Enumeration and analysis of antigen-specific T cells play a central role in cellular immunology. While many methodologies have been established for the analysis of humoral responses, until recently the direct analysis of antigen-specific T-cell responses was extremely difficult. Difficulty in analyzing T cells was partially due to the fact that T lymphocytes recognize cell surface peptide-major histocompatibility complex (MHC) complexes and also due to the intrinsic low affinity of T-cell receptors (TCR) for cognate ligand. In this review, we discuss some new methodologies for visualizing antigen-specific T cells and how they have impacted our understanding of cellular immune responses.
In the past, several methods were used to quantitate antigen-specific T-cell responses. Traditionally, these assays were functional assays, dependent on the proliferation or lytic activity of antigen-specific T cells in vitro. To improve the sensitivity of these assays, it was necessary to expand the population of antigen-specific T cells in vitro before measuring effector functions, such as cytolysis, proliferative responses, or cytokine release. Serial limiting-dilution assays (LDA) were the old “gold standard” for quantitative analysis of antigen-specific cytotoxic T lymphocytes (CTL) (19). This assay depended on the ability of antigen-specific T cells to survive, function, and proliferate in vitro upon stimulation. Therefore, the number of antigen-specific CTL determined by LDA significantly underestimated the true number of antigen-specific CTL and also lacked further detailed information about the characteristics of antigen-specific CD8+ T cells in vivo.
Recently, new assays based on cytokine induction and/or release have been developed. These assays do not require in vitro proliferation of antigen-specific T cells but do depend on peptide-induced production of cytokines. Cytokine production can be detected in an ELISPOT assay (34), intracellularly (29, 31), or captured on the cell surface (8) and analyzed by flow cytometry. Analysis for cytokine-producing cells has influenced our estimate of antigen-specific T-cell precursor frequency. While these methods have been useful in visualizing cells on the basis of effector cytokine release, there is also a skewing that occurs depending on which cytokine is being assayed that is not present if one can visualize T cells solely based on TCR specificity. In addition, due to technical aspects of the assays, one cannot keep cells alive for additional studies after ELISPOT assay or intracellular cytokine staining.
Anticlonotypic TCR-specific antibodies have also been useful in analyzing the antigen-specific T-cell response in vivo. However, different T cells can use a variety of TCR to recognize the same peptide-MHC complex; therefore, anticlonotypic TCR, by definition, underestimate the polyclonal nature of the T-cell response.
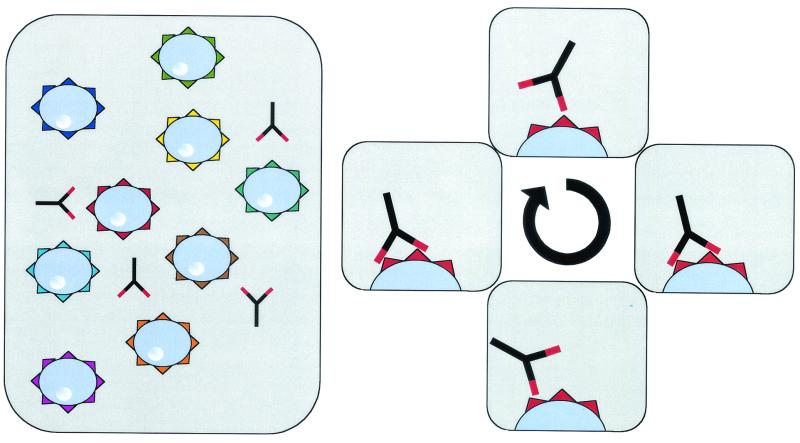
Recent advances have enabled the use soluble multivalent analogs of MHC complexes to study T-cell specificity. While it has been possible to analyze TCR-peptide-MHC interactions using recombinant soluble monovalent MHC molecules, these molecules do not bind T cells stably and allow flow cytometry-based visualization of antigen-specific cells. TCR have a low binding affinity (Kd) for peptide MHC complexes that is much lower than that of antibodies for their antigen (39) and also a very short dwell time (11, 51), making it difficult to analyze the interaction of soluble monovalent MHC with cognate TCR by standard flow cytometry-based assays. Therefore, recombinant monomeric MHC molecules have not been used to detect antigen-specific T cells. However, multimerized MHC molecules have a significantly increased avidity for their TCR. Multivalent MHC molecules bind with their different arms independently to the same cell, resulting in a stable binding even if individual TCR-peptide-MHC complex interactions are unstable (Fig. 1).
