Abstract
Zinc is a vital micronutrient to all organisms and it is therefore very important to determine the mechanisms that regulate cellular zinc uptake. Previously, we reported on zinc uptake transporters from zebrafish (Danio rerio; DrZIP1) and Fugu pufferfish (Takifugu rubripes; FrZIP1) that facilitated cellular zinc uptake of high affinity (Km<0.5 μM) in both CHSE214 [chinook salmon (Oncorhynchus tshawytscha) embryonic 214] cells and Xenopus laevis oocytes. To investigate additional biochemical pathways of zinc uptake in fish, we molecularly cloned the second fish member (FrZIP2) of the SLC39 subfamily II from Fugu pufferfish gill. Functional characterization suggests that FrZIP2 stimulated zinc uptake in a temperature-, time-, concentration- and pH-dependent manner when overexpressed in MDCK cells (Madin–Darby canine kidney cells). In comparison with FrZIP1 and DrZIP1 (<0.5 μM), FrZIP2 appears to represent a low-affinity zinc uptake transporter (Km=13.6 μM) in pufferfish. FrZIP2 protein was selective for zinc, but it might also transport Cu2+, since 20 times excess of Cu2+ completely abolished its zinc uptake activity. The zinc uptake by FrZIP2 was stimulated in a slightly acidic medium (pH 5.5–6.5) and was completely blocked at pH 7.5 and above, suggesting that an inward H+ gradient might provide a driving force for zinc transport by FrZIP2. Furthermore, FrZIP2-mediated zinc uptake activity was slightly inhibited by 0.5 mM HCO3−, indicating that FrZIP2 may employ a different mechanism of zinc translocation from the assumed HCO3−-coupled zinc transport used by human SLC39A2. The FrZIP2 gene was expressed in all the tissues studied herein, with especially high levels in the ovary and intestines. Thus FrZIP2 may be a prominent zinc uptake transporter of low affinity in many cell types of Fugu pufferfish.
Keywords: copper, influx, MDCK cell, pufferfish, SLC39A3, zinc uptake transporter
Abbreviations: CHSE214, chinook salmon (Oncorhynchus tshawytscha) embryonic 214; ECaC, epithelial calcium channel; MDCK cells, Madin–Darby canine kidney cells; ORF, open reading frame; RACE, rapid amplification of cDNA ends; RT, reverse transcriptase; SLC39A, solute carrier family 39A; TM, transmembrane
INTRODUCTION
Zinc is an essential micronutrient to all organisms by participating in protein, nucleic acid, carbohydrate and lipid metabolism, as well as in the control of gene transcription and co-ordination of other biological processes [1]. Due to its functional importance, zinc balance has to be precisely regulated to satisfy the proper demands of the normal physiological functions for zinc, and the loss of zinc balance can result in serious damaging consequences. It has been found that even moderate zinc deficiency can cause problems, including anaemia, loss of appetite, immune system defects, developmental problems and teratogenesis [2]. Conversely, excessive zinc accumulation can be toxic and has been linked to neurodegeneration [3]. Furthermore, zinc pollution is an environmental problem with the high frequency of water quality criteria violation in Europe and the U.S.A.
Consistent with the essential yet toxic nature of zinc, cells harbour mechanisms for homoeostatic zinc control with respect to uptake, excretion and distribution. Zinc uptake across the apical membrane of absorptive epithelial cells is the first gate to regulate zinc homoeostasis in vertebrates [4,5]. Uptake of zinc in fish much resembles that in mammals [5]. There is, however, at least one notable difference between the two groups in this regard. Whereas the intestine is an important zinc uptake site in both fish and mammals, fish also take up zinc across their gills. Like in mammals, the dietary zinc absorption in fish intestine is a carrier-mediated process that follows Michaelis–Menten kinetics [5]. The branchial zinc uptake is also a carrier-mediated transcellular process, thought to primarily take place in discrete ‘mitochondria-rich’ ionocytes, called chloride cells [4,5]. Correspondingly, a group of transporter proteins exist to mediate cellular zinc uptake in the evolutionarily diverse organisms (yeast, plants and mammals) [6,7]. These proteins are from the large ZIP family of transporters [derived from ZRT1-, IRT1 (iron-regulated transporter 1)-like protein] [8], and play roles in transport of zinc from outside the cell into the cytoplasm [6,7]. ZIP transporters have also been found to mobilize stored zinc by transporting the metal from endosomes or vacuoles into the cytosol [9,10]. The ZIP family has recently been given a systematic name, SLC39A (solute carrier family 39A), which is composed of four separate subfamilies of transporter proteins [11]. Subfamily I contains mainly fungal and plant sequences, while subfamily II consists of mammalian, nematode and insect genes [6,7]. Subfamily III (gufA subfamily), is a group of prokaryote and eukaryote proteins related to the gufA gene of Myxococcus xanthus, which has unknown function. Most ZIP subfamily III proteins have not been functionally characterized except the yeast ZRT3, which translocates zinc from the vacuole to the cytoplasm in yeast [9]. The fourth subfamily, also named LIV-1 subfamily of ZIP zinc transporters (LZT), is related to the oestrogen-regulated gene LIV-1 that facilitates cellular zinc uptake in glandular tissues [7]. Thus ZIP proteins appear to represent an impressive array of molecular mechanisms in zinc homoeostasis at diverse phylogenetic levels.
In mammals, SLC39A4 (ZIP4), a member of the LZT subfamily, is a likely mediator of dietary zinc uptake in the intestines and is dynamically regulated by dietary zinc at both transcriptional and post-translational levels [12–14]. It remains to be established whether or not there are orthologues of SLC39A4 in fish. Mutations of SLC39A4 in humans are related to the disease AE (acrodermatitis enteropathica), which is characterized by decreased zinc uptake in the small intestine and other cells of the body and an autosomal recessive pattern of inheritance [13,14]. However, the dysfunctional SLC39A4 only reduces zinc uptake and does not completely abrogate the dietary zinc absorption [13,14], suggesting that there are probably alternative pathways for zinc uptake in the intestines. Three members of the ZIP subfamily II (SLC39A1, SLC39A2 and SLC39A3) have so far been functionally characterized in mammals [11,15–17]. The relative abundance of the respective mRNA for these proteins in intestine of mouse does not seem to be dramatically changed in response to zinc deficiency [17,18]. However, zinc supplementation in human subjects was found to reduce SLC39A4 protein in the intestine [19]. Recent evidence suggests that post-translational events may play an important role in controlling cellular zinc influx. Zinc uptake mediated by SLC39A1, SLC39A3 and SLC39A4 appears to be regulated through trafficking of these transporters between the plasma membrane and intracellular compartments [20,21]. Lowering the zinc availability to cultured mouse cells resulted in a decreased rate of endocytosis of these proteins and a resulting increased localization to the plasma membrane. Two closely related members of the ZIP subfamily II, DrZIP1 and FrZIP1, have recently been molecularly cloned from the gills of zebrafish and pufferfish respectively [22]. Both of these are similar to human and mouse SLC39A1 and SLC39A2. DrZIP1 acted as a high-affinity zinc uptake transporter when expressed in either CHSE214 cells [chinook salmon (Oncorhynchus tshawytscha) embryonic 214 cells] or Xenopus laevis oocytes [22]. As both DrZIP1 and FrZIP1 genes are expressed in the gill and the intestine, their proteins may mediate a pathway of cellular zinc absorption in these tissues [22]. ECaC (epithelial calcium channel; TRPV6) seems to be another pathway of zinc uptake from the water in fish gills [23]. ECaC mediates a shared route for zinc and calcium uptake [23] that was previously inferred in the gill, but not the intestine, by using physiological methodologies [4]. This is consistent with the finding that expression of ECaC in fish is high in gills and low in intestine [23]. In the present study, we identified and functionally characterized a second member of the ZIP subfamily II (FrZIP2) from the gill of pufferfish, Takifugu rubripes. The results suggest that FrZIP2 protein is similar to human and mouse SLC39A3 and that it mediated low-affinity zinc uptake when expressed in MDCK (Madin–Darby canine kidney) cells. Although a large number of slc39A genes have now been identified, there is little information on the biochemical mechanisms by which their respective proteins operate. We present novel functional information showing that the transport activity of the Fugu SLC39A3 orthologue, FrZIP2, is inhibited by HCO3− and by pH above neutral. Consistent with published information on mouse ZIP3 [20], zinc transport by FrZIP2 was markedly inhibited by copper.
