Abstract
UV radiation suppresses the immune response, a fact which raises the question of whether the phenomenon may find practical applications in the outcome of infectious diseases. In this study, BALB/c mice were exposed to low-dose UVB (250 J/m2) from Dermaray M-DMR-100 for 4 consecutive days. Twelve hours after the last UV exposure, groups of mice were injected with 2 × 106 Leishmania amazonensis promastigotes. The development of skin lesions, as assessed by measurement of visible cutaneous lesions, was significantly suppressed in low-dose UVB-irradiated mice compared to nonirradiated controls. In order to characterize the cytokines involved in this phenomenon, BALB/c mice were irradiated with identical doses of UVB, and gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin 4 cytokine levels in blood serum and skin were examined at different times by a sandwich enzyme-linked immunosorbent assay, immunohistochemical analysis, and reverse transcription (RT)-PCR. Upregulated expression of serum IFN-γ and TNF-α was observed from 6 to 24 h. Positive results for IFN-γ and TNF-α in UVB-irradiated mice were obtained by immunohistochemical analysis. By RT-PCR, the mRNA expression of both IFN-γ and TNF-α cytokines was detected in a time-dependent manner only in UVB-irradiated mice. Histopathological analysis and electron microscopy revealed that cellular infiltration, tissue parasitism, and parasitophorus vacuoles in irradiated mice were markedly less noticeable than those in nonirradiated controls. These results suggested that low-dose UVB irradiation played a pathogen-suppressing role in Leishmania-susceptible BALB/c mice via systemic and local upregulation of Th1 (IFN-γ and TNF-α) cytokines.
Leishmania species have a worldwide distribution and can infect humans, causing a variety of clinical appearances ranging from small cutaneous lesions to disseminated visceral leishmaniasis. Characteristically, Leishmania parasites multiply exclusively in host macrophages (6), leading to highly polarized Th1 and Th2 immune responses in resistant (C57BL/6) and susceptible (BALB/c) mice, respectively (38). A model of infection with Leishmania parasites inducing cutaneous lesions in susceptible mice results in a disseminated and lethal infection accompanied by an immune response dominated by CD4+ Th2 cells secreting interleukin 4 (IL-4), IL-5, and IL-10 (37, 38). In contrast, resistant strains of mice which exhibit a self-limiting infection develop an immune response dominated by CD4+ Th1 cells secreting gamma interferon (IFN-γ), IL-2, and tumor necrosis factor alpha (TNF-α) (34).
Resistance obtained in BALB/c mice after immunization, CD4+-cell depletion, or anti IL-4 treatment correlates with a decrease in the Th2 response and a concomitant increase in the Th1 response (39, 42). The characteristic of these immunological manipulations is that, in most cases, they can be effective only when performed before or soon after the infection. For the early containment of parasite replication, IFN-γ synthesis (initially by natural killer cells and later by Th1 lymphocytes), leading to macrophage activation, is crucial (15).
The UV radiation found in sunlight is the primary environmental agent responsible for inducing skin cancer. UVB (290 to 320 nm) exhibits high biological activity, and while it is mostly absorbed by the epidermis, small but significant quantities can penetrate to the dermis. In contrast, 35 to 50% of incident UVA radiation (320 to 400 nm) penetrates to the dermis (26). Exposure of humans (49) and mice (30) to suberythemal doses of UVB (280 to 320 nm) light interferes with the production of the cell-mediated immune response to contact-sensitizing haptens applied epicutaneously at the site of irradiation. The mechanism by which UVB irradiation activates the suppressor, rather than the effector, arm of the immune response is not fully understood; however, the release of cytokines and an alteration in the activity of epidermal Langerhans cells following UVB irradiation have been shown to be involved (32).