FIG. 1.
Multivalent molecules bind stably to T cells. Different arms from the same molecule bind independently to the same cell. Therefore, the avidity of these complexes is increased, inducing a stable binding of multimeric molecules to the TCR.
There are two broad approaches for generating multivalent MHC complexes. Altman et al. (2) first described the use of HLA-peptide tetrameric complexes. Peptide-MHC tetrameric complexes depend on the production of soluble peptide-MHC tagged with a biotin residue and complexed through fluorescent labeled strepavidin. The modified MHC heavy-chain extracellular domain sequences are expressed in Escherichia coli and refolded around a specific peptide and β2m. These molecules bind stably to T cells displaying the cognate TCR and have been successfully used to directly visualize antigen-specific cells ex vivo by flow cytometry (2). Tetramer technology has recently been reviewed (5, 54).
Multimeric peptide-MHC complexes can also be formed by using immunoglobulin as a molecular scaffold (13). In this system, the extracellular domains of MHC molecules are fused with the constant region of an immunoglobulin heavy chain separated by a short amino acid linker. In contrast to tetrameric MHC complexes, MHC-immunoglobulin G (MHC-Ig) fusion proteins are expressed in eukaryotic cells, making it unnecessary to refold the denatured MHC molecule. They can be easily loaded with the peptide of interest and are stable at 4°C. Peptide-loaded MHC-IgG dimers can be used for the identification of antigen-specific CTL as first described by Greten et al. in 1998 (22). A number of currently available techniques for monoclonal antibodies can be easily combined with these MHC-Ig fusion antibodies (such as binding antibodies to specific beads, etc.) to phenotypically characterize antigen-specific CTL populations.
Use of multimeric MHC complexes in analysis of the cellular immune response.
Some of the first insights into the robust nature of the CD8 portion of the immune response were found when multimeric MHC were used to analyze virus-specific CD8+ T cells. Many studies have shown virus-specific T-cell frequencies of between 0.1 and 15% of the CD8+ T cells. T-cell responses to major viral infections, including human immunodeficiency virus, Epstein-Barr virus, human T-cell leukemia virus type 1 (HTLV-1), hepatitis B virus, and hepatitis C virus, have been evaluated. The fact that up to 10 to 15% of all CD8+ cells are directed at one viral epitope raised the question of how the T-cell memory repertoire can accommodate new infections. A recent analysis of this issue indicates plasticity in the repertoire of memory T cells that changes upon new infection (46). Upon infection with a new virus, the memory compartment changes and cross-reactive T-cell epitope responses become more prominent. Thus, individuals accommodate to new viral infections by changes in the TCR repertoire in memory T-cell population (46).
Multimeric MHC complexes have also been used to identify tumor-specific CTL in melanoma patients (4, 27, 35, 56). High frequencies of Melan-A specific CD8+ T cells can also be found in healthy individuals (44). Others have investigated the T-cell response to different vaccination approaches in vivo (14, 26, 37). Experiments using animal tumor models are just beginning to shed some more light on the role of antigen-specific CTL in tumor immunology (28, 49).
Multimeric MHC class I complexes are also used to analyze the antigen-specific T-cell response to malaria (6), cytomegalovirus (20, 47), Mycobacterium tuberculosis (50), and simian immunodeficiency virus (15, 32, 42). Others have used tetrameric MHC molecules to analyze minor MHC-restricted CTL (41) and have used nonclassical MHC tetramers, which bind to NK cells (1, 7).
Multimeric MHC molecules allow not only for enumeration and characterization of the antigen-specific T-cell response in the peripheral blood or spleens of mice but also in local anatomic niches and sites of inflammatory pathology. This information can be valuable for the understanding of the pathology of immunology-related disorders. Greten et al. were able to demonstrate a threefold enrichment of HTLV-1-specific CTL cells in the cerebrospinal fluid of patients with HAM/TSP, a demyelinating disorder (22). Others have analyzed antigen-specific T cells in melanoma tumors (3), directly isolated from the livers of patients with hepatitis (25), and in the synovial fluid of patients with treatment-resistant Lyme arthritis (40). All these studies show that a higher frequency of the presumptive pathogenic cells accumulate at the site of pathology and strongly implicate these cells in the disease process.
