studies using human umbilical endothelial cells (58), that G-CSF increases the adhesion of neutrophils from healthy subjects to both resting and TNF-stimulated microvascular endothelial cells. Differences in phenotype and patterns of expression of adhesion molecules between macro- and microvascular endothelial cells could possibly explain this finding (23, 50). Our data are also consistent with the evidence that G-CSF upregulates expression of neutrophil adhesion molecules in mature neutrophils in vitro (10). Furthermore, intravenous administration of G-CSF to healthy controls resulted in a rapid decline in the absolute neutrophil count that is most probably due to a transient increase in endothelial binding in vivo (16). It is interesting that in our model the addition of G-CSF to neutrophils from cirrhotic patients did not significantly increase their already high adhesion to HMEC-1 cells. We cannot determine whether this is a consequence of the anti-inflammatory of effect of G-CSF as observed in septic neutropenic patients (28) or a defective neutrophil response.
Our data also demonstrate that C-GSF increases neutrophil migration across TNF-α-stimulated HMEC-1 monolayers following a chemotactic gradient in both healthy controls and cirrhotic patients. This effect is most likely mediated by G-CSF interaction with neutrophils, as G-CSF fails to enhance the secretion of proinflammatory cytokines such as TNF-α and granulocyte-macrophage CSF or upregulate the expression of adhesion molecules in endothelial cells (9, 57). Cirrhotic patients with advanced liver disease and portal hypertension frequently present with neutropenia. Although several mechanisms are implicated in the pathogenesis of this neutropenia, neutrophils from cirrhotic patients clearly have a shorter life span with accelerated apoptosis in vitro (29). G-CSF decreases apoptosis in neutrophils while enhancing motility and chemotaxis at low concentrations (13, 37), thereby increasing neutrophil ability to migrate across vascular endothelium. G-CSF-treated neutrophils also display higher levels of interleukin 8 receptor, which might promote chemotaxis and increased neutrophil recruitment into sites of infection during G-CSF treatment (32). Interestingly, administration of human recombinant G-CSF to healthy controls causes decreased influx of neutrophils to skin windows, suggesting that the role of G-CSF in cell recruitment in infected and healthy individuals may be different (42).
A major concern about G-CSF therapy is its potential role for producing tissue injury (22). In our model, addition of G-CSF failed to increased neutrophil adhesion in cirrhotic patients to resting and TNF-activated microvascular endothelium. These data suggest that G-CSF does not increase neutrophil-adherent mediated endothelial injury in cirrhotic patients. G-CSF has already been used in several clinical trials in combination with gamma interferon as an antiviral drug in the treatment of cirrhotic patients with hepatitis C virus infection. In these patients, G-CSF increased the mean white count and was well tolerated, with minor side effects such as headache, anorexia, and bone pain (41, 53).
Further investigation is needed to determinate a possible beneficial effect of G-CSF administration to cirrhotic patients to improve their host defense mechanism against bacterial infections. Although G-CSF is routinely used to accelerate the recovery of neutrophil counts after chemotherapy and as adjuvant therapy in severe infections in neutropenic patients (4, 8), its use in nonneutropenic septic patients or as a prophylactic agent against infection remains controversial (14, 25, 44, 48, 55). Our findings suggest the attractive hypothesis that G-CSF could increase neutrophil recruitment to the infective focus in cirrhotic patients and become a possible adjuvant nonantibiotic therapy in prophylaxis and treatment of severe bacterial infections in cirrhotic patients with advanced liver disease.
Acknowledgments
This study was supported in part by the Postgraduate Fellowship grants BAE 96/5240 and 97/5406 (Ministry of Health, FIS, Madrid, Spain).
We thank Edwin W. Ades (Chief, Biological Products Branch, CCID, CDC, Atlanta) for providing the cellular line HMEC-1. We also acknowledge the help of Francisco Sanchez-Madrid, M. A. del Pozo, and Carmen Dominguez (Department of Immunology, Hospital La Princesa, Madrid, Spain) in establishing the neutrophil transendothelial migration assay.
