Abstract
Hormone receptor HR-positive/HER2-negative (HR+/HER2−) breast cancer is the most common subtype in China, representing 60–70% of cases, with a rising incidence due to aging demographics and lifestyle changes. CDK4/6 inhibitors such as palbociclib, ribociclib and abemaciclib have been proven effective in treating HR+/HER2 − advanced or metastatic breast cancer (ABC/MBC), though they may increase healthcare costs. This study aims to compare the efficacy, safety and cost-effectiveness of CDK4/6 inhibitors for the second-line treatment of HR+/HER2 − ABC/MBC from the Chinese healthcare perspective. A cohort-based partitioned survival model was utilized, drawing on the survival data published from PALOMA-3, MONALEESA-3 and MONARCH-2 trials. Costs, and quality-adjusted life years (QALYs) were used to calculate the incremental cost-effectiveness ratio (ICER) over a 15-year time horizon. Deterministic and probabilistic sensitivity analyses were performed to assess the robustness of the model results. In the base-case analysis, the model estimated health benefits to be 2.10 QALYs for palbociclib plus fulvestrant (PAL + FUL), 2.55 QALYs for ribociclib plus fulvestrant (RIB + FUL), and 2.60 QALYs for abemaciclib plus fulvestrant (ABE + FUL), with corresponding costs of $34,423, $41,119, and $48,019. Compared with PAL + FUL, the ICERs were $27,161 per QALY for ABE + FUL and $15,073 per QALY for RIB + FUL. The robustness of these findings was confirmed through uncertainty analyses. Among the three strategies, the most cost-effective probabilities of PAL + FUL, RIB + FUL and ABE + FUL were 0%, 99.8%, and 0.2% under the willingness-to-pay (WTP) threshold of 3 times per-capita gross domestic product ($37,738) in China. This study indicated that both RIB + FUL and ABE + FUL are cost-effective at the WTP threshold compared with PAL + FUL. Notably, RIB + FUL offers the greatest cost-effective advantage for the second-line treatment of HR+/HER2 − ABC/MBC among these three CDK4/6 inhibitors.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-97504-3.
Keywords: CDK4/6 inhibitors, Fulvestrant, Cost-effectiveness, HR-positive/HER2-negative, Breast cancer, China
Subject terms: Cancer, Health care
Introduction
Breast cancer, the most common and malignant tumor, recorded 2.3 million new cases in 2022, making it the second leading cause of global cancer incidence worldwide, following lung cancer1. The age-standardized incidence and mortality rates of breast cancer have significantly increased in China over the past decade, taking a substantial burden on the Chinese healthcare and economic systems2,3. In China, it is estimated that 357,200 people were diagnosed with breast cancer and 75,000 died from the disease in 20222. HR+/HER2 − breast cancer is the most common subtype in China, representing 60–70% of cases, with a rising incidence due to aging demographics and lifestyle changes4. Prior to the advent of cyclin-dependent kinase 4/6 (CDK4/6) inhibitors, endocrine therapy was the primary treatment for HR+/HER2 − breast cancer. However, approximately one-quarter of HR+/HER2 − breast cancer patients experienced recurrence or metastasis and progressed to advanced breast cancer due to primary resistance or secondary resistance5. Primary resistance occurs when cancer cells inherently evade treatment due to preexisting genetic or molecular alterations, such as mutations in drug targets or defects in apoptotic pathways. In contrast, secondary resistance develops during treatment as a result of tumor evolution under therapeutic pressure, involving mechanisms such as acquired mutations, epigenetic modifications, or activation of alternative survival pathways. The development of CDK4/6 inhibitors has revolutionized the clinical management of HR+/HER2 − breast cancer, offering a new therapeutic strategy to overcome endocrine resistance and improve patient outcomes6.
CDK4/6 inhibitors, with different pharmacological properties but the same mechanism of action, can inhibit the phosphorylation of tumor suppressor retinoblastoma protein by preventing CDK4/6 from binding to cyclin D, and then arrests the G1 to S phase progression in cell cycle regulation7. They effectively inhibit tumor cell proliferation, and delay or overcome the development of endocrine resistance8. Currently, CDK4/6 inhibitors combined with fulvestrant have been recommended as the standard treatment for second-line and subsequent therapy for HR+/HER2 − advanced or metastatic breast cancer in guidelines such as National Comprehensive Cancer Network (NCCN) and Chinese Society of Clinical Oncology (CSCO) clinical practice guidelines. Studies on CDK4/6 inhibitors − palbociclib, ribociclib, and abemaciclib − plus fulvestrant for the second-line treatment of HR+/HER2 − ABC/MBC including the PALOMA-3 trial9–11, MONALEESA-3 trial12–14, and MONARCH-2 trial15,16, have all shown significant improvements in the progression-free survival and overall survival results. In addition to three aforementioned CDK4/6 inhibitors marketed in China, dalpiciclib as a new CDK4/6 inhibitor based on the efficacy and safety data from the DAWNA-1 trial, has also been approved by the NMPA in China. However, the overall survival (OS) analysis has not yet been published, and the follow-up is ongoing.
Breast cancer is a highly prevalent and incident disease, and therefore an increase in treatment costs resulting from the incorporation of these targeted therapeutic drugs could significantly impact health care budgets, especially in low- and middle-income countries17. China’s healthcare system faces pressures from rapid population aging, fragmented insurance coverage, and disparities in access to high-cost therapies. Unlike high-income countries, out-of-pocket expenses remain substantial for patients, even under national insurance schemes18. The cost-effectiveness of the three CDK4/6 inhibitor plus fulvestrant therapies in China has yet to be determined. In this study, we evaluate the cost-effectiveness of palbociclib, ribociclib, and abemaciclib plus fulvestrant in the second-line treatment of HR+/HER2 − advanced or metastatic breast cancer from the Chinese healthcare perspective. Our pharmacoeconomic assessment aims to support decision-making for policymakers, medical teams and patients, ensuring the rational allocation of medication resources and healthcare expenditure.
Materials and methods
Model structure
A cohort-based partitioned survival analysis (PartSA) model with three mutually exclusive health states (progression-free survival (PFS), progressive disease (PD), and death) was constructed to ascertain the costs and outcomes of patients with HR+/HER2 − advanced or metastatic breast cancer that progressed during prior endocrine therapy (Fig. 1). PFS and OS curves were utilized to directly estimate the proportion of patients in each health state over time based on standard survival parametric functions fitted to the original PFS and OS data19.