These profound effects of UV radiation on the development of chronic hypersensitivity have raised the question of whether the phenomenon may have practical consequences for infectious diseases, in addition to its relevance for cutaneous carcinogenesis. A variety of infectious disease models, including herpes simplex virus types 1 and 2, Candida albicans, Mycobacterium bovis BCG, Mycobacterium lepraemurium, and Borrelia burgdorferi infections in mice and Trichinella spiralis infections in rats, indicated that exposing these animals to UV radiation before infection abrogated the development of delayed-type hypersensitivity and resulted in a more severe disease outcome (7, 10, 18, 20, 21, 33, 48). In contrast, Giannini reported that, in the mouse model of Leishmania major, cutaneous lesion development was significantly suppressed by UVB irradiation (13). These controversial results raised the question of how UVB may affect immunity to infectious agents and, by that action, increase the incidence or severity of infections.
In our previous study (22), it was shown that low-dose UVA irradiation (10 and 30 J/cm2) prior to Leishmania infection significantly suppressed cutaneous lesion development in BALB/c mice infected with Leishmania amazonensis. It was concluded that the suppression was due to Th1 induction by low-dose UVA irradiation. In the present study, to elucidate the effects of low-dose UVB irradiation, we observed its effects on Leishmania infection. We show that BALB/c mice previously irradiated with low-dose UVB become protected against cutaneous lesion development from infection with L. amazonensis. The basis of this low-dose UVB irradiation-induced protection was evaluated by examining the production of IFN-γ, TNF-α, and IL-4 cytokines.
MATERIALS AND METHODS
Mice and parasites.
Specific-pathogen-free, male BALB/c mice were obtained from the Animal Center of the Faculty of Medicine, University of the Ryukyus (Okinawa, Japan). Mice were 6 to 8 weeks old at the beginning of each experiment. Within each experiment, the mice were age matched. All experiments were approved ethically by the University of the Ryukyus. The animals were housed in a specific-pathogen-free barrier facility and were given free access to National Institutes of Health Formula 31 mouse food and sterilized water. Ambient lighting was automatically controlled to provide 12-h light-12-h dark cycles. A total of 20 mice in each group were used. L. amazonensis (MHOM/BR/73/M2269) parasites were cultured in vitro as reported previously (45).
UVB irradiation.
The UVR source was Dermaray M-DMR-100 (SBLB; Toshiba, Tokyo, Japan), having a bank of five fluorescent tubes (FL20S.E-30; Toshiba) that emitted an average irradiation of 0.45 mW/cm2 over the wavelength range of 280 to 370 nm, as measured with Torex UV radiometer 305/365-DII (Topcon, Tokyo, Japan). For local UVB irradiation, hairs from the dorsal skin were shaved. Experimental mice were restrained in specially constructed wire cages and irradiated at a distance of 30 cm from the tube lights. The dose of UVB received by each mouse averaged approximately 250 J/m2 at each exposure for 4 consecutive days. Control mice were shaved and restrained in the same manner but were not exposed to UVB irradiation.
Leishmania infection and disease parameters.
At 12 h after the last UVB exposure, irradiated and control mice were injected intradermally with 2 × 106 L. amazonensis promastigotes suspended in 0.05 ml of normal saline. The extent of cutaneous lesion development was recorded weekly. The size of a lesion was calculated from the average diameter approximating a circle by measurement of length and width (x axis multiplied by y axis). All mice were killed at 12 weeks after infection because control mice developed large ulcers. Biopsy samples were taken and processed for routine histopathologic analysis and transmission electron microscopic examination as reported previously (23).
Cytokine assays.
To examine the effects of low-dose UVB irradiation prior to Leishmania infection, systemic and local expression of IFN-γ, TNF-α, and IL-4 was examined. Six- to 8-week-old male BALB/c mice (six mice per group) were irradiated at the shaved skin of the back with UVB 250 J/m2 per day for 4 consecutive days. Nonirradiated mice served as controls. Tissue and blood samples were collected at 6, 12, and 24 h after the last irradiation and processed for immunohistochemical analysis, mRNA extraction, and enzyme-linked immunosorbent assay (ELISA), respectively. Serum was obtained from blood after centrifugation at 2,000 × g for 20 min at 4°C. Serum cytokine levels were measured by a sandwich ELISA according to the protocol provided by the manufacturer with a commercially available murine IFN-γ Quantikine M kit (R&D Systems, Minneapolis, Minn.) or mouse TNF-α and IL-4 kits (Endogen Inc., Woburn, Mass.). All measurements were determined in duplicate.