EXPERIMENTAL
Cloning of the ZIP homologue from Fugu pufferfish gill
Total RNA from gill, intestine, kidney and ovary of Fugu pufferfish was obtained from HGMP-RC (Human Genome Mapping Project Resource Centre) of the Medical Research Council (MRC), Cambridge, U.K. The first-strand cDNA was synthesized with Powerscript™ RT (reverse transcriptase) (ClonTech Laboratories, Basingstoke, U.K.) and the primer ADP1 (Table 1).
Table 1. Primers used in reverse transcription and PCRs.
V=A, G or C.
| Primer | Nucleotide sequence (5′→3′) |
|---|---|
| FrZIP2-F | GCAGCTTCAGGATGGACATC |
| FrZIP2-R | TCACCACTTGATGAACACGAG |
| 5GSP | TGAGCAGCGGCAGAGACCTC |
| 5NGSP | AGCCGAGTCGGAGCTGAGCAG |
| 3GSP | GCAGCTTCAGGATGGACATC |
| 5NGSP | GTCGGTGGTGCTGCAGGGAC |
| FrZIP2(H)6-R | TACAGGATCCTCAATGGTGATGGTGATGATGCCACTTGATGAACACGAGC |
| FrORF-F | TGACCTCGAGCACCATGGACATCCTGGTGGCCAAG |
| FrORF-R | TACAGGATCCTCACCACTTGATGAACACGAG |
| ADP1 | CTGATCTAGAATTCGCGAAGC(T)17V |
| ADP2 | AGCAGTGGTATCAACGCAGAGT(T)17V |
| UAP1 | TGATCTAGAATTCGCGAAGC |
| UAP2 | AAGCAGTGGTATCAACGCAGAGT |
Mining of MRC-HGMP Fugu genome database [24] was performed with the predicted Fugu pufferfish ZIP1 protein (FrZIP1, Genbank® accession no. AAS21267) as the query. Consequently, two genomic sequences, M001226 and S001063, were identified to contain segments with marked homology to FrZIP1 amino acid sequence. Primers FrZIP2-F and FrZIP2-R (Table 1) were then synthesized according to the sequences flanking the predicted FrZIP2 ORF (open reading frame), and PCR was subsequently performed.
The 3′- and 5′-ends of FrZIP2 cDNA were amplified by RACE (rapid amplification of cDNA ends)-PCR by the method of Dolphin et al. [25]. The 5′-end of FrZIP2 cDNA was amplified by PolyA tailing and subsequent 5′-RACE-PCR with gene-specific primers (5GSP and 5NGSP, Table 1) and universal primers (ADP2 and UAP2, Table 1). The 3′-RACE-PCR was performed to amplify the 3′-end of FrZIP2 cDNA with gene-specific primers (3GSP and 3NGSP) and UAP1 (Table 1). The full-length FrZIP2 ORF was amplified with Platinum™ Pfx Taq polymerase (Invitrogen), and both of its strands were thoroughly sequenced on a capillary DNA sequencer (CEQ2000; Beckman Coulter, High Wycombe, Buckinghamshire, U.K.) to verify its sequence.
Computational analysis
The alignment of the ZIP proteins and the prediction of phylogenetic relationship were generated with the CLUSTAL W method [26]. The hydrophobicity of FrZIP2 protein was analysed as described by Moller et al. [27]. Potential phosphorylation sites in FrZIP2 protein were predicted with [28].
Expression constructs
For functional analysis, the full-length FrZIP2 ORF was amplified with Platinum™ Pfx Taq polymerase with primers FrORF-F and FrORF-R (Table 1) and subsequently inserted into the XhoI and BamHI sites of the mammalian expression plasmid vector, pIRES2EGFP (ClonTech), to generate pIRES2EGFP-FrZIP2. The FrZIP2 ORF was tagged at its C-terminus with a His6 epitope by PCR with the primers FrZIP2-F and FrZIP2(H)6-R (Table 1) and cloned into pIRES2EGFP to generate pIRES2EGFP-FrZIP2(H)6. The inserted genes in the plasmid constructs above were thoroughly sequenced to verify the reading direction and sequence correctness.
Cell culture
CHSE214 cells (from A.T.C.C., Manassas, VA, U.S.A.) and MDCK cells (gift from Dr A. T. Mckie, King's College London) were grown in Eagle's minimum essential medium (Eagle's salts, L-glutamine and sodium bicarbonate) supplemented with 10% (v/v) fetal bovine serum (Sigma–Aldrich), 1% non-essential amino acids and 2% (v/v) penicillin–streptomycin (Gibco BRL, Paisley, Renfrewshire, Scotland, U.K.). MDCK cells were cultured at 37 °C in 5% CO2, while CHSE214 cells were grown at 20 °C in 5% CO2.
SDS-urea/PAGE and Western blotting
Expression plasmids, pIRES2EGFP-FrZIP2(H)6 and pIRES2EGFP, were transiently transfected into CHSE214 and MDCK cells respectively with Lipofectamine™ reagent (Invitrogen). After 48 h, the transfected cells were lysed in lysis buffer {10 mM Tris/HCl, pH 7.5, 150 mM NaCl, 2% (w/v) Chaps, 2 mM PMSF, 0.5 mM 4-[2-aminoethyl(1-benzenesulphonyl)-fluoride], 20 μg/ml leupeptin, 10 μg/ml pepstatin and 10 μg/ml aprotinin} and subsequently sonicated. Urea was then added to the cell lysate to a final concentration of 8 M. Protein samples (20 μg of total protein/sample) were resolved by SDS-urea/PAGE (containing 8 M urea). The proteins were blotted on to a nitrocellulose membrane (Schleicher and Schuell, Dassel, Germany) from the gel, and His6-tagged FrZIP2 proteins were immunodetected by using mouse anti-His antibody solution (1:2000 dilution) and secondary rabbit anti-mouse IgG–horseradish peroxidase conjugate (1:8000 dilution). ECL® Western Blotting System (Amersham Biosciences) was used for the luminol-based detection of horseradish peroxidase-conjugated antimouse antibodies on the membrane.
Stable transfection and assays of cellular 65Zn2+ uptake
Expression plasmids, pIRES2EGFP-FrZIP2 and pIRES2EGFP, were transfected into MDCK cells with Lipofectamine™ reagent (Invitrogen), and clonally derived stable transfectant MDCK cell lines were generated by combining G418 and green fluorescence selection.
Both MDCK-FrZIP2 and MDCK-Vec cells were grown in 500 ml flasks to approx. 80% confluence. These cells were then mildly trypsinized and suspended. The suspended cells were added into 6 ml Falcon tubes (∼4×106/tube), and washed twice with 1 ml of prewarmed uptake buffer (150 mM KCl, 100 mM glucose and 10 mM Hepes, pH 7.0; 37 °C) [16] by centrifugation at 800 g for 5 min. After incubation in 0.5 ml of prewarmed uptake buffer in a shaking water bath at 37 °C for 5 min, each was added with 0.5 ml of prewarmed uptake buffer containing 65Zn2+ plus a certain concentration of ZnCl2, and incubated for a further 20 min unless indicated otherwise.