Fig. 1.
Model structure of the PartSA model.
According to the study of tevaarwerk et al.20, the survival probability of advanced breast cancer patients at the 15-year was approximately 5%. We adopted a 15-year time horizon with each simulation cycle of 4 weeks in the base case analysis. Over a 15-year time horizon, the probabilities of death for abemaciclib, palbociclib, and ribociclib were 99.0%, 95.3%, and 92.3% in our study, respectively. The WTP threshold was set at three times the GDP ($37,7378 in 2023), in accordance with the Chinese pharmacoeconomic evaluation guide21. The primary outcomes of our model included total costs, life-years (LYs), quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs), all of which were discounted by 5% 21, aligned with the standard pharmacoeconomics modeling practices in China. Additionally, a half-cycle correction was applied to both cost and effectiveness calculations to account for the assumption that transitions occur at the midpoint of each cycle rather than solely at the beginning or end. By mitigating time discretization bias, this correction enhances the accuracy of cumulative cost and QALYs estimations in cost-effectiveness analysis.
Patients and treatment
The target population for the economic evaluation consists of HR+/HER2 − advanced or metastatic breast cancer who experienced progression during prior endocrine therapy. In China, over 60% of breast cancer cases occur in women aged ≥ 50 years, the majority of which (approximately 60–70%) are HR+/HER2 − subtype. Our study specifically focuses on HR+/HER2 − advanced or metastatic breast cancer, with median ages of 57, 63, and 59 years in the PALOMA-3, MONALEESA-3, and MONARCH-2 trials, respectively. This demographic profile closely mirrors the broader Chinese population affected by this disease. Notably, this population faces a high risk of endocrine therapy resistance, as well as significant comorbidities such as osseous metastasis and visceral metastasis. Characteristics of the comparable population enrolled in three randomized controlled trials are detailed in Supplementary Table S1.
The treatment regimens for the three CDK4/6 inhibitors used in the study align with those specified in the PALOMA-3, MONALEESA-3, and MONARCH-2 trials: (1) palbociclib 125 mg, once daily for 3 weeks, followed by one-week break in each 28-day cycle; (2) abemaciclib 150 mg, twice daily throughout each 28-day cycle; (3) ribociclib 600 mg per day for 3 weeks followed by a week break. In addition, patients also received 500 mg fulvestrant via intramuscular injection on days 1 and 15 of the first cycle, and on day 1 of each subsequent cycle. Treatment continued until disease progression or death.
Clinical data inputs
The clinical efficacy and safety data were extracted from clinical trials, with detailed information provided in Supplementary Table S2. Pseudo-individual patient data (pseudo-IPD) reconstruction enables a more precise estimation of survival functions by deriving individual-level event times from Kaplan-Meier graphs. This is crucial in calculating more accurate mean survival times, which are fundamental for cost-effectiveness analysis. Pseudo-IPD were extracted using Engauge Digitizer 4.1 from these trials, reconstructed through R version 4.3.2 following the methodology recommended by Guyot and fitted using standard parametric models in IPDfromKM22,23. The standard parametric models including log-normal, gamma, weibull, gompertz, exponential, log-logistic, and generalized gamma functions, were explored to fit the OS and PFS curves24. The Akaike information criterion (AIC) and Bayesian information criterion (BIC) are commonly used criteria for model selection, where AIC balances model fit and complexity with a focus on predictive accuracy, while BIC penalizes complexity more heavily, favoring simpler models, especially with larger sample sizes. The optimal fitting distribution was selected based on the lowest value of the AIC and BIC, as well as through visual inspection. All survival curve simulation results are summarized in Table 1. The best-fitting parametric functions for PFS were lognormal across all strategies. For OS, the optimal distributions were log-logistic for palbociclib, weibull for abemaciclib, and log-logistic for ribociclib. Detailed values of AIC and BIC and Kaplan-Meier curves fittings and extrapolations for PFS and OS are available in the Supplementary Materials (Supplementary Table S3, S4; Supplementary Figures S1, S2).
Table 1.
Survival parameters for three CDK4/6 inhibitors, Palbociclib, abemaciclib, and ribociclib combination therapies.
| Strategies | PFS | OS | ||
|---|---|---|---|---|
| Best fitting | Survival parameters | Best fitting | Survival parameters | |
| Palbociclib + fulvestrant | Lognormal |
Meanlog = 2.2345763; sdlog = 1.126612 |
Loglogistic |
Shape = 1.762026; scale = 32.63381 |
| Abemaciclib + fulvestrant | Lognormal |
Meanlog = 3.120524; sdlog = 1.377591 |
Weibull |
Shape = 1.38068; scale = 60.06384 |
| Ribociclib + fulvestrant | Lognormal |
Meanlog = 2.579618; sdlog = 1.327546 |
Loglogistic |
Shape = 1.678677; scale = 41.21087 |
PFS progression free survival, OS overall survival.
Cost inputs
This study adopts the perspective of the Chinese healthcare system, only direct medical care costs were included in the cost-effectiveness analysis. These costs comprised drug acquisition, follow-up costs, best supportive care (BSC), subsequent therapy after progression, end-of-life care, and costs associated with severe adverse events (SAEs, grade ≥ 3) as detailed in Table 2. The protocols for subsequent therapy following disease progression were derived from PALOMA-3, MONALEESA-3, and MONARCH-2 trials. Detailed cost calculations and the incidence of SAEs are also presented in Table 2. The body surface area (BSA) of Chinese women aged 55–59 years was calculated as 1.63 m2, based on an average height of 1.57 m and a weight of 60.7 kg, as reported in the Chinese Fifth National Physical Fitness Monitoring Communique 202225. This BSA was used to calculate the dose of the agents. The YAOZH (yaozh.com) database was used in this study as it provides comprehensive and up-to-date information on drug pricing, procurement costs, and reimbursement policies in China. Given the importance of accurate local cost data for cost-effectiveness analysis, YAOZH serves as a reliable source reflecting real-world pharmaceutical expenditures in the Chinese healthcare system. Drug unit costs were obtained from the average bidding prices listed on YAOZH (yaozh.com) and converted to 2024 USD at an exchange rate of $1 = 7.1036 CNY, as of 9 February 2024. Additional costs and the duration of subsequent therapy after progression were sourced from published literature26–28.