REFERENCES
- 1.Ades, E. W., F. J. Candal, R. A. Swerlick, V. G. George, S. Summers, D. C. Bosse, and T. J. Lawley. 1992. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J. Invest. Dermatol. 99:683-690. [DOI] [PubMed] [Google Scholar]
- 2.Altin, M., I. A. Rajkovic, R. D. Hughes, and R. Williams. 1983. Neutrophil adherence in chronic liver disease and fulminant hepatic failure. Gut 24:746-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andus, T., J. Bauer, and W. Gerok. 1991. Effects of cytokines on the liver. Hepatology 13:364-375. [PubMed] [Google Scholar]
- 4.Azzara, A., and G. Carulli. 1997. Morphological features following G-CSF treatment. Haematologica 82:504-505. [PubMed] [Google Scholar]
- 5.Barnes, P. F., C. Arevalo, L. S. Chan, S. F. Wong, and T. B. Reynolds. 1988. A prospective evaluation of bacteremic patients with chronic liver disease. Hepatology 8:1099-1103. [DOI] [PubMed] [Google Scholar]
- 6.Biasi, D., A. Carletto, C. Dell'Agnola, P. Caramaschi, F. Montesanti, G. Zavateri, S. Zeminian, P. Bellavite, and L. M. Bambara. 1996. Neutrophil migration, oxidative metabolism, and adhesion in elderly and young subjects. Inflammation 20:673-681. [DOI] [PubMed] [Google Scholar]
- 7.Bosse, D., V. George, F. J. Candal, T. J. Lawley, and E. W. Ades. 1993. Antigen presentation by a continuous human microvascular endothelial cell line, HMEC-1, to human T cells. Pathobiology 61:236-238. [DOI] [PubMed] [Google Scholar]
- 8.Brugger, W., S. Heimfeld, R. J. Berenson, R. Mertelsmann, and L. Kanz. 1995. Reconstitution of hematopoiesis after high-dose chemotherapy by autologous progenitor cells generated ex vivo. N. Engl. J. Med. 333:283-287. [DOI] [PubMed] [Google Scholar]
- 9.Bussolino, F., M. Ziche, J. M. Wang, D. Alessi, L. Morbidelli, O. Cremona, A. Bosia, P. C. Marchisio, and A. Mantovani. 1991. In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J. Clin. Invest. 87:986-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carulli, G. 1997. Effects of recombinant human granulocyte colony-stimulating factor administration on neutrophil phenotype and functions. Haematologica 82:606-616. [PubMed] [Google Scholar]
- 11.Casafont, F., E. Sanchez, L. Martin, J. Aguero, and F. P. Romero. 1997. Influence of malnutrition on the prevalence of bacterial translocation and spontaneous bacterial peritonitis in experimental cirrhosis in rats. Hepatology 25:1334-1337. [DOI] [PubMed] [Google Scholar]
- 12.Corberand, J. X., P. F. Laharrague, and G. Fillola. 1986. Neutrophils of healthy aged humans are normal. Mech. Ageing Dev. 36:57-63. [DOI] [PubMed] [Google Scholar]
- 13.Dale, D. C., W. C. Liles, W. R. Summer, and S. Nelson. 1995. Granulocyte colony-stimulating factor--role and relationships in infectious diseases. J. Infect. Dis. 172:1061-1075. [DOI] [PubMed] [Google Scholar]
- 14.Davis, K. A., T. C. Fabian, D. N. Ragsdale, L. L. Trenthem, M. A. Croce, and K. G. Proctor. 1999. Granulocyte colony-stimulating factor and neutrophil-related changes in local host defense during recovery from shock and intra-abdominal sepsis. Surgery 126:305-313. [PubMed] [Google Scholar]
- 15.Dawson, C. D., J. A. Scheppler, J. K. Nicholson, and R. C. Holman. 1991. Enumeration of antigen sites on cells by flow cytometry. Pathobiology 59:57-61. [DOI] [PubMed] [Google Scholar]
