Abstract
HNSCC remains a substantial health issue, with treatment options including surgery, radiation, and platinum-based chemotherapy. Unfortunately, despite progress in research, only modest gains have been made in disease control, with existing treatments resulting in significant functional and quality-of-life issues. The introduction of immunotherapy in the treatment of HNSCC has resulted in some improvements in outlook for patients and is now standard of care for populations with both recurrent and metastatic disease. However, despite the early successes, responses to immune checkpoint inhibition (ICI) remain modest to low, approaching 14%–22% objective response rates. Challenges to the effectiveness of ICI and other immunotherapies are complex, including the diverse and dynamic molecular plasticity and heterogeneity of HNSCCs; lack of immunogenic antigens; accumulated suppressive immune populations such as myeloid cells and dysfunctional T cells; nutrient depletion; and metabolic dysregulation in the HNSCC tumor microenvironment. In this Review, we explore the mechanisms responsible for immunotherapy resistance, dissect these challenges, and discuss potential opportunities for overcoming hurdles to the development of successful immunotherapy for HNSCC.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer globally, with approximately 800,000 new cases and approximately 450,000 deaths annually (1). Treatment of HNSCC has made modest advances over the course of decades, consisting of cytotoxic platinum-based chemoradiation (CRT) or primary surgical management with risk-adapted adjuvant therapy (i.e., radiation and/or chemotherapy). With current treatment strategies, more than 50% of patients experience recurrence within 3 years, while more than 10% experience distant failures (2–4). Moreover, surgery, radiation, and chemotherapy all harbor significant side effects. Unfortunately, HNSCC lacks actionable genomic targets due to the complex and evolving genomic landscape, with limited success in targeting EGFR and PIK3CA (5, 6). Therefore, understanding the molecular mechanisms responsible for HNSCC pathogenesis and development of effective therapeutic strategies are substantial hurdles in the management of HNSCC.
The emergence of immune checkpoint inhibition (ICI) represents a major shift in the treatment paradigm of primary and recurrent/metastatic (R/M) HNSCC. ICI is now the standard of care (SoC) for R/M HNSCC (7), but response rates remain modest, around 14%–22% (8, 9). Other immunotherapeutic strategies, including tumor vaccines and adoptive transfer of antigen-specific T cells, have also been explored in clinical trials involving patients with HNSCC with limited response rates (10, 11). Several potential factors contribute to these modest responses, including tumor-intrinsic molecular heterogeneity and metabolic adaptations, which diminish antitumor immunity, tumor antigen escape, influx of suppressive immune cell populations, dysregulated metabolism, and development of dysfunctional antitumor T cells (12–16). To overcome these formidable obstacles and advance the outlook for patients, better understanding of the mechanisms driving resistance to antitumor immunity is urgently needed.
In this Review, we provide an update on the current landscape of immunotherapy in patients with HNSCC, explore potential mechanisms driving resistance to immunotherapy, discuss major challenges in the immunotherapy of HNSCC patients, and offer our perspective on prioritizing development of targets influencing immunotherapy outcomes.
Current immunotherapeutic strategies in HNSCC
While current immunotherapeutic strategies for HNSCC primarily leverage ICIs, such as α–PD-1 therapies, a variety of approaches including T cell–based immunotherapy are emerging. The advent of ICIs, particularly PD-1 inhibitors such as nivolumab and pembrolizumab, has transformed the treatment landscape for HNSCC. While the impact of ICIs in HNSCC has not matched that in melanoma (17) or cutaneous squamous cell carcinoma (18), the landmark trials CheckMate 141 and KEYNOTE-040 galvanized the use of ICIs in the treatment of R/M HNSCC (8, 9). Moreover, forthcoming data from the KEYNOTE-689 trial will be paradigm-shifting (19). Critical questions remain at the forefront of ongoing research: What is the optimal timing for immunotherapy administration — adjuvant or/and neoadjuvant? sequential or concurrent? How can predictive biomarkers refine patient selection? And how can immunotherapies best integrate with established SoC? These challenges reflect the rapidly evolving landscape of HNSCC immunotherapy. An overview of current evidence and future directions of immunotherapy is provided in Tables 1–5, setting the stage for the next chapter in this transformative field.
Table 1. Immune checkpoint blockade therapy in HNSCC.
Combination ICI.
Initial ICI trials in HNSCC demonstrated durable treatment responses and overall survival (OS), suggesting maintenance of immune equilibrium (Table 1). Combination ICI with multiple coinhibitory molecules may amplify the treatment response by differentially regulating various cell populations in the tumor microenvironment (TME) (20). The CheckMate 651 study, combining α–PD-1 and α–CTLA-4 blockade in R/M HNSCC, observed no change in objective response rate (ORR) or OS (21). Similarly, CheckMate 714 observed no change in ORR with α–PD-1/α–CTLA-4 inhibition over α–PD-1-alone in platinum-refractory R/M HNSCC (22). The phase III EAGLE trial evaluated durvalumab, an α–PD-L1 monoclonal antibody, versus durvalumab plus tremelimumab (α–CTLA-4) versus SoC in patients with R/M HNSCC. OS did not differ across groups relative to SoC (23). In parallel, KESTREL found no benefit to single-agent or combination α–PD-L1 with or without α–CTLA-4, even noting that patients with high PD-L1 expression receiving SoC had better ORR compared with durvalumab alone or durvalumab plus tremelimumab (24). While reinvigoration of cytotoxic T cell function through coregulatory signal pathway modulation is effective, deeper understanding of the mechanisms regulating the fate and response of effector lymphocytes in TME is critically needed.
Radiation therapy in combination with ICI.
Ionizing radiation is under active investigation for enhancing immunotherapeutic responses. Putative mechanisms supporting this approach are the activation of cytotoxic lymphocytes, DC activation and T cell priming, activation of pro-death signaling in tumor cells, and release of damage-associated molecular patterns (DAMPs) (25). A phase I study testing the safety of partial tumor irradiation with stereotactic body radiotherapy to oligometastatic disease coupled with pembrolizumab in advanced solid tumors demonstrated encouraging results (Table 2) (26). In contrast, NRG-HN004 (ClinicalTrials.gov NCT03258554) compared radiotherapy with concurrent plus adjuvant durvalumab versus RT/cetuximab, observing no improvement in progression-free survival (PFS) (27). However, irradiation also induces deleterious effects, including increased Treg and myeloid-derived suppressor cell (MDSC) infiltration, PD-L1 induction, and activation of prosurvival mechanisms via chronic IFN signaling (25, 28). The role of radiotherapy in modulating antitumor immunity remains to be elucidated as a tool for enhancing immunotherapy effectiveness.
Table 2. Chemoradiotherapy coupled with immune checkpoint blockade in HNSCC.
Chemoradiotherapy combined with ICI.
Building on the advances of KEYNOTE-040 and CheckMate 141 in R/M HNSCC, exciting advances are on the horizon integrating immunotherapy with definitive CRT (Table 2). The role of SoC cisplatin chemotherapy for enhancing ICI is under active investigation, with support from preclinical data (29, 30). Preclinical and clinical data suggest that cisplatin promotes immunogenic tumor cell death (30), DC activation, and antigen-specific T cell killing (31). Data evaluating concurrent versus sequential pembrolizumab in the definitive treatment of HNSCC with CRT highlight better outcomes with sequential immunotherapy (32). The phase III KEYNOTE-048 trial comparing pembrolizumab with and without chemotherapy with cetuximab plus chemotherapy for R/M HNSCC revealed a lack of PFS benefit with ICI (33). Pembrolizumab with chemotherapy improved OS compared with cetuximab plus chemotherapy in all subpopulations, independent of PD-L1 combined positive score (CPS) status. The phase III KEYNOTE-412 (NCT03040999) trial evaluated pembrolizumab plus CRT versus CRT in locally advanced HNSCC (LA-HNSCC), finding no difference in event-free survival (34). The JAVELIN trial comparing avelumab plus CRT versus CRT in LA-HNSCC also observed no difference in PFS (35). Further work is warranted to elucidate the extent to which chemotherapy can enhance ICI effectiveness.
RTK inhibition and ICI.
Combining ICI with RTK inhibition is promising, though recent trial results were modest (Table 3). EGFR is an established therapeutic target in HNSCC. There is a propensity for EGFR copy number amplification and overexpression in carcinogen-driven, HPV– HNSCCs. Cetuximab, an EGFR-targeting mAb, was one of the first immunotherapies approved for HNSCC, marking a pivotal moment in systemic cancer therapy. Cetuximab can augment antitumor immunity, promoting DC maturation, CD8+ T cell priming, NK cell functions, and antibody-dependent cell-mediated cytotoxicity (ADCC) (36, 37). Compared with SoC cisplatin, cetuximab offers unique benefits, particularly for patients unable to tolerate cisplatin, though its utility has been limited by the side effect of acneiform rash (38). Initial studies demonstrated the advantages of combining cetuximab with radiotherapy, showing improved outcomes compared with radiotherapy alone (39). Promising results were also observed with pembrolizumab and cetuximab in combination in a phase II trial among patients with platinum-ineligible or -resistant R/M HNSCC (40). However, GORTEC-REACH — comparing concurrent cisplatin or cetuximab radiotherapy versus radiotherapy with concurrent weekly cetuximab and avelumab (α–PD-L1) — failed to meet its primary end point (41). Despite these limitations, cetuximab facilitated broader adoption of immunotherapies in HNSCC and bridged the gap to the next generation of immunotherapies, leaving a lasting legacy in HNSCC treatment.
Table 3. Tyrosine kinase inhibition coupled with immune checkpoint blockade in HNSCC.
In addition to cetuximab, VEGF inhibitors, including tyrosine kinase inhibitors (TKIs), have immunomodulatory properties. A phase II trial combining pembrolizumab and cabozantinib, a multikinase TKI, observed a partial response or stable disease in over half the cohort in conjunction with increased CD8+ T cell infiltrates in responders (42). The ALPHA study combining pembrolizumab with afatinib, an irreversible TKI, observed a promising ORR (43). The KEYNOTE-146 phase IB/II trial of lenvatinib plus pembrolizumab found an encouraging response rate in the phase II expansion cohort (44). However, the LEAP-010 phase III study (NCT04199104) combining first-line pembrolizumab with or without lenvatinib was discontinued after OS failed to improve (45). Given the variability in these results, a biological approach to identify and overcome barriers to effective antitumor immunity in HNSCC is warranted.
Antitumor vaccine therapy.
