Abstract
Some postmenopausal women suffer from genital and urinary symptoms, while others do not. Therefore, the hypoestrogenic status cannot entirely explain the occurrence of the genitourinary syndrome in menopause (GSM). Differences in the urinary microbiome might play a role in bladder function and vulnerability to urinary symptoms. This study aimed to compare characterization urinary microbiome in postmenopausal women who experienced GSM with urinary symptoms with that in those without urinary symptoms. Forty participants were screened for genital symptoms of GSM and then divided into the urinary symptoms group and the non-urinary symptoms group on the basis of a validated questionnaire. 16 S rRNA gene sequencing was performed to investigate microbial diversity. The alpha diversity was used to evaluate the species richness and evenness, while the beta diversity was used to estimate the differences in the urinary microbiome between the groups. Differential abundance analysis was used to investigate biomarkers in the groups by linear discriminant analysis effect size. The relationship between the urinary microbiome and urinary symptoms was assessed using Spearman’s correlation analysis. The characteristics of the participants were not different between the groups. Gardnerella was found in 22.2% (4/18) and 11.1% (2/18) of participants in the urinary symptoms group and in the non-urinary symptoms group, respectively (p > 0.05). Alpha diversity was less in the urinary symptoms group than in the non-urinary symptoms group, but this was not significant. Beta diversity of the urinary microbiome was not significantly different between the two groups. A differential abundance analysis showed that the genus Prevotella was significantly dominant in postmenopausal women with GSM who reported urinary symptoms. Prevotella was marginally correlated with voiding symptoms (r2 = 0.44; p = 0.01). The bladder or urinary microbiome is closely related to urinary symptoms of GSM. Species richness and diversity are not significantly different between postmenopausal women with GSM with and without urinary symptoms. Prevotella is dominant in symptomatic women and slightly correlated with voiding symptoms.
Keywords: Genitourinary syndrome, Menopause, Microbiome, Urinary symptoms, Genital symptoms, 16S rRNA, Prevotella
Subject terms: Bladder, Microbial communities
Introduction
The physiological aging process and menopause with low estrogen levels can negatively change the urogenital system. Vaginal atrophy secondary to a decline in estrogen, involves thinning of the vaginal walls, changing bacterial flora and increasing pH, is associated with vaginal burning, itching, irritation, and dyspareunia1,2. The urinary tract is also affected by a decline in estrogen, thinning of the bladder and urethral lining may lead to symptoms of dysuria, urgency, urinary incontinence (UI), and an increased incidence of urinary tract infection (UTI)3.
A collection of urinary and genital symptoms resulting from diminished estrogenic stimulation to the vulvovaginal or lower urinary tract is defined as genitourinary syndrome of menopause (GSM)4. Dysuria and urgency or urgency urinary incontinence are the most frequently reported lower urinary tract symptoms (LUTSs), with rates of 29% and 28% in women with GSM, respectively5. Some postmenopausal women suffer from both genital and urinary symptoms, while others do not6,7. Therefore, the hypoestrogenic status cannot entirely explain the occurrence of GSM in postmenopausal women.
The potential influence of the microbiome in the urinary tract on the pathobiology of genitourinary diseases has recently begun to be realized. Evolving studies have shown that the urinary microbiota and microbiome can affect urinary symptoms or disorders. The variability of the urinary microbiome (urotype) has been reported previously, and may relate to hormonal effects in the genitourinary tract8–11. Changes in the urinary microbiome or microbial dysbiosis appear to be a factor in some individuals who have the symptom of overactive bladder, urgency urinary incontinence (UUI), or recurrent UTI9,12–15.
In addition, Microbial dysbiosis is linked to urinary symptoms. Specific bacteria genera may play a more significant role in urinary symptoms. Studies that have employed 16 S rRNA gene sequencing to investigate the urinary microbiome have demonstrated variations in microbiome characteristics among asymptomatic individuals and various subtypes of urinary incontinence (UI)11. For example, analyses of urine collected through catheterization from women with and without urgency urinary incontinence (UUI) revealed differences in their urinary microbiome signatures9,11. Furthermore, an investigation that utilized 16 S rRNA analysis to perform a case-control study showed that polymicrobial communities were present in the urine of both UUI cases and asymptomatic controls11.
However, the relative abundance of 14 bacterial species was significantly differed between the control and UUI samples. In another study, researchers observed that women with UUI had a greater abundance of the Gardnerella genus and a lower abundance of the Lactobacillus genus in their urinary microbiome in comparison to asymptomatic women9. A case-controlled study16 conducted on women with overactive bladder syndrome. The result showed Proteus was statistically more commonly growth in the mid steam urine of patients with OAB than urine of control group. While some studies suggest a possible link between Prevotella and urinary symptoms, including Prevotella, were decreased in the urine of overactive bladder (OAB) patients17. Some research was interesting in the relationship between menopause and the urine microbiome, suggesting that hormonal changes associated with menopause can influence the composition and diversity of the female urinary microbiota18.
