Abstract
Leptospirosis is a common zoonosis seen worldwide, but it is rare in our locality (Hong Kong). Clinical manifestations of leptospirosis are variable and may range from subclinical infection to fever, jaundice, hemorrhagic tendency, and fulminant hepato-renal failure. Severe hyperbilirubinemia and acute renal failure have been associated with high mortality. We report our experience with a patient who developed severe Weil's syndrome with marked conjugated hyperbilirubinemia and oliguric acute renal failure. These complications persisted despite treatment with penicillin and hemodiafiltration. Plasma exchange was instituted in view of the severe hyperbilirubinemia (970 μmol/liter). This was followed by prompt clinical improvement, with recovery of liver and renal function. The beneficial effects of plasma exchange could be attributed to amelioration of the toxic effects of hyperbilirubinemia on hepatocyte and renal tubular cell function. We conclude that plasma exchange should be considered as an adjunctive therapy for patients with severe icteric leptospirosis complicated by acute renal failure who have not shown rapid clinical response to conventional treatment.
Leptospirosis is a common zoonosis seen worldwide which may occur as seasonal outbreaks in areas of endemicity, but it is a rare disease in our locality (Hong Kong), and only sporadic cases are seen. It is caused by a spirochete, Leptospira sp., that infects mammals, in particular, rodents. Animals excrete infected urine and remain infectious for years. Humans may contract the disease through contact with infected urine. Subclinical infection is common, and evidence of infection can only be detected by a positive serological response. On the other hand, fulminant infection may occur, resulting in septic shock and severe multiorgan failure (4). In particular, Weil's syndrome is a severe form of leptospirosis characterized by fever, jaundice, hemorrhagic tendency, and hepato-renal failure (1). Conjugated hyperbilirubinemia with variable derangement of liver function is common in most cases of leptospirosis, whereas patterns of renal involvement include interstitial nephritis, acute renal failure, and tubular dysfunction (18). Severe jaundice and oliguric renal failure are important prognostic markers which are associated with a high mortality (7).
Apart from appropriate antibiotic therapy for leptospirosis, supportive measures for multiorgan failure are also important. Optimal management of acute renal failure usually requires intensive hemodialysis pending spontaneous recovery of renal function. It has been suggested that plasma exchange may confer beneficial effects through the removal of bilirubin (10). We report the case of a patient with severe hyperbilirubinemia and acute renal failure complicating leptospirosis. In addition to penicillin and continuous venovenous hemodiafiltration, this patient was treated with plasma exchange and his condition showed prompt improvement afterwards, with resolution of hyperbilirubinemia and renal dysfunction.
A 39-year-old driver with good past health except for asymptomatic HBsAg seropositivity presented with fever and bilateral calf pain and swelling. Physical examination showed a febrile gentleman with jaundice, conjunctival suffusion, and bilateral lower limb superficial abrasions. There was no lymphadenopathy. The liver and the spleen were not palpable. His blood pressure was 120/80 mm Hg. Initial investigations showed thrombocytopenia (platelet count, 35 × 109/liter), a white cell count of 8.7 × 109/liter (neutrophils, 89.5%; lymphocytes, 4.7%; monocytes, 5.7%; eosinophils, 0.1%), a hemoglobin level of 13.9 g/dl, and a serum creatinine level of 91 μmol/liter (normal range, 75 to 110 μmol/liter), while that of serum bilirubin was 22 μmol/liter (normal range, 5 to 20 μmol/liter). The patient's general condition deteriorated rapidly with spiking fever and hypotension. His platelet count decreased to 14 × 109/liter, the hemoglobin level was 10.0 g/dl, and the serum creatinine was raised to 251 μmol/liter. Urine microscopy showed the absence of red blood cells or casts. Serum bilirubin increased to 86 μmol/liter and the alanine transaminase level was elevated to 96 U/liter (normal range, 10 to 57 U/liter). Ultrasonogram of the hepatobiliary system and kidneys showed normal findings. Chest radiograph showed diffuse bilateral pulmonary infiltrates. Antineutrophil