Table 2.
Basic parameters input to the model and the ranges of the sensitivity analyses. AEs adverse events, PFS progression free survival, PD progressive disease.
| Model input | Base case | Distribution | Low | High | References |
|---|---|---|---|---|---|
| Drug costs per cycle ($) | |||||
| Abemaciclib | 670.08 | Gamma | 536.07 | 670.08 | YAOZH |
| Ribociclib | 628.79 | Gamma | 503.04 | 628.79 | YAOZH |
| Palbociclib | 509.07 | Gamma | 407.25 | 509.07 | YAOZH |
| Follow-up cost | 125.60 | Gamma | 94.20 | 157.00 | 29 |
| Best supportive care cost | 783.64 | Gamma | 587.73 | 979.55 | 30 |
| End-of-life care cost | 1867.62 | Gamma | 1400.72 | 2334.53 | 31 |
| Costs of AEs per event | |||||
| Neutropenia | 114.41 | Gamma | 91.53 | 137.29 | 28 |
| Leucopenia | 114.41 | Gamma | 91.53 | 137.29 | 28 |
| Diarrhea | 44.30 | Gamma | 35.44 | 53.16 | 27 |
| Anemia | 138.03 | Gamma | 110.42 | 165.64 | 28 |
| Hepatobiliary toxicity | 87.30 | Gamma | 69.84 | 104.76 | 28 |
| Infection | 225.24 | Gamma | 180.19 | 270.29 | Consulting expert |
| Proportion of patients received subsequent treatment (%) | |||||
| Abemaciclib | 0.762 | Beta | 0.716 | 0.808 | 15,16 |
| Ribociclib | 0.815 | Beta | 0.766 | 0.864 | 12,14 |
| Palbociclib | 0.769 | Beta | 0.723 | 0.815 | 9,11 |
| Risk of AEs in Palbociclib | |||||
| Neutropenia | 0.696 | Beta | 0.557 | 0.835 | 9,11 |
| Leucopenia | 0.383 | Beta | 0.306 | 0.46 | 9,11 |
| Infections | 0.052 | beta | 0.042 | 0.062 | 9,11 |
| Anemia | 0.043 | Beta | 0.034 | 0.052 | 9,11 |
| Risk of AEs in Ribociclib | Beta | ||||
| Neutropenia | 0.571 | Beta | 0.457 | 0.685 | 12,14 |
| Leucopenia | 0.155 | Beta | 0.124 | 0.186 | 12,14 |
| Hepatobiliary toxicity | 0.137 | Beta | 0.11 | 0.164 | 12,14 |
| Infections | 0.077 | Beta | 0.062 | 0.092 | 12,14 |
| Anemia | 0.039 | Beta | 0.031 | 0.047 | 12,14 |
| Risk of AEs in Abemaciclib | Beta | ||||
| Neutropenia | 0.297 | Beta | 0.238 | 0.356 | 15,16 |
| Leucopenia | 0.111 | Beta | 0.089 | 0.133 | 15,16 |
| Diarrhea | 0.145 | Beta | 0.116 | 0.174 | 15,16 |
| Anemia | 0.09 | Beta | 0.072 | 0.108 | 15,16 |
| Utility | |||||
| PFS | 0.837 | Beta | 0.753 | 0.921 | 32,33 |
| PD | 0.443 | Beta | 0.399 | 0.487 | 34 |
| Disutilities of AEs | |||||
| Neutropenia | −0.13 | Constant | −0.104 | −0.156 | 35,36 |
| Leucopenia | −0.13 | Constant | −0.104 | −0.156 | 35,36 |
| Diarrhea | −0.103 | Constant | −0.077 | −0.129 | 34 |
| Anemia | −0.073 | Constant | −0.058 | −0.088 | 35,36 |
| Infections | −0.15 | Constant | −0.12 | −0.18 | 35,36 |
| Hepatobiliary toxicity | −0.12 | Constant | −0.096 | −0.144 | 34 |
| Discount | 0.05 | Constant | 0 | 0.08 | |
Utility inputs
The healthsrelated utility values applied to the PFS and PD health states were detailed in Table 2. The utility value for the PFS state was derived from previous studies32,33, which was calculated from EuroQol 5-dimension 5-level (EQ-5D-5 L) data collected in the MONALEESA-2 trial.
The utility value for the PD state was sourced from the study of Lloyd et al.34, which estimated health-state utility values using the standard gamble technique. Disutility values associated with SAEs were also included, based on data from published sources34–36. The disutility values applied in our model correspond to SAEs with grade 3/4 toxicities reported in PALOMA-3, MONALEESA-3, and MONARCH-2 trials, as these are the most severe and clinically relevant adverse events that significantly affect patients’ health-related quality of life. The disutility values for adverse events, including neutropenia, leucopenia, diarrhea, anemia, infection, and hepatobiliary toxicity, were derived from studies that specifically assessed the impact of severe toxicities on utility scores.
Uncertainty analyses
Deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA) were carried out to verify the robustness and reliability of the baseline conclusions. DSA was typically performed as one-way sensitivity analysis, where parameters were estimated based on their 95% confidence intervals (CIs) or through plausible variations, defined by upper and lower values. ICERs were generated with different values, and parameters that exerted the greatest influences on the ICER at the WTP threshold of $37,738 were presented using a tornado diagram. In the PSA, cost parameters were modeled as gamma distribution, while the incidence of AEs and utility parameters were modeled as beta distribution, referring to the model book37 and the published cost-effectiveness analysis papers38,39. A total of 5,000 Monte Carlo iterations were performed to assess overall model uncertainty and ensure probabilistic sensitivity analysis convergence while maintaining computational efficiency. This iteration count strikes a balance between precision and feasibility and aligns with commonly adopted practices in health economic evaluations. The resulting incremental ICER scatter plots and cost-effectiveness acceptability curves illustrated the model’s uncertainty. This analysis adhered to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline40, as detailed in Supplemental table S7.