Differences in the urinary microbiome might play a role in bladder function and vulnerability to urinary conditions. In addition, there is a lack of studies on the relationship between GSM and the urinary microbiome in postmenopausal women. Therefore, the present study aimed to compare the characterize urinary microbiome of postmenopausal women who had genital and urinary symptoms and that of those who did not have urinary symptoms.
Methods
Subjects and study design
This cross-sectional study was conducted in a university hospital in Bangkok, Thailand after the approval of the Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University (protocol number: MURA 2021/947). Eligible participants were postmenopausal women (aged 50–80 years) who had been attending Gynecologic health services between January 2022 and May 2022. Women who had experienced at least one genital symptom with and without urinary symptoms of GSM were recruited. The inclusion criteria were (1) natural or surgical menopause for at least 1 year; and (2) the ability to communicate fluently in Thai. The exclusion criteria were as follows: (1) local estrogen therapy use within 3 months; (2) systemic antibiotic use in the previous 7 days; (3) current vaginal probiotic use; (4) existing therapeutic catheterization; (5) UTI (based on a history and urine dipstick); and (6) withdrawal from participation. Written informed consent was obtained from all women who participated in the study.
Participants
All participants were screened for genital symptoms of GSM using the validated revised urogenital atrophy questionnaire19. The participants were then divided into the urinary symptoms group and the non-urinary symptoms group on the basis of the validated Thai version of the International Consultation on Incontinence Questionnaire-Female Lower Urinary Tract Symptoms (ICIQ-FLUTS)20.
The ICIQ-FLUTS is a self-administered tool that allows evaluation and quantification of LUTSs and their effect on quality of life. The ICIQ-FLUTS includes three domains of abnormal bladder filling, abnormal voiding, and urinary incontinence21,22. The reference period for symptom assessment was the 4 weeks preceding the day that the questionnaire was administered. In the assessment of urinary symptoms, each question provides the patient with five response options indicating the increasing frequency of the specific symptom: ‘never’ (0), ‘occasionally’ (1), ‘sometimes’ (2), ‘most of the time’ (3), and ‘always’ (4). The ICIQ-FLUTS does not have a predefined scoring system or established thresholds. For our study, we defined a score of ≥ 2 (indicating at least occasional presence of the symptom) as a positive indication of experiencing the symptom. Demographic information and clinical characteristics, such as age, body mass index (BMI), parity, medical comorbidities, menopausal status, and previous gynecological surgery, were also collected. All participants were also asked to complete the validated Thai version of the Female Sexual Function Index (FSFI) questionnaire to measure sexual function23. A pelvic examination was completed, including an evaluation for the vulva health index. Urethral estrogenization was then evaluated using the urethral maturation index after a gentle swab from the distal urethra.
Specimen collection
The transurethral catheterization technique was used for urine specimen collection. This procedure was performed by the principal investigator (KC) in accordance with the standard clinical protocols to represent bladder specimens24. Urine specimens were examined with the dipstick test to exclude the presence of UTI. All urine specimens obtained from catheterization were then immediately stored at 4 °C and transferred to the laboratory. After preserving urine with urine stabilizing buffer (Zymo Research, Irvine, CA) with the optimal ratio (recommended by the manufacturer), urine specimens were centrifuged at 3500 × g for 4 min at 4 °C. The supernatant was then discarded, and the pellet was stored at  80°C until further use.
80°C until further use.
DNA isolation and 16 S rRNA amplicon sequencing.
DNA extraction and 16 S ribosomal RNA gene sequencing were performed by Zymo Research. Briefly, DNA extraction was performed using the ZymoBIOMICS® DNA Miniprep Kit (Zymo Research) according to the manufacturer’s instructions. Library preparation was conducted using the Quick-16 S™ NGS Library Prep Kit (Zymo Research) with 16 S primers that cover the V3–V4 region of the 16 S ribosomal RNA gene. The final pooled library was quantified with TapeStation® (Agilent Technologies, Santa Clara, CA) and Qubit® (Thermo Fisher Scientific, Waltham, WA). Sequencing was performed on an Illumina® MiSeq™ platform (Illumina, San Diego, CA) with a v3 reagent kit with 10% PhiX spike-in.
Bioinformatics analysis.
Raw reads were qualified using FastQC25. The analysis for unique amplicon sequence variants and the removal of errors and chimeric sequences were performed using the DADA2 pipeline.24 Taxonomy assignment, alpha diversity, and beta diversity were determined with Qiime v.1.9.1.25 The analysis of microbial similarity was performed by principle co-ordinate analysis (PCoA) on the basis of Bray–Curtis dissimilarity, and implemented using Vegan v2.5-3 package in R (https://cran.r-project.org/package=vegan). Finally, linear determinant analysis effect size (LEfSe)26 was conducted if the abundance of taxonomy was significant between the groups. The sequence reads obtained from this study were deposited at NCBI’s GenBank with the accession PRJNA970510.