For assessment of the specificity of the FrZIP2-dependent activity for zinc over other possible metal substrates, the cells were incubated in prewarmed uptake buffer containing 1.5 μM plus 0.028 μCi 65Zn2+ with or without added competitor metals (10 μM LaCl3, 1 mM CaCl2, 30 μM CdCl2, 30 μM CoCl2, 30 μM CuCl2, 30 μM FeCl3, 30 μM FeCl2 or 30 μM ZnCl2) at 37 °C for 20 min. Stock solutions of the chloride salts of various metals (LaCl3, CaCl2, CdCl2, CoCl2, CuCl2, FeCl3 and FeCl2) were prepared at 10 mM concentration in distilled water, while ZnCl2 stock solution was prepared at 10 mM in 0.02 M HCl. For pH effects on 65Zn2+ uptake, cells were incubated in prewarmed uptake buffer of different pH values (pH 5.5, pH 6, pH 6.5, pH 7, pH 7.5 or pH 8) containing 1.5 μM plus 0.03 μCi 65Zn2+ at 37 °C for 20 min. pH levels in uptake buffer were adjusted with 1 M HCl or 1 M NaOH. For assaying the effects of HCO3− on 65Zn2+ uptake, cells were incubated in prewarmed uptake buffer containing 1.5 μM plus 0.03 μCi 65Zn2+ and either 0 or 0.5 mM NaHCO3 at 37 °C for 20 min. Control cells were incubated in uptake buffer containing 0.5 mM NaHCO3 at 37 °C for 20 min to examine if the addition of HCO3− could change the pH level of uptake buffer.
Assays were stopped by washing cells three times in ice-cold washing buffer (150 mM KCl, 100 mM glucose, 1 mM EDTA and 10 mM Hepes, pH 7.0), and cells were lysed in 1 ml of 1 M NaOH. After thoroughly mixing, 20 μl of cell lysate from each assay was diluted 50 times for protein quantification with Bradford reagent (Sigma–Aldrich) and 0.9 ml was taken for radioactivity counting in a LKB1282 CompuGamma counter.
RT–PCR
To examine the tissue distribution of FrZIP2 mRNA, 2 μg of total RNA from Fugu pufferfish gill, gut, kidney and ovary was treated with DNase I (Promega, Chilworth, Southampton, U.K.), repurified with TRI reagent (Sigma–Aldrich), and reverse-transcribed to the first-strand cDNA with Powerscript™ RT (Clontech). FrZIP2 was amplified by PCR with primers FrZIP2-F and FrZIP2-R (Table 1). The cycle conditions were an initial denaturation of 2 min at 94 °C, 30 cycles of 0.5 min at 94 °C, 0.5 min at 58 °C, 2 min at 72 °C, followed by a final extension cycle of 2 min at 72 °C. As a control, a 0.5 kb fragment of β-actin (Ensembl ID: SINFRUG00000081338) was amplified by PCR with the primers ACT-F and ACT-R (Table 1; a gift from Dr M. S. Clark, HGMP-RC, MRC, Cambridge, U.K.), which are specific to sequences in exons 3 and 5 respectively. The β-actin fragment was amplified for 25 cycles of 0.5 min at 94 °C, 0.5 min at 60 °C, 2 min at 72 °C, followed by a final extension cycle of 2 min at 72 °C.
The PCR products were resolved in 1.5% agarose gels, and the levels of FrZIP2 mRNA were semi-quantitatively normalized to the corresponding β-actin mRNA levels by using SigmaGel software (Jandel Scientific, Erkrath, Germany).
RESULTS
Structural characterization of FrZIP2
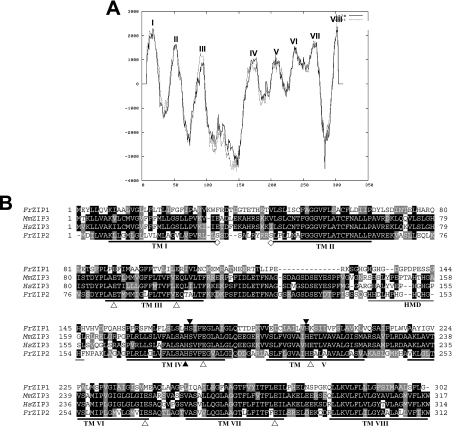
The cDNA for a ZIP homologue was molecularly cloned from Fugu pufferfish gill with a combination of database mining and PCR techniques, and herein named FrZIP2. FrZIP2 cDNA is 1573 bp in length including a PolyA tail at its 3′-end, and contains an ORF encoding a protein of 312 amino acids (Figure 1B) with the predicted molecular mass of 33 kDa. The FrZIP2 cDNA sequence has been submitted to GenBank® database (NCBI) with accession no. AY529486. This sequence appears to correspond to Ensembl gene ID SINFRUG00000155304 in Fugu Build 2d.
Figure 1. Structural characterization of FrZIP2 protein.
(A) Hydrophobicity analysis of pufferfish ZIP transporter 2 (FrZIP2). Eight TM domains (TM I–VIII) of FrZIP2 protein are predicted with TMpred (http://www.ch.embnet.org/software/TMPRED_form.html). TM IV and V have low hydrophobicity in comparison with other TMs. The dotted line indicates outside-to-inside topology (N-terminus outside) that is predicted to be the strongly preferred model, and the straight line indicates inside-to-outside orientation (N-terminus inside). (B) Alignment of amino acid sequences of FrZIP2, FrZIP1 (accession no. AY529485) and mammalian ZIP3 (SLC39A3). FrZIP2 was aligned with FrZIP1, human and mouse ZIP3 [accession numbers BC005869 (HsZIP3) and BC005502 (MmZIP3)]. Shaded residues represent positions of identity or similarity among the sequences. The eight predicted TM domains are underlined and numbered I–VIII. A potential histidine-rich metal-binding motif between TM III and TM IV is underlined in grey. ▲, conserved functional histidine and serine residues; △, glutamate residues conserved between FrZIP2 and mammalian ZIP3 proteins; and ◇, potential phosphorylation sites of protein kinase A. Signature sequence of the ZIP family in TM IV of FrZIP2 is boxed with a grey line. HMD, histidine-rich metal-binding domain.
FrZIP2 protein shares significant identity with functionally characterized vertebrate members of the ZIP subfamily II, e.g. zebrafish and Fugu pufferfish ZIP1 (DrZIP1 and FrZIP1; ∼30%), human SLC39A1, SLC39A2 (31%) and SLC39A3 (60%). The regions of identity were scattered throughout the length of these predicted proteins (Figure 1B). In contrast, FrZIP2 protein shares relatively low identity with members of ZIP subfamily I (<25%) and very low identity with members of ZIP subfamilies III and IV (<15%). Thus FrZIP2 is a piscine member of the ZIP subfamily II. Accordingly, many conserved structural features in most other members of the ZIP subfamily II can also be found in FrZIP2 protein, including eight TM (transmembrane) domains and a potential metal-binding motif with a series of histidine repeats (HHDLHPHGHQHGH) (Figures 1A and 1B). Furthermore, both TM IV and V of FrZIP2 protein contain histidine residues that are functionally conserved among most ZIP family members (Figure 1B), and a serine residue in TM IV of FrZIP2 is conserved in almost all the characterized members of the ZIP subfamilies I and II (Figure 1B). Consistent with the existence of charged and polar residues, TM IV and V of FrZIP2 are predicted to be amphipathic due to a relatively low hydrophobicity (Figure 1A), which has been thought to be very important for zinc transport mediated by IRT1 [29]. Additionally, there are two glutamate residues in TM II and one in TM IV, V, VI and VII of FrZIP2 protein (Figure 1B). All of these glutamate residues are surprisingly well conserved in mammalian SLC39A3 proteins (Figure 1B), and may contribute to zinc specificity since glutamate is a good ligand for zinc binding [30]. FrZIP2 protein also contains two predicted cAMP-dependent protein kinase (protein kinase A) phosphorylation sites (residues 27–33 and 38–43; Figure 1B).
FrZIP2 protein has very short N- and C-termini with predicted location on the luminal/outer side of the targeted membrane that is also a conserved feature of most functionally characterized ZIP proteins (Figure 1A), and a typical signature sequence of the ZIP family (FALSAHSVFEGVALG) is identified within TM IV of FrZIP2 protein (Figure 1B).