cytoplasmic antibody and antiglomerular basement membrane antibody were not detected. Immunoglobulin M anti-hepatitis A virus and anti-hepatitis C virus were negative while HBeAg was found to be positive. Peripheral blood smear demonstrated no schistocytes. Sepsis workup including blood, urine, sputum, and stool cultures were negative. Intravenous levofloxacin was empirically administered without obvious clinical improvement. The patient developed septic shock and oliguric acute renal failure. Serological tests for infection by hantavirus, dengue virus, and members of the genera Mycoplasma, Chlamydia, Salmonella, Legionella, Rickettsia, and Leptospira were performed and his serum tested strongly positive for immunoglobulin M against Leptospira interrogans by enzyme-linked immunosorbent assay. On further inquiry, the patient recalled immersion up to the level of the waist during a recent flood affecting the village in which he was residing and he noted dead rats in the vicinity. The skin abrasions over the lower limbs were sustained during escape from the flood at that time. We suspected that he might have contracted Leptospira infection through contact with contaminated water.
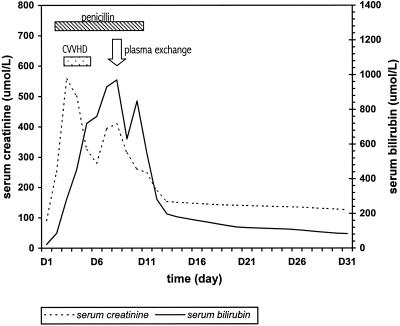
Treatment with penicillin was started intravenously with 1 MU every 6 h on the 3rd day after hospitalization. The patient's fever resolved. Nevertheless, the renal failure persisted, with a peak serum creatinine level of 570 μmol/liter. Venovenous hemodiafiltration was commenced, with a resultant transient fall in serum creatinine, but the conjugated hyperbilirubinemia continued to worsen. Moderate impairment of liver function was noted, with prothrombin time prolonged to 14.5 s and serum albumin decreased to 19 g/liter. Plasma exchange with a Haemanetics MCS 3p+ machine was performed on day 8 when bilirubin increased to 970 μmol/liter. Two liters of plasma was removed and replaced by same volume of plasma. The bilirubin level immediately after plasma exchange was 630 μmol/liter and was 849 μmol/liter after equilibrium on the next day, from which point it showed a steady decrease. There was concomitant progressive improvement in serum creatinine and urine output (Fig. 1). The patient's general condition improved rapidly. Penicillin therapy was stopped after 8 days. He was discharged on day 15. At the time of writing, 31 days after admission, his serum creatinine and bilirubin levels were 126 and 83 μmol/liter, respectively.
FIG. 1.
Serial levels of bilirubin and creatinine in relation to treatment.
Clinical manifestations of leptospirosis are variable and can include fever, myalgia, conjunctival suffusion, jaundice, renal failure, and gastrointestinal disturbances (14). Classical Weil's syndrome involving the kidney and the liver has been estimated to occur in 15.6% of cases of leptospirosis (13). Other reported organ-specific complications include pulmonary infiltrates with acute respiratory distress syndrome, myocarditis, pancreatitis, uveitis and aseptic meningoencephalitis. Endotoxemia with direct vasculotoxic effect has been incriminated as the major mechanism responsible for causing the multiorgan damage associated with full-blown disease. It is of interest that the characteristic conjugated hyperbilirubinemia associated with leptospirosis is usually out of proportion to the degree of elevation of liver parenchymal enzymes. Failure of bilirubin excretion due to microcirulatory abnormalities and intrahepatic biliary obstruction in addition to hepatocellular damage have been proposed to be the underlying mechanisms for this unique feature of leptospirosis (8). On the other hand, the pathogenesis of leptospiral interstitial nephritis is related to direct invasion of the organism into the kidney, but it may also be immunologically mediated (9). Variable degrees of acute renal insufficiency are commonly observed. Other contributing factors for acute renal insufficiency complicating leptospirosis include excessive hyperbilirubinemia, circulating endotoxins, azotemia related to hypotension, and rhabdomyolysis. Penicillin is an effective treatment for leptospirosis and should be given intravenously for 1 week (19). A Jerish-Hexheimer reaction similar to that encountered in the treatment of syphilis may be seen (5) and should be differentiated from allergic drug reaction. Doxycycline is an alternative for patients who are allergic to penicillin (12).