Detection of cytokines by immunohistochemical analysis.
The avidin-biotin-peroxidase complex technique was used for immunohistochemical analysis with an LSAB kit (Dako, Kyoto, Japan). Fresh tissue samples were immediately embedded in Tissue-Tek optimal-cutting-temperature compound (Sakura Finetechnical Co., Ltd., Tokyo, Japan). Four-micrometer-thick sections were cut in a cryostat, fixed in cold acetone for 10 min, and then air dried. Endogenous peroxidase activity was quenched by incubation in 0.3% hydrogen peroxide for 10 min. The sections were treated with 1% normal serum at room temperature for 30 min to remove nonspecific binding sites for the second antibodies. The sections were then incubated with primary antibodies against IFN-γ (hamster anti-mouse IFN-γ, 1:100; PharMingen, San Diego, Calif.) and TNF-α (rabbit anti-mouse TNF-α, 1:250; Serotec Ltd., Kidlington, Oxford, United Kingdom), diluted in phosphate-buffered saline (PBS), and allowed to stand overnight at 4°C. Anti-IFN-γ and TNF-α antibodies are monoclonal and are specific for the corresponding cytokine only, and there is no cross-reaction with cytokine receptors, according to information from the manufacturers. Biotinylated species-specific immunoglobulin G (hamster anti-mouse IFN-γ, 1:200, PharMingen), prediluted rabbit anti-mouse TNF-α antibody (LSAB kit), and avidin-biotin-peroxidase complex were each applied for 10 min. The sections then were treated with streptavidin in Tris-HCl buffer for 10 min and washed between steps with PBS for 10 min. The peroxidase binding sites were revealed with freshly prepared 3-amino-9-ethylcarbazole solution for 3 min, the reaction was stopped by submerging the slides in PBS, and the sections were counterstained with Mayer’s hematoxylin and mounted for light microscopy.
RNA extraction and reverse transcription (RT)-PCR.
Total RNA was extracted from fresh skin samples with RNA zol B (Tel-Test B, Friendswood, Tex.) as described previously (22). cDNAs were synthesized from 5 μg (2 μl) of total RNA by using Moloney murine leukemia virus reverse transcriptase (Toyobo, Osaka, Japan) and oligo(dT)12-18 primers (Gibco-BRL Products, Gaithersburg, Md.). Two microliters (5 μg) of total RNA was reverse transcribed by specific priming to first-strand cDNA as described by Inafuku et al. (19). PCR amplification was performed with a PT-100 programmable thermal cycler (MJ Research, Inc., Watertown, Mass.). The reaction mixture consisted of 5 μl of 10× reaction buffer (500 mM KCl, 100 mM Tris-HCl [pH 8.3], 1.5 mM MgCl2), 4 μl of deoxynucleoside triphosphate (2.5 mM), 0.25 μl of Taq DNA polymerase (Takara Co., Ltd., Shiga, Japan), and 100 pmol (1 μl) each of forward and reverse primers; to make a final volume of 50 μl, 33.75 μl of distilled water was added. PCR cycles were run under the following conditions: DNA denaturation at 94°C for 1 min, primer annealing at 60°C, and DNA extension at 72°C for 2 min. After 45 cycles for TNF-α, IFN-γ, IL-4, and housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH), the reaction was stopped. Primers were made to order by Hokkaido System Science Co., Sapporo, Japan. The sequences of the PCR primers and the product sizes for IFN-γ, TNF-α, IL-4, and GAPDH are given in Table 1. A 100-bp ladder (Toyobo) was used as a size marker. PCR products were subjected to electrophoresis in a 2.5% agarose gel containing 0.8 mg/ml ethidium bromide and photographed.