Outcome measures
The primary outcome was to identify urinary microbiota characteristics in postmenopausal women who experienced GSM with and without urinary symptoms. The secondary outcomes were as follows: (1) the characteristics and diversity of the urinary microbiome, which included microbiome composition, alpha diversity, beta diversity, and differential abundance analysis; (2) the urogenital estrogen status (vulva health index and urethral maturation index), and (3) the prevalence of sexual dysfunction.
Statistical analysis
Continuous variables with a normal distribution are shown as the mean ± standard deviation. If continuous variables did not have a normal distribution, median and quartiles were used. These data were then compared between the urinary symptoms and the non-urinary symptoms groups using the chi-square or exact test where appropriate. All analyses were performed using StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC27. A p value < 0.05 was considered statistically significant.
The study evaluated alpha diversity, which included various indices such as observed species, Chao1, Shannon, and Simpson using QIIME software. Richness was calculated using Chao1 and observed species indices, and a larger value indicated a more diverse sample.
The Shannon and Simpson indices combined the interaction between richness and evenness. The Wilcoxon rank-sum test was applied to identify significant differences in alpha diversity. Beta diversity, which was measured by calculating the Bray–Curtis and weighted Uni-Frac and unweighted Uni-Frac distances, was used to compare microbial composition between the samples. Additionally, taxa summaries were reformatted and analyzed using Linear Discriminant analysis effect size (LEfSe) to identify significant biomarkers between groups at the genus level. The Mann–Whitney U test was used to identify specific bacteria that were significantly different between groups, and their effect size was estimated using linear discriminant analysis. A logarithmic linear discriminant analysis score threshold value of 2.0 was used to identify discriminative features.
Sample size estimation.
The two independent proportions formula was used to calculate the appropriate sample size. Based on the proportion of G. vaginalis in the urinary microbiome of women who have genital symptoms of GSM with or without urinary symptoms from a previous study9,16 participants were required in each group. Forty participants were enrolled, considering an additional 10% data loss.
Results
Demographic and clinical characteristics of the participants
Forty eligible postmenopausal women were enrolled in the study. Three women were excluded because of incomplete questionnaires and one woman had been using local estrogen. After the participants completed the ICIQ-FLUTS, 36 participants were divided into the urinary symptoms group (N = 18) and the non-urinary symptoms group (N = 18) based on their reported scores. None of the participants had a positive dipstick urinalysis. Table 1 presents the demographic characteristics of participants across the two groups. No significant differences were observed between the groups in terms of age, body mass index, type of menopause, years since menopause, underlying medical conditions, and sexual status. Additionally, the genital scores from the revised UAQ showed no differences between the groups. Furthermore, the prevalence of urinary tract infections (UTIs) over the past year was not different between the groups.
Table 1.
Participant demographics.
| GSM with urinary symptoms (n = 18) | GSM without urinary symptoms (n = 18) | p-value | |
|---|---|---|---|
| Age (years), Mean (SD) | 61.22 (7.27) | 60.50 (6.06) | 0.7480 |
| BMI (kg/m2), Mean (SD) | 26.65 (5.59) | 24.24 (4.90) | 0.1776 |
| Type of menopause, frequency (%) | 1.0000 | ||
| Natural menopause | 17 (94.44%) | 16 (88.89%) | |
| Surgical menopause | 1 (5.56%) | 2 (11.11%) | |
| Amenorrhea (years), Median (Q1-Q3) | 10 (6–15) | 9 (4–16) | 0.8239 |
| Parity, frequency (%) | 0.4020 | ||
| Nulliparous | 2 (11.1 1%) | 5 (27.78%) | |
| Parous | 16 (88.89%) | 13 (72.22%) | |
| Underlying diseases, frequency (%) | |||
| Hypertension | 9 (50.00%) | 6 (33.33%) | 0.310 |
| Diabetes | 5 (27.78%) | 4 (22.22%) | 1.0000 |
| Dyslipidemia | 8 (44.44%) | 6 (33.33%) | 0.4940 |
| History of UTI in 1 years ago | 7 (38.89%) | 2 (11.11%) | 0.1210 |
| Revised UAQ score, Mean (SD) | |||
| Genital symptoms | 15.44 (0.7) | 15.16 (0.78) | 0.6584 |
| Sexually active, frequency (%) | 0.2748 | ||
| Yes | 11 (6.11%) | 14 (77.78%) | |
| No | 7 (38.89%) | 4 (22.22%) | |
| History of UTI, frequency (%) | 7 (38.89%) | 4 (22.22%) | 0.4750 |
The vulva health index score and the urethral maturation index were not significantly different between the two groups (Table 2). Mild vulva atrophy was observed in 77.78% (14/18) and 88.89% (16/18) of participants in the urinary symptoms group and in the non-urinary symptoms group, respectively (p = 0.66). The mean number of parabasal cells was also similar in both groups. No significant difference in the FSFI was found between the groups (total FSFI score: 17.44 versus 18.85; p = 0.13).