Vaccine-based immunotherapy for HPV+ HNSCC is a logical intervention to target tumor cells expressing viral antigens (e.g., E6/E7) (46). Several strategies — including live-vector vaccines (e.g., axalimogene filolisbac secreting the Lm-LLO-HPV E7 fusion protein), peptide vaccines such as ISA101 in combination with ICI (NCT03669718, NCT04398524, and NCT04369937), and the DNA vaccine MEDI0457 — have been developed and tested in patients with HPV+ cervical and oropharyngeal squamous cell carcinoma (OPSCC), with modest results (Table 4). One hurdle is overcoming T cell dysfunction in the TME with vaccine-mediated approaches. Several groups are examining combinations of ICI with anticancer vaccines, including a vaccinia virus encoding E6/E7 combined with IL-2 plus α–PD-L1 (NCT03260023) (47); a liposomal-based HPV16 E6/E7 peptide vaccine (PDS0101) in combination with pembrolizumab (NCT04260126 and NCT05232851) (48); and the SQZ-PBMC-HPV vaccine in combination with atezolizumab, ipilimumab, and nivolumab in patients with R/M HPV16+ solid tumors (NCT04084951). Additionally, a novel fusion protein in combination with pembrolizumab (HPV16 E7-pHLA-IL2-Fc) is under investigation at several centers (NCT03978689), with results suggesting expansion of E711–20–specific clonotypes (49). Identifying high-affinity tumor antigens while avoiding cross-reactivity with host proteins and emergence of poorly immunogenic neoantigens remains a challenge in vaccine-based therapy in HNSCC.
Table 4. Other immunotherapeutic strategies in HNSCC.
Adoptive T cell therapy.
Adoptive T cell therapy (ACT) — infusing tumor-reactive T cells, expanded tumor-infiltrating T cells (TILs), gene-engineered T cell receptor T (TCR-T) cells, and CAR T cells — represents an opportunity to leverage antigen specificity in HNSCC treatment, though there is a paucity of known antigens in HNSCC (Table 4). Previously, Hinrichs’s group tested ACT using TILs selected for HPV E6 and E7 reactivity (11). TIL therapy is limited by the lengthy process of isolating and expanding TILs, as well as the need for surgical tumor excision from patients. Alternatively, TCR-T cell manufacturing decreases production time. TCR-T cell therapy has been accomplished in HPV-related cancers including cervical cancer and OPSCC using autologous E7 TCR-T cells (50). In a phase I trial of HPV16 E7 TCR-T cell therapy, 50% of patients responded, including several with α–PD-1–refractory disease (50). However, limited progress has been made in the development of CAR T–based therapies in HNSCCs. Although ACT offers the advantage of specifically targeting tumor cells compared with the other immunotherapy strategies, it still faces formidable challenges in the suppressive TME, such as nutrient deprivation, suppressive metabolites, and regulatory immune cell interactions. Combining tumor-specific T cell therapies with agents that overcome these limitations in the TME should increase the effectiveness of this approach.
Mechanisms diminishing the response to current immunotherapeutic strategies
Given the limited success with current immunotherapies in HNSCC, identification of the mechanisms responsible for immunotherapeutic resistance is urgently needed. Potential mechanisms are summarized below and shown in Figure 1.
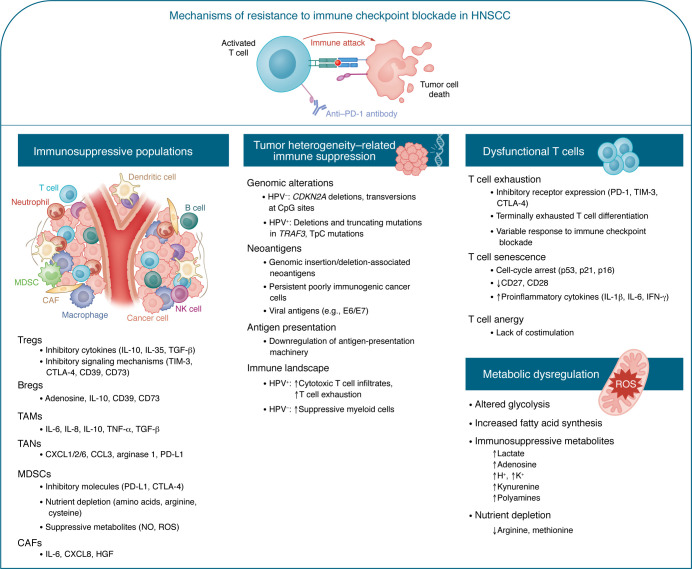
Figure 1. Mechanisms driving resistance to immune checkpoint blockade therapy in HNSCC.
The potential mechanisms driving resistance to antitumor immune responses are illustrated. Overcoming immune-suppressive populations including Tregs, Bregs, tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), myeloid-derived suppressor cells (MDSCs), and cancer-associated fibroblasts (CAFs), as well as immune plasticity, will be critical for enhancing the response to immunotherapy and antitumor immunity. Defining cell-intrinsic features of HPV-related and carcinogen-driven (e.g., smoking) HNSCC will also be fundamental for reducing their tumor-intrinsic immune-suppressive capacity and immune escape mechanisms. Dysfunctional T cells generated by chronic antigen stimulation or T cell senescence induced by the tumor metabolome, proteome, and chemokine/cytokine milieu also impair the effectiveness of the immune response in HNSCC. Inadequate T cell costimulation drives T cell anergy, further impairing this response. Less-well-understood mechanisms driven by metabolic dysregulation impact the antitumor immune response in the tumor microenvironment, for which further work is warranted.
Molecular and immune heterogeneity of HNSCC.
HNSCC tumorigenesis is driven by HPV (HPV+) and/or carcinogens (e.g., smoking and alcohol). Carcinogen-driven (i.e., HPV–) HNSCC is largely mediated by loss-of-function mutations in tumor-suppressor genes (e.g., TP53 and CDKN2A), whereas HPV+ HNSCC carcinogenesis is driven by viral oncoprotein–mediated inactivation of tumor-suppressor genes (6). Tumor molecular heterogeneity drives different immune pathogeneses via several mechanisms (Figures 1 and 2). While mutation rates do not differ by HPV status, there are differences in where mutations tend to occur (e.g., CpG sites) (6). Genomic heterogeneity and instability potentially drive additional mechanisms promoting immune escape (51). Prevalent somatic mutations and indel-derived tumor-specific neoantigens partially account for this heterogeneity. Implications for antitumor immunity include the emergence of dominant tumor antigens that suppress the function of other TCR clonotypes (52). Moreover, cancer immunoediting results in persistent poorly immunogenic cancer cells that can escape the equilibrium phase (53, 54).
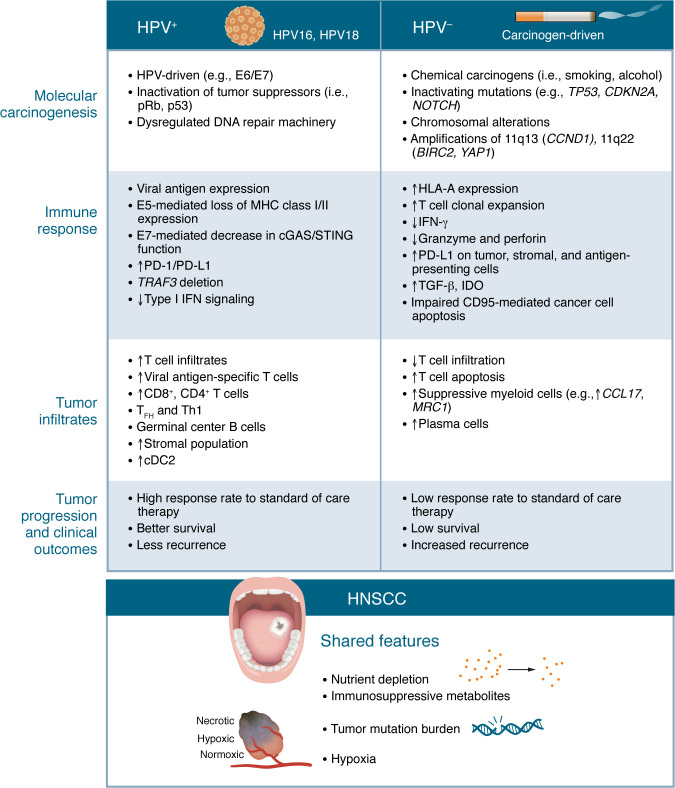
Figure 2. Features driving distinct immune pathogenesis in HPV+ versus HPV– HNSCC.
Unique etiologies of HNSCC drive differential immune responses and pathogenesis. HPV+ HNSCCs are driven by dominant viral antigens (e.g., E6/E7), altered immune checkpoint signaling, and diminished cytosolic DNA–sensing functionality resulting in high levels of T lymphocyte infiltrates and germinal center B cells. In comparison, carcinogen-driven HNSCCs harbor a myeloid-rich, immune-suppressive tumor microenvironment driven by release of regulatory signaling molecules, genomic heterogeneity, and a lack of highly immunogenic neoepitopes. In HPV+ HNSCCs, viral oncoproteins promote loss of tumor suppressor gene expression and viral protein–mediated impairment of immunogenicity and antigen presentation. In HPV– HNSCCs, carcinogens such as those found in tobacco smoke impair CD8+ T cell function. Collectively, these differences are associated with generally better survival outcomes in HPV+ HNSCC compared with HPV– HNSCC. cDC2, type 2 conventional DCs.
More nuanced differences between HPV+ and HPV– HNSCCs also drive variable immune phenotypes and responses, as illustrated in Figure 2. HPV+ and HPV– HNSCCs share some immune features, such as a metabolic milieu detrimental to antitumor immunity (55), chronic antigen stimulation, and variable Treg infiltrates (55–57), but also possess distinct features. HPV– HNSCCs have a more immune-suppressed TME, with high frequencies of PD-1–expressing CD4+ Th1 cells, accumulation of tumor-associated macrophages (TAMs) and MDSCs, and high MHC expression and tumor immunogenicity of tumor antigens (56–61). HPV+ HNSCCs are unique in that the causal agent also accounts for immunogenic antigens provoking tumor-specific responses. HPV+ tumors are notable for enrichment of conventional CD4+ and CD8+ T cells, B cell subsets, and stromal cells and enriched HPV-specific T cells, as well as exhausted T cells (62–66) (Figure 2). Emerging data reveal that HPV gene expression is variable across HPV+ HNSCCs, which may represent another mechanism of immune evasion by these tumors (12). Further work is needed to define the unique molecular and biochemical features driving immune pathogenesis in HPV+ and HPV– HNSCCs.
Suppressive tumor-infiltrating immune cell populations.
Malignant tumors can recruit and/or develop different types of suppressive cells in the TME, such as Tregs (14), tumor-associated neutrophils (TANs) (67), MDSCs (15), TAMs (16), and cancer-associated fibroblasts (CAFs), which promote cancer progression and immune escape, and induce immunotherapy resistance in HNSCC (Figure 1).
CAFs.