- 16.de Haas, M., J. M. Kerst, C. E. van der Schoot, J. Calafat, C. E. Hack, J. H. Nuijens, D. Roos, R. H. van Oers, and A. E. von dem Borne. 1994. Granulocyte colony-stimulating factor administration to healthy volunteers: analysis of the immediate activating effects on circulating neutrophils. Blood 84:3885-3894. [PubMed] [Google Scholar]
- 17.de la Ossa, J. C., M. Malago, and B. L. Gewertz. 1992. Neutrophil-endothelial cell binding in neutrophil-mediated tissue injury. J. Surg. Res. 53:103-107. [DOI] [PubMed] [Google Scholar]
- 18.Feliu, E., M. A. Gougerot, J. Hakim, E. Cramer, C. Auclair, B. Rueff, and P. Boivin. 1977. Blood polymorphonuclear dysfunction in patients with alcoholic cirrhosis. Eur. J. Clin. Invest. 7:571-577. [DOI] [PubMed] [Google Scholar]
- 19.Fiuza, C., M. Salcedo, G. Clemente, and J. M. Tellado. 2000. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J. Infect. Dis. 182:526-533. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert, J. A., and P. S. Kamath. 1995. Spontaneous bacterial peritonitis: an update. Mayo Clin. Proc. 70:365-370. [DOI] [PubMed] [Google Scholar]
- 21.Gomez, F., P. Ruiz, and A. D. Schreiber. 1994. Impaired function of macrophage Fc gamma receptors and bacterial infection in alcoholic cirrhosis. N. Engl. J. Med. 331:1122-1128. [DOI] [PubMed] [Google Scholar]
- 22.Gross-Weege, W., M. Weiss, M. Schneider, M. Wenning, B. Harms, K. Dumon, C. Ohmann, and H. D. Roher. 1997. Safety of a low-dosage Filgrastim (rhG-CSF) treatment in non-neutropenic surgical intensive care patients with an inflammatory process. Intensive Care Med. 23:16-22. [DOI] [PubMed] [Google Scholar]
- 23.Haraldsen, G., D. Kvale, B. Lien, I. N. Farstad, and P. Brandtzaeg. 1996. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J. Immunol. 156:2558-2565. [PubMed] [Google Scholar]
- 24.Haslett, C., L. A. Guthrie, M. M. Kopaniak, R. B. Johnston, Jr., and P. M. Henson. 1985. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am. J. Pathol. 119:101-110. [PMC free article] [PubMed] [Google Scholar]
- 25.Held, T. K., and A. S. Cross. 1999. Role of hematopoietic growth factors in non-neutropenic infections and sepsis. Curr. Opin. Hematol. 6:176-183. [DOI] [PubMed] [Google Scholar]
- 26.Homann, C., K. Varming, K. Hogasen, T. E. Mollnes, N. Graudal, A. C. Thomsen, and P. Garred. 1997. Acquired C3 deficiency in patients with alcoholic cirrhosis predisposes to infection and increased mortality. Gut 40:544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu, C. C., and C. M. Leevy. 1971. Inhibition of PHA-stimulated lymphocyte transformation by plasma from patients with advanced alcoholic cirrhosis. Clin. Exp. Immunol. 8:749-760. [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa, K., H. Tanaka, T. Matsuoka, T. Shimazu, T. Yoshioka, and H. Sugimoto. 1998. Recombinant human granulocyte colony-stimulating factor attenuates inflammatory responses in septic patients with neutropenia. J. Trauma 44:1047-1054. [DOI] [PubMed] [Google Scholar]
- 29.Kusaba, N., R. Kumashiro, H. Ogata, M. Sata, and K. Tanikawa. 1998. In vitro study of neutrophil apoptosis in liver cirrhosis. Intern. Med. 37:11-17. [DOI] [PubMed] [Google Scholar]
- 30.Laffi, G., M. Foschi, E. Masini, A. Simoni, L. Mugnai, G. La Villa, G. Barletta, P. F. Mannaioni, and P. Gentilini. 1995. Increased production of nitric oxide by neutrophils and monocytes from cirrhotic patients with ascites and hyperdynamic circulation. Hepatology 22:1666-1673. [PubMed] [Google Scholar]