Table 2.
Participants estrogenic status and sexual functioning (%) (n = 36).
| Signs | GSM with urinary symptoms (n = 18)) | GSM without urinary symptoms (n = 18) | p-value |
|---|---|---|---|
| Vulva health index score, frequency (%) | |||
| Mild vulva atrophy | 16 (88.89%) | 14 (77.78%) | 0.6580 |
| Moderate vulva atrophy | 2 (11.11%) | 4 (22.22%) | |
| GSM assessment tool, frequency (%) | |||
| Urethra | 1.000 | ||
| Normal to slightly prominent | 16 (88.89%) | 17 (94.44%) | |
| Moderately prominent urethral meatus | 2 (11.11%) | 1 (5.56%) | |
| Urethral maturation index | |||
| Parabasal cell, Mean (SD) | 84.73 (29.37) | 92.78 (16.99) | 0.3288 |
| Total FSFI score, Mean (SD) | 18.85 (2.54) | 17.44 (3.43) | 0.1333 |
The severity of LUTS symptoms was significantly higher in the urinary symptoms group than in the non-urinary symptoms group (filling symptoms score 5 versus 1, voiding symptoms score 2 versus 0, incontinence symptoms score 7 versus 0; p < 0.05, Table 3).
Table 3.
Urinary symptoms of GSM; number (%) (n = 36).
| ICIQ – FLUTS Questionnaire | GSM with urinary symptoms (n = 18) | GSM without urinary symptoms (n = 18) | p-value |
|---|---|---|---|
| Filling symptoms (score 0–16) | < 0.001 | ||
| Median | 5.00 | 1.00 | |
| IQR (Q1-Q3) | 5.00–6.00 | 0.00–1.00 | |
| Mean (SD) | 5.50 (2.03) | 0.72 (0.82) | |
| Voiding symptoms (score 0–12) | 0.0002 | ||
| Median | 2.00 | 0.00 | |
| IQR (Q1-Q3) | 0.00–3.00 | 0.00 | |
| Mean (SD) | 1.89 (1.71) | 0.16 (0.38) | |
| Incontinence symptoms (score 0–20) | < 0.001 | ||
| Median | 0.00 | 0.00 | |
| IQR (Q1-Q3) | 5.00–9.00 | 0.00 | |
| Mean (SD) | 6.89 (2.39) | 0.00 | |
Comparison of urotypes
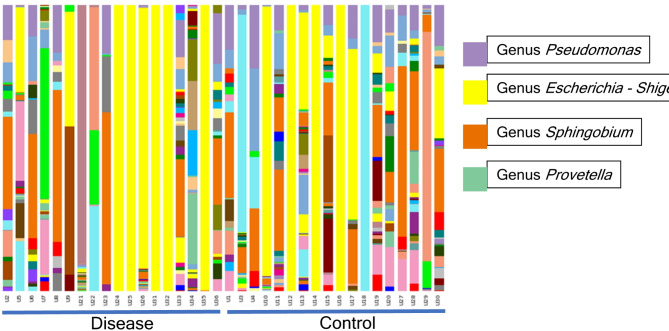
Overview of urinary microbiome of participants, the relative abundance of corresponding bacteria in the sample of each group at the genus level are shown in Fig. 1.
Fig. 1.
Microbial composition. Stacked bar plots depict the sequence abundances of genus- or family-level taxa in both groups. Taxa were ranked according to mean abundance across all samples. The y axis represents the percentage of sequences for a particular bacterial taxa; the x axis represents the study participants separated by cohort. Overview of urinary microbiome of participants. Each bar corresponds to a subject and each colored box represents a genus. The height of each colored box indicates the relative abundance of corresponding bacteria in the sample.
The urinary microbiome was characterized (Table 4). The most common urotype in both groups was Lactobacillus. Gardnerella was found in 22.2% (4/18) and 11.1% (2/18) of participants in the urinary symptoms group and in the non-urinary symptoms group, respectively, but there was no significant difference (p > 0.05). In contrast, in Fig. 1Prevotella was significantly more predominant in the urinary symptoms group than in the non-urinary symptoms group (p < 0.05).
Table 4.