An abundance of CAFs is found in the stroma, constituting up to 80% of the cellular composition in late-stage HNSCC (68). CAFs play an important role in HNSCC tumor growth, facilitating proliferation, invasion, migration, and angiogenesis, and promoting treatment resistance (13). Several subtypes of CAFs accumulate in HNSCCs, with most exhibiting protumoral function, such as myofibroblasts with high α–smooth muscle actin (α-SMA) expression, extracellular matrix–expressing (ECM-expressing) CAFs, and MHC II+ CAFs (69, 70). Furthermore, exploration of another phenotype of CAFs expressing elastic fiber differentiation genes revealed a negative prognostic impact on HPV+ HNSCC (69, 70). CAFs can affect tumor cells and immune cells in the TME via multiple mechanisms: (i) The signaling regulatory loop of CAF-derived HGF and HNSCC-derived basic FGF (bFGF) increases oxidative phosphorylation (OXPHOS) in CAFs and glycolysis in HNSCC cells (68); (ii) CAFs and their supernatants suppress T cell proliferation and promote Treg functions (71); (iii) CAFs induce immunotherapy resistance via CD8+ T cell exclusion (72); (iv) CAFs secrete a number of factors that induce protumoral and immunosuppressive macrophage differentiation from monocytes, which suppresses T cell proliferation (73); and (v) CAF-derived TGF-β promotes cetuximab resistance in HNSCC preclinical models (74).
TAMs.
TAMs are a major tumor-infiltrating immune cell subset in HNSCC, playing a key role in tumor growth (75, 76). M2 macrophage infiltrates correlate with aggressive tumor features, lymph node metastases, and poor prognosis in HNSCC (76–78). TAMs also correlate with aggressive clinicopathologic features in HNSCC (16). Under hypoxic stress, TAMs secrete TNF-α, IL-1, IL-6, IL-8, VEGF, GM-CSF, TGF-β, and MMP, promoting tumor angiogenesis and invasion (79). TAMs are the major source of PD-L1 and other immune checkpoint ligands in the HNSCC TME (69). PD-L1+ TAMs are closely associated with CD8+ T cell function, suggesting regulatory cell-cell interactions in HNSCC (69). In addition, TAMs express PD-1, which decreases their phagocytic and cytotoxic potency (80).
MDSCs.
Infiltration of MDSCs is increased in oral cavity squamous cell carcinoma (OCSCC) and correlates with pathological markers and prognosis (81). The inhibitory molecules PD-L1 and CD155 are highly coexpressed on MDSCs from HNSCC patients and associated with tumor progression and decreased cytotoxic T cell infiltrates (82). MDSCs can be phenotypically subdivided into two groups, polymorphonuclear MDSC (PMN-MDSC) and monocytic MDSC (M-MDSC). Increased M-MDSC infiltrates are associated with tumor burden after boron neutron capture therapy for HNSCC (83). PD-L1 is expressed to a greater degree on M-MDSCs than on PMN-MDSCs (84).
TANs.
Neutrophils play a crucial role in HNSCC (67). However, the prognostic significance of TANs in HNSCC is poorly understood (85), which may be related to the variability neutrophil phenotypes, including a cytotoxic antitumor “N1” state and an immunosuppressive protumor “N2” state. The diversity and plasticity of neutrophils contribute to variable immune control, though there is much to be learned.
Tregs and Bregs.
Tregs are present in the systemic circulation and tumors of patients with HNSCC, and are associated with HNSCC outcomes (86–88). A spectrum of Treg phenotypes likely exists in HNSCC. Neuropilin 1 (NRP1) is preferentially expressed on intratumor Tregs in HNSCC, and NRP1+ Tregs are more suppressive and associated with worse outcomes (86). TIM3+ Tregs inhibit T cell proliferation, while TIM3 antagonism relieves Treg-mediated immunosuppression in HNSCC (89, 90). CTLA-4 and CD39 are coexpressed on the majority of tumor-infiltrating Tregs, with a greater capacity for suppression than circulating Tregs in HNSCC. CTLA-4+ Tregs can suppress cetuximab-mediated ADCC, while their depletion restores NK cytolytic function (14, 91). In addition to Tregs, Bregs with potent immunosuppressive function were identified in HNSCC (discussed below) (92).
T cell exhaustion.
Exhausted T cell infiltrates are associated with poor outcomes in HNSCCs (93, 94). PD-1– and CTLA-4–expressing T cells are increased in the systemic circulation of HNSCC patients (95). Two subsets of exhausted T cells, CD8+PD1+TCF1+ progenitor exhausted T cells (Texprog) and CD8+PD-1+TCF1– terminally exhausted T cells (Texterm) have been identified in HNSCCs. Texterm T cells were associated with Treg abundance in TME (96). Furthermore, HPV status correlates with PD-L1 expression and T cell exhaustion in HNSCC (Figure 2). T cells in HPV+ HNSCCs express higher levels of exhaustion markers, including PD-1, TIM3, LAG3, and TIGIT, compared with those in HPV– HNSCC (97). PD-1+ T cells are associated with a favorable outcome in HPV+ HNSCC, perhaps serving as a proxy for activated infiltrating T cells responding to the viral antigens (98, 99). In contrast, HPV– HNSCCs tend to have a higher frequency of dysfunctional PD-1+ TILs, correlating with a worse overall prognosis (94).
T cell senescence.
T cell senescence is another important dysfunctional state with a distinct phenotype and function in chronic infections and cancers (100, 101). Senescent T cells highly express senescence associated β-gal but downregulate the costimulatory molecules CD27 and CD28. Senescent T cells are in a state of cell-cycle arrest, with increased cell cycle–regulatory molecules p16, p21, and p53; however this cell population remains metabolically active, producing high amounts of the proinflammatory cytokines IL-2, TNF, and IFN-γ, as well as the suppressive cytokines IL-10 and TGF-β (101). Senescent T cells have been found among TILs in HNSCCs and other cancers (101–103). HNSCC cell lines can directly induce T cell senescence in vitro (103). Importantly, these tumor-induced senescent T cells exert potent suppressive effects on T cell proliferation and function (103). The phenotypic and functional characteristics of senescent T cells may contribute to diminished ICI responses in HNSCC. Improving our understanding of the mechanisms involved in the induction and regulatory role of senescent T cells in HNSCC may lead to novel immunotherapies.
Metabolic dysregulation in the TME.
Metabolic dysfunction in cancer impacts both tumor and immune cells in HNSCC (104), with the TME characterized by nutrient depletion (105), hypoxia (55), acidity, and suppressive metabolites (106, 107). Tumors manipulate central carbon metabolism, producing lactate through aerobic glycolysis, which can support suppressive Tregs (104) while blunting T and NK cell immune surveillance (108). Tumors also exploit glutamine and lipid metabolism, creating vulnerabilities that hinder T cell function. Furthermore, dysregulated metabolism of glycerolipids, glycerophospholipids, and sphingolipids and upregulation of cholesterol synthesis in HNSCC affect antitumor immune responses (109–111). Tumors can leverage homeostatic mechanisms by release of immunosuppressive metabolites and ions (e.g., spermidine, K+) (112, 113), favoring regulatory immune cells, and suppress antitumor responses (55, 104, 114, 115). Glutaminase and glutamate are enriched in HNSCCs (116) and can be targeted with differential effects on cancer cell and immune function, given the metabolic plasticity of T cells (115). Competition for fatty acids and dysregulated lipid metabolites in the TME diminish CD8+ T cell responses (109–111, 117). Knowledge of differential dependencies on metabolites and nutrients in the TME could equip us with tools to impair tumor cell viability while enhancing antitumor immunity and abrogating immunotherapeutic resistance.
Emerging challenges impacting HNSCC response to immunotherapeutic strategies
Potential mechanisms diminishing immunotherapy responses in HNSCC are discussed above. However, several emerging concepts in tumor immunity are being uncovered, which are critical for enhancing immunotherapy against HNSCC.
Characterizing novel tumor-infiltrating immune cell populations.
Accumulating studies have uncovered the function of certain immune cell types in HNSCC, but there are still cell types whose functions or identity remain unknown or controversial. For example, several studies have demonstrated the existence of γδ T cells in the HNSCC TME, which are associated with poor survival in patients with HNSCC (118–120). However, others found that higher levels of γδ T cells were correlated with lower clinical stages and better OS in HNSCC patients (121). Thus, the precise role of γδ T cells in HNSCC pathogenesis has yet to be elucidated.
Bregs are another poorly defined cell population in HNSCC. Bregs primarily drive immunosuppression, but their effects on tumor progression depend on their phenotypes. Tumor-infiltrating Bregs with a CD19+CD38+CD1d+IgM+CD147+ phenotype have been identified and express key regulatory molecules including IL-10, CD25, and IDO, contributing to suppression of antitumor immune responses (122). CD19+CD24hiCD38hi Bregs preferentially localize in tumor tissue rather than peripheral blood and exhibit higher density in the HNSCC TME relative to CD16+ B cells (123). Adenosine-producing Bregs (CD39+CD73+) suppress effector B cells by inhibiting Bruton’s tyrosine kinase phosphorylation via adenosine (124). Notably, increased CD19+IL-10+ Bregs in OCSCC were associated with CD4+ T cell differentiation into Tregs and worse survival outcomes in patients (125). Increased frequencies of atypical memory (CD27–IgM–IgD–) B cells in OCSCC were associated with lower lymph node metastasis, while CD24hiCD38hi Bregs were associated with higher stage and nodal metastases (126). Deeper understanding of their function is necessary to develop potential treatment combinations that could improve HNSCC outcomes.
Dynamics and plasticity of immune cell subsets.
Tregs exhibit a range of phenotypes and variable associations with outcomes in HNSCC patents (56, 127). Various Treg subsets are found in the TME, including thymic selection–derived Tregs (tTregs), peripherally converted Tregs (pTregs), tr-Tregs (tissue-resident Tregs), and follicular Tregs (Tfr Tregs). The functions of these Treg subsets on antitumor responses remain unclear (128). However, the TME augments Treg phenotype, stability, and plasticity, enabling them to switch phenotypic and functional states. Hypoxia in TME affects Treg function and stability. HIF-1α can repolarize Tregs into Th17 cells by upregulating RORγt while attenuating Treg development (129). Nrp1 is required to maintain the stability and function of tumor-infiltrating Tregs. Nrp1–/– Tregs produce IFN-γ, which undermines the function of WT Tregs. Hypoxia in the HNSCC TME may drive IFN-γ–induced Treg fragility through HIF-1α (130). Comprehensive understanding of the immunological mechanisms responsible for the control of Treg functionality, plasticity, and instability in TME represents a challenge for HNSCC immunotherapy.
TAMs and TANs exhibit phenotypic plasticity, which can be detrimental to tumor control (131). TME induces the polarization of macrophages and TAMs predominantly exhibiting an M1 phenotype at early tumor stages, when antigen presentation drives antitumor CD8+ T cell and NK cell recruitment (132). In the HNSCC TME, tumor cell–derived cytokines and chemokines including IL-6, IL-10, and CCL2 can drive polarization of TAMs toward the M2 phenotype (133). Increased TANs and neutrophil-to-lymphocyte ratio (NLR) were associated with poor prognosis in patients with HNSCC (134, 135). TANs exhibit phenotypic plasticity regulated by TME factors and can be distinguished by an antitumorigenic N1 phenotype or protumorigenic N2 phenotype. TGF-β stimulates N2 and inhibits N1 polarization, while IFN-β promotes N1 and inhibits N2 polarization in the TME. Migration of neutrophils to tumor-draining lymph nodes in HNSCC shapes antitumor immunity in a stage-dependent manner (136). In N0 (without lymph node metastasis) HNSCC, neutrophils can prime T cells, with neutrophil accumulation in T cell–rich zones associated with improved survival. In contrast, neutrophils become immunosuppressive in patients with lymph node metastases and are associated with a poor prognosis. Further understanding of how TAMs and TANs dynamically regulate antitumor immunity is needed to strategically target these cells for enhancing immunotherapeutic responses in HNSCC.