Multimeric MHC molecules can be used to analyze antigen-specific T cells from tissue in situ. Several groups have tried using multimeric MHC complexes in immunohistochemistry analysis. However, the model of how multimeric MHC molecules bind stably to antigen-specific T cells (see above) suggests that these reagents may only work on viable cell membranes. It is very difficult to imagine that different MHC complexes on one molecule find different TCR on a cell membrane in a fixed tissue section. Therefore, initial experiments used a technique with viable 200- to 500-μm tissue sections (23, 48). The ability to identify antigen-specific T cells directly in situ will provide important insights into the anatomic localization of antigen-specific T cells during both normal and pathological immune responses.
Recent studies have also highlighted the use of multimeric MHC in analysis of the maturation of immune responses. Investigators have shown, by using multimeric MHC, that with there is an avidity maturation of immune responses (9, 45). This has been interpreted as a focusing of affinity during the maturation of immune responses. Focusing of affinity was thought to primarily occur through selection of specific high-affinity TCR clones. However, recent work has shown that the state of activation of the T cell has a dramatic impact on its ability to bind multivalent MHC (21). This occurs by the process of TCR reorganization (21) and occurs even though TCR do not increase their sensitivity by undergoing a process of gene mutations that mature to become high-affinity receptors for antigen (reviewed in reference 38). This process is of avidity maturation can ensure that T cells, once activated, readily recognize the low density of antigen in the periphery even in the presence of a relatively modest intrinsic affinity of the TCR for a specific peptide-MHC complex. A recent follow-up study also showed that tetramer binding was sensitive to cholesterol levels (16). This may be reflected in different cholesterol levels associated with different states of activation that affect multimeric MHC binding. Thus, alternate states of activation could also explain the difference seen in avidity during the maturation of the immune response in vivo.
Differences in effector function have been reported since not all multimer-positive cells express effector functions such as the lysis of target cells or the release of cytokines such as interferon gamma. This has been observed in multiple viral systems (10, 22, 55, 57) and was recently reviewed (52). The appearance of these dysfunctional CD8 cells may relate to magnitude of antigen presented to T cells and/or to the loss of CD4 population. It appears that the dysfunctional state can be reversed by the addition of exogenous cytokines. The physiologic role of antigen-specific cells that do not produce “normal” effector cytokines is obviously an area of great interest.
Potential therapeutic uses of multimeric MHC.
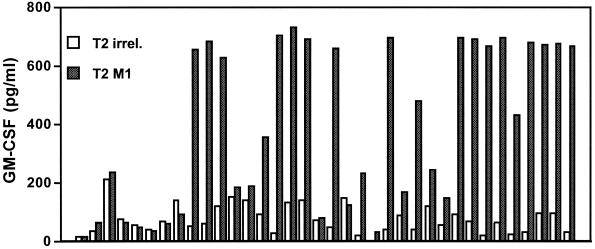
While many investigators have used multimeric peptide-MHC complexes to enumerate T cells, these molecules are potentially valuable therapeutic tools for the generation of antigen-specific T cells. After one or two in vitro stimulations of peripheral blood mononuclear cells (PBMC) with a specific peptide, antigen-specific CTL cells can be identified by multimeric peptide-MHC complexes and directly isolated from mixed CTL bulk cultures by cell sorting (17, 18). This method clearly simplifies the generation of antigen-specific CTL and may facilitate adoptive T-cell therapies. More interestingly, investigators have been able to separate antigen-specific CTL with high-affinity TCR from those with low-affinity TCR by using peptide-MHC tetramers (53, 56). Sorted T cells can further be amplified in vitro and used therapeutically. A simpler technique with MHC-immunoglobulin complexes does not even require a cell sorter instrument but instead combines the use of multimeric MHC complexes with magnetic beads specific for murine IgG molecules to enrich for antigen-specific cells. Using this approach, we have been able to enrich influenza-specific T cells (Fig. 2). PBMC from an HLA-A2-positive donor were stimulated with the influenza M1 peptide for 8 days in the presence of recombinant interleukin-2 (IL-2). PBMC were incubated with M1 peptide-loaded HLA-A2-IgG. Excess MHC-IgG protein was washed off, and anti-IgG1-coated magnetic beads were added for the isolation of M1-specific CD8+ T cells by using an MACS column. Purified M1-specific T cells were subcloned into 96-well plates in different dilutions and nonspecifically restimulated using phytohemagglutinin and IL-2. Analysis of T-cell clones by cytokine release with peptide-loaded T2 cells as targets revealed that a high percentage of these clones was specific for the influenza M1 peptide. This clearly demonstrates the power of this technique to isolate peptide-specific T cells from in vitro cultures to generate T-cell clones.