Despite penicillin therapy, to which the infection responded as evidenced by resolution of fever, the organ-specific complications continued to deteriorate in our patient. His acute renal failure persisted and serum bilirubin level continued to increase. Extreme hyperbilirubinemia has been reported to exert multiple cellular toxic effects, including effects on cellular respiration, membrane integrity, and transport functions, and excessive accumulation of bilirubin has been incriminated as causing renal tubular damage, thereby contributing to persistence of renal failure (6). Variable degrees of renal tubular dysfunction have been demonstrated in patients with jaundice due to biliary obstruction or liver failure (2, 16). Removal of serum bilirubin by plasma separation or bilirubin adsorption has led to favorable clinical outcomes in patients with severe hyperbilirubinemia after liver transplantation (15). Treatment of the hyperbilirubinemia per se may therefore be beneficial in reducing toxic insults to kidney and liver cells. Furthermore, plasma exchange may have additional benefits in removing circulating endotoxins, catabolic products, and inflammatory mediators (17). In this context, it is of interest that following plasma exchange, the bilirubin level in our patient started to decline spontaneously, with concomitant improvement in renal function and subsequent favorable clinical outcome. The sequence of events suggested a turning point in the patient's clinical course following plasma exchange.
The role of plasmapheresis in the treatment of leptospirosis has not been defined, although it has anecdotally been used in patients with favorable clinical outcome (3, 11, 20). Our observations show that plasma exchange may indeed be a useful adjunctive therapy in patients with severe prolonged hyperbilirubinemia. Amelioration of hyperbilirubinemia might have contributed to the resolution of acute renal failure consequent to the reduced toxic effect on renal tubular cells. Furthermore, a similar beneficial effect on hepatocytes would also facilitate their excretion of conjugated bilirubin, the disturbance of which represents a cardinal abnormality in leptospirosis. We conclude that plasma exchange should be considered in patients with leptospirosis complicated by severe hyperbilirubinemia and renal failure who have not shown rapid clinical response to conventional treatment.
REFERENCES
- 1.Alston, J. M., and J. C. Broom. 1958. Leptospirosis in man and animals, p. 4-12. E&S Livingstone Ltd., London, United Kingdom.