Distinct metabolic dysregulation in tumor and immune cells.
While we provided a broad overview of metabolic features of TME that impair antitumor immune responses and immunotherapy effectiveness above, several unknowns persist. For example, glycolysis, pentose phosphate metabolism, tricarboxylic acid cycle, and glutamine metabolism are upregulated in HNSCC (137), but we do not know how specific cells such as TILs use these metabolites and what functions they serve in immune evasion. Moreover, tumors can leverage homeostatic metabolites to dysregulate antitumor immunity through unclear mechanisms (114). Distinct metabolic spatial phenotypes have been identified (138); however, the role of these spatial features in driving metastasis, treatment resistance, and immune evasion in HNSCC is unknown. Dysregulated lipid metabolism is also present in HNSCC (139–141). Preclinical data suggest that inhibiting cholesterol synthesis may enhance immunotherapy responses (141). Therefore, additional work is needed to parse the specific mechanisms, substrates, and enzyme kinetics involved in cell-intrinsic metabolism, immune evasion, and response to immunotherapies in HNSCC.
HPV and HPV-specific immunity in HNSCC pathogenesis.
While much is known about the pathogenesis, progression, and therapeutic outcomes in HPV+ HNSCC (142), the molecular processes regulating HPV-mediated immune evasion and responses to immunotherapy remain under investigation (Figure 2). Although HPV viral antigens represent a tumor-specific biomarker, the distribution of viral antigen expression in tumor cells and molecular controls of viral antigen expression must be defined (143, 144). Furthermore, there are several challenges to targeting viral antigens, including MHC-restricted cytotoxic T cell dysfunction and viral molecular mimicry of human proteins (e.g., of HPV16 E7), which could be due to HPV-mediated disruption of antigen processing and presentation (145, 146) and disruption of chemokine and cytokine expression (147, 148). There are likely numerous other undiscovered mechanisms through which HPV drives immune escape, such as metabolic dysregulation, chronic T cell stimulation, impaired coactivation, or genomic alterations. Identifying these mechanisms will equip us to strategically target HPV-intrinsic mechanisms for HNSCC treatment (149).
Novel therapeutic strategies and combinations
Despite the aforementioned challenges in enhancing antitumor immune responses to immunotherapy, forthcoming therapeutic options target specific cell populations, patient- and TME-specific immunotherapy combinations, and metabolic reprogramming (Figure 3).
Figure 3. Novel strategies for overcoming immunotherapy resistance in HNSCC.
(A) ICI can be enhanced by decreasing inhibitory T cell receptor signaling, reinvigorating dysfunctional T cells, and modulating metabolic pathways in cancer cells and suppressive immune populations. Neoadjuvant ICI can increase the antitumor immune response and debulk tumors prior to ablative surgery or cytotoxic therapy. Combining cytotoxic therapies with immunotherapy may also improve the antitumor immune response via dendritic cell activation and T cell priming, activation of pro-death signaling in tumor cells, and release of DAMPs. (B) Reversing T cell senescence may also be accomplished through MAPK pathway inhibition, lipid metabolism modulation, and DNA damage blockade. Inhibiting the ability of cancer cells and Tregs to induce T cell senescence offers a novel opportunity for increasing ICI responses. (C) Metabolic reprogramming of tumor cells and Tregs also provides novel strategies for HNSCC treatment. (D) Targeting suppressive immune and stromal populations will be critical for altering the overall balance of cytotoxic/effector to regulatory responses in the TME.
Neoadjuvant immunotherapy.
Neoadjuvant immunotherapy is poised to revolutionize HNSCC management in the definitive and R/M treatment settings, with the groundbreaking phase III KEYNOTE-689 trial setting the stage for the next era of HNSCC therapy. Multiple neoadjuvant immunotherapy studies have been completed or are ongoing (150–153) (Table 5). Neoadjuvant ICI may enhance long-term tumor control by (i) rejuvenating tumor-specific cytotoxic lymphocytes and trafficking to micrometastatic deposits while enhancing DC presentation of tumor antigens to T cells (154); (ii) increasing antigen-specific responses to a diverse neoantigen repertoire (52); and (iii) increasing systemic immunity (155). Patients in KEYNOTE-689 (NCT03765918) were randomized to receive either neoadjuvant pembrolizumab followed by surgery and adjuvant pembrolizumab or standard surgical resection with adjuvant SoC therapy (156). Unpublished findings prelude improvements in key primary and secondary outcomes (19). Other innovative approaches such as neoadjuvant bintrafusp alfa, an engineered fusion protein targeting PD-L1 and TGF-β signaling, demonstrate enhanced systemic immunity and antigen-specific T cell responses (155). Neoadjuvant α–PD-1/α–CTLA4 therapy using HNSCC samples from the IMCISION trial identified a decrease in activated Tregs and dysfunctional CD8+ T cells (157). Leveraging samples from a phase II trial of neoadjuvant nivolumab or nivolumab/ipilimumab in patients with untreated oral OCSCC illustrated increased local and systemic antitumor immunity (151, 158). Neoadjuvant immunotherapy (159), neoadjuvant chemoimmunotherapy (160, 161), and neoadjuvant radiation coupled with immunotherapy (NCT03635164, NCT03247712) (162, 163) in combination are under investigation. Further studies are urgently needed to dissect the mechanisms and optimize treatment combinations driving responses to neoadjuvant immunotherapy in HNSCC.
Table 5. Neoadjuvant immunotherapy in HNSCC.
Immunotherapy combinations.
Strategically targeting multiple limbs of the immune response may enhance outcomes. Trials testing inhibition of immune-regulatory signaling molecules (e.g., LAG3, TIGIT, TIM-3, CD266, PVRIG, STING, and CD96) are currently underway (Figure 3). These approaches are intended to overcome compensatory upregulation of known and unknown immune-regulatory checkpoint molecules, which may drive adaptive resistance to ICI (164). In one clinical case report, a patient with SoC-refractory HNSCC was successfully treated with the combination of nivolumab and ipilimumab (165). Clinical studies of eftilagimod and relatlimab (LAG-3 inhibitors) for HNSCC are underway. The TACTI-002 trial (NCT03625323) combining eftilagimod with pembrolizumab in second-line metastatic HNSCC observed encouraging antitumor activity (166). Two clinical studies (NCT04080804 and NCT04326257) are treating HNSCC patients with the combination of relatlimab and nivolumab, or nivolumab and ipilimumab, to assess clinical activity. Combined TIGIT and PD-1/PD-L1 blockade is also under investigation, including the combination of neoadjuvant tiragolumab and atezolizumab (NCT03708224 and NCT04665843); α-TIGIT humanized mAbs MK-7684 (NCT05007106) and ASP8374 (NCT03260322) in combination with pembrolizumab; and BMS-986207 in combination with nivolumab (NCT02913313). Additional novel immunotherapy strategies have targeted TGF-β or combined ICI with histone deacetylase inhibition (167, 168) (Table 4). A deeper understanding of T cell activation signaling should drive the rational implementation of forthcoming trials to test for evidence of optimized combinations in HNSCC immunotherapy.
Targeting senescent T cells in the TME.
Targeting T cell senescence is an emerging concept in tumor immunotherapy (Figure 3) (100, 101, 169). Tregs and tumor cells can induce T cell senescence by triggering effector T cell DNA damage (169–173). Blockage of DNA damage in T cells can prevent T cell senescence and enhance antitumor immunity in both melanoma and breast cancer tumor models (172). Importantly, combining α–PD-L1 checkpoint blockade with DNA damage inhibition to abrogate T cell senescence can synergistically enhance antitumor immunity in those models (172). In addition, activation of MAPK signaling is another important molecular process responsible for development of T cell senescence induced by Tregs and tumor cells in the TME (170–172). Blocking MAPK signaling can also prevent T cell senescence and promote the antitumor efficacy of α–PD-L1 therapy in melanoma and breast cancer tumor models (172). These studies indicate that prevention of senescence in T cells could be an important checkpoint and effective strategy for enhancing HNSCC immunotherapy.
Targeting suppressive myeloid cell and stromal cell populations.
Immunosuppressive myeloid cell populations can promote tumorigenesis and contribute to the therapy resistance in HNSCC. SX-682, an inhibitor of the myeloid chemokine receptors CXCR1 and CXCR2, inhibited MDSC trafficking and accumulation but enhanced NK cell infiltration, activation, and function in a mouse HNSCC model (174). Immunosuppressive neutrophils upregulate CD36 and fatty acid transport protein 2 (FATP2), which are involved in lipid trafficking in the tumor-bearing mice and human HNSCC. Targeting neutrophil lipid metabolism through FATP2 inhibition reduces the suppressive activity of neutrophils in preclinical models (175). Targeting of the CD47/SIRPa axis on TAMs is another novel opportunity to promote antitumor immunity (176). This is currently being assessed in phase II clinical trials in HNSCC (NCT04854499 and NCT04675294) and oropharynx cancer (NCT05787639). Strategies targeting CAFs in HNSCC are also developing, including CAF reversion or normalization, CAF depletion, targeting ECM, and blocking the immunomodulatory effect of secreted molecules from CAFs or relevant downstream pathways (177). Collectively, these suppressive myeloid and stromal cell–mediated therapeutic responses and should be considered in personalized treatment of HNSCC.
Leveraging metabolism to reprogram the TME.
A major challenge in treating HNSCC is overcoming metabolic changes in the TME that promote cancer cell–intrinsic treatment resistance and impair antitumor immunity (178, 179). The increased glucose uptake and enhanced glycolysis characteristic of HNSCC appear to make HNSCC susceptible to targeted therapies involving glycolytic inhibitors (179). Key molecules in glycolysis such as HK, PKM2, and GLUT are promising targets for HNSCC treatment. The HK2 inhibitor 2-DG decreases glycolysis and inhibits cell proliferation in HNSCC cell lines (180). Upregulated mTOR signaling is associated with metabolic dysregulation and increased expression of PKM2, PDK1, HIF-1α, LDH, and GLUT1 in HNSCC (181). Inhibition of mTOR signaling with rapamycin reduces tumor growth in HNSCC (182). An oral antihyperglycemic agent, metformin, can affect cell proliferation and antitumor activity in HNSCC through AMPK activation and mTOR inhibition by targeting mitochondrial complex I in HNSCC cells (183). Altering tumor cell metabolism in combination with immune modulation may enhance ICI in HNSCC. A phase II therapeutic trial (NCT04114136) of metformin or rosiglitazone combined with α–PD-1 therapy in solid tumors is currently recruiting participants, aiming to determine whether these compounds synergize with α–PD-1 therapy. Notably, more-selective strategies specifically targeting metabolism in tumor cells will need to avoid inadvertent impairment of tumor-specific cytolytic T cell function. Furthermore, effective metabolic interventions can synergize with immunotherapy and offer novel and promising strategies for enhancing the effectiveness of ICI.