FIG. 2.
Using magnetically labeled anti-IgG antibodies, influenza M1-specific CTL were enriched from peripheral blood, restimulated in vitro and tested for specificity in a granulocyte-macrophage colony-stimulating factor secretion assay. A total of 36 independent clones were analyzed, and 22 of these demonstrated clear specificity for the influenza M1 peptide.
MHC class II tetramer and dimer.
Similar to the analysis of CD8+ CTL, a number of groups have begun to use class II dimers and tetramers to analyze the antigen-specific CD4+ T-cell response. Multimeric MHC class II complexes differ from MHC class I constructs due to structural differences between MHC class I and class II molecules. Whereas MHC class I molecules consist of a single chain (α1-α3, transmembrane and intracellular domain), MHC class II molecules consist of two transmembrane glycoprotein chains, which are noncovalently bound to each other. Currently, two general approaches exist for the generation of multimeric class II MHC complexes (12, 24, 40). The two chains of the MHC molecule are expressed in different cell systems and are either fused to a biotinylation site to make tetravalent molecules or fused to the heavy and light chain of an immunoglobulin sequence for the generation of dimeric MHC-immunoglobulin molecules. In most multimeric MHC class II constructs, the antigenic peptide has to be covalently bound to the beta chain of the class II MHC complex. This affords additional stability to the complex. To facilitate chain pairing in these constructs, a rate-limiting step, Kalandadze et al. have included c-jun and c-fos zippers sites at the carboxy-terminal end of the alpha and beta chains to improve the stability of the MHC class II molecules (30).
Current data on MHC class II-specific T cells suggest that the number of antigen-specific CD4+ T cells are very low and might actually be under the detection limit of flow cytometry in many cases. In many systems examined so far, multimeric MHC class II constructs can only detect specific CD4+ T cells after one in vitro stimulation (33, 43). A number of studies with MHC class II tetramers indicate that the frequency of antigen-specific T lymphocytes, which is in the range of 0.2 to 0.6% of the T cells, is under the detection limit of flow cytometry. Combining the dimer and/or tetramer technology with the use of a fluorescent dye (carboxyfluorescein succinimide esther [CSFE]) (36) allows for the quantitative analysis after cell division. The CSFE technique is applicable to in vitro cell division, as well as to in vivo division of adoptively transferred cells, and can resolve multiple successive generations by using flow cytometry. Each time a cell divides, CSFE is apportioned equally among the two daughter cells, which contain half of the fluorescence. The number of cell divisions can be determined by comparing the resultant CSFE fluorescence to the original fluorescence of the parent population. Using this approach, the absolute number of precursor frequencies can be estimated, although this type of assay still involves a single in vitro stimulation, which may influence TCR repertoire usage and T-cell function.
In summary, recent findings in molecular and structural immunology have led to the development of a new technique in cellular immunology. Multimeric MHC molecules have not only brought new insights into direct cellular T-cell responses in infectious disease but also into different aspects of tumor immunology. Apart from simple counting of antigen-specific CTL, these reagents can also be used for qualitative analysis, and recent findings suggest that they might even have an effect as an immunomodulating reagent.
Acknowledgments
Support for this work was provided by NIH grants AI-29575 and AI-44129 and by NMSS (RG 2637 A2/1). Additional funding for this study was provided by Pharmingen. Under licensing agreements between Pharmingen and Johns Hopkins University, J.P.S. and T.F.G. are entitled to a share of the royalty received by the university on sales of DimerX products. J.P.S. is also a paid consultant to Pharmingen. The terms of this agreement are managed by Johns Hopkins University in accordance with its conflict-of-interest policies. T.F.G. is supported by a grant from the Deutsche Forschungsgemeinschaft (Gr 1511 1-1 and 2-1) and the Wilhelm Sander Stiftung. Additional details about the Schneck lab are available online (http://pathology2.jhu.edu/schnecklab).