TABLE 1.
Primer sequences and amplified product sizes
| Cytokine or protein | Type of primer | Sequence (5′ to 3′) | Amplified band size (bp) |
|---|---|---|---|
| INF-γ | Sense | GGCTGTTTCTGGCTGTTACTGC | 426 |
| Antisense | GACTCCTTTTCCGCTTCCTGA | ||
| TNF-α | Sense | CCTCCCTCTCATCAGTTCTA | 501 |
| Antisense | GCAATGACTCTAAAGTAGACCTG | ||
| IL-4 | Sense | GACGGCACAGAGCTATTGATGGGTC | 411 |
| Antisense | TAGGCTTTCCAGGAAGTCTTTCAGTGATGTG | ||
| GAPDH | Sense | TGGCCAAGGTCATCCATGAC | 210 |
| Antisense | AGGCCATGCCAGTGAGCTTC |
Statistical analysis.
Each experiment was repeated three times. When appropriate, Student's unpaired t test was used to determine the statistical significance of differences between groups. P values of <0.05 were considered significant.
RESULTS
Effects of low-dose UVB irradiation on the course of Leishmania infection.
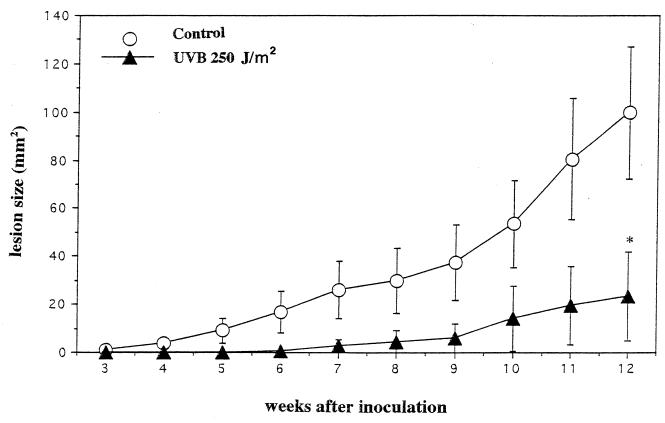
When we examined the course of L. amazonensis infection in nonirradiated and low-dose UVB-irradiated BALB/c mice, different patterns were observed. Normal control BALB/c mice are susceptible to this parasite and develop nonhealing lesions at the sight of parasite inoculation. At 3 weeks of inoculation, nonirradiated mice showed visible lesions at the injection site; in contrast, low-dose UVB-irradiated mice remained resistant to infection. After 6 weeks of infection, 12 of 20 (60%) of nonirradiated mice had lesions, while 3 of 20 (14.2%) of mice irradiated with low-dose UVB had small papules. After 12 weeks of infection, 8 of 20 (40%) of mice irradiated with low-dose UVB had small nodules, while 100% of nonirradiated mice had large ulcers and had to be sacrificed. The lesion size difference between low-dose UVB-irradiated and nonirradiated control mice was statistically significant (P < 0.0008) (Fig. 1).
FIG. 1.
Low-dose UVB irradiation suppresses the lesion development of cutaneous L. amazonensis infection. BALB/c mice were irradiated with UVB at 250 J/m2 for 4 consecutive days; 12 h later, control and irradiated mice were injected with 2 × 106 L. amazonensis promastigotes. Three weeks after inoculation, visible skin lesions were measured weekly, and size was calculated by measuring length and width. Nonirradiated and irradiated mice were killed at 12 weeks after inoculation. Data presented here are the means and standard deviations from a representative experiment repeated three times with the same results. Statistically, the significance of the difference between the groups was analyzed by Student's unpaired t test. An asterisk indicates a P value of <0.0008.