Urotype; frequency (%).
| Genus | GSM with urinary symptoms (n = 18) | GSM without urinary symptoms (n = 18) | p-value |
|---|---|---|---|
| Gardnerella | 4 (22.2) | 2 (11.1) | 0.6580 |
| Lactobacillus | 18 (100) | 16 (88.9) | 0.486 |
| Enterobacteriaceae | 5 (27.8) | 2 (11.1) | 0.402 |
| Provetella | 17 (94.4) | 1 (5.5) | < 0.05 |
| Aquabacterium | 2 (11.1) | 8 (44.4) | < 0.05 |
| Rhodococus | 5 (27.8) | 1 (5.5) | < 0.05 |
| Enterobacter-Kluyvera | 5 (27.8) | 1 (5.5) | < 0.05 |
| Pseudomonas aeruginosa | 6 (33.3) | 1 (5.5) | < 0.05 |
The bacterial compositions of urine samples were analyzed using multiple measures of alpha diversity. Overall, alpha diversity was less in the urinary symptoms group than in the non-urinary symptoms group, but this difference was not significant (Table 5; Fig. 2A-D). PCoA showed partial clustering between the two groups (Fig. 2E-F), but this did not reach significance. LEfSe showed that the genus Prevotella was significantly dominant in postmenopausal women with GSM who reported urinary symptoms. The genera Aquabacterium, Rhodococus, and Enterobacter-Kluyvera, and Pseudomonas aeruginosa were dominant in the women who did not experience urinary symptoms (Fig. 2G).
Table 5.
Richness and diversity measures of urinary Microbiome.
| Metrics | Total [mean (SD)] | GSM with urinary symptoms (n = 18) | GSM without urinary symptoms (n = 18) | p-value | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Max-min | Mean (SD) | Max-min | |||
| No. of observed OTUS | 26.80(7.47) | 27.77(7.59) | 5.40-31.24 | 25.82(7.58) | 4.33–30.27 | 0.4230 |
| Chao1estimator | 28.44(6.46) | 29.10(638) | 9.98–31.49 | 27.79(6.79) | 7.59–30.85 | 0.3228 |
| Shannon index | 2.62(0.41) | 2.92(0.31) | 1.98–3.03 | 2.32(0.24) | 1.57–2.41 | 0.2218 |
| Inverse Simpson index | 5.88(1.36) | 7.03(0.90) | 4.31–7.34 | 4.74(0.47) | 3.32–4.91 | 0-2441 |
Fig. 2.
Bioinformatic analysis of the urinary microbiome in both groups. (A–D) are the alpha diversity analysis of the species in both groups. (E) and (F) are the beta diversity analysis of the species in both groups. G is the linear discriminant analysis efect size (LEfSe) for the biomarker species.
Correlation analysis
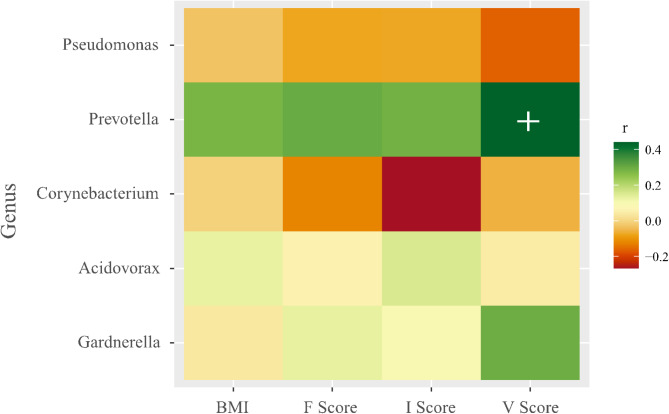
Spearman’s correlation analysis showed a marginal correlation between Prevotella and voiding symptoms (V score) (r2 = 0.44; p = 0.01, Fig. 3). Interestingly, Prevotella appeared to be correlated with the body mass index, incontinence (I Score), and filling (F Score), but this was not significant.
Fig. 3.
Correlation analysis between Genus Prevotella and body mass index (BMI), filling (F Score), incontinence (I Score) and Voiding symptoms (V score) (r2 = 0.44; p = 0.01).
Discussion
Main findings
While hypoestrogenic changes could be a primary cause of the occurrence of GSM in postmenopausal women, other conditions can also increase the risk of developing these symptoms. One potential explanation for this finding is that differences in the urinary microbiome might play a role in bladder function and vulnerability to urinary conditions. Several studies have investigated the impact of the microbiota and the balance between the host and resident microbiota on the human body28,29. In addition to the gut microbiota, the vaginal microbiota and urine microbiota also affect urinary diseases, but this process is poorly understood. A previous study reported the relationship between the bladder microbiome and the occurrence and development of LUTS causing chronic inflammation30. In this study, we describe the urinary microbiome in postmenopausal women who had genital symptoms of GSM with or without urinary symptoms. Gardnerella was rare in both groups, whereas Prevotella was more prevalent in postmenopausal women who reported urinary symptoms. Nevertheless, our results suggest that the detected differences in the urinary microbiome are more related to urinary symptoms than hormonal effects in the urogenital tract based on the equivalent estrogenic status in both groups.