Expression of FrZIP2 proteins in cultured cells
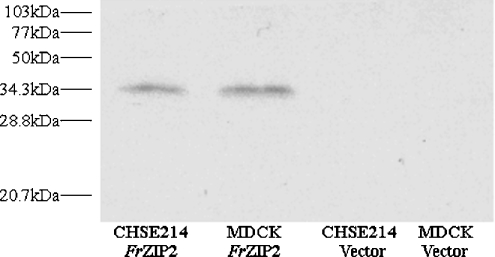
Functional characterization of FrZIP2 in cultured cells depends on whether the full-size FrZIP2 protein can be successfully expressed in these cells. To examine the expression of FrZIP2 protein in cultured cells, we labelled FrZIP2 ORF with a His6 epitope at its C-terminus, and FrZIP2(His)6 was subsequently inserted into the mammalian expression plasmid vector, pIRES2EGFP, to generate pIRES2EGFP-FrZIP2(H)6. After pIRES2EGFP-FrZIP2(H)6 was transfected into CHSE214 or MDCK cells, SDS-urea/PAGE and Western blotting were performed to immunodetect FrZIP2(H)6 protein that is expected to be transcriptionally expressed from the human CMV (cytomegalovirus) immediate-early enhancer/promoter and subsequently translated. As shown in Figure 2, a single protein band was detected in lanes containing protein samples from pIRES2EGFP-FrZIP2(H)6-transfected MDCK and CHSE214 cells, whereas there were no visible protein bands in other lanes containing protein samples from MDCK or CHSE214 cells with the empty plasmid vector, pIRES2EGFP (Figure 2). When being compared with protein markers, the molecular mass of the detected FrZIP2(H)6 protein is close to 34.3 kDa (Figure 2), which is similar to the predicted FrZIP2 protein of 34 kDa. The existence of FrZIP2(H)6 proteins suggests that FrZIP2(H)6 can be successfully expressed as the full-size protein when transfected into MDCK and CHSE214 cells. Since the only difference between the expression constructs pIRES2EGFP-FrZIP2(H)6 and pIRES2EGFP-FrZIP2 is that an epitope of His6 was added at the C-terminus of FrZIP2 ORF, it can be deduced that FrZIP2 can be successfully expressed as the full-size protein in both MDCK and CHSE214 cells.
Figure 2. Protein expression of FrZIP2(H)6 in CHSE214 and MDCK cells.
Expression plasmid pIRES2EGFP-FrZIP2(H)6 was transfected into either CHSE214 or MDCK cells, and the expressed FrZIP2(H)6 proteins in both cell lines were then detected by SDS-urea/PAGE and Western blotting.
Functional characterization of FrZIP2 in MDCK cells
To understand the roles of FrZIP2 in cellular zinc uptake, FrZIP2 was heterologously expressed and functionally characterized in MDCK cells. This particular cell line was chosen because of its epithelial properties that may be appropriate for functional studies of ZIP zinc uptake transporters (FrZIP2) in epithelial cells. For the functional expression of FrZIP2, the FrZIP2 ORF was cloned into pIRES2EGFP to generate pIRES2EGFP-FrZIP2 for expression from the CMV promoter. A single bicistronic mRNA containing both FrZIP2 ORF and EGFP with an internal ribosome entry site in the middle were produced when transfected into cultured cells, and FrZIP2 and EGFP ORFs were subsequently translated as separate polypeptides. Hence, EGFP could be used as a marker of the stable expression of FrZIP2 in MDCK cells. Stable MDCK transfectants were generated with the expression of either FrZIP2 or empty vector, hereafter referred to as MDCK-FrZIP2 and MDCK-Vec respectively. The stable transfected cells were monitored for green fluorescence using an inverted phase-contrast fluorescence microscope (Nikon Eclipse 200) to ensure the stable expression of FrZIP2 before 65Zn2+ flux assays.
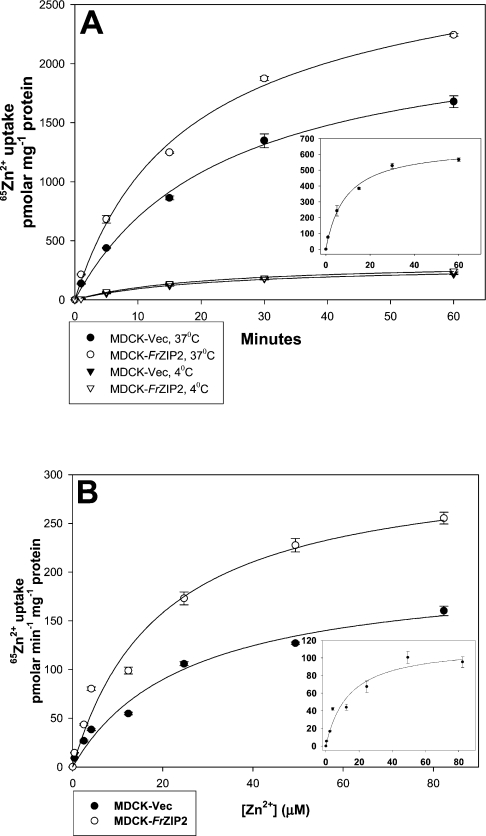
Zinc accumulation in MDCK-Vec cells suggests the existence of an endogenous zinc uptake activity that displays temperature- and time-dependent properties. When MDCK-Vec cells were incubated with uptake buffer containing 3 μM ZnCl2 plus 0.028 μCi 65Zn2+, only very low levels of 65Zn2+ accumulation were detected at 4 °C, whereas at 37 °C, 65Zn2+ accumulation was significantly increased in a typical time-dependent manner (Figure 3A). Similarly, 65Zn2+ accumulation in MDCK-FrZIP2 cells was also temperature- and time-dependent. At 4 °C, there was no difference of 65Zn2+ accumulation between MDCK-FrZIP2 and MDCK-Vec cells (Figure 3A). However, at 37 °C, MDCK-FrZIP2 cells accumulated more 65Zn2+ over a 60 min period than did the MDCK-Vec cells (Figure 3A). Thus the expression of FrZIP2 in MDCK cells increased the cellular zinc accumulation in a temperature- and time-dependent way.
Figure 3. Functional expression of FrZIP2 in MDCK cells.
(A) Time and temperature dependences of zinc accumulation assayed in MDCK-FrZIP2 (open symbols) and MDCK-Vec (filled symbols) cells. The cells were incubated in uptake buffer containing 3 μM ZnCl2 plus 0.028 μCi 65Zn2+ at 4 °C (triangles) or 37 °C (circles) for 1, 5, 15, 30 or 60 min. The inset shows the time-dependent zinc uptake via FrZIP2. Values are means±S.E.M. (n=3). (B) Concentration-dependent zinc uptake of MDCK-FrZIP2 and MDCK-Vec cells. Zinc uptake rate was determined by incubating cells in uptake buffer containing a range of ZnCl2 concentrations at 37 °C for 20 min. The concentration-dependent zinc uptake via FrZIP2 is shown in the inset. Values are means±S.E.M. (n=3).
Both the endogenous and FrZIP2-mediated zinc uptake activities in MDCK cells were concentration-dependent and saturable processes. When assayed over a range of zinc concentrations, the endogenous system in MDCK-Vec cells displayed Michaelis–Menten kinetics with an apparent Km of 25 μM zinc and a Jmax of 203 pmol zinc·min−1·(mg of protein)−1 (Figure 3B). The specific contribution of FrZIP2 to zinc uptake was estimated by subtracting the uptake rates in vector-only control cells from the corresponding values in MDCK-FrZIP2 cells, and the FrZIP2-dependent zinc uptake activity had an apparent Km of 13.6 μM zinc and a Jmax of 115 pmol zinc·min−1·(mg of protein)−1 (Figure 3B). Thus, based on its temperature-, time- and concentration-dependent properties of zinc uptake, we suggest that FrZIP2 functions as a zinc uptake transporter when expressed in MDCK cells.