- 2.Bairaktari, E., G. Liamis, O. Tsolas, and M. Elisaf. 2001. Partially reversible renal tubular damage in patients with obstructive jaundice. Hepatology 33:1365-1369. [DOI] [PubMed] [Google Scholar]
- 3.Bourdais, A., B. Lonjon, R. Vergez-Pascal, A. Fournier, and W. A. Lo. 1988. Respiratory complications of leptospirosis. Apropos of 6 cases, 3 of which show hemodynamic studies. Med. Trop. 48:149-160. [PubMed] [Google Scholar]
- 4.Farr, R. W. 1995. Leptospirosis. Clin. Infect. Dis. 21:1-6. [DOI] [PubMed] [Google Scholar]
- 5.Friedland, J. S., and D. A. Warrell. 1991. The Jarisch-Herxheimer reaction in leptospirosis: possible pathogenesis and review. Rev. Infect. Dis. 13:1245-1246. [DOI] [PubMed]
- 6.Geiger, H., J. Klepper, P. Lux, and A. Heidland. 1992. Biochemical assessment and clinical evaluation of a bilirubin adsorbent column (BR-350) in critically ill patients with intractable jaundice. Int. J. Artif. Organs 15:35-39. [PubMed] [Google Scholar]
- 7.Heath, C. W., Jr., A. D. Alexander, and M. M. Galton. 1965. Leptospirosis in the United States: analysis of 483 cases in man 1949-1961. N. Engl. J. Med. 273:857-915. [DOI] [PubMed] [Google Scholar]
- 8.Higgin, R., and G. Cousineau. 1977. The pathogenesis of leptospirosis II. Jaundice in experimental leptospirosis in guinea pigs. Can. J. Comp. Med. 41:182-187. [PMC free article] [PubMed] [Google Scholar]
- 9.Lai, K. N., I. Aarons, A. J. Woodroffe, and A. R. Clarkson. 1982. Renal lesions in leptospirosis. Aust. N. Z. J. Med. 12:276-279. [DOI] [PubMed] [Google Scholar]
- 10.Landini, S., U. Coli, S. Lucatello, and G. Bazzato. 1981. Plasma exchange in severe leptospirosis. Lancet ii:1119-1120. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence, I. G., R. J. Dalby, N. R. Lad, and R. J. Shepherd. 1996. An atypical case of atypical pneumonia. Br. J. Clin. Pract. 50:346-348. [PubMed] [Google Scholar]
- 12.McClain, J. B., W. R. Ballou, S. M. Harrison, and D. L. Steinweg. 1984. Doxycycline therapy for leptospirosis. Ann. Intern. Med. 100:696-698. [DOI] [PubMed] [Google Scholar]
- 13.Merien, F., and P. Perolat. 1996. Public health importance of human leptospirosis in the South Pacific: a five-year study in New Caledonia. Am. J. Trop. Med. Hyg. 55:174-178. [DOI] [PubMed] [Google Scholar]
- 14.Muthusethupathi, M. A., S. Shivakumar, R. Suguna, M. Jayakumar, R. Vijayakumar, C. O. Everard, and D. G. Carrington. 1995. Leptospirosis in Madras: a clinical and serological study. J. Assoc. Physicians India 43:456-458. [PubMed] [Google Scholar]
- 15.Ott, R., H. Rupprecht, G. Born, et al. 1998. Plasma separation and bilirubin adsorption after complicated liver transplantation. Transplantation 65:434-453. [DOI] [PubMed] [Google Scholar]
- 16.Rector, W. G., Jr., G. C. Kanel, J. Rakela, and T. B. Reynolds. 1985. Tubular dysfunction in the deeply jaundiced patient with hepatorenal syndrome. Hepatology 5:321-326. [DOI] [PubMed] [Google Scholar]
- 17.Scharfman, W. B., J. R. Tillotson, E. G. Taft, and E. Wright. 1979. Plasmapheresis for meningococcemia with disseminated intravascular coagulation. N. Engl. J. Med. 300:1277-1278. [PubMed] [Google Scholar]
- 18.Sitprija, V., V. Pipatanagul, K. Mertowidjojo, V. Boonpucknavig, and S. Boonpucknavig. 1980. Pathogenesis of renal disease in leptospirosis: clinical and experimental studies. Kidney Int. 17:827-836. [DOI] [PubMed] [Google Scholar]
- 19.Watt, G., L. P. Padre, M. L. Tuazon, et al. 1988. Placebo-controlled trial of intravenous penicillin for severe and late leptospirosis. Lancet i:433-435. [DOI] [PubMed] [Google Scholar]
- 20.Yokouchi, K., H. Mikawa, M. Yamamoto, et al. 1990. A case of Weil's disease complicated with liver disorder, acute kidney failure and myocarditis treated by hemodialysis and plasma exchange. Nippon Naika Gakkai Zasshi 79:674-675. [DOI] [PubMed] [Google Scholar]