Urinary microbiomes and urinary symptoms
As widely known that change of Gardnerella and Lactobacillus are associated with individuals with UUI, our work did not identify any difference in the relative abundance of Gardnerella and Lactobacillus between two groups. Furthermore, the prevalence of G. vaginalis was relatively low in our study population with or without urinary symptoms. It is important to note that a previous study included “estrogen-positive” participants9, whereas our study focused on postmenopausal participants only. Interestingly, one study showed that Gardnerella was more common in younger women31. This suggests that the difference in hormonal background may indicate different urinary microbiome. A previous study reported the strong association between hormonal status and bacterial community that hormone exposure group had higher frequency of Lactobacillus and Gardnerella18.
Looking at diversity, alpha diversity was not significantly different in our study. This lack of finding is consistent with Pearce et al.’s study9, and our diversity estimates were within the range of their study. There was a lot of individual variability in the diversity and richness from each sample. Therefore, this high level of individual variability emphasizes the need to examine much larger cohorts to detect group differences. In the present study, we did not detect large differences in sequence-based diversity between the two groups. This lack of finding might be due to the lack of power of our small sample size or laboratory techniques. Alternatively, the amount of diversity within the urinary microbiome of women who experienced urinary symptoms may not differ from that in those without urinary symptoms.
Regarding the importance of laboratory techniques, our study performed DNA extraction directly from preserved clinical specimens, in which bacteria could not grow. Therefore, the proportion of microbial DNA could reflect an actual proportion in the urine. A study utilizing enhanced culture method called expanded quantitative urine culture coupled with Matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry31. found that alpha diversity indices were positively correlated with the Urinary Distress Inventory subscale score32. , while a decrease in microbial diversity in women with UUI was associated with increased more severe symptom11,32. It is noteworthy that prior culture method could enrich certain bacterial species, especially pathogenic bacteria, resulting in difference in diversity between groups.
The analysis of the relationship between prior UTIs, the urinary microbiome, and the presence of urinary symptoms in postmenopausal women with GSM was performed using Fisher’s exact test. The results showed no significant association between UTI history and the presence of urinary symptoms (p = 0.4705). The small sample size (n = 36) in our study may have limited the statistical power to detect a meaningful association. The observed lack of association, therefore, may not preclude a subtle influence of prior UTIs on the urinary microbiome and its subsequent contribution to the risk of urinary symptoms. Future studies incorporating larger cohorts and longitudinal data are needed to further investigate this complex relationship. Our findings suggest that alterations in urinary microbiome composition, particularly predominant genera, are potentially more directly associated with urinary symptoms than prior UTI history alone.
Prevotella and urinary symptoms
Among postmenopausal women with GSM and urinary symptoms in our study, one or two genera dominated the majority of the sequence profiles. The most frequently detected genus, as determined by sequencing, was Prevotella. Prevotella comprises anaerobic Gram-negative bacteria of the phylum Bacteroidetes, which also includes the clinically important genera Bacteroides and Porphyromonas. Species of these genera are mainly found in the oral cavity and the upper respiratory tract or urogenital tract33. Prevotella is commonly isolated from the female genital tract and can cause urinary tract infections and pelvic inflammatory disease34. Furthermore, Si et al.35showed that Prevotella is strongly associated with postmenopausal status. A recent finding showed that the presence of Prevotella in pre-operative vaginal swab was associated with UTI after vaginal hysterectomy with transvaginal pelvic reconstruction due to pelvic organ prolapse36. Even though, there is no direct evidence that Prevotella cause bladder hyperresponsiveness, there are some studies paving the way that Prevotella might be involved with incontinence.
Prevotella has been found to induce inflammatory process. Preclinical studies suggest that Prevotella is causal in human disease and inflammatory processes from colonization experiments33. Prevotella can induce inflammation through several mechanisms, such as activating Toll-like receptor 2 (TLR2) on antigen-presenting cells (APCs). This activation promotes the production of Th17 cytokines, including interleukin-IL-23 and IL-1, that affect responses and recruitment of neutrophils, leading to T helper type 17 (Th17) -mediated mucosal inflammation37 Some studies indicate that Prevotella can effectively induce neutrophil dysfunction and death, facilitating the establishment of infections; this process may lead to systemic inflammation due to the influence of bacteria and bacterial products33.
This inflammatory capacity may contribute to bladder dysfunction from urothelium cell injury from inflammatory cytokine38. In clinical study Li et al.30showed that Prevotella was positively correlated with the degree of nocturia (correlation coefficient = 0.291; p = 0.015). Pearce9 and Nardos et al.39similarly found enrichment of Prevotella in patients with UUI. With regard to recurrent UTI, the following organisms were detected at a higher rate than that with acute uncomplicated cystitis: the phylum Bacteroidetes, class Bacteroidia, order Bacteroidales, family Prevotellaceae, and phylum Firmicutes35. on the others hand P. Wu et al. conducted the study characterize the female urinary microbiome associated with OAB. The result shown that Prevotella, were reduced in the urine of overactive bladder patients compared to controls17.