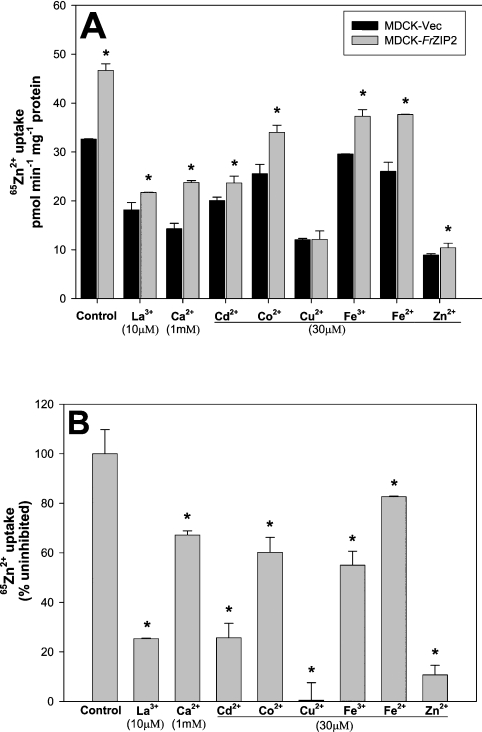
To examine the specificity of the FrZIP2-dependent activity for zinc over other possible metal substrates, we tested the effects of various metal ions on zinc uptake by MDCK-FrZIP2 cells. As a control, the metal ion specificity of the endogenous zinc uptake system was also determined in MDCK-Vec cells. The endogenous zinc uptake activity in MDCK-Vec cells was markedly inhibited by 30 μM non-radioactive Zn2+, Cu2+, Cd2+, 1 mM Ca2+ or 10 μM La3+, and to a far less extent, by 30 μM Co2+, Fe2+ or Fe3+ (Figure 4A). In contrast, FrZIP2-dependent uptake activity was strongly inhibited by excess non-radioactive Zn2+ and, surprisingly, completely blocked by Cu2+ (Figures 4A and 4B). Ca2+ (1 mM) only slightly inhibited the FrZIP2-dependent zinc uptake activity (Figures 4A and 4B). Like the endogenous zinc uptake system, FrZIP2-dependent uptake activity was also significantly inhibited by La3+ and Cd2+, and to a lesser extent by other metals such as Co2+, Fe2+ and Fe3+ (Figures 4A and 4B). Thus the FrZIP2-dependent activity showed a degree of selectivity for zinc, but it seems possible that FrZIP2 may transport Cu2+ and other cations as well.
Figure 4. Characterization of metal specificity of FrZIP2-dependent and endogenous zinc uptake systems in MDCK cells.
Excess of the indicated non-radioactive metal ions was added to uptake buffer containing 1.5 μM ZnCl2 plus 0.028 μCi 65Zn2+, and the cells were incubated under these conditions at 37 °C for 20 min before washing and counting. (A) Zinc uptake of MDCK-Vec and MDCK-FrZIP2 cells was measured and compared with cells incubated in the absence of inhibitor (control). Values are means±S.E.M. (n=3). (B) Inhibition of FrZIP2-dependent zinc uptake activity by indicated excessive metals. The percentage zinc uptake for each condition was compared with uptake in uncompeted cells (control). Significant differences between experimental groups were tested using non-parametric ANOVA followed by post-hoc test. *P<0.05, significant differences from mock-transfected cells (A) or control 65Zn2+ uptake (B).
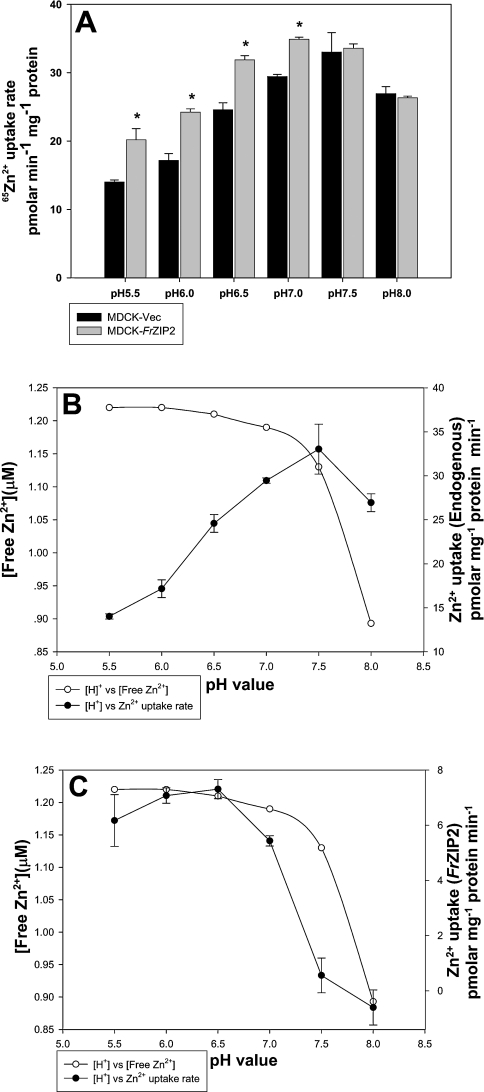
To understand the biochemical properties of FrZIP2, we investigated the effects of pH levels in uptake buffer on zinc uptake mediated by both endogenous and FrZIP2-mediated zinc uptake systems. The endogenous zinc uptake activity peaked at pH between 5.5 and 7.5, and then decreased slightly at pH 8.0 in comparison with those at pH 7.5 and 7.0 (Figure 5A). In marked contrast, FrZIP2-mediated zinc uptake was completely inhibited at pH levels above 7.0, and reached a peak at pH 6.0–6.5 (Figure 5A).
Figure 5. Effects of pH on the endogenous and FrZIP2-dependent zinc uptake activities in MDCK cells.
(A) Zinc uptake rate was assayed in MDCK-Vec (black bars) and MDCK-FrZIP2 cells (grey bars) with 1.5 μM ZnCl2 plus 0.03 μCi 65Zn2+ in uptake buffer adjusted to the indicated pH. Values are means±S.E.M. (n=3). Significant differences between experimental groups were tested using non-parametric ANOVA followed by post-hoc test. *P<0.05, significant differences from control values. (B) Effects of pH on free Zn2+ concentration and the endogenous zinc uptake activities. (C) Effects of pH on free Zn2+ concentration and the FrZIP2-mediated zinc uptake activity that was calculated by subtracting MDCK-Vec rates from the corresponding MDCK-FrZIP2 values. The free zinc concentration in uptake buffer with different pH values was analysed with MINEQL+ software.
As a change in pH influences zinc speciation in the uptake buffer, we calculated the concentration of Zn2+ ion at each pH using MINEQL+ software (4.0). As shown in Figures 5(B) and 5(C), the free Zn2+ ion concentration in uptake buffer decreased with increase in pH. The endogenous zinc uptake activity in MDCK cells was highest between pH 5.5 and 7.5, and did not follow changes of the free Zn2+ concentrations (Figure 5B). In contrast, the FrZIP2-mediated zinc uptake was entirely blocked in uptake buffer at pH 7.5 and 8.0 although the uptake buffer still contained 1.13 and 0.893 μM Zn2+ ions respectively (Figure 5C). Conversely, the FrZIP2-mediated zinc uptake was higher at pH 6.0 and 6.5 than at pH 7.0, while the free Zn2+ concentration in uptake buffer was just slightly elevated from 1.19 μM (pH 7.0) to 1.21 μM (pH 6.5) and 1.22 μM (pH 6.0) (Figure 5C). According to the concentration-dependent properties of FrZIP2-dependent zinc uptake, this slight change of free Zn2+ concentration seemed unlikely to influence significantly the zinc accumulation via FrZIP2, and thus the elevation of FrZIP2-mediated zinc uptake at pH 6.0 and 6.5 in comparison with pH 7.0 was likely due to the increased acidity in uptake buffer rather than the slight changes of free Zn2+ concentration.