Given that the utility values for PFS and PD in the base case analysis were derived from different sources, we selected the utility values (PFS utility = 0.76, PD utility = 0.55) reported by Beauchemin et al.41 which were obtained from an exhaustive review of the literature for scenario analysis. Additionally, we evaluated the impact of the time horizon (5-year and 10-year) on the findings, as cost-effectiveness analyses are influenced by the evolution of treatment benefits, cost accumulation, and discounting over time.
Results
Base-case cost-effectiveness analysis
In the PartSA model, the estimated health benefits for PAL + FUL, RIB + FUL, and ABE + FUL were 4.00, 4.91, and 4.55 LYs, and 2.10, 2.55 and 2.60 QALYs, with associated costs of $34,423, $41,119 and $48,019, respectively. Compared with PAL + FUL, ABE + FUL and RIB + FUL demonstrated incremental gains of 0.55 and 0.91 LYs, and 0.50 and 0.44 QALYs, respectively. The incremental costs were $13,596 for ABE + FUL and $6,696 for RIB + FUL, leading to ICERs of $27,161 per QALY and $15,073 per QALY, respectively. When compared with RIB + FUL, ABE + FUL yielded an additional 0.056 QALYs at an incremental cost of $6,900, resulting in an ICER of $123,215 per QALY. The base case results of the cost-effectiveness analysis were detailed in Table 3.
Table 3.
Base case results of the cost-effectiveness analysis. LYs, life years, QALYs, qualityadjusted life years, ICER, incremental cost-effectiveness ratio.
| Outcomes | Palbociclib plus fulvestrant | Ribociclib plus fulvestrant | Abemaciclib plus fulvestrant |
|---|---|---|---|
| LYs | 4.00 | 4.91 | 4.55 |
| QALYs | 2.10 | 2.55 | 2.60 |
| Cost, $ | 34,423 | 41,119 | 48,019 |
| Incremental cost, $ | – | 6696 | 13,596 |
| Incremental LYs | – | 0.91 | 0.55 |
| Incremental QALYs | – | 0.44 | 0.50 |
| ICER, $ | – | 15,073 | 27,161 |
|
ICER (abemaciclib vs. ribociclib), $ |
– | – | 123,215 |
Deterministic sensitivity analysis
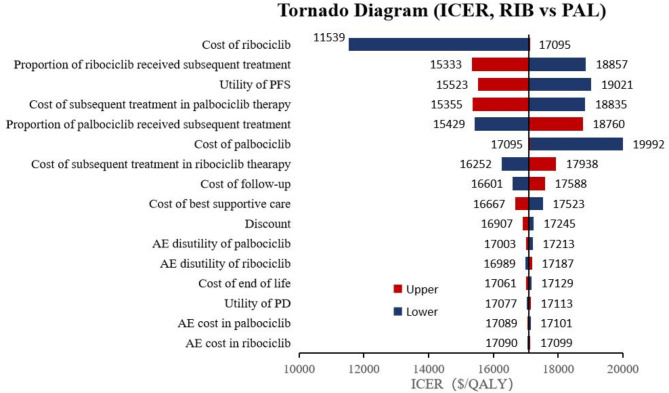
A series of one-way deterministic sensitivity analyses were conducted to identify key drivers of the ICER. For the comparison of ABE + FUL versus PAL + FUL, utility of PFS had the greatest impact on the outcomes, as illustrated in Fig. 2. Specifically, as the utility value of PFS decreased to 0.753, the ICER increased to $34,474 per QALY, which remained below the WTP threshold of $37,738 in China. In the case of RIB + FUL versus PAL + FUL, the cost of ribociclib emerged as the critical model factor, as depicted in Fig. 3, with other parameters showing medium or minor influences on the outcome. Furthermore, for ABE + FUL versus RIB + FUL, the discount rate had the most significant effect on the results, as shown in Fig. 4. Regardless of how the parameters varied within the established range, the ICERs consistently exceeded the WTP threshold of $37,738.
Fig. 2.
Tornado diagram of abemaciclib plus fulvestrant versus palbociclib plus fulvestrant. ABE, abemaciclib, PAL, palbociclib.
Fig. 3.
Tornado diagram of ribociclib plus fulvestrant versus palbociclib plus fulvestrant. RIB, ribociclib, PAL, palbociclib.
Fig. 4.
Tornado diagram of abemaciclib plus fulvestrant versus ribociclib plus fulvestrant. ABE, abemaciclib, RIB, ribociclib.
Probabilistic sensitivity analysis
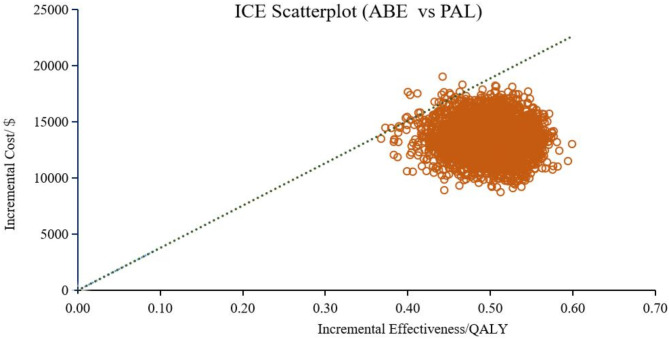
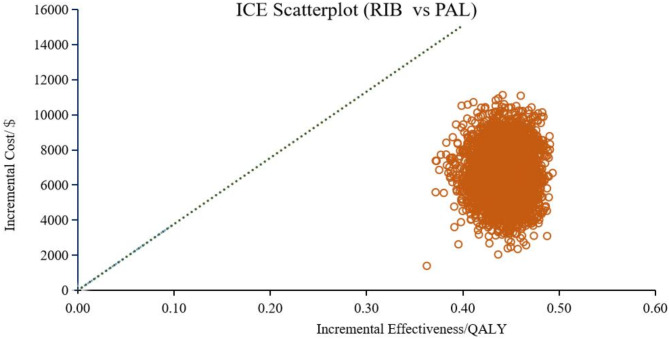
In addition to the DSA, we conducted PSA to further evaluate the robustness of our model. Figure 5 displays scatter plots from 5000 Monte Carlo iteration, showing the costs and effectiveness of different treatment options with CDK4/6 inhibitors plus fulvestrant. Compared with PAL + FUL, both RIB + FUL and ABE + FUL acquired more QALYs and incurred higher costs.