Taken altogether, these findings suggest that some Prevotella strains may act as pathogens on urogenital health involve several factors, including chronic inflammation and host interactions. There is conflicting information regarding Prevotella’ s role in urinary symptoms. Prevotella is not the only genus implicated in inflammatory diseases, as different species can have varying effects. This suggests that only specific strains may exhibit pathobiontic properties40. Despite this potential association between Prevotella and urinary symptoms, further studies are required to investigate the biological association between specific strains of bacterium and the bladder environment.
Strengths
To the best of our knowledge, this is the first study of the urinary microbiome in postmenopausal women. The catheterized urine specimens were used instead of voided midstream urine. Wolfe and his co-workers found that the microbiomes in voided urine and urine collected by transurethral catheters were significantly different. In addition, the microbiome in catheterized urine specimens was closer to the bladder microbiome due to the lower rates of contamination of microbial flora from the genital tract24. We assessed signs of vulva atrophy and the maturation index of the urethra to address the estrogenic status of urogenital tissue. We report the causative agents of urinary symptoms of GSM. The information obtained on the urinary microbiome may lead to a better understanding of the pathophysiology of lower urinary tract symptoms and appropriate therapy in the future.
Limitations
There are some limitations to this study. First, sample size in this study was small but it was large enough to infer the result to population. Second, we did not analyze the relationship between the urinary microbiome and the vaginal microbiome, which could demonstrate the interplay between micro-organisms originating from both locations. Moreover, we did not use standard urine culture to eliminate UTI. However, routine bacterial urine cultures are not always necessary in the evaluation of outpatients with uncomplicated UTIs and simple lower UTIs. Due to the laboratory technique, we were unable to identify the microbiome species level in this study. Finally, this was a cross-sectional study in which the causal relationship between urinary symptoms and urinary microbiome is difficult to determine. Further longitudinal studies on microbial metabolic activity and the host immune environment could help identify mechanisms to further explain these findings.
Conclusion
This study suggests that the bladder or urinary microbiome is intimately related to urinary symptoms of GSM. Species richness and diversity are not significantly different between women with GSM and urinary symptoms and those without urinary symptoms. The genus Prevotella is dominant in women with GSM and urinary symptoms and is correlated with voiding symptoms. Eventually, the urinary microbiome may have clinical Urogynecology diagnostic and therapeutic implications.
Acknowledgements
We would like to thank all staffs for their contribution in the study.
Author contributions
Conceptualization, methodology design, and writing-original draft: KC, PP, and JM. Formal analysis and visualization: KC and PP. Writing—Review & Editing: KC, PP, RW, CT, and JMKC, PP, and JM are equally contributed to this study.
Funding
This work was funded by Faculty of Medicine Ramathibodi Hospital (grant number: RF_65055).
Data availability
The sequence reads obtained from this study were deposited at NCBI’s GenBank with the accession number PRJNA970510.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Palacios, S. Managing urogenital atrophy. Maturitas63 (4), 315–318 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Portman, D. J. & Gass, M. L. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the international society for the study of women’s sexual health and the North American menopause society. Menopause21 (10), 1063–1068 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Panay, N. Genitourinary syndrome of the menopause–dawn of a new era? Climacteric18 (Suppl 1), 13–17 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Phillips, N. A. & Bachmann, G. A. The genitourinary syndrome of menopause. Menopause28 (5), 579–588 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Angelou, K., Grigoriadis, T., Diakosavvas, M., Zacharakis, D. & Athanasiou, S. The genitourinary syndrome of menopause: an overview of the recent data. Cureus12 (4), e7586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srisukho, S., Pantasri, T., Piyamongkol, W., Phongnarisorn, C. & Morakote, N. The experience of genitourinary syndrome of menopause (GSM) among Thai postmenopausal women: the non-reporting issue. Int. Urogynecol. J.30 (11), 1843–1847 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Shardell, M., Gravitt, P. E., Burke, A. E., Ravel, J. & Brotman, R. M. Association of vaginal microbiota with signs and symptoms of the genitourinary syndrome of menopause across reproductive stages. J. Gerontol. Biol. Sci. Med. Sci.76 (9), 1542–1550 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearce, M. M. et al. The female urinary Microbiome in urgency urinary incontinence. Am. J. Obstet. Gynecol.213 (3), 347e1–34711 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearce, M. M. et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio5 (4), e01283–e01214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komesu, Y. M. et al. The urinary Microbiome in women with mixed urinary incontinence compared to similarly aged controls. Int. Urogynecol. J.29 (12), 1785–1795 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karstens, L. et al. Does the urinary Microbiome play a role in urgency urinary incontinence and its severity?? Front. Cell. Infect. Microbiol.6(78). (2016). [DOI] [PMC free article] [PubMed]