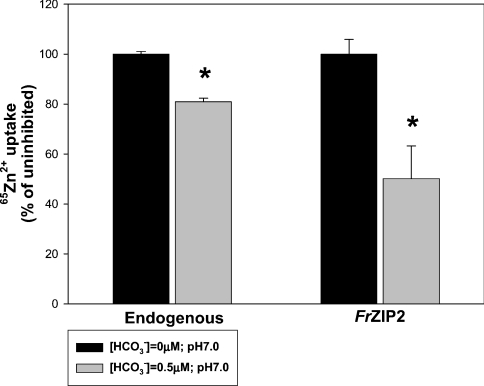
Both the endogenous and FrZIP2-mediated zinc uptake activities were inhibited by HCO3− (0.5 mM) in uptake buffer (Figure 6). Control experiments showed that this level of HCO3− did not change the pH (pH 7.0) in uptake buffer over the duration of the experiment. The addition of 0.5 mM HCO3− caused no major change in the free Zn2+ ion concentration (1.17 μM) in uptake buffer when compared with that (1.19 μM) in control uptake buffer according to the zinc speciation analysis with MINEQL+ software. This slight change of Zn2+ ion concentration by the addition of HCO3− seemed unlikely to be the cause of the significant decrease of either endogenous or FrZIP2-dependent zinc uptake after the addition of HCO3− (Figure 6). Thus the inhibition of endogenous and FrZIP2-mediated zinc uptake activities by the addition of 0.5 mM HCO3− probably represented true functional properties of these two zinc uptake systems.
Figure 6. HCO3− effects on endogenous and FrZIP2-mediated zinc uptake activities in MDCK cells.
Zinc uptake was assayed in MDCK-Vec (black bars) and MDCK-FrZIP2 cells (grey bars) with flux buffer containing 1.5 μM ZnCl2 plus 0.03 μCi 65Zn2+ and either 0 mM (control) or 0.5 mM NaHCO3. Percentage zinc uptake with 0.5 mM NaHCO3 was compared with uptake in cells without added NaHCO3. Values are means±S.E.M. (n=3). The statistical significance of differences was determined using Mann–Whitney U-test. *Significant differences from control values (P<0.05).
Tissue distribution of FrZIP2 mRNA
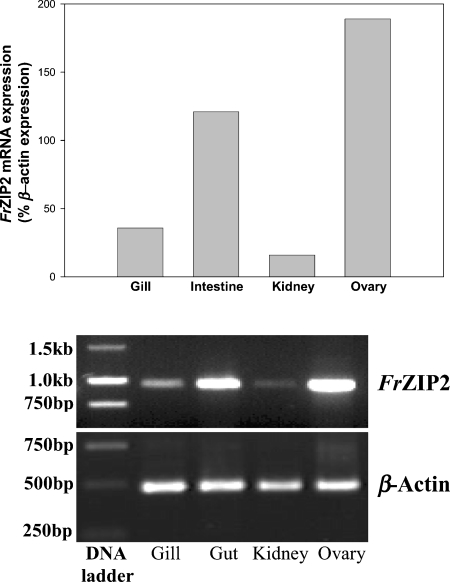
RT–PCR was performed to examine the expression levels of FrZIP2 mRNA in the gill, intestine, kidney and ovary of Fugu pufferfish. According to our results, the highest level of FrZIP2 mRNA was detected in the ovary and intestine, and the lowest in the kidney (Figure 7). Pufferfish gill contains an intermediate amount of FrZIP2 mRNA (Figure 7).
Figure 7. Distribution of FrZIP2 mRNA in various tissues of pufferfish by RT–PCR.
Upper panel: relative levels of FrZIP2 mRNA in pufferfish gill, intestine, kidney and ovary normalized by β-actin mRNA using SigmaGel software (Jandel Scientific). Lower panel: expression pattern of FrZIP2 and β-actin mRNA in agarose gels.
DISCUSSION
Zinc uptake across the apical membrane of absorptive epithelial cells is the first gate to regulate zinc homoeostasis, and thus it is very important to understand the molecular mechanisms of cellular zinc uptake. In mammals, SLC39A4 seems to mediate dietary zinc uptake in the intestine [12–14,18,19]. However, probably there are alternative pathways for zinc uptake in mammalian intestines as dysfunctional ZIP4 only impairs zinc uptake and does not completely abrogate the dietary zinc absorption [18,19]. While the existence of a fish SLC39A4 orthologue remains uncertain, ZIP1 protein seems to be a constitutive high-affinity zinc uptake transporter in various tissues of both zebrafish and Fugu pufferfish [22], and ECaC appears to be the candidate protein mediating the reciprocally competitive uptake of calcium and zinc in the gill [23]. In the present study, to understand other zinc uptake pathways that mediate zinc uptake in fish gill and intestine, we molecularly cloned and functionally characterized a second fish member of the ZIP subfamily II, FrZIP2, from Fugu pufferfish gill.
Structural characterization suggested that FrZIP2 is a fish member of the ZIP subfamily II. Just like most of the functionally characterized ZIP protein in fungi, plants and mammals [11], FrZIP2 protein exhibits all the conserved features of ZIP subfamilies I and II, including eight TM domains, a potential metalbinding domain between TM III and IV, functionally important histidine residues in the amphipathic TM IV and V etc. Furthermore, FrZIP2 shares much higher amino acid identity with individual members of the ZIP subfamily II than those in the ZIP subfamilies I, III and IV, suggesting that it belongs to the ZIP subfamily II. In particular, the highest amino acid identity (∼60%) exists throughout FrZIP2 and mammalian SLC39A3 proteins, especially in the regions of eight TM domains. Thus, together with the observation that mouse SLC39A3 functions as a zinc uptake transporter when expressed in HEK-293 cells [17], these structural properties support our initial hypothesis that FrZIP2 is another zinc uptake transporter in pufferfish along with FrZIP1.
Functional characterization in MDCK cells renders further supportive evidence that FrZIP2 can mediate uptake of zinc. FrZIP2 facilitated cellular zinc uptake in a temperature-, time-, concentration- and pH-dependent manner in MDCK cells. In comparison, the apparent Km of FrZIP2 for zinc uptake (13.6 μM Zn2+) is significantly lower than the endogenous uptake system of MDCK cells (25 μM Zn2+) was similar to that of the yeast low-affinity zinc uptake transporter ZRT2 [31], and markedly higher than those of the yeast and zebrafish high-affinity zinc uptake transporters ZRT1 (1 μM Zn2+) [32] and DrZIP1 (<0.5 μM Zn2+) [22], human SLC39A1 and SLC39A2 (3 μM Zn2+) and mouse SLC39A1–SLC39A3 (1.5 μM Zn2+) [15–17]. Furthermore, we recently showed that FrZIP2 protein displayed much lower zinc binding affinity than the orthologue of FrZIP1 in zebrafish, DrZIP1 (eight times lower) [22]. Thus FrZIP2 appears to be a low-affinity zinc uptake transporter in Fugu pufferfish.
As would be expected from a zinc influx protein, FrZIP2-mediated uptake of 65Zn in transfected MDCK cells was greatly inhibited by 20-fold excess of non-radioactive zinc. Surprisingly however, FrZIP2-mediated 65Zn uptake was completely quenched by 20-fold excess of Cu2+. This suggests that FrZIP2 might transport Cu2+ as well as Zn2+. A similar phenomenon was also observed in Arabidopsis ZIP2 (AtZIP2) and human SLC39A2. A 10-fold excess of Cu2+ inhibited zinc uptake of AtZIP2 to the same extent as a 10-fold of non-radioactive Zn2+ [33], and zinc uptake by human SLC39A2 was also greatly inhibited by Cu2+ [15]. Interestingly, a distinct pathway of copper transport (Cu2+) was identified in Ctr1-homozygous knockout mouse embryonic cells, which exhibited approx. 30% residual copper transport activity that was a saturable process with a Km of approx. 10 μM Cu2+ and with biochemical features distinct from that of Ctr1 [34]. Furthermore, 50-fold excess of zinc markedly inhibited this Cu2+ uptake pathway, while it exhibited no effects on the Cu+ uptake mediated by Ctr1 [34], suggesting that this copper uptake pathway may be related to cellular zinc uptake. Consistently, physiological evidence indicates that the intestinal zinc uptake pathway in mammals and fish may also be used for Cu2+ transport as Cu2+ status has a considerable effect on zinc absorption and vice versa [35–37], which is further supported by the observation that the endogenous zinc uptake in MDCK cells was also inhibited by Cu2+. Human SLC39A1, SLC39A2 and the divalent metal transporter (DMT1, SLC40A1) were considered unlikely to have a role in mediating Cu2+ uptake [34], but SLC39A3 remains a candidate. Thus it would be interesting to investigate if FrZIP2 and its close homologues in mammals, SLC39A3, can mediate cellular Cu2+ uptake.