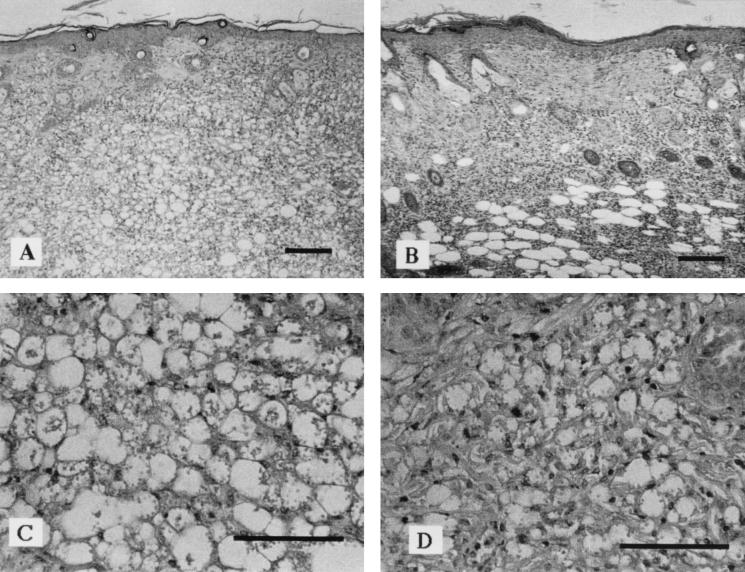
The results shown in Fig. 2 illustrate the tissue pathology seen in nonirradiated mice infected with L. amazonensis only (Fig. 2A and C) or infected with L. amazonensis 12 h after the last UVB exposure (Fig. 2B and D). Nonirradiated control mice, infected with L. amazonensis, showed an intense diffuse inflammatory infiltrate, tissue necrosis, and areas of ulceration with intense tissue parasitism. In contrast, mice preirradiated with low-dose UVB showed a small infiltrate with little tissue destruction, lymphocytes, plasma cells, few polymorphonuclear cells, many noninfected macrophages, and few infected macrophages. No parasites were observed in the viscera of irradiated or control mice (data not shown).
FIG. 2.
Low-dose UVB irradiation decreases parasite burden at the parasite injection site. Hematoxylin-eosin-stained tissue specimens were from BALB/c mice injected with 2 × 106 L. amazonensis promastigotes. (A and C) Tissue samples taken from control mice. (B and D) Tissue samples taken from mice irradiated with low-dose UVB. Bars, 100 μm.
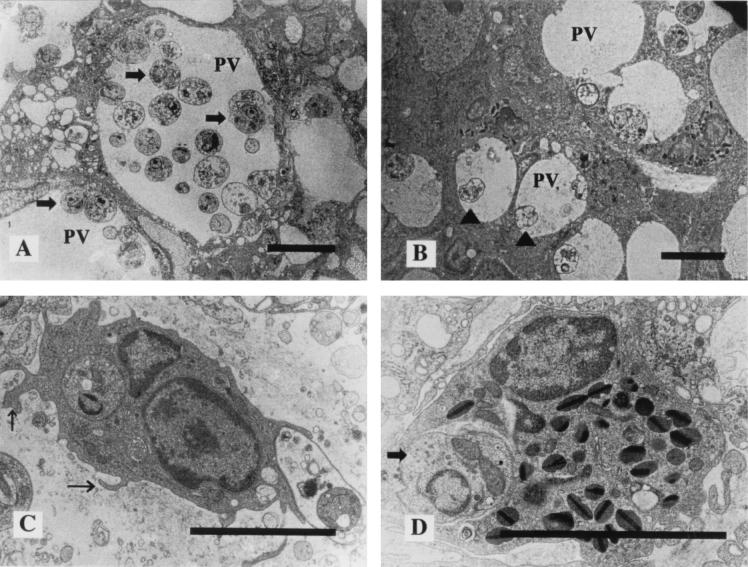
Electron microscopic observation of control mouse lesion revealed that the dermal macrophages had large parasitophorus vacuoles (PV) and contained many Leishmania parasites. These parasites were round and had well-developed cell organelles (Fig. 3A). In the dermis, many Leishmania parasites were also seen outside macrophages. In contrast, specimens obtained from low-dose UVB-irradiated mice showed smaller PV and a few Leishmania parasites phagocytized by dermal macrophages (Fig. 3B). Signs of macrophage activation were seen in the majority of these cells, which exhibited characteristics of intense activity, such as the presence of evident nucleoli, increases in cytoplasmic volume associated with a large number of lysosomes, and numerous microvilli (Fig. 3C). Interestingly, in low-dose UVB-irradiated mice, Leishmania parasites were also phagocytized by dermal eosinophils (Fig. 3D).