Fig. 5.
Scatter plots of costs and effectiveness for different treatment options.
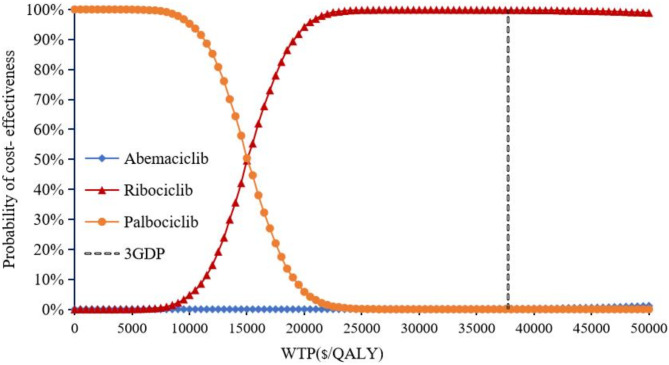
Cost-effectiveness scatter plots showed that compared with PAL + FUL, the probability of ABE + FUL being cost-effective was 99.58% (shown in Fig. 6), while RIB + FUL achieved a probability of 100% (shown in Fig. 7). Compared with RIB + FUL, the probability of ABE + FUL being cost-effective was 0.42% (shown in Supplementary Figure S3). The cost-effectiveness acceptability curve further demonstrated that the probabilities of PAL + FUL, RIB + FUL and ABE + FUL being the most cost-effective were 0%, 99.8%, and 0.2% respectively, when assessed under the WTP threshold of $37,738 as shown in Fig. 8. The cost-effectiveness acceptability curves of ABE + FUL versus PAL + FUL, and RIB + FUL versus PAL + FUL are available in the Supplementary Figure S4 and Figure S5.
Fig. 6.
Scatter plots of costs and effectiveness of abemaciclib plus fulvestrant versus palbociclib plus fulvestrant. ABE, abemaciclib, PAL, palbociclib.
Fig. 7.
Scatter plots of costs and effectiveness of ribociclib plus fulvestrant versus palbociclib plus fulvestrant. RIB, ribociclib, PAL, palbociclib.
Fig. 8.
Cost-effectiveness acceptability curve.
Scenario analysis results
The scenario analysis used utility values of 0.76 for PFS and 0.55 for PD. The results indicated an ICER of $32,130 per QALY for ABE + FUL versus PAL + FUL, and $15,108 per QALY for RIB + FUL versus PAL + FUL. In this scenario, ABE + FUL, yielding 2.59 QALYs at a cost of $48,019, was dominated by RIB + FUL, which provided 2.61 QALYs for $41,119, making ribociclib plus fulvestrant the most cost-effective treatment option.
When the time horizon was reduced to a 5-year horizon for the comparison between ABE + FUL and PAL + FUL, the ICER decreased to $26,422 per QALY. Extending the time horizon to a 10-year horizon resulted in a further decrease in the base case ICER to $25,898 per QALY. For RIB + FUL versus PAL + FUL, shortening the time horizon to a 5-year horizon increased the ICER to $17,770 per QALY, and extending it to a 10- year horizon resulted in an increase from the base case ICER to $15,618 per QALY. However, for ABE + FUL versus RIB + FUL, the ICER significantly decreased from $123,215 over a 15-year horizon, to $53,261 over a 10-year horizon, and $39,141 over a 5-year horizon.
Discussion
Reports on the clinical benefits of CDK4/6 inhibitors such as palbociclib, ribociclib, and abemaciclib combined with fulvestrant in the second-line treatment for HR+/HER2 − advanced or metastatic breast cancer, have significantly piqued the interest of oncologists and healthcare decision-makers. However, the high cost of CDK4/6 inhibitors has dramatically increased healthcare expenditures. Economic evaluations of the CDK4/6 inhibitors are particularly crucial for resource-limited countries like China. This study assessed the cost-effectiveness of the three aforementioned CDK4/6 inhibitors in second-line treatment from the Chinese healthcare perspective, marking the first comparative analysis in China.
In accordance with the recommendations from the 2020 edition of the Chinese pharmacoeconomic evaluation guide21, this study adopted three times the GDP per capita as the threshold for WTP. The results showed that, within the WTP threshold of $37,738 in China, RIB + FUL and ABE + FUL were cost-effective compared to PAL + FUL. However, ABE + FUL was not cost-effective when compared with RIB + FUL. Notably, ribociclib plus fulvestrant emerged as the most cost-effective, with a 99.8% probability of being the most cost-effective option, far exceeding the probabilities for palbociclib (0%) and abemaciclib (0.2%). The sensitivity analysis identified PFS utility, ribociclib cost, and discount rate as the key drivers of ICERs variation across comparisons. Despite fluctuations in these parameters within the set ranges, the ICER for ABE + FUL vs. PAL + FUL remained below the WTP threshold, while for ABE + FUL vs. RIB + FUL, it consistently exceeded the threshold, indicating limited cost-effectiveness.
Since utility values can vary across regions and cultures, we conducted a scenario analysis using utility values of 0.76 for PFS and 0.55 for PD. In this scenario, the health gains of ABE + FUL slightly decreased from 2.60 to 2.59 QALYs, indicating a negligible impact. In contrast, the health gains of PAL + FUL and RIB + FUL increased from 2.10 to 2.17 QALYs and 2.55 to 2.61 QALYs, respectively. This difference may be attributed to the unique survival curve characteristics of the palbociclib and ribociclib regimens, which resulted in greater long-term incremental gains in QALYs. This variation had minimal impact on the ICER, and the cost-effectiveness outcomes remained consistent with the base model. These findings confirm that regional variations in utility values did not alter our cost-effectiveness conclusions, demonstrating the robustness of the model.