- 31.Lahnborg, G., L. Friman, and L. Berghem. 1981. Reticuloendothelial function in patients with alcoholic liver cirrhosis. Scand. J. Gastroenterol. 16:481-489. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd, A. R., A. Biragyn, J. A. Johnston, D. D. Taub, L. Xu, D. Michiel, H. Sprenger, J. J. Oppenheim, and D. J. Kelvin. 1995. Granulocyte-colony stimulating factor and lipopolysaccharide regulate the expression of interleukin 8 receptors on polymorphonuclear leukocytes. J. Biol. Chem. 270:28188-28192. [DOI] [PubMed] [Google Scholar]
- 33.Lumsden, A. B., J. M. Henderson, and M. H. Kutner. 1988. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology 8:232-236. [DOI] [PubMed] [Google Scholar]
- 34.Macey, M. G., D. A. McCarthy, S. Vordermeier, A. C. Newland, and K. A. Brown. 1995. Effects of cell purification methods on CD11b and L-selectin expression as well as the adherence and activation of leucocytes. J. Immunol. Methods 181:211-219. [DOI] [PubMed] [Google Scholar]
- 35.MacGregor, R. R. 1990. In vivo neutrophil delivery in men with alcoholic cirrhosis is normal despite depressed in vitro chemotaxis. Alcohol Clin. Exp. Res. 14:195-199. [DOI] [PubMed] [Google Scholar]
- 36.Navasa, M., A. Rimola, and J. Rodes. 1997. Bacterial infections in liver disease. Semin. Liver Dis. 17:323-333. [DOI] [PubMed] [Google Scholar]
- 37.Nelson, S., and G. J. Bagby. 1996. Granulocyte colony-stimulating factor and modulation of inflammatory cells in sepsis. Clin. Chest Med. 17:319-332. [DOI] [PubMed] [Google Scholar]
- 38.Nolan, J. P. 1981. Endotoxin, reticuloendothelial function, and liver injury. Hepatology 1:458-465. [DOI] [PubMed] [Google Scholar]
- 39.Norman, D. A., J. M. Atkins, L. L. Seelig, Jr., C. Gomez-Sanchez, and G. J. Krejs. 1980. Water and electrolyte movement and mucosal morphology in the jejunum of patients with portal hypertension. Gastroenterology 79:707-715. [PubMed] [Google Scholar]
- 40.Nouri-Aria, K. T., G. J. Alexander, B. C. Portmann, J. E. Hegarty, A. L. Eddleston, and R. Williams. 1986. T and B cell function in alcoholic liver disease. J. Hepatol. 2:195-207. [DOI] [PubMed] [Google Scholar]
- 41.Pardo, M., I. Castillo, S. Navas, and V. Carreno. 1995. Treatment of chronic hepatitis C with cirrhosis with recombinant human granulocyte colony-stimulating factor plus recombinant interferon-alpha. J. Med. Virol. 45:439-444. [DOI] [PubMed] [Google Scholar]
- 42.Price, T. H., G. S. Chatta, and D. C. Dale. 1996. Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. Blood 88:335-340. [PubMed] [Google Scholar]
- 43.Rajkovic, I. A., and R. Williams. 1986. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology 6:252-262. [DOI] [PubMed] [Google Scholar]
- 44.Root, R. K., and D. C. Dale. 1999. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor: comparisons and potential for use in the treatment of infections in nonneutropenic patients. J. Infect. Dis. 179(Suppl. 2):S342-S352. [DOI] [PubMed] [Google Scholar]
- 45.Rossi, A., and P. Hellewell. 1994. Mechanisms of neutrophil accumulation in tissues, p. 223-243. In P. Hellewell and T. Williams (ed.), Immunopharmacology of neutrophils. Academic Press Ltd., London, United Kingdom.