Both MDCK endogenous and FrZIP2-mediated zinc uptake activities were inhibited by La3+ and Cd2+. La3+ is known to be a calcium channel blocker. It blocks ECaC-mediated calcium transport and inhibits both calcium and zinc uptake in rainbow-trout gill [4,38]. From the present results, it appears that La3+ blocks the FrZIP2-mediated zinc uptake activity as well. The inhibitory effect of Cd2+ on FrZIP2-mediated zinc uptake is consistent with the fact that waterborne Cd2+ reduces branchial zinc influx in fish and competitively inhibits zinc uptake in cultured mammalian cells [39].
Ca2+ (1 mM) had little effect on FrZIP2-mediated zinc uptake while it inhibited the endogenous zinc uptake activity in MDCK cells by approx. 50%. A common pathway for zinc and calcium uptake has been identified in fish gills [38] and in rat, piglet and pig enterocytes [40–42]. ECaC appears to be the candidate protein mediating the common pathway for zinc and calcium uptake in fish gill [23], but it still remains unknown if the mammalian ECaC orthologues fulfil the same functions in mammalian cells as well.
Like human SLC39A1 [16], zinc uptake via FrZIP2 was inhibited by 0.5 mM HCO3−. This property is different from that of human SLC39A2 that zinc uptake activity of human SLC39A2 was stimulated by 0.5 mM HCO3− [15], suggesting that FrZIP2 may employ a different mechanism of zinc translocation from human SLC39A2. A Zn2+/HCO3− symport mechanism has been proposed on the zinc transport activity of human SLC39A2 [15]. This does not appear to be the mechanism by which zinc is translocated across the cell membrane by FrZIP2. Instead, experimental data from the present study indicate that a proton-driven mechanism of zinc transport may be a possibility.
FrZIP2 also exhibited a different pH dependence from human SLC39A2. Zinc uptake by human SLC39A2 was inhibited at pH levels below 7.0 and stimulated at higher pH [15]. In marked contrast, FrZIP2-mediated zinc uptake was markedly reduced at pH levels above 7.0, and reached a peak at about pH 6.0 and 6.5. This different pH dependence further suggests that FrZIP2 employs a different mechanism of zinc transport from that of human SLC39A2, and that the H+ gradient across the cell membrane might provide partial driving force for zinc translocation via FrZIP2. In accordance with the pH dependence of FrZIP2, a slightly reduced water pH has been observed to increase the movement of zinc across the gill into the body in fish [43]. Coupled with the expression of FrZIP2 mRNA in pufferfish gill, this concurrence of pH dependence of FrZIP2 and physiology of branchial zinc uptake in fish suggests that FrZIP2 may be a molecular mechanism of branchial zinc acquisition in Fugu pufferfish.
Substantial expression of FrZIP2 mRNA was detected in the intestine, suggesting that FrZIP2 may be an important pathway of cellular zinc uptake in the Fugu pufferfish intestine as well. Consistent with the consensus that fish intestine is the bulk pathway of zinc absorption under standard dietary conditions and gills may act to supplement absorption when required [4], pufferfish intestine contains a higher level of FrZIP2 mRNA than the gill. In contrast, FrZIP2 mRNA was detected at a very low level in pufferfish kidney. In marine teleosts, the kidneys mainly function to excrete bivalent ions such as calcium, magnesium and phosphate [44]. Although the renal zinc metabolism in fish is still unknown, the low level of renal FrZIP2 mRNA is consistent with the major roles of kidney in ion homoeostasis in marine fish. The highest level of FrZIP2 mRNA was found in the ovary among the four pufferfish tissues herein studied. This is consistent with previous findings that considerable quantities of zinc are needed for the developing oocytes [45,46]. In accordance with this, mouse slc39A1 and slc39A2 are expressed at relatively high levels in the ovary [17]. Interestingly, mouse slc39A3 seems to be expressed preferentially in testes [17]. Slc39A3 is also expressed in mammary glands of the rat during mid lactation [47]. Furthermore, as the zinc concentration of the milk decreased during the course of lactation, levels of slc39A3 mRNA and SLC39A3 protein decreased concomitantly, indicating that rat SLC39A3 may be involved in the transport of zinc into milk. Clearly, zinc transporters are important throughout development and different members of the slc30A and slc39A families are expressed in a time- and tissue-specific manner. Such a subfunctionalization may have provided the evolutionary advantage that allowed the many members of these families to co-exist.
Acknowledgments
This work was supported by K. C. Wong Education Foundation (The landmark square, Hong Kong, People's Republic of China), A.Q. was supported by a Chinese Scholarship council (Beijing, People's Republic of China) and an U.S. EPA grant R 826104-01-1 (to C.H.).
References
- 1.Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990;29:5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- 2.MacDiarmid C. W., Gaither L. A., Eide D. J. Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J. 2000;19:2845–2855. doi: 10.1093/emboj/19.12.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frederickson C. J., Bush A. I. Synaptically released zinc: physiological functions and pathological effects. Biometals. 2001;14:353–366. doi: 10.1023/a:1012934207456. [DOI] [PubMed] [Google Scholar]
- 4.Hogstrand C., Wood C. M. The physiology and toxicology of zinc in fish. In Toxicology of Aquatic Pollution. In: Taylor E. W., editor. Cambridge: Cambridge University Press; 1996. pp. 61–84. [Google Scholar]
- 5.Bury N. R., Walker P. A., Glover C. N. Nutritive metal uptake in teleost fish. J. Exp. Biol. 2003;206:11–23. doi: 10.1242/jeb.00068. [DOI] [PubMed] [Google Scholar]
- 6.Gaither L. A., Eide D. J. Eukaryotic zinc transporters and their regulation. Biometals. 2001;14:251–270. doi: 10.1023/a:1012988914300. [DOI] [PubMed] [Google Scholar]
- 7.Taylor K. M., Nicholson R. I. The LZT proteins; the LIV-1 subfamily of zinc transporters. BBA Biomembranes. 2003;1611:16–30. doi: 10.1016/s0005-2736(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 8.Lioumi M., Ferguson C. A., Sharpe P. T., Freeman T., Marenholz I., Mischke D., Heizmann C., Ragoussis J. Isolation and characterization of human and mouse ZIRTL, a member of the IRT1 family of transporters, mapping within the epidermal differentiation complex. Genomics. 1999;62:272–280. doi: 10.1006/geno.1999.5993. [DOI] [PubMed] [Google Scholar]
- 9.MacDiarmid C. W., Gaither L. A., Eide D. J. Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J. 2000;19:2845–2855. doi: 10.1093/emboj/19.12.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor K. M., Morgan H. E., Johnson A., Nicholson R. I. Structure-function analysis of HKE4, a member of the new LIV-1 subfamily of zinc transporters. Biochem. J. 2003;377:131–139. doi: 10.1042/BJ20031183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eide D. J. The SLC39 family of metal ion transporters. Pflugers Arch. Eur. J. Physiol. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- 12.Dufner-Beattie J., Wang F. D., Kuo Y. M., Gitschier J., Eide D., Andrews G. K. The Acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc regulated zinc transporter in mice. J. Biol. Chem. 2003;278:33474–33481. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- 13.Wang K., Zhou B., Kuo Y. M., Zemansky J., Gitschier J. A novel member of a zinc transporter family is defective in Acrodermatitis enteropathica. Am. J. Hum. Genet. 2002;71:66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Küry S., Dréno B., Bézieau S., Giraudet S., Kharfi M., Kamoun R., Moisan J. P. Identification of SLC39A4, a gene involved in Acrodermatitis enteropathica. Nat. Genet. 2002;31:239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 15.Gaither L. A., Eide D. J. Functional expression of the human hZIP2 zinc transporter. J. Biol. Chem. 2000;275:5560–5564. doi: 10.1074/jbc.275.8.5560. [DOI] [PubMed] [Google Scholar]