- 12.Brubaker, L. & Wolfe, A. J. The female urinary microbiota, urinary health and common urinary disorders. Ann. Transl Med.5 (2), 34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govender, Y., Gabriel, I., Minassian, V. & Fichorova, R. The current evidence on the association between the urinary Microbiome and urinary incontinence in women. Front. Cell. Infect. Microbiol.9(133). (2019). [DOI] [PMC free article] [PubMed]
- 14.Petersen, C. & Round, J. L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol.16 (7), 1024–1033 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas-White, K., Brady, M., Wolfe, A. J. & Mueller, E. R. The bladder is not sterile: history and current discoveries on the urinary Microbiome. Curr. Bladder Dysfunct. Rep.11 (1), 18–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtiss, N. et al. A case controlled study examining the bladder Microbiome in women with overactive bladder (OAB) and healthy controls. Eur. J. Obstet. Gynecol. Reprod. Biol.214, 31–35 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Wu, P. et al. Urinary Microbiome and psychological factors in women with overactive bladder. Front. Cell. Infect. Microbiol.7, 488 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas-White, K. J. et al. Evaluation of the urinary microbiota of women with uncomplicated stress urinary incontinence. Am. J. Obstet. Gynecol.216 (1), 55.e1–55.e16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lester, J., Bernhard, L. & Ryan-Wenger, N. A self-report instrument that describes urogenital atrophy symptoms in breast cancer survivors. West. J. Nurs. Res.34 (1), 72–96 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chattrakulchai, K., Manonai, J., Silpakit, C. & Wattanayingcharoenchai, R. Validation of the Thai version of the international consultation on incontinence Questionnaire-Female lower urinary tract symptoms (ICIQ-FLUTS). Int. Urogynecol. J.31 (12), 2603–2610 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Brookes, S. T., Donovan, J. L., Wright, M., Jackson, S. & Abrams, P. A scored form of the Bristol female lower urinary tract symptoms questionnaire: data from a randomized controlled trial of surgery for women with stress incontinence. Am. J. Obstet. Gynecol.191 (1), 73–82 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Jackson, S. et al. The Bristol female lower urinary tract symptoms questionnaire: development and psychometric testing. Br. J. Urol.77 (6), 805–812 (1996). [DOI] [PubMed] [Google Scholar]
- 23.Rosen, R. et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J. Sex. Marital Ther.26 (2), 191–208 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Wolfe, A. J. et al. Evidence of uncultivated bacteria in the adult female bladder. J. Clin. Microbiol.50 (4), 1376–1383 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews, S. FastQC: a quality control tool for high through-put sequence data: Babraham Institute. http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
- 26.Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol.12 (6), R60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.StataCorp Stata Statistical Software: Release 17. College Station. (StataCorp LLC, 2021).
- 28.Nelson, D. E. et al. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLoS One5 (11), e14116 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostaff, M. J., Stange, E. F. & Wehkamp, J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol. Med.5 (10), 1465–1483 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, K. et al. Interplay between bladder microbiota and overactive bladder symptom severity: a cross-sectional study. BMC Urol.22 (1), 39 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price, T. K. et al. The urobiome of continent adult women: a cross-sectional study. Bjog127 (2), 193–201 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi, W. et al. The effect of pathophysiological changes in the vaginal milieu on the signs and symptoms of genitourinary syndrome of menopause (GSM). Menopause28 (1), 102–108 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Larsen, J. M. The immune response to prevotella bacteria in chronic inflammatory disease. Immunology151 (4), 363–374 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikamo, H. et al. In vitro bactericidal activities of antimicrobial agents and morphologic changes on prevotella Bivia. Chemotherapy45 (5), 342–348 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Yoo, J. J. et al. Urinary Microbiome characteristics in female patients with acute uncomplicated cystitis and recurrent cystitis. J. Clin. Med.10(5). (2021). [DOI] [PMC free article] [PubMed]
- 36.Occhino, J. A., Byrnes, J. N., Wu, P. Y., Chen, J. & Walther-Antonio, M. R. Preoperative vaginal Microbiome as a predictor of postoperative urinary tract infection. Sci. Rep.14 (1), 28990 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Si, J., You, H. J., Yu, J., Sung, J. & Ko, G. Prevotella as a hub for vaginal microbiota under the influence of host genetics and their association with obesity. Cell. Host Microbe21 (1), 97–105 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Grover, S., Srivastava, A., Lee, R., Tewari, A. K. & Te, A. E. Role of inflammation in bladder function and interstitial cystitis. Ther. Adv. Urol.3 (1), 19–33 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nardos, R. et al. Network-Based differences in the vaginal and bladder microbial communities between women with and without urgency urinary incontinence. Front. Cell. Infect. Microbiol.12, 759156 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Aquino, S. G. et al. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J. Immunol.192 (9), 4103–4111 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequence reads obtained from this study were deposited at NCBI’s GenBank with the accession number PRJNA970510.