REFERENCES
- 1.Allan, D. S., M. Colonna, L. L. Lanier, T. D. Churakova, J. S. Abrams, S. A. Ellis, A. J. McMichael, and V. M. Braud. 1999. Tetrameric complexes of human histocompatibility leukocyte antigen (HLA)-G bind to peripheral blood myelomonocytic Cells. J. Exp. Med. 189:1149-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman, J. D., P. Moss, P. Goulder, D. H. Barouch, W. M. McHeyzer, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 3.Anichini, A., A. Molla, R. Mortarini, G. Tragni, I. Bersani, M. Di Nicola, A. M. Gianni, S. Pilotti, R. Dunbar, V. Cerundolo, and G. Parmiani. 1999. An expanded peripheral T cell population to a cytotoxic T lymphocyte (CTL)-defined, melanocyte-specific antigen in metastatic melanoma patients impacts on generation of peptide-specific CTLs but does not overcome tumor escape from immune surveillance in metastatic lesions. J. Exp. Med. 190:651-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baurain, J. F., D. Colau, N. van Baren, C. Landry, V. Martelange, M. Vikkula, T. Boon, and P. G. Coulie. 2000. High frequency of autologous anti-melanoma CTL directed against an antigen generated by a point mutation in a new helicase gene. J. Immunol. 164:6057-6066. [DOI] [PubMed] [Google Scholar]
- 5.Bercovici, N., M. T. Duffour, S. Agrawal, M. Salcedo, and J. P. Abastado. 2000. New methods for assessing T-cell responses. Clin. Diagn. Lab. Immunol. 7:859-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonelo, A., D. Valmori, F. Triponez, J. M. Tiercy, G. Mentha, J. Oberholzer, P. Champagne, J. F. Romero, F. Esposito, I. Nebie, C. Barbey, P. Romero, S. Herrera, G. Corradin, and J. A. Lopez. 2000. Generation and characterization of malaria-specific human CD8+ lymphocyte clones: effect of natural polymorphism on T cell recognition and endogenous cognate antigen presentationby liver cells. Eur. J. Immunol. 30:3079-3088. [DOI] [PubMed] [Google Scholar]
- 7.Braud, V. M., D. S. Allan, C. A. O'Callaghan, K. Soderstrom, A. D'Andrea, G. S. Ogg, S. Lazetic, N. T. Young, J. I. Bell, J. H. Phillips, L. L. Lanier, and A. J. McMichael. 1998. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391:795-799. [DOI] [PubMed] [Google Scholar]
- 8.Brosterhus, H., S. Brings, H. Leyendeckers, R. A. Manz, S. Miltenyi, A. Radbruch, M. Assenmacher, and J. Schmitz. 1999. Enrichment and detection of live antigen-specific CD4+ and CD8+ T cells based on cytokine secretion. Eur. J. Immunol. 29:4053-4059. [DOI] [PubMed] [Google Scholar]
- 9.Busch, D. H., and E. G. Pamer. 1999. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 189:701-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, J., A. Srikiatkhachorn, and T. J. Braciale. 2001. Visualization and characterization of respiratory syncytial virus F-specific CD8+ T cells during experimental virus infection. J. Immunol. 167:4254-4260. [DOI] [PubMed] [Google Scholar]
- 11.Corr, M., A. E. Slanetz, L. F. Boyd, M. T. Jelonek, S. Khilko, B. K. al-Ramadi, Y. S. Kim, S. E. Maher, A. L. Bothwell, and D. H. Margulies. 1994. T cell receptor-MHC class I peptide interactions: affinity, kinetics, and specificity. Science 265:946-949. [DOI] [PubMed] [Google Scholar]
- 12.Crawford, F., H. Kozono, J. White, P. Philippa Marrack, and J. Kappler. 1998. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity 8:675-682. [DOI] [PubMed] [Google Scholar]
- 13.Dal Porto, J., T. E. Johansen, B. Catipovic, D. J. Parfiit, D. Tuveson, U. Gether, S. Kozlowski, D. T. Fearon, and J. P. Schneck. 1993. A soluble divalent class I major histocompatibility complex molecule inhibits alloreactive T cells at nanomolar concentrations. Proc. Natl. Acad. Sci. USA 90:6671-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhodapkar, M. V., R. M. Steinman, M. Sapp, H. Desai, C. Fossella, J. Krasovsky, S. M. Donahoe, P. R. Dunbar, V. Cerundolo, D. F. Nixon, and N. Bhardwaj. 1999. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J. Clin. Investig. 104:173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donahoe, S. M., W. J. Moretto, R. V. Samuel, P. A. Marx, T. Hanke, R. I. Connor, and D. F. Nixon. 2000. Direct measurement of CD8+ T cell responses in macaques infected with simian immunodeficiency virus. Virology 272:347-356. [DOI] [PubMed] [Google Scholar]