Scenario analyses exploring the impact of different time horizons (5-year and 10-year) on the main outcomes indicated that time horizons had minimal influence on the ICER for ABE + FUL versus PAL + FUL and RIB + FUL versus PAL + FUL. The minimal influence of the time horizon on the ICERs for these comparisons can be attributed to two factors. First, the clinical benefits of the treatment strategies are primarily concentrated in the PFS phase, which predominantly occurs within the first 5 years of treatment. Beyond this period, due to disease progression and similar subsequent management, the survival curves of the strategies gradually converge, limiting additional QALY gains from extending the time horizon. Second, the cost structure is front-loaded, with most expenses (e.g., drug acquisition) incurred during the initial treatment phase. In contrast, post-progression costs (e.g., palliative care) are similar between the strategies, resulting in a limited impact on the long-term incremental differences in ICERs. However, for the comparison of ABE + FUL versus RIB + FUL, the ICER increased significantly as the time horizon extended, rising from $39,141 per QALY over a 5-year horizon to $123,215 per QALY over a 15-year horizon. This trend was driven by a decrease in incremental QALYs (from 0.15 to 0.056) and an increase in incremental costs (from $6012 to $6900) over the longer time horizon. This discrepancy can be attributed to the differing distribution of health gains between the two groups, while the cost differences remain relatively modest due to slow cost growth and the impact of discounting. The RIB + FUL group demonstrated greater health gains during PD phase but relatively fewer gains during the PFS phase compared to the ABE + FUL group. As the study time horizon lengthened, the extended PD phase in the RIB + FUL group gradually showed its advantages, increasing its overall QALYs gains. Nevertheless, the improved long-term survival benefits underscore why ribociclib plus fulvestrant (RIB + FUL) remains the most cost-effective option.
From the perspective of the USA for the second-line treatment of HR+/HER2 − advanced or metastatic breast cancer, Wang et al.32 found that palbociclib, ribociclib and abemaciclib combined with fulvestrant yielded 1.92, 2.33, and 2.36 QALYs at costs of $441,194, $541,372, and $541,890, respectively. They suggested that abemaciclib combined with fulvestrant might be cost-effective compared with ribociclib combined with fulvestrant, yet not be cost-effective when compared with palbociclib combined with fulvestrant in the USA. This study aligns with the findings of Wang et al.32, where ribociclib combined with fulvestrant therapy also showed the most life-years. The difference in QALYs may be attributed to the fact that Wang et al. obtained health benefit data through indirect comparison, whereas this study utilized the newly published data on progression-free survival and overall survival.
Previous studies, primarily published before 2021, focused on the cost-effectiveness of CDK4/6 inhibitors in first-line treatment of HR+/HER2 − metastatic breast cancer, with limited studies evaluating second-line treatment. Among the few second-line analyses, Stellato et al.42 assessed cost-effectiveness from a Canadian healthcare perspective, while Wang et al.32 and Jiang et al.43 focused on the U.S. system. Zhang et al.44 conducted a comparative study between the U.S. and China, specifically evaluating palbociclib plus fulvestrant versus fulvestrant alone. Notably, palbociclib entered the Chinese market in 2018 at an initial price of $1924 per cycle, but with the introduction of generic versions, its median price has since dropped significantly to $509. Moreover, earlier studies such as Zhang et al.44 were limited by immature OS data at the time of publication. For example, the final OS analysis of the MONARCH-2 trial was published in October 2022, and updated OS results of the PALOMA-3 trial were reported in August 2022, underscoring the need for reassessing cost-effectiveness with contemporary clinical evidence. Earlier studies suggested that CDK4/6 inhibitors combined with fulvestrant were not cost-effective due to high drug costs32,42–44. However, these studies were based on U.S. pricing data, making direct comparisons with China inapplicable. Pharmacoeconomic evaluations are highly time-sensitive and country-specific, as healthcare costs, reimbursement structures, and pricing regulations vary significantly. In contrast, our study found these therapies to be cost-effective, largely due to significant drug price reductions and lower medical costs, achieved through strategic healthcare negotiations. For instance, ribociclib’s price dropped nearly 70%, from $1,881 to $629 per package in 2023, slightly lower than the $732 predicted by Wan et al.45. Similar pricing strategies led to the inclusion of palbociclib and abemaciclib in China’s national medical insurance list, substantially improving affordability. Additionally, at the time of this study, the entry of multiple generic versions of palbociclib further reduced its market price. The cost data used in our analysis reflect the most recent and relevant pricing information available in the Chinese healthcare system.
Our study benefits from the use of mature survival data in RCTs, as the extended OS follow-up data were publicized. OS is most challenging outcome to achieve in a clinical trial, and the most desirable in oncology study17. Although palbociclib combined with fulvestrant did not demonstrate a statistically significant OS benefit in the PALOMA-3 trial9, it achieved a clinically meaningful improvement, with median PFS extended from 4.6 to 11.2 months (hazard ratio 0.497), supporting its role as a standard-of-care treatment. Abemaciclib showed statistically significant OS only in second-line therapy or in early relapses due to resistant to endocrine therapy15. Ribociclib was the only CDK4/6 inhibitor associated with significant OS gains in both first-line46 and second-line treatments12,14, observed irrespective of the combination partner (aromatase inhibitors or fulvestrant) or menopause status (pre/peri or postmenopause). Thus, it was not surprising that ribociclib plus fulvestrant therapy had the greatest cost-effectiveness advantage among the three CDK4/6 inhibitors in second-line treatment. Our study also has the strength that a robust model developed using a cohort-based partitioned survival approach, a method used in 73% of the proposals submitted to NICE in the oncology field, especially in metastatic setting47.
A few potential limitations of this study should be recognized. First, the utility data, a crucial parameter in pharmacoeconomic evaluation, may vary according to different regions, races, religious and cultural beliefs, etc. The utility data in this study were derived from published literature32,34, which may introduce a certain degree of bias. However, the robustness of the model results was confirmed by the sensitivity analysis and scenario analysis. Second, the cost data in this study, including routine follow-up costs, supportive treatment costs and costs associated with adverse events, were all derived from literature data rather than real-world data, which may lead to some discrepancies between the study results and the real-world outcomes. The real-world pharmacoeconomic evaluations of CDK4/6 inhibitors in China are needed in the future. If real-world disease management costs or adverse event management costs are higher, the ICER may increase, reducing the cost-effectiveness of these treatment strategies. However, one-way deterministic sensitivity analyses showed that these costs were not the key drivers of the cost-effectiveness.Third, the costs of drugs may be overestimated, as we could not consider dose reductions and drug wastage in the model because the time for dose modifications in the treatment course for adverse event management was not specified in the clinical trials. If dose reductions are frequent and substantial, they may lead to lower per-patient drug costs than those estimated from clinical trials, therefore real-world data is needed to evaluate their impact in Chinese clinical practice. However, unlike other markets, China’s centralized procurement system and dose packaging regulations help minimize drug wastage. Hospitals and pharmacies dispense medications according to precise treatment regimens, reducing unnecessary waste and optimizing resource utilization.