- 46.Rotrosen, D., and J. I. Gallin. 1986. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J. Cell Biol. 103:2379-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Runyon, B. A., S. Squier, and M. Borzio. 1994. Translocation of gut bacteria in rats with cirrhosis to mesenteric lymph nodes partially explains the pathogenesis of spontaneous bacterial peritonitis. J. Hepatol. 21:792-796. [DOI] [PubMed] [Google Scholar]
- 48.Schafer, H., K. Hubel, H. Bohlen, G. Mansmann, K. Hegener, B. Richarz, F. Oberhauser, G. Wassmer, A. H. Holscher, H. Pichlmaier, V. Diehl, and A. Engert. 2000. Perioperative treatment with filgrastim stimulates granulocyte function and reduces infectious complications after esophagectomy. Ann. Hematol. 79:143-151. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz, A., E. Fernandez Repollet, R. Vogt, and J. W. Gratama. 1996. Standardizing flow cytometry: construction of a standardized fluorescence calibration plot using matching spectral calibrators. Cytometry 26:22-31. [DOI] [PubMed] [Google Scholar]
- 50.Swerlick, R. A., K. H. Lee, L. J. Li, N. T. Sepp, S. W. Caughman, and T. J. Lawley. 1992. Regulation of vascular cell adhesion molecule 1 on human dermal microvascular endothelial cells. J. Immunol. 149:698-705. [PubMed] [Google Scholar]
- 51.Tanji-Matsuba, K., S. F. van Eeden, Y. Saito, M. Okazawa, M. E. Klut, S. Hayashi, and J. C. Hogg. 1998. Functional changes in aging polymorphonuclear leukocytes. Circulation 97:91-98. [DOI] [PubMed] [Google Scholar]
- 52.Tilg, H., W. Vogel, C. J. Wiedermann, L. Shapiro, M. Herold, G. Judmaier, and C. A. Dinarello. 1993. Circulating interleukin-1 and tumor necrosis factor antagonists in liver disease. Hepatology 18:1132-1138. [PubMed] [Google Scholar]
- 53.Van Thiel, D. H., H. Faruki, L. Friedlander, S. Fagiuoli, P. Caraceni, P. J. Molloy, R. J. Kania, and H. I. Wright. 1995. Combination treatment of advanced HCV associated liver disease with interferon and G-CSF. Hepatogastroenterology 42:907-912. [PubMed] [Google Scholar]
- 54.Wardlaw, A., and G. Walsh. 1994. Neutrophil adhesion receptors, p. 133-148. In P. G. Hellewell, and T. J. Williams (ed.), Immunopharmacology of neutrophils. Academic Press Ltd., London, United Kingdom.
- 55.Winston, D. J., P. F. Foster, K. A. Somberg, R. W. Busuttil, M. F. Levy, P. A. Sheiner, K. R. Reddy, N. Fotheringham, M. Armstrong, and E. Logan. 1999. Randomized, placebo-controlled, double-blind, multicenter trial of efficacy and safety of granulocyte colony-stimulating factor in liver transplant recipients. Transplantation 68:1298-1304. [DOI] [PubMed] [Google Scholar]
- 56.Wyke, R. J. 1989. Bacterial infections complicating liver disease. Baillieres Clin. Gastroenterol. 3:187-210. [DOI] [PubMed] [Google Scholar]
- 57.Xu, S., M. Hoglund, L. Hakansson, and P. Venge. 2000. Granulocyte colony-stimulating factor (G-CSF) induces the production of cytokines in vivo. Br. J. Haematol. 108:848-853. [DOI] [PubMed] [Google Scholar]
- 58.Yong, K. L. 1996. Granulocyte colony-stimulating factor (G-CSF) increases neutrophil migration across vascular endothelium independent of an effect on adhesion: comparison with granulocyte-macrophage colony-stimulating factor (GM-CSF). Br. J. Haematol. 94:40-47. [DOI] [PubMed] [Google Scholar]
- 59.Youssef, P. P., B. X. Mantzioris, P. J. Roberts-Thomson, M. J. Ahern, and M. D. Smith. 1995. Effects of ex vivo manipulation on the expression of cell adhesion molecules on neutrophils. J. Immunol. Methods 186:217-224. [DOI] [PubMed] [Google Scholar]