- 16.Drake, D. R. R., and T. J. Braciale. 2001. Cutting edge: lipid raft integrity affects the efficiency of MHC class I tetramer binding and cell surface TCR arrangement on CD8+ T cells. J. Immunol. 166:7009-7013. [DOI] [PubMed] [Google Scholar]
- 17.Dunbar, P. R., J. L. Chen, D. Chao, N. Rust, H. Teisserenc, G. S. Ogg, P. Romero, P. Weynants, and V. Cerundolo. 1999. Cutting edge: rapid cloning of tumor-specific CTL suitable for adoptive immunotherapy of melanoma. J. Immunol. 162:6959-6962. [PubMed] [Google Scholar]
- 18.Dunbar, P. R., G. S. Ogg, J. Chen, N. Rust, P. van der Bruggen, and V. Cerundolo. 1998. Direct isolation, phenotyping and cloning of low-frequency antigen-specific cytotoxic T lymphocytes from peripheral blood. Curr. Biol. 8:413-416. [DOI] [PubMed] [Google Scholar]
- 19.Eichmann, K., I. Falk I. Melchers, and M. M. Simon. 1980. Quantitative studies on T cell diversity. I. Determination of the precursor frequencies for two types of streptococcus A-specific helper cells in nonimmune, polyclonally activated splenic T cells. J. Exp. Med. 152:477-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engstrand, M., C. Tournay, M. A. Peyrat, B. M. Eriksson, J. Wadstrom, B. Z. Wirgart, F. Romagne, M. Bonneville, T. H. Totterman, and O. Korsgren. 2000. Characterization of CMVpp65-specific CD8+ T lymphocytes using MHC tetramers in kidney transplant patients and healthy participants. Transplantation 69:2243-2250. [DOI] [PubMed] [Google Scholar]
- 21.Fahmy, T. M., J. G. Bieler, M. Edidin, and J. P. Schneck. 2001. Increased TCR avidity after T cell activation: a mechanism for sensing low-density antigen. Immunity 14:135-143. [PubMed] [Google Scholar]
- 22.Greten, T. F., J. E. Slansky, R. Kubota, S. S. Soldan, E. M. Jaffee, T. P. Leist, D. M. Pardoll, S. Jacobson, and J. P. Schneck. 1998. Direct visualization of antigen-specific T cells: HTLV-1 Tax11-19-specific CD8+ T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc. Natl. Acad. Sci. USA 95:7568-7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haanen, J. B., M. G. van Oijen, F. Tirion, L. C. Oomen, A. M. Kruisbeek, F. A. Vyth-Dreese, and T. N. Schumacher. 2000. In situ detection of virus- and tumor-specific T-cell immunity. Nat. Med. 6:1056-1060. [DOI] [PubMed] [Google Scholar]
- 24.Hamad, A. R., S. M. O'Herrin, M. S. Lebowitz, A. Srikrishnan, J. Bieler, J. Schneck, and D. Pardoll. 1998. Potent T cell activation with dimeric peptide-major histocompatibility complex class II ligand: the role of CD4 coreceptor. J. Exp. Med. 188:1633-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He, X. S., B. Rehermann, F. X. Lopez-Labrador, J. Boisvert, R. Cheung, J. Mumm, H. Wedemeyer, M. Berenguer, T. L. Wright, M. M. Davis, and H. B. Greenberg. 1999. Quantitative analysis of hepatitis C virus-specific CD8+ T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci. USA 96:5692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jager, E., S. Gnjatic, Y. Nagata, E. Stockert, D. Jager, J. Karbach, A. Neumann, J. Rieckenberg, Y. T. Chen, G. Ritter, E. Hoffman, M. Arand, L. J. Old, and A. Knuth. 2000. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc. Natl. Acad. Sci. USA 97:12198-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jager, E., Y. Nagata, S. Gnjatic, H. Wada, E. Stockert, J. Karbach, P. R. Dunbar, S. Y. Lee, A. Jungbluth, D. Jager, M. Arand, G. Ritter, V. Cerundolo, B. Dupont, Y. T. Chen, L. J. Old, and A. Knuth. 2000. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc. Natl. Acad. Sci. USA 97:4760-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ju, D. W., Y. Yang, Q. Tao, W. G. Song, L. He, G. Chen, S. Gu, C. C. Ting, and X. Cao. 2000. Interleukin-18 gene transfer increases antitumor effects of suicide gene therapy through efficient induction of antitumor immunity. Gene Ther. 7:1672-1679. [DOI] [PubMed] [Google Scholar]