Conclusion
In conclusion, our cost-effectiveness analysis and sensitivity analyses indicated that both ribociclib plus fulvestrant and abemaciclib plus fulvestrant were cost-effective compared with palbociclib plus fulvestrant at the WTP threshold of $37,738 for the second-line treatment of HR+/HER2 − ABC/MBC from the Chinese healthcare perspective. However, compared directly with ribociclib plus fulvestrant, abemaciclib plus fulvestrant did not show cost-effectiveness. Among the three CDK4/6 inhibitors combination therapies evaluated, ribociclib plus fulvestrant emerged as the most cost-effective option for the second-line treatment of postmenopausal women with HR+/HER2 − ABC/MBC in China.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
Caifeng Jia:Conceptualization、Data curation、Formal Analysis、Methodology、Writing – original draft; Sen Zhang:Data curation、Methodolog、Investigation; Jie Wang: Software、Methodology、Data curation; Bing Feng: Software、Methodology、Visualization; Fenghao Shi:Software、Methodology、Visualization; Meiqi Wang:Conceptualization、Data curation; Sainan Li:Conceptualization、Data curation; Hao Xu: Data curation、Investigation; Mingxia Wang:Supervision、Project administration、Writing – review & editing. All authors reviewed the manuscript.
Funding
This work was supported by the Medical Science Research Project of Hebei (20240243).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The data of the PFS/OS was based on previously published trials, and ethics approval or specific consent procedures were not applicable for this study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/20/2025
The original online version of this Article was revised: The Funding section in the original version of this Article was incorrect. The Funding section now reads: “This work was supported by the Medical Science Research Project of Hebei (20240243).”
References
- 1.Bray, F. A. O. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin.74, 1542–4863. 10.3322/caac.21834 (2024). [DOI] [PubMed] [Google Scholar]
- 2.Han, B. et al. Cancer incidence and mortality in China, 2022. J. Natl. Cancer Center4, 47–53 10.1016/j.jncc.2024.01.006 (2024). [DOI] [PMC free article] [PubMed]
- 3.Lei, S. et al. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. (London England). 41, 1183–1194. 10.1002/cac2.12207 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan, L. et al. Breast cancer in China. Lancet Oncol.15, e279–289. 10.1016/s1470-2045(13)70567-9 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Hamilton, E. & Infante, J. R. Targeting CDK4/6 in patients with cancer. Cancer Treat. Rev.45, 129–138. 10.1016/j.ctrv.2016.03.002 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Asghar, U., Witkiewicz, A. K., Turner, N. C. & Knudsen, E. S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov.. 14, 130–146. 10.1038/nrd4504 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finn, R. S., Aleshin, A. & Slamon, D. J. Targeting the cyclin-dependent kinases (CDK) 4/6 in Estrogen receptor-positive breast cancers. Breast Cancer Res.18, 17. 10.1186/s13058-015-0661-5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang, S., Xu, Q., Sun, W., Zhou, J. & Zhou, J. Immunomodulatory effects of CDK4/6 inhibitors. Biochim. Et Biophys. Acta Rev. Cancer. 1878, 188912. 10.1016/j.bbcan.2023.188912 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Cristofanilli, M. et al. Overall survival with Palbociclib and fulvestrant in women with HR+/HER2- ABC: Updated exploratory analyses of PALOMA-3, a double-blind, phase III randomized study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res.28, 3433–3442. 10.1158/1078-0432.Ccr-22-0305 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cristofanilli, M. et al. Fulvestrant plus Palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol.17, 425–439. 10.1016/s1470-2045(15)00613-0 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Turner, N. C. et al. Overall survival with Palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med.379, 1926–1936. 10.1056/NEJMoa1810527 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Slamon, D. J. et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N. Engl. J. Med.382, 514–524. 10.1056/NEJMoa1911149 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Slamon, D. J. et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-Positive, human epidermal growth factor receptor 2-Negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. Off.J. Am. Soc. Clin. Oncol.36, 2465–2472. 10.1200/jco.2018.78.9909 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Slamon, D. J. et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: Updated overall survival. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol.32, 1015–1024. 10.1016/j.annonc.2021.05.353 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Sledge, G. W. Jr. et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: A randomized clinical trial. JAMA Oncol.6, 116–124. 10.1001/jamaoncol.2019.4782 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sledge, G. W. Jr. et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol.35, 2875–2884. 10.1200/jco.2017.73.7585 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Buehler, A. M., Castilho, G., Dionne, P. A. & Stefani, S. Cost-effectiveness of ribociclib plus letrozole versus Palbociclib plus letrozole or letrozole as monotherapy in first-line treatment of postmenopausal women with HR+/HER2- locally advanced or metastatic breast cancer: A Brazilian private payer perspective. Therapeutic Adv. Med. Oncol.13, 17588359211000593. 10.1177/17588359211000593 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yip, W. et al. 10 years of health-care reform in China: Progress and gaps in universal health coverage. Lancet (London England). 394, 1192–1204. 10.1016/s0140-6736(19)32136-1 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Woods, B. S., Sideris, E., Palmer, S., Latimer, N. & Soares, M. Partitioned survival and state transition models for healthcare decision making in oncology: Where are we now? Value Health J. Int. Soc. Pharmacoecon. Outcomes Res.23, 1613–1621. 10.1016/j.jval.2020.08.2094 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Tevaarwerk, A. J. et al. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: Little evidence of improvement over the past 30 years. Cancer119, 1140–1148. 10.1002/cncr.27819 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, G., Wu, H. S., Wu, J., Dong, J. & Li, C. H. China Guidelines for Pharmacoeconomic Evaluations (China Market, 2020). [Google Scholar]
- 22.Guyot, P., Ades, A. E., Ouwens, M. J. & Welton, N. J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol.12, 9. 10.1186/1471-2288-12-9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, N., Zhou, Y. & Lee, J. J. IPDfromKM: Reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol.21, 111. 10.1186/s12874-021-01308-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latimer, N. R. in Survival analysis for economic evaluations alongside clinical Trials - Extrapolation with Patient-Level data (National Institute for health and care Excellence, 2013) (NICE) copyright © 2013 National Institute for health and clinical excellence, unless otherwise stated. All Rights Reserved. [PubMed]
- 25.National Physical Fitness Monitoring Center Issued the Fifth National Physical Fitness Monitoring Communique (General administration of sport of China, 2022). www.sport.gov.cn/n315/n329/c24335066/content.html.