Lipid metabolism reprogramming is also a critical hallmark of HNSCC linked to the carcinogenesis and development of HNSCC (109). Fatty acid synthase (FASN) is overexpressed and associated with aggressiveness, prognosis, and risk of metastasis in OCSCC. Inhibitors targeting FASN, including TVB-3166, C75, and triclosan, have anticancer effects on OCSCC cell lines, with decreased proliferation, migration, and invasion (184, 185). The FASN inhibitor orlistat reduces the volume of primary tumors and lymph node metastases in an orthotopic OCSCC mouse model (186). Notably, metabolic differences between HPV-related and carcinogen-driven HNSCCs should be taken into account to identify the optimal metabolic treatment strategy, although targeting energetic metabolism is a promising anticancer therapy for HNSCC treatment (187).
Future perspectives
Immunotherapy is a promising strategy in HNSCC. However, several hurdles remain. Potential mechanisms of ICI resistance include molecular and immune heterogeneity coupled with high levels of regulatory immune cell populations, T cell dysfunction, and metabolic dysregulation in the TME. To enhance ICI, more effort is needed to define the function and role of novel tumor-infiltrating immune cell populations, the tumor-intrinsic adaptations that promote immune suppression, the developmental trajectories and plasticity of immune cell populations, and strategies for overcoming metabolic dysregulation. Overcoming challenges to ICI by targeting novel combinations of immune checkpoint molecules and reinvigorating dysfunctional T cells will also be important. Leveraging novel techniques, including single-cell RNA sequencing, spatial multispectral imaging, and multiomics strategies to better understand the TME at a single-cell and molecular resolution will aid these endeavors.
Identification of reliable biomarkers for predicting immunotherapy response in HNSCC remains a critical area of research. PD-L1 expression has been the most widely studied biomarker and is routinely used to guide the use of ICIs. However, its utility is limited due to variable expression thresholds and response rates. Tumor mutational burden (TMB) and microsatellite instability (MSI) are additional biomarkers that have shown promise in other cancer types, but their predictive value in HNSCC has been less robust (188, 189). Emerging biomarkers, such as circulating tumor DNA (ctDNA), and the composition of the TME, including density and activity of infiltrating T cells and regulatory cells, are being investigated. Additionally, expression of HPV-related viral antigens may guide immunotherapies such as tumor vaccines or engineered TCR T cells (50). Advances in multiomics profiling and artificial intelligence are facilitating the discovery of composite biomarkers that integrate genetic, transcriptomic, and proteomic data, which may enhance the precision of immunotherapy in HNSCC.
Developing novel combination therapies targeting regulatory cell subsets, dysfunctional T cells, and the dysregulated metabolite and nutrient milieu of the TME will also be important for advancing immune responses and long-lasting systemic immunity to HNSCC. Reversing T cell exhaustion secondary to chronic antigen stimulation will require a detailed understanding of how the frequency of antigen-positive tumor cells and strength of the peptide/MHC-TCR interaction influence T cell fate (190). Developing computational and bench models to identify and quantify putative neoantigens and test their binding affinity may help us understand which T cell infiltrates require reinvigoration via immune checkpoint antagonism versus those that would benefit from targeting regulatory cell subsets. Furthermore, overcoming the nutrient-depleted, acidic, hypoxic TME will further optimize responses to these therapies. In addition, dissecting the fundamental immune microenvironment differences and heterogeneity between HPV+ and HPV– HNSCC will elucidate the mechanisms by which tumors evade the endogenous immune response. In parallel, it will be important to define how other viruses such as Epstein-Barr virus influence the tumor immune landscape in HNSCC. Finally, targeting metabolic vulnerabilities such as lactate, glutamine, polyamine, and lipid metabolism is urgently needed in HNSCC (114, 115, 171). Mounting data highlight the plasticity and adaptability of immune cells — such as Tregs’ use of lactate as a fuel source and alternative T cell nutrient utilization — while tumor cells succumb to metabolic disruption (115). Identifying how metabolites and nutrients are differentially used by tumors and immune cells will permit strategic development of novel therapies.
Acknowledgments
This work was partially supported by grants from the Melanoma Research Alliance (No. 621833 to GP), the NIH (CA184379, CA242188, CA237149, AI173260, AG078822, and AG067441 to GP), the American Head and Neck Society/American Academy of Otolaryngology–Head and Neck Surgery Foundation (AHNS/AAO-HNSF) Young Investigator Combined Award (to RAH), and the Dean’s Scholar Burroughs Wellcome Fund Physician-Scientist Institutional Award (to RAH).
Version 1. 04/15/2025
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2025, Liu et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2025;135(8):e188128. https://doi.org/10.1172/JCI188128.
Contributor Information
Xia Liu, Email: lxia@wustl.edu.
R. Alex Harbison, Email: hrichard@wustl.edu.
Mark A. Varvares, Email: mark_varvares@meei.harvard.edu.
Sidharth V. Puram, Email: sidpuram@wustl.edu.
Guangyong Peng, Email: pengg@wustl.edu.
References
- 1.Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 Cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Pignon JP, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Bernier J, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JS, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 5.Gillison ML, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393(10166):40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Network TCGA. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Comprehensive Cancer Network. Head and Neck Cancers, Version 3.2024). https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1437 Accessed March 20, 2025.
- 8.Ferris RL, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen EEW, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal C, et al. Safety and efficacy of MEDI0457 plus durvalumab in patients with human papillomavirus-associated recurrent/metastatic head and neck squamous cell carcinoma. Clin Cancer Res. 2023;29(3):560–570. doi: 10.1158/1078-0432.CCR-22-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevanović S, et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin Cancer Res. 2019;25(5):1486–1493. doi: 10.1158/1078-0432.CCR-18-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puram SV, et al. Cellular states are coupled to genomic and viral heterogeneity in HPV-related oropharyngeal carcinoma. Nat Genet. 2023;55(4):640–650. doi: 10.1038/s41588-023-01357-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puram SV, et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell. 2017;171(7):1611–1624. doi: 10.1016/j.cell.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jie H-B, et al. CTLA-4+ regulatory T cells increased in cetuximab-treated head and neck cancer patients suppress nk cell cytotoxicity and correlate with poor prognosis. Cancer Res. 2015;75(11):2200–2210. doi: 10.1158/0008-5472.CAN-14-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen WC, et al. Inflammation-induced myeloid-derived suppressor cells associated with squamous cell carcinoma of the head and neck. Head Neck. 2017;39(2):347–355. doi: 10.1002/hed.24595. [DOI] [PubMed] [Google Scholar]
- 16.Kumar AT, et al. Prognostic significance of tumor-associated macrophage content in head and neck squamous cell carcinoma: a meta-analysis. Front Oncol. 2019;9:656. doi: 10.3389/fonc.2019.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Migden MR, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 19. Merck. Merck’s KEYTRUDA (pembrolizumab) Met Primary Endpoint of Event-Free Survival (EFS) as Perioperative Treatment Regimen in Patients With Resected, Locally Advanced Head and Neck Squamous Cell Carcinoma. https://www.merck.com/news/mercks-keytruda-pembrolizumab-met-primary-endpoint-of-event-free-survival-efs-as-perioperative-treatment-regimen-in-patients-with-resected-locally-advanced-head-and-neck-squamous-c Updated October 8, 2024. Accessed February 17, 2025.
- 20.Franken A, et al. CD4+ T cell activation distinguishes response to anti-PD-L1+anti-CTLA4 therapy from anti-PD-L1 monotherapy. Immunity. 2024;57(3):541–558. doi: 10.1016/j.immuni.2024.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Haddad RI, et al. Nivolumab plus ipilimumab versus EXTREME regimen as first-line treatment for recurrent/metastatic squamous cell carcinoma of the head and neck: the final results of Checkmate 651. J Clin Oncol. 2023;41(12):2166–2180. doi: 10.1200/JCO.22.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrington KJ, et al. Efficacy and safety of nivolumab plus ipilimumab vs nivolumab alone for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck: the phase 2 CheckMate 714 Randomized Clinical Trial. JAMA Oncol. 2023;9(6):779–789. doi: 10.1001/jamaoncol.2023.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferris RL, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020;31(7):942–950. doi: 10.1016/j.annonc.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Psyrri A, et al. Durvalumab with or without tremelimumab versus the EXTREME regimen as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck: KESTREL, a randomized, open-label, phase III study. Ann Oncol. 2023;34(3):262–274. doi: 10.1016/j.annonc.2022.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Pitroda SP, et al. Integration of radiotherapy and immunotherapy for treatment of oligometastases. Lancet Oncol. 2019;20(8):e434–e442. doi: 10.1016/S1470-2045(19)30157-3. [DOI] [PubMed] [Google Scholar]
- 26.Korpics MC, et al. Partial tumor irradiation plus pembrolizumab in treating large advanced solid tumor metastases. J Clin Invest. 2023;133(10):e162260. doi: 10.1172/JCI162260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mell LK, et al. Radiotherapy with durvalumab vs. Cetuximab in patients with locoregionally advanced head and neck cancer and a contraindication to cisplatin: phase II results of NRG-HN004. Int J Radiat Oncol Biol Phys. 2022;114(5):1058. doi: 10.1016/j.ijrobp.2022.09.003. [DOI] [Google Scholar]
- 28.Marciscano AE, et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin Cancer Res. 2018;24(20):5058–5071. doi: 10.1158/1078-0432.CCR-17-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spanos WC, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135(11):1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 30.Park SJ, et al. Cisplatin and oxaliplatin induce similar immunogenic changes in preclinical models of head and neck cancer. Oral Oncol. 2019;95:127–135. doi: 10.1016/j.oraloncology.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galsky MD, et al. Immunomodulatory effects and improved outcomes with cisplatin- versus carboplatin-based chemotherapy plus atezolizumab in urothelial cancer. Cell Rep Med. 2024;5(2):101393. doi: 10.1016/j.xcrm.2024.101393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zandberg DP, et al. A randomized phase II study of concurrent vs. sequential pembrolizumab with chemoradiation (CRT) in locally advanced head and neck cancer (LA HNSCC): 4-year results and tumor-immune microenvironment analysis. Ann Oncol. 2023;34:S554–S93. doi: 10.1016/j.annonc.2023.09.2002. [DOI] [Google Scholar]
- 33.Burtness B, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 34.Machiels J-P, et al. Pembrolizumab plus concurrent chemoradiotherapy versus placebo plus concurrent chemoradiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck (KEYNOTE-412): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2024;25(5):572–587. doi: 10.1016/S1470-2045(24)00100-1. [DOI] [PubMed] [Google Scholar]