- 29.Jung, T., U. Schauer, C. Heusser, C. Neumann, and C. Rieger. 1993. Detection of intracellular cytokines by flow cytometry. J. Immunol. Methods 159:197-207. [DOI] [PubMed] [Google Scholar]
- 30.Kalandadze, A., M. Galleno, L. Foncerrada, J. L. Strominger, and K. W. Wucherpfennig. 1996. Expression of recombinant HLA-DR2 molecules. Replacement of the hydrophobic transmembrane region by a leucine zipper dimerization motif allows the assembly and secretion of soluble DR alpha beta heterodimers. J. Biol. Chem. 271:20156-20162. [DOI] [PubMed] [Google Scholar]
- 31.Kern, F., I. P. Surel, C. Brock, B. Freistedt, H. Radtke, A. Scheffold, A. Blasczyk, P. Reinke, J. Schneider-Mergener, A. Radbruch, P. Walden, and H.-D. Volk. 1998. T-cell epitope mapping by flow cytometry. Nat. Med. 4:975-978. [DOI] [PubMed] [Google Scholar]
- 32.Kuroda, M. J., J. E. Schmitz, D. H. Barouch, A. Craiu, T. M. Allen, A. Sette, D. I. Watkins, M. A. Forman, and N. L. Letvin. 1998. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J. Exp. Med. 187:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwok, W. W., A. W. Liu, E. J. Novak, J. A. Gebe, R. A. Ettinger, G. T. Nepom, S. N. Reymond, and D. M. Koelle. 2000. HLA-DQ tetramers identify epitope-specific T cells in peripheral blood of herpes simplex virus type 2-infected individuals: direct detection of immunodominant antigen-responsive cells. J. Immunol. 164:4244-4249. [DOI] [PubMed] [Google Scholar]
- 34.Lalvani, A., R. Brookes, S. Hambleton, W. J. Britton, A. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, P. P., C. Yee, P. A. Savage, L. Fong, D. Brockstedt, J. S. Weber, D. Johnson, S. Swetter, J. Thompson, P. D. Greenberg, M. Roederer, and M. M. Davis. 1999. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 5:677-685. [DOI] [PubMed] [Google Scholar]
- 36.Lyons, A. B., and C. R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171:131-137. [DOI] [PubMed] [Google Scholar]
- 37.Marchand, M., N. van Baren, P. Weynants, V. Brichard, B. Dreno, M. H. Tessier, E. Rankin, G. Parmiani, F. Arienti, Y. Humblet, A. Bourlond, R. Vanwijck, D. Lienard, M. Beauduin, P. Y. Dietrich, V. Russo, J. Kerger, G. Masucci, E. Jager, J. De Greve, J. Atzpodien, F. Brasseur, P. G. Coulie, P. van der Bruggen, and T. Boon. 1999. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int. J. Cancer 80:219-230. [DOI] [PubMed] [Google Scholar]
- 38.Margulies, D. H. 2001. TCR avidity: It's not how strong you make it, it's how you make it strong. Nat. Immunol. 2:669-670. [DOI] [PubMed] [Google Scholar]
- 39.Matsui, K., J. J. Boniface, P. A. Reay, H. Schild, B. Fazekas de St. Groth, and M. M. Davis. 1991. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science 254:1788-1791. [DOI] [PubMed] [Google Scholar]
- 40.Meyer, A. L., C. Trollmo, F. Crawford, P. Marrack, A. C. Steere, B. T. Huber, J. Kappler, and D. A. Hafler. 2000. Direct enumeration of borrelia-reactive CD4 T cells ex vivo by using MHC class II tetramers. Proc. Natl. Acad. Sci. USA 97:11433-11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutis, T., G. Gillespie, E. Schrama, J. H. Falkenburg, P. Moss, and E. Goulmy. 1999. Tetrameric HLA class I-minor histocompatibility antigen peptide complexes demonstrate minor histocompatibility antigen-specific cytotoxic T lymphocytes in patients with graft-versus-host disease. Nat. Med. 5:839-842. [DOI] [PubMed] [Google Scholar]