- 26.Jeong, E., Wang, C., Wilson, L. & Zhong, L. Cost-effectiveness of adding ribociclib to endocrine therapy for patients with HR-positive, HER2-negative advanced breast cancer among premenopausal or perimenopausal women. Front. Oncol.11, 658054. 10.3389/fonc.2021.658054 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhan, M. et al. Cost-effectiveness analysis of trastuzumab Deruxtecan in patients with HER2-low advanced breast cancer based on DESTINY-Breast04. Front. Public. Health. 11, 1049947. 10.3389/fpubh.2023.1049947 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou, T. et al. Economic evaluation of sintilimab plus bevacizumab versus Sorafenib as a first-line treatment for unresectable hepatocellular carcinoma. Adv. Ther.39, 2165–2177. 10.1007/s12325-022-02079-4 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Wang, H., Wang, Y., Gong, R., Geng, Y. & Li, L. Cost-effectiveness of Pertuzumab and trastuzumab as a first-line treatment of HER2-positive metastatic breast cancer in China. Ann. Palliat. Med.10, 11382–11393. 10.21037/apm-21-2412 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Ding, H. et al. Cost-effectiveness analysis of fulvestrant versus anastrozole as first-line treatment for hormone receptor-positive advanced breast cancer. Eur. J. Cancer Care. 10.1111/ecc.12733 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Wu, B., Zhang, Q. & Sun, J. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J. Immunother. Cancer. 6, 124. 10.1186/s40425-018-0440-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, Y., Rui, M., Guan, X., Cao, Y. & Chen, P. Cost-effectiveness analysis of abemaciclib plus fulvestrant in the second-line treatment of women with HR+/HER2- advanced or metastatic breast cancer: A US payer perspective. Front. Med.8, 658747. 10.3389/fmed.2021.658747 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mistry, R. et al. Cost-effectiveness of ribociclib plus letrozole versus palbociclib plus letrozole and letrozole monotherapy in the first-line treatment of postmenopausal women with HR+/HER2- advanced or metastatic breast cancer: A U.S. Payer perspective. J. Managed Care Specialty Pharm.24, 514–523. 10.18553/jmcp.2018.24.6.514 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd, A., Nafees, B., Narewska, J., Dewilde, S. & Watkins, J. Health state utilities for metastatic breast cancer. Br. J. Cancer. 95, 683–690. 10.1038/sj.bjc.6603326 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang, X. et al. First-line treatment with ribociclib plus endocrine therapy for premenopausal women with Hormone-receptor-positive advanced breast cancer: A cost-effectiveness analysis. Clin. Breast. Cancer.21, e479–e488. 10.1016/j.clbc.2021.01.019 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Launois, R., Reboul-Marty, J., Henry, B. & Bonneterre, J. A cost-utility analysis of second-line chemotherapy in metastatic breast cancer. Docetaxel versus Paclitaxel versus Vinorelbine. PharmacoEconomics10, 504–521. 10.2165/00019053-199610050-00008 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Briggs, A., Sculpher, M. & Claxton, K. Modelling methods for health economic evaluation. (2006).
- 38.Masurkar, P. P., Damgacioglu, H., Deshmukh, A. A. & Trivedi, M. V. Cost effectiveness of CDK4/6 inhibitors in the first-line treatment of HR+/HER2- metastatic breast cancer in postmenopausal women in the USA. PharmacoEconomics41, 709–718 10.1007/s40273-023-01245-y (2023). [DOI] [PubMed] [Google Scholar]
- 39.Shi, F., He, Z., Su, H., Wang, L. & Han, S. Economic evaluation of Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma in China. 13, 961347. 10.3389/fphar.2022.961347 (2022). [DOI] [PMC free article] [PubMed]
- 40.Husereau, D. et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. BMJ (Clin. Res. ed.). 376, e067975. 10.1136/bmj-2021-067975 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beauchemin, C., Letarte, N., Mathurin, K., Yelle, L. & Lachaine, J. A global economic model to assess the cost-effectiveness of new treatments for advanced breast cancer in Canada. J. Med. Econ.19, 619–629. 10.3111/13696998.2016.1151431 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Stellato, D., Thabane, M. E., Park, J., Chandiwana, D. & Delea, T. E. Cost effectiveness of ribociclib in combination with fulvestrant for the treatment of postmenopausal women with HR+/HER2- advanced breast cancer who have received no or only one prior line of endocrine therapy: A Canadian healthcare perspective. PharmacoEconomics39, 1045–1058 10.1007/s40273-021-01027-4 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Jiang, W. et al. Cost-effectiveness analysis of ribociclib plus fulvestrant for hormone receptor-positive/human EGF receptor 2-negative breast cancer. Immunotherapy13, 661–668. 10.2217/imt-2020-0237 (2021). [DOI] [PubMed] [Google Scholar]
- 44.Zhang, Y. et al. Cost-effectiveness analysis of adding Palbociclib as a second-line endocrine therapy for HR(+)/HER2(-) metastatic breast cancer from the US and Chinese perspectives. Clin. Ther.41, 1175–1185. 10.1016/j.clinthera.2019.04.033 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Wan, X. et al. Ribociclib in hormone-receptor-positive advanced breast cancer: Establishing a value-based cost in China. Breast (Edinburgh Scotland). 43, 1–6. 10.1016/j.breast.2018.10.004 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Im, S. A. et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N. Engl. J. Med.381, 307–316. 10.1056/NEJMoa1903765 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Woods, B., Palmer, S. E., Latimer, S. & Soares, N. M. NICE DSU technical support document 19: Partitioned survival analysis for decision modelling in health care: A critical review. (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.