- 35.Lee NY, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22(4):450–462. doi: 10.1016/S1470-2045(20)30737-3. [DOI] [PubMed] [Google Scholar]
- 36.Taylor RJ, et al. Ex vivo antibody-dependent cellular cytotoxicity inducibility predicts efficacy of cetuximab. Cancer Immunol Res. 2015;3(5):567–574. doi: 10.1158/2326-6066.CIR-14-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srivastava RM, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19(7):1858–1872. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonner JA, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 39.Bonner JA, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 40.Sacco AG, et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021;22(6):883–892. doi: 10.1016/S1470-2045(21)00136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourhis J, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Ann Oncol. 2021;32 doi: 10.1016/j.annonc.2021.08.2112. [DOI] [PubMed] [Google Scholar]
- 42.Saba NF, et al. Pembrolizumab and cabozantinib in recurrent metastatic head and neck squamous cell carcinoma: a phase 2 trial. Nat Med. 2023;29(4):880–887. doi: 10.1038/s41591-023-02275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kao HF, et al. Afatinib and pembrolizumab for recurrent or metastatic head and neck squamous cell carcinoma (ALPHA Study): a phase II study with biomarker analysis. Clin Cancer Res. 2022;28(8):1560–1571. doi: 10.1158/1078-0432.CCR-21-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor MH, et al. Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J Clin Oncol. 2020;38(11):1154–1163. doi: 10.1200/JCO.19.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siu LL, et al. Phase III LEAP-010 study: first-line pembrolizumab with or without lenvatinib in recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) J Clin Oncol. 2020;38(15_suppl):TPS6589. doi: 10.1200/JCO.2020.38.15_suppl.TPS6589. [DOI] [Google Scholar]
- 46.Song S, et al. Human papillomavirus type 16 E6 and E7 oncogenes abrogate radiation-induced DNA damage responses in vivo through p53-dependent and p53-independent pathways. Proc Natl Acad Sci U S A. 1998;95(5):2290–2295. doi: 10.1073/pnas.95.5.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Tourneau C, et al. Phase Ib/II trial of TG4001 (Tipapkinogene sovacivec), a therapeutic HPV-vaccine, and Avelumab in patients with recurrent/metastatic (R/M) HPV-16+ cancers. Ann Oncol. 2019;30:v494–v5. doi: 10.1093/annonc/mdz253.035. [DOI] [Google Scholar]
- 48.Wood L, et al. Preliminary Safety of PDS0101 (Versamune +HPVmix) and Pembrolizumab Combination Therapy in Subjects with Recurrent/Metastatic Human Papillomavirus-16 Positive Oropharyngeal Squamous Cell Carcinoma (OPSCC) Int J Radiat Oncol Biol Phys. 2022;112(5):e37–e8. doi: 10.1016/j.ijrobp.2021.12.087. [DOI] [Google Scholar]
- 49.Chung CH, et al. A phase 1 dose-escalation and expansion study of CUE-101, a novel HPV16 E7-pHLA-IL2-Fc fusion protein, given as monotherapy and in combination with pembrolizumab in patients with recurrent/metastatic HPV16+ head and neck cancer. J Clin Oncology. 2023;41(16_suppl):6013. doi: 10.1200/JCO.2023.41.16_suppl.6013. [DOI] [Google Scholar]
- 50.Nagarsheth NB, et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat Med. 2021;27(3):419–425. doi: 10.1038/s41591-020-01225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mroz EA, Rocco JW. MATH, a novel measure of intratumor genetic heterogeneity, is high in poor-outcome classes of head and neck squamous cell carcinoma. Oral Oncol. 2013;49(3):211–215. doi: 10.1016/j.oraloncology.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedman J, et al. Neoadjuvant PD-1 immune checkpoint blockade reverses functional immunodominance among tumor antigen-specific T cells. Clin Cancer Res. 2020;26(3):679–689. doi: 10.1158/1078-0432.CCR-19-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schreiber RD, et al. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 54.Thorsson V, et al. The immune landscape of cancer. Immunity. 2018;48(4):812–830. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zandberg DP, et al. Tumor hypoxia is associated with resistance to PD-1 blockade in squamous cell carcinoma of the head and neck. J Immunother Cancer. 2021;9(5):e002088. doi: 10.1136/jitc-2020-002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mandal R, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1(17):e89829. doi: 10.1172/jci.insight.89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muijlwijk T, et al. Comparative analysis of immune infiltrates in head and neck cancers across anatomical sites. J Immunother Cancer. 2024;12(1):e007573. doi: 10.1136/jitc-2023-007573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Desrichard A, et al. Tobacco smoking-associated alterations in the immune microenvironment of squamous cell carcinomas. J Natl Cancer Inst. 2018;110(12):1386–1392. doi: 10.1093/jnci/djy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hernandez CP, et al. Effects of cigarette smoke extract on primary activated T cells. Cell Immunol. 2013;282(1):38–43. doi: 10.1016/j.cellimm.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saloura V, et al. Characterization of the T-cell receptor repertoire and immune microenvironment in patients with locoregionally advanced squamous cell carcinoma of the head and neck. Clin Cancer Res. 2017;23(16):4897–4907. doi: 10.1158/1078-0432.CCR-17-0103. [DOI] [PubMed] [Google Scholar]
- 61.Hayes DN, et al. Genetic landscape of human papillomavirus-associated head and neck cancer and comparison to tobacco-related tumors. J Clin Oncol. 2015;33(29):3227–3234. doi: 10.1200/JCO.2015.62.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cillo AR, et al. Immune landscape of viral- and carcinogen-driven head and neck cancer. Immunity. 2020;52(1):183–199. doi: 10.1016/j.immuni.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ashrafi GH, et al. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int J Cancer. 2005;113(2):276–283. doi: 10.1002/ijc.20558. [DOI] [PubMed] [Google Scholar]
- 64.Luo X, et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J Clin Invest. 2020;130(4):1635–1652. doi: 10.1172/JCI129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaikh MH, et al. cGAS-STING responses are dampened in high-risk HPV type 16 positive head and neck squamous cell carcinoma cells. Microb Pathog. 2019;132:162–165. doi: 10.1016/j.micpath.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 66.Liu C, et al. Increased expression of PD‑L1 by the human papillomavirus 16 E7 oncoprotein inhibits anticancer immunity. Mol Med Rep. 2017;15(3):1063–1070. doi: 10.3892/mmr.2017.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sounbuli K, et al. Diverse neutrophil functions in cancer and promising neutrophil-based cancer therapies. Int J Mol Sci. 2022;23(24):15827. doi: 10.3390/ijms232415827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar D, et al. Cancer-associated fibroblasts drive glycolysis in a targetable signaling loop implicated in head and neck squamous cell carcinoma progression. Cancer Res. 2018;78(14):3769–3782. doi: 10.1158/0008-5472.CAN-17-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kürten CHL, et al. Investigating immune and non-immune cell interactions in head and neck tumors by single-cell RNA sequencing. Nat Commun. 2021;12(1):7338. doi: 10.1038/s41467-021-27619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q, et al. Integrated analysis of single-cell RNA-seq and bulk RNA-seq reveals distinct cancer-associated fibroblasts in head and neck squamous cell carcinoma. Ann Transl Med. 2021;9(12):1017. doi: 10.21037/atm-21-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi H, et al. Immunosuppressive activity of cancer-associated fibroblasts in head and neck squamous cell carcinoma. Cancer Immunol Immunother. 2015;64(11):1407–1417. doi: 10.1007/s00262-015-1742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ford K, et al. NOX4 inhibition potentiates immunotherapy by overcoming cancer-associated fibroblast-mediated CD8 T-cell exclusion from tumors. Cancer Res. 2020;80(9):1846–1860. doi: 10.1158/0008-5472.CAN-19-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takahashi H, et al. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget. 2017;8(5):8633–8647. doi: 10.18632/oncotarget.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yegodayev KM, et al. TGF-beta-activated cancer-associated fibroblasts limit cetuximab efficacy in preclinical models of head and neck cancer. Cancers (Basel) 2020;12(2):339. doi: 10.3390/cancers12020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Utispan K, Koontongkaew S. Fibroblasts and macrophages: Key players in the head and neck cancer microenvironment. J Oral Biosci. 2017;59(1):23–30. doi: 10.1016/j.job.2016.11.002. [DOI] [Google Scholar]
- 76.Hu Y, et al. Tumor-associated macrophages correlate with the clinicopathological features and poor outcomes via inducing epithelial to mesenchymal transition in oral squamous cell carcinoma. J Exp Clin Cancer Res. 2016;35:12. doi: 10.1186/s13046-015-0281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marcus B, et al. Prognostic factors in oral cavity and oropharyngeal squamous cell carcinoma. Cancer. 2004;101(12):2779–2787. doi: 10.1002/cncr.20701. [DOI] [PubMed] [Google Scholar]
- 78.Weber M, et al. Small oral squamous cell carcinomas with nodal lymphogenic metastasis show increased infiltration of M2 polarized macrophages – An immunohistochemical analysis. J Craniomaxillofac Surg. 2014;42(7):1087–1094. doi: 10.1016/j.jcms.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 79.Vaupel P, Multhoff G. Hypoxia-/HIF-1α-driven factors of the tumor microenvironment impeding antitumor immune responses and promoting malignant progression. Adv Exp Med Biol. 2018;1072:171–175. doi: 10.1007/978-3-319-91287-5_27. [DOI] [PubMed] [Google Scholar]
- 80.Gordon SR, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pang X, et al. Myeloid derived suppressor cells contribute to the malignant progression of oral squamous cell carcinoma. PLoS One. 2020;15(2):e0229089. doi: 10.1371/journal.pone.0229089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mao L, et al. TIGIT/CD155 blockade enhances anti-PD-L1 therapy in head and neck squamous cell carcinoma by targeting myeloid-derived suppressor cells. Oral Oncol. 2021;121:105472. doi: 10.1016/j.oraloncology.2021.105472. [DOI] [PubMed] [Google Scholar]
- 83.Chang CH, et al. Targeting M-MDSCs enhances the therapeutic effect of BNCT in the 4-NQO-induced murine head and neck squamous cell carcinoma model. Front Oncol. 2023;13:1263873. doi: 10.3389/fonc.2023.1263873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cassetta L, et al. Differential expansion of circulating human MDSC subsets in patients with cancer, infection and inflammation. J Immunother Cancer. 2020;8(2):e001223. doi: 10.1136/jitc-2020-001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brunkhorst H, et al. Neutrophils in HNSCC can be associated with both a worse or favorable prognosis. Biomolecules. 2024;14(2):205. doi: 10.3390/biom14020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chuckran CA, et al. Prevalence of intratumoral regulatory T cells expressing neuropilin-1 is associated with poorer outcomes in patients with cancer. Sci Transl Med. 2021;13(623):eabf8495. doi: 10.1126/scitranslmed.abf8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strauss L, et al. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13(21):6301–6311. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]