- 16.Gaither L. A., Eide D. J. The human ZIP1 transporter mediates zinc uptake in human K562 erythroleukemia cells. J. Biol. Chem. 2001;276:22258–22264. doi: 10.1074/jbc.M101772200. [DOI] [PubMed] [Google Scholar]
- 17.Dufner-Beattie J., Langmade S. J., Wang F., Eide D. J., Andrews G. K. Structure, function, and regulation of a subfamily of mouse zinc transporter genes. J. Biol. Chem. 2003;278:50142–50150. doi: 10.1074/jbc.M304163200. [DOI] [PubMed] [Google Scholar]
- 18.Liuzzi J. P., Bobo J. A., Lichten L. A., Samuelson D. A., Cousins R. J. Responsive transporter genes within the murine intestinal–pancreatic axis form a basis of zinc homeostasis. Proc. Natl. Acad. Sci. U.S.A. 2004;101:14355–14360. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cragg R. A., Philips S. R., Piper J. M., Varma J. S., Campbell J. C., Mathers J. C., Ford D. Homeostatic regulation of zinc transporters in the human small intestine by dietary zinc supplementation. Gut. 2005;54:469–478. doi: 10.1136/gut.2004.041962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F. D., Dufner-Beattie J., Kim B. E., Petris M. J., Andrews G., Eide D. J. Zinc stimulated endocytosis controls activity of the mouse ZIP1 and ZIP3 zinc uptake transporters. J. Biol. Chem. 2004;279:24631–24639. doi: 10.1074/jbc.M400680200. [DOI] [PubMed] [Google Scholar]
- 21.Kim B. E., Wang F., Dufner-Beattie J., Andrews G. K., Eide D. J., Petris M. J. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J. Biol. Chem. 2004;279:4523–4530. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- 22.Qiu A., Shayeghi M., Hogstrand C. Molecular cloning and functional characterisation of a high affinity zinc importer (DrZIP1) from zebrafish, Danio rerio. Biochem. J. 2005;388:745–754. doi: 10.1042/BJ20041807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu A., Hogstrand C. Functional characterisation and genomic analysis of an epithelial calcium channel (ECaC) from pufferfish, Fugu rubripes. Gene. 2004;342:113–123. doi: 10.1016/j.gene.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 24.Aparicio S., Chapman J., Stupka E., Putnam N., Chia J.-M., Dehal P., Christoffels A., Rash S., Hoon S., Smit A., et al. Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science. 2002;297:1301–1310. doi: 10.1126/science.1072104. [DOI] [PubMed] [Google Scholar]
- 25.Dolphin C. T., Cullingford T. E., Shephard E. A., Smith R. L., Phillips I. R. Differential developmental and tissue-specific regulation of expression of the genes encoding three members of the flavin-containing monooxygenase family of man, FMO1, FM03 and FM04. Eur. J. Biochem. 1996;235:683–689. doi: 10.1111/j.1432-1033.1996.00683.x. [DOI] [PubMed] [Google Scholar]
- 26.Higgins D., Thompson J., Gibson T., Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moller S., Croning M. D. R., Apweiler R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics. 2001;17:646–653. doi: 10.1093/bioinformatics/17.7.646. [DOI] [PubMed] [Google Scholar]
- 28.Kreegipuu A., Blom N., Brunak S. PhosphoBase, a database of phosphorylation sites: release 2.0. Nucleic Acids Res. 1999;27:237–239. doi: 10.1093/nar/27.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerinot M. L. The ZIP family of metal transporters. BBA Biomembranes. 2000;1465:190–198. doi: 10.1016/s0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 30.Berg J. M., Shi Y. G. The galvanisation of biology: a growing application for the roles of zinc. Science. 1996;271:1081–1085. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 31.Zhao H., Eide D. The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J. Biol. Chem. 1996;271:23203–23210. doi: 10.1074/jbc.271.38.23203. [DOI] [PubMed] [Google Scholar]
- 32.Zhao H., Eide D. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2454–2458. doi: 10.1073/pnas.93.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grotz N., Fox T., Connolly E., Park W., Guerinot M. L., Eide D. J. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7220–7224. doi: 10.1073/pnas.95.12.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J., Petris M. J., Thiele D. J. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter: identification of a ctr1-independent copper transport system. J. Biol. Chem. 2002;277:40253–40259. doi: 10.1074/jbc.M208002200. [DOI] [PubMed] [Google Scholar]
- 35.Mills C. F. The influence of chemical species on the adsorption and physiological utilization of trace elements from the diet or environment. In: Bernhard M., Brinckman F. E., Sadler P. J., editors. The Importance of Chemical ‘Speciation’ in Environmental Processes. Berlin/Heidelberg: Springer-Verlag; 1986. pp. 71–83. [Google Scholar]
- 36.Linder M. C. New York: Plenum Press; 1991. Biochemistry of Copper; pp. 15–161. [Google Scholar]
- 37.Glover C. N., Hogstrand C. Effects of dissolved metals and other hydrominerals on in vivo intestinal zinc uptake in freshwater rainbow trout. Aquat. Toxicol. 2003;62:281–293. doi: 10.1016/s0166-445x(02)00108-x. [DOI] [PubMed] [Google Scholar]
- 38.Hogstrand C., Verbost P. M., Bonga S. E. W., Wood C. M. Mechanisms of zinc uptake in gills of freshwater rainbow trout: interplay with calcium transport. Am. J. Physiol. Reg. 1996;270:R1141–R1147. doi: 10.1152/ajpregu.1996.270.5.R1141. [DOI] [PubMed] [Google Scholar]
- 39.Reyes J. G. Zinc transport in mammalian cells. Am. J. Physiol. Cell Physiol. 1996;39:C401–C410. doi: 10.1152/ajpcell.1996.270.2.C401. [DOI] [PubMed] [Google Scholar]
- 40.Rothbassell H. A., Clydesdale F. M. The influence of zinc, magnesium, and iron on calcium uptake in brush-border membrane-vesicles. J. Am. Coll. Nutr. 1991;10:44–49. doi: 10.1080/07315724.1991.10718125. [DOI] [PubMed] [Google Scholar]
- 41.Gunshin H., Noguchi T., Naito H. Effect of calcium on the zinc uptake by brush-border membrane-vesicles isolated from the rat small intestine. Agr. Biol. Chem. Tokyo. 1991;55:2813–2816. [Google Scholar]
- 42.Bertolo R. F., Brttger W. J., Atkinson S. A. Calcium competes with zinc for a channel mechanism on the brush border membrane of piglet intestine. J. Nutr. Biochem. 2001;12:66–72. doi: 10.1016/s0955-2863(00)00125-x. [DOI] [PubMed] [Google Scholar]
- 43.Bradley R. W., Sprague J. B. Accumulation of zinc by rainbow-trout as influenced by pH, water hardness and fish size. Environ. Toxicol. Chem. 1985;4:685–694. [Google Scholar]
- 44.Nishimura H., Imai M. Control of renal-function in fresh-water and marine teleosts. Fed. Proc. 1982;41:2355–2360. [PubMed] [Google Scholar]
- 45.Fletcher G. L., King M. J. Seasonal dynamics of Cu2+, Zn2+, Ca2+, and Mg2+ in gonads and liver of winter flounder (Pseudopleuronectes americanus) – evidence for summer storage of Zn2+ for winter gonad development in females. Can. J. Zool. 1978;56:284–290. [Google Scholar]
- 46.Thompson E. D., Mayer G. D., Balesaria S., Glover C. N., Walsh P. J., Hogstrand C. Physiology and endocrinology of zinc accumulation during the female squirrelfish reproductive cycle. Comp. Biochem. Physiol. A Mol. Integ. Physiol. 2003;134:819–828. doi: 10.1016/s1095-6433(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 47.Kelleher S. L., Lonnerdal B. Zn transporter levels and localization change throughout lactation in rat mammary gland and are regulated by Zn in mammary cells. J. Nutr. 2003;133:3378–3385. doi: 10.1093/jn/133.11.3378. [DOI] [PubMed] [Google Scholar]