- 42.Nixon, D. F., S. M. Donahoe, W. M. Kakimoto, R. V. Samuel, K. J. Metzner, A. Gettie, T. Hanke, P. A. Marx, and R. I. Connor. 2000. Simian immunodeficiency virus-specific cytotoxic T lymphocytes and protection against challenge in rhesus macaques immunized with a live attenuated simian immunodeficiency virus vaccine. Virology 266:203-210. [DOI] [PubMed] [Google Scholar]
- 43.Novak, E. J., A. W. Liu, G. T. Nepom, and W. W. Kwok. 1999. MHC class II tetramers identify peptide-specific human CD4+ T cells proliferating in response to influenza A antigen. J. Clin. Investig. 104:R63-R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pittet, M. J., D. Valmori, P. R. Dunbar, D. E. Speiser, D. Lienard, F. Lejeune, K. Fleischhauer, V. Cerundolo, J. C. Cerottini, and P. Romero. 1999. High frequencies of naive Melan-A/MART-1-specific CD8+ T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J. Exp. Med. 190:705-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savage, P. A., J. J. Boniface, and M. M. Davis. 1999. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity 10:485-492. [DOI] [PubMed] [Google Scholar]
- 46.Selin, L. K., M. Y. Lin, K. A. Kraemer, D. M. Pardoll, J. P. Schneck, S. M. Varga, P. A. Santolucito, A. K. Pinto, and R. M. Welsh. 1999. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity 11:733-742. [DOI] [PubMed] [Google Scholar]
- 47.Singhal, S., J. C. Shaw, J. Ainsworth, M. Hathaway, G. M. Gillespie, H. Paris, K. Ward, D. Pillay, P. A. Moss, and D. J. Mutimer. 2000. Direct visualization and quantitation of cytomegalovirus-specific CD8+ cytotoxic T-lymphocytes in liver transplant patients. Transplantation 69:2251-2259. [DOI] [PubMed] [Google Scholar]
- 48.Skinner, P. J., M. A. Daniels, C. S. Schmidt, S. C. Jameson, and A. T. Haase. 2000. Cutting edge: In situ tetramer staining of antigen-specific T cells in tissues. J. Immunol. 165:613-617. [DOI] [PubMed] [Google Scholar]
- 49.Slansky, J. E., F. M. Rattis, L. F. Boyd, T. Fahmy, E. M. Jaffee, J. P. Schneck, D. H. Margulies, and D. M. Pardoll. 2000. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity 13:529-538. [DOI] [PubMed] [Google Scholar]
- 50.Smith, S. M., R. Brookes, M. R. Klein, A. S. Malin, P. T. Lukey, A. S. King, G. S. Ogg, A. V. Hill, and H. M. Dockrell. 2000. Human CD8+ CTL specific for the mycobacterial major secreted antigen 85A. J. Immunol. 165:7088-7095. [DOI] [PubMed] [Google Scholar]
- 51.Sykulev, Y., A. Brunmark, M. Jackson, R. J. Cohen, P. A. Peterson, and H. N. Eisen. 1994. Kinetics and affinity of reactions between an antigen-specific T cell receptor and peptide-MHC complexes. Immunity 1:15-22. [DOI] [PubMed] [Google Scholar]
- 52.Welsh, R. M. 2001. Assessing CD8 T cell number and dysfunction in the presence of antigen. J. Exp. Med. 193:F19-F22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whelan, J. A., P. R. Dunbar, D. A. Price, M. A. Purbhoo, F. Lechner, G. S. Ogg, G. Griffiths, R. E. Phillips, V. Cerundolo, and A. K. Sewell. 1999. Specificity of CTL interactions with peptide-MHC class I tetrameric complexes is temperature dependent. J. Immunol. 163:4342-4348. [PubMed] [Google Scholar]
- 54.Whiteside, T. L. 2000. Monitoring of antigen-specific cytolytic T lymphocytes in cancer patients receiving immunotherapy. Clin. Diagn. Lab. Immunol. 7:327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiong, Y., M. A. Luscher, J. D. Altman, M. Hulsey, H. L. Robinson, M. Ostrowski, B. H. Barber, and K. S. MacDonald. 2001. Simian immunodeficiency virus (SIV) infection of a rhesus macaque induces SIV-specific CD8+ T cells with a defect in effector function that is reversible on extended interleukin-2 incubation. J. Virol. 75:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yee, C., P. A. Savage, P. P. Lee, M. M. Davis, and P. D. Greenberg. 1999. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J. Immunol. 162:2227-2234. [PubMed] [Google Scholar]
- 57.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]