- 88.Seminerio I, et al. Infiltration of FoxP3+ regulatory T cells is a strong and independent prognostic factor in head and neck squamous cell carcinoma. Cancers (Basel) 2019;11(2):227. doi: 10.3390/cancers11020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Z, et al. Novel effector phenotype of Tim-3+ regulatory T cells leads to enhanced suppressive function in head and neck cancer patients. Clin Cancer Res. 2018;24(18):4529–4538. doi: 10.1158/1078-0432.CCR-17-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu J-F, et al. Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer. J Exp Clin Cancer Res. 2018;37(1):44. doi: 10.1186/s13046-018-0713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okuyama K, et al. Tumor microenvironmental modification by the current target therapy for head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2023;42(1):114. doi: 10.1186/s13046-023-02691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gavrielatou N, et al. The role of B cells in head and neck cancer. Cancers (Basel) 2021;13(21):5383. doi: 10.3390/cancers13215383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao Y, et al. Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment. Cell Mol Immunol. 2020;17(1):27–35. doi: 10.1038/s41423-019-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kansy BA, et al. PD-1 status in CD8+ T cells associates with survival and anti-PD-1 therapeutic outcomes in head and neck cancer. Cancer Res. 2017;77(22):6353–6364. doi: 10.1158/0008-5472.CAN-16-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lechner A, et al. Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget. 2017;8(27):44418–44433. doi: 10.18632/oncotarget.17901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang D, et al. A comprehensive profile of TCF1+ progenitor and TCF1– terminally exhausted PD-1+CD8+ T cells in head and neck squamous cell carcinoma: implications for prognosis and immunotherapy. Int J Oral Sci. 2022;14(1):8. doi: 10.1038/s41368-022-00160-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gameiro SF, et al. Treatment-naïve HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV- counterparts that has implications for immunotherapy. Oncoimmunology. 2018;7(10):e1498439. doi: 10.1080/2162402X.2018.1498439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Badoual C, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73(1):128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 99.Partlová S, et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology. 2015;4(1):e965570. doi: 10.4161/21624011.2014.965570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ye J, et al. Human regulatory T cells induce T-lymphocyte senescence. Blood. 2012;120(10):2021–2031. doi: 10.1182/blood-2012-03-416040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu X, et al. Senescent T cells within suppressive tumor microenvironments: emerging target for tumor immunotherapy. J Clin Invest. 2020;130(3):1073–1083. doi: 10.1172/JCI133679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsukishiro T, et al. Rapid turnover of the CD8(+)CD28(–) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52(10):599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ye J, et al. TLR8 signaling enhances tumor immunity by preventing tumor-induced T-cell senescence. EMBO Mol Med. 2014;6(10):1294–1311. doi: 10.15252/emmm.201403918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Watson MJ, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. 2021;591(7851):645–651. doi: 10.1038/s41586-020-03045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bian Y, et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature. 2020;585(7824):277–282. doi: 10.1038/s41586-020-2682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Notarangelo G, et al. Oncometabolite d-2HG alters T cell metabolism to impair CD8+ T cell function. Science. 2022;377(6614):1519–1529. doi: 10.1126/science.abj5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi D, et al. USP14 promotes tryptophan metabolism and immune suppression by stabilizing IDO1 in colorectal cancer. Nat Commun. 2022;13(1):5644. doi: 10.1038/s41467-022-33285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brand A, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24(5):657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 109.Liang J, et al. Lipid metabolism reprogramming in head and neck cancer. Front Oncol. 2023;13:1271505. doi: 10.3389/fonc.2023.1271505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Y, et al. High SQLE expression and gene amplification correlates with poor prognosis in head and neck squamous cell carcinoma. Cancer Manag Res. 2021;13:4709–4723. doi: 10.2147/CMAR.S305719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu X, et al. Targeting epigenetic modulation of cholesterol synthesis as a therapeutic strategy for head and neck squamous cell carcinoma. Cell Death Dis. 2021;12(5):482. doi: 10.1038/s41419-021-03760-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Medina CB, et al. Metabolites released from apoptotic cells act as tissue messengers. Nature. 2020;580(7801):130–135. doi: 10.1038/s41586-020-2121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vodnala SK, et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science. 2019;363(6434):eaau0135. doi: 10.1126/science.aau0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harbison RA, et al. Interrogation of T cell-enriched tumors reveals prognostic and immunotherapeutic implications of polyamine metabolism. Cancer Res Commun. 2022;2(7):639–652. doi: 10.1158/2767-9764.CRC-22-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leone RD, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366(6468):1013–1021. doi: 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kamarajan P, et al. Head and neck squamous cell carcinoma metabolism draws on glutaminolysis, and stemness is specifically regulated by glutaminolysis via aldehyde dehydrogenase. J Proteome Res. 2017;16(3):1315–1326. doi: 10.1021/acs.jproteome.6b00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ringel AE, et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell. 2020;183(7):1848–1866. doi: 10.1016/j.cell.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bas M, et al. Gamma-delta T-cells in patients with squamous cell carcinoma of the head and neck. Oral Oncol. 2006;42(7):691–697. doi: 10.1016/j.oraloncology.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 119.Mito I, et al. Comprehensive analysis of immune cell enrichment in the tumor microenvironment of head and neck squamous cell carcinoma. Sci Rep. 2021;11(1):16134. doi: 10.1038/s41598-021-95718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parikh AS, et al. Immune cell deconvolution reveals possible association of γδ T cells with poor survival in head and neck squamous cell carcinoma. Cancers (Basel) 2023;15(19):4855. doi: 10.3390/cancers15194855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu H, et al. High abundance of intratumoral γδ T cells favors a better prognosis in head and neck squamous cell carcinoma: a bioinformatic analysis. Front Immunol. 2020;11:573920. doi: 10.3389/fimmu.2020.573920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lindner S, et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res. 2013;73(8):2468–2479. doi: 10.1158/0008-5472.CAN-12-3450. [DOI] [PubMed] [Google Scholar]
- 123.Lechner A, et al. Tumor-associated B cells and humoral immune response in head and neck squamous cell carcinoma. Oncoimmunology. 2019;8(3):1535293. doi: 10.1080/2162402X.2018.1535293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jeske SS, et al. Adenosine-producing regulatory B cells in head and neck cancer. Cancer Immunol Immunother. 2020;69(7):1205–1216. doi: 10.1007/s00262-020-02535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou X, et al. CD19(+)IL-10(+) regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4(+) T cells to CD4(+)Foxp3(+) regulatory T cells. Oral Oncol. 2016;53:27–35. doi: 10.1016/j.oraloncology.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 126.Norouzian M, et al. Atypical memory and regulatory B cell subsets in tumor draining lymph nodes of head and neck squamous cell carcinoma correlate with good prognostic factors. Head Neck Pathol. 2020;14(3):645–656. doi: 10.1007/s12105-019-01095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cho JH, Lim YC. Prognostic impact of regulatory T cell in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2021;112:105084. doi: 10.1016/j.oraloncology.2020.105084. [DOI] [PubMed] [Google Scholar]
- 128.Kang JH, Zappasodi R. Modulating Treg stability to improve cancer immunotherapy. Trends Cancer. 2023;9(11):911–927. doi: 10.1016/j.trecan.2023.07.015. [DOI] [PubMed] [Google Scholar]
- 129.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Overacre-Delgoffe AE, et al. Interferon-γ drives Treg fragility to promote anti-tumor immunity. Cell. 2017;169(6):1130–1141. doi: 10.1016/j.cell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 2021;22(13):6995. doi: 10.3390/ijms22136995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li B, et al. Targeting tumor-associated macrophages in head and neck squamous cell carcinoma. Oral Oncol. 2020;106:104723. doi: 10.1016/j.oraloncology.2020.104723. [DOI] [PubMed] [Google Scholar]
- 134.Haddad CR, et al. Neutrophil-to-lymphocyte ratio in head and neck cancer. J Med Imaging Radiat Oncol. 2015;59(4):514–519. doi: 10.1111/1754-9485.12305. [DOI] [PubMed] [Google Scholar]
- 135.Rodrigo JP, et al. Neutrophil to lymphocyte ratio in oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis. Cancers (Basel) 2023;15(3):802. doi: 10.3390/cancers15030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pylaeva E, et al. During early stages of cancer, neutrophils initiate anti-tumor immune responses in tumor-draining lymph nodes. Cell Rep. 2022;40(7):111171. doi: 10.1016/j.celrep.2022.111171. [DOI] [PubMed] [Google Scholar]
- 137.Ohashi T, et al. Metabolic profiling analysis of head and neck squamous cell carcinoma. Oral Dis. 2022;30(2):342–352. doi: 10.1111/odi.14432. [DOI] [PubMed] [Google Scholar]
- 138.Curry JM, et al. Tumor microenvironment in head and neck squamous cell carcinoma. Semin Oncol. 2014;41(2):217–234. doi: 10.1053/j.seminoncol.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 139.Wang L, et al. Plasma lipid profiling and diagnostic biomarkers for oral squamous cell carcinoma. Oncotarget. 2017;8(54):92324–92332. doi: 10.18632/oncotarget.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dickinson A, et al. Mass spectrometry-based lipidomics of oral squamous cell carcinoma tissue reveals aberrant cholesterol and glycerophospholipid metabolism - A Pilot study. Transl Oncol. 2020;13(10):100807. doi: 10.1016/j.tranon.2020.100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kansal V, et al. Statin drugs enhance responses to immune checkpoint blockade in head and neck cancer models. J Immunother Cancer. 2023;11(1):e005940. doi: 10.1136/jitc-2022-005940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fakhry C, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32(30):3365–3373. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang JW, Roden RB. L2, the minor capsid protein of papillomavirus. Virology. 2013;445(1-2):175–186. doi: 10.1016/j.virol.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Florin L, et al. Assembly and translocation of papillomavirus capsid proteins. J Virol. 2002;76(19):10009–10014. doi: 10.1128/JVI.76.19.10009-10014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cromme FV, et al. Differences in MHC and TAP-1 expression in cervical cancer lymph node metastases as compared with the primary tumours. Br J Cancer. 1994;69(6):1176–1181. doi: 10.1038/bjc.1994.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Keating PJ, et al. Frequency of down-regulation of individual HLA-A and -B alleles in cervical carcinomas in relation to TAP-1 expression. Br J Cancer. 1995;72(2):405–411. doi: 10.1038/bjc.1995.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huang SM, McCance DJ. Down regulation of the interleukin-8 promoter by human papillomavirus type 16 E6 and E7 through effects on CREB binding protein/p300 and P/CAF. J Virol. 2002;76(17):8710–8721. doi: 10.1128/JVI.76.17.8710-8721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kleine-Lowinski K, et al. Selective suppression of monocyte chemoattractant protein-1 expression by human papillomavirus E6 and E7 oncoproteins in human cervical epithelial and epidermal cells. Int J Cancer. 2003;107(3):407–415. doi: 10.1002/ijc.11411. [DOI] [PubMed] [Google Scholar]
- 149.Ruffin AT, et al. Improving head and neck cancer therapies by immunomodulation of the tumour microenvironment. Nat Rev Cancer. 2023;23(3):173–188. doi: 10.1038/s41568-022-00531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Uppaluri R, et al. Neoadjuvant and adjuvant pembrolizumab in resectable locally advanced, human papillomavirus-unrelated head and neck cancer: a multicenter, phase II trial. Clin Cancer Res. 2020;26(19):5140–5152. doi: 10.1158/1078-0432.CCR-20-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schoenfeld JD, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in untreated oral cavity squamous cell carcinoma: a phase 2 open-label randomized clinical trial. JAMA Oncol. 2020;6(10):1563–1570. doi: 10.1001/jamaoncol.2020.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ferrarotto R, et al. Impact of neoadjuvant durvalumab with or without tremelimumab on CD8+ tumor lymphocyte density, safety, and efficacy in patients with oropharynx cancer: CIAO trial results. Clin Cancer Res. 2020;26(13):3211–3219. doi: 10.1158/1078-0432.CCR-19-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vos JL, et al. Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat Commun. 2021;12(1):7348. doi: 10.1038/s41467-021-26472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Topalian SL, et al. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367(6477):eaax0182. doi: 10.1126/science.aax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sievers C, et al. Phenotypic plasticity and reduced tissue retention of exhausted tumor-infiltrating T cells following neoadjuvant immunotherapy in head and neck cancer. Cancer Cell. 2023;41(5):887–902.e5. doi: 10.1016/j.ccell.2023.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cohen E, et al. Neoadjuvant and adjuvant pembrolizumab (pembro) plus standard of care (SOC) in patients (pts) with resectable locally advanced (LA) head and neck squamous cell carcinoma (HNSCC): The phase III KEYNOTE-689 study. Ann Oncol. 2019;30:ix106. doi: 10.1093/annonc/mdz428.029. [DOI] [Google Scholar]
- 157.van der Leun AM, et al. Dual immune checkpoint blockade induces analogous alterations in the dysfunctional CD8+ T-cell and activated Treg compartment. Cancer Discov. 2023;13(10):2212–2227. doi: 10.1158/2159-8290.CD-22-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Luoma AM, et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell. 2022;185(16):2918–2935. doi: 10.1016/j.cell.2022.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ferris RL, et al. Neoadjuvant nivolumab alone or in combination with relatlimab or ipilimumab in resectable head and neck squamous cell carcinoma (HNSCC) J Clin Oncol. 2023;41(16_suppl):6018. doi: 10.1200/JCO.2023.41.16_suppl.6018. [DOI] [Google Scholar]
- 160.Zinner R, et al. Neoadjuvant nivolumab (N) plus weekly carboplatin (C) and paclitaxel (P) in resectable locally advanced head and neck cancer. J Clin Oncol. 2020;38(15_suppl):6583. doi: 10.1200/JCO.2020.38.15_suppl.6583. [DOI] [Google Scholar]
- 161.Rosenberg A, et al. Nivolumab, nabpaclitaxel, and carboplatin followed by risk/response adaptive de-escalated locoregional therapy for HPV-associated oropharyngeal cancer: OPTIMA II trial. J Clin Oncol. 2021;39(15_suppl):6011. doi: 10.1200/JCO.2021.39.15_suppl.6011. [DOI] [Google Scholar]
- 162.Darragh LB, et al. A phase I/Ib trial and biological correlate analysis of neoadjuvant SBRT with single-dose durvalumab in HPV-unrelated locally advanced HNSCC. Nat Cancer. 2022;3(11):1300–1317. doi: 10.1038/s43018-022-00450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leidner R, et al. Neoadjuvant immunoradiotherapy results in high rate of complete pathological response and clinical to pathological downstaging in locally advanced head and neck squamous cell carcinoma. J Immunother Cancer. 2021;9(5):e002485. doi: 10.1136/jitc-2021-002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Koyama S, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schwab KS, et al. Successful treatment of refractory squamous cell cancer of the head and neck with nivolumab and ipilimumab. Case Rep Oncol. 2018;11(1):17–20. doi: 10.1159/000485562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Doger de Spéville B, et al. Final results from TACTI-002 Part C: A phase II study of eftilagimod alpha (soluble LAG-3 protein) and pembrolizumab in patients with metastatic 2nd line head and neck squamous cell carcinoma unselected for PD-L1. J Clin Oncol. 2023;41(16_suppl):6029. doi: 10.1200/JCO.2023.41.16_suppl.6029. [DOI] [Google Scholar]
- 167.Redman JM, et al. Enhanced neoepitope-specific immunity following neoadjuvant PD-L1 and TGF-β blockade in HPV-unrelated head and neck cancer. J Clin Invest. 2022;132(18):e161400. doi: 10.1172/JCI161400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rodriguez CP, et al. A phase II trial of pembrolizumab and vorinostat in recurrent metastatic head and neck squamous cell carcinomas and salivary gland cancer. Clin Cancer Res. 2020;26(4):837–845. doi: 10.1158/1078-0432.CCR-19-2214. [DOI] [PubMed] [Google Scholar]
- 169.Ye J, et al. Tumor-derived γδ regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J Immunol. 2013;190(5):2403–2414. doi: 10.4049/jimmunol.1202369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu X, et al. Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition. Nat Commun. 2018;9(1):249. doi: 10.1038/s41467-017-02689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu X, et al. Reprogramming lipid metabolism prevents effector T cell senescence and enhances tumor immunotherapy. Sci Transl Med. 2021;13(587):eaaz6314. doi: 10.1126/scitranslmed.aaz6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu X, et al. Blockades of effector T cell senescence and exhaustion synergistically enhance antitumor immunity and immunotherapy. J Immunother Cancer. 2022;10(10):e005020. doi: 10.1136/jitc-2022-005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li L, et al. TLR8-mediated metabolic control of human treg function: a mechanistic target for cancer immunotherapy. Cell Metab. 2019;29(1):103–123. doi: 10.1016/j.cmet.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Greene S, et al. Inhibition of MDSC trafficking with SX-682, a CXCR1/2 inhibitor, enhances NK-Cell immunotherapy in head and neck cancer models. Clin Cancer Res. 2020;26(6):1420–1431. doi: 10.1158/1078-0432.CCR-19-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Veglia F, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. 2019;569(7754):73–78. doi: 10.1038/s41586-019-1118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huang C, et al. Sirpα on tumor-associated myeloid cells restrains antitumor immunity in colorectal cancer independent of its interaction with CD47. Nat Cancer. 2024;5(3):500–516. doi: 10.1038/s43018-023-00691-z. [DOI] [PubMed] [Google Scholar]
- 177.Hu C, et al. Heterogeneity of cancer-associated fibroblasts in head and neck squamous cell carcinoma: opportunities and challenges. Cell Death Discov. 2023;9(1):124. doi: 10.1038/s41420-023-01428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vyas A, et al. Recurrent human papillomavirus-related head and neck cancer undergoes metabolic reprogramming and is driven by oxidative phosphorylation. Clin Cancer Res. 2021;27(22):6250–6264. doi: 10.1158/1078-0432.CCR-20-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yamamoto M, et al. Targeting metabolic pathways for head and neck cancers therapeutics. Cancer Metastasis Rev. 2017;36(3):503–514. doi: 10.1007/s10555-017-9691-z. [DOI] [PubMed] [Google Scholar]
- 180.Sandulache VC, et al. Glucose, not glutamine, is the dominant energy source required for proliferation and survival of head and neck squamous carcinoma cells. Cancer. 2011;117(13):2926–2938. doi: 10.1002/cncr.25868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chandel V, et al. Metabolic regulation in HPV associated head and neck squamous cell carcinoma. Life Sci. 2020;258:118236. doi: 10.1016/j.lfs.2020.118236. [DOI] [PubMed] [Google Scholar]
- 182.Day TA, et al. Inhibition of mTOR signaling and clinical activity of rapamycin in head and neck cancer in a window of opportunity trial. Clin Cancer Res. 2019;25(4):1156–1164. doi: 10.1158/1078-0432.CCR-18-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wu X, et al. Metformin inhibits progression of head and neck squamous cell carcinoma by acting directly on carcinoma-initiating cells. Cancer Res. 2019;79(17):4360–4370. doi: 10.1158/0008-5472.CAN-18-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Boelcke WP, et al. Pharmacological fatty acid synthase inhibitors differently affect the malignant phenotype of oral cancer cells. Arch Oral Biol. 2022;135:105343. doi: 10.1016/j.archoralbio.2021.105343. [DOI] [PubMed] [Google Scholar]
- 185.Aquino IGd, et al. Anticancer properties of the fatty acid synthase inhibitor TVB-3166 on oral squamous cell carcinoma cell lines. Arch Oral Biol. 2020;113:104707. doi: 10.1016/j.archoralbio.2020.104707. [DOI] [PubMed] [Google Scholar]
- 186.Agostini M, et al. The fatty acid synthase inhibitor orlistat reduces the growth and metastasis of orthotopic tongue oral squamous cell carcinomas. Mol Cancer Ther. 2014;13(3):585–595. doi: 10.1158/1535-7163.MCT-12-1136. [DOI] [PubMed] [Google Scholar]
- 187.Prusinkiewicz MA, et al. Survival-associated metabolic genes in human papillomavirus-positive head and neck cancers. Cancers (Basel) 2020;12(1):253. doi: 10.3390/cancers12010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sha D, et al. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020;10(12):1808–1825. doi: 10.1158/2159-8290.CD-20-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Stewart M, et al. High Tumor Mutational Burden May Predict Improved Response to Immunotherapy in Head and Neck Cancer Patients. Int J Radiat Oncol Biol Phys. 2024;118(5):e43. doi: 10.1016/j.ijrobp.2024.01.097. [DOI] [Google Scholar]
- 190.Gottschalk RA, et al. Distinct influences of peptide-MHC quality and quantity on in vivo T-cell responses. Proc Natl Acad Sci U S A. 2012;109(3):881–886. doi: 10.1073/pnas.1119763109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bauml J, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35(14):1542–1549. doi: 10.1200/JCO.2016.70.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.McBride S, et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2021;39(1):30–37. doi: 10.1200/JCO.20.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bourhis J, et al. LBA35 Avelumab-cetuximab-radiotherapy versus standards of care in patients with locally advanced squamous cell carcinoma of head and neck (LA-SCCHN): Randomized phase III GORTEC-REACH trial. Ann Oncol. 2021;32:S1310. doi: 10.1016/j.annonc.2021.08.2112. [DOI] [Google Scholar]
- 194.Massarelli E, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(1):67–73. doi: 10.1001/jamaoncol.2018.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hanna GJ, et al. Neoadjuvant and adjuvant nivolumab and lirilumab in patients with recurrent, resectable squamous cell carcinoma of the head and neck. Clin Cancer Res. 2022;28(3):468–478. doi: 10.1158/1078-0432.CCR-21-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Patel SA, et al. A phase 2 study of neoadjuvant chemotherapy plus durvalumab in resectable locally advanced head and neck squamous cell carcinoma. Cancer. 2023;129(21):3381–3389. doi: 10.1002/cncr.34930. [DOI] [PubMed] [Google Scholar]
- 197.Li X, et al. Induction chemotherapy combined with immunotherapy in locally advanced head and neck squamous cell carcinoma. BMC Cancer. 2021;21(1):622. doi: 10.1186/s12885-021-08373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang Z, et al. Neoadjuvant chemoimmunotherapy for the treatment of locally advanced head and neck squamous cell carcinoma: a single-arm phase 2 clinical trial. Clin Cancer Res. 2022;28(15):3268–3276. doi: 10.1158/1078-0432.CCR-22-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]