FIG. 3.
Transmission electron micrographs of parasite inoculation sites after 12 weeks. (A) A specimen from a control mouse shows large PV, containing many Leishmania parasites (arrows). (B) A specimen from a low-dose UVB-irradiated mouse shows small PV, containing few Leishmania parasites; some show degenerative changes (arrowheads). (C) In a low-dose UVB-irradiated mouse, signs of macrophage activation, a large number of lysosomes, and many microvilli (arrows) are seen. (D) Leishmania parasites are phagocytized by eosinophils in a low-dose UVB-irradiated mouse (arrow). Scale bars, 5 μm.
Time-dependent induction of IFN-γ and TNF-α cytokines.
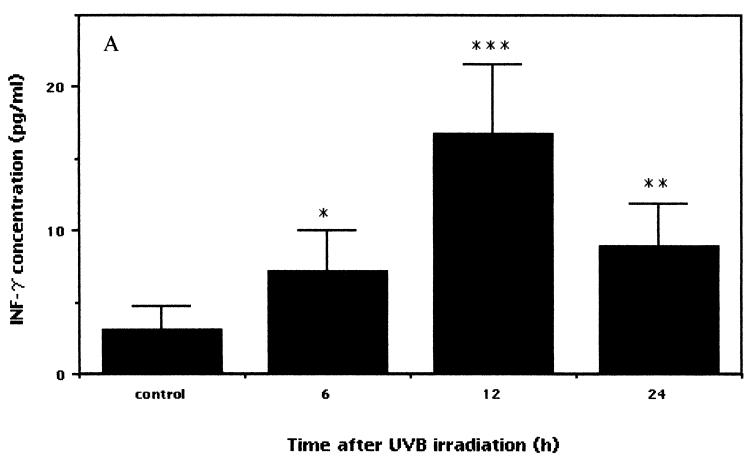
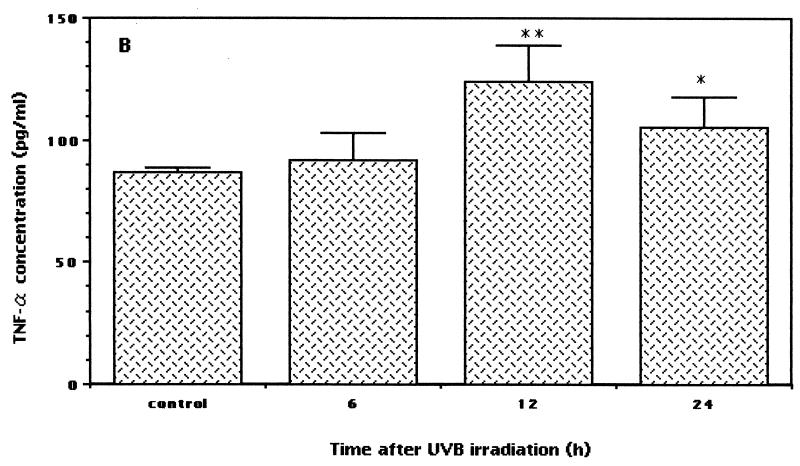
In order to examine the immune responses in nonirradiated mice versus those preirradiated with low-dose UVB, blood samples were collected at different times. IFN-γ, TNF-α, and IL-4 responses were evaluated by a sandwich ELISA. The IFN-γ level in low-dose UVB-irradiated mice was significantly upregulated compared to that in control mice (Fig. 4A). Differences were observed at 6 h after irradiation, and the levels of serum IFN-γ between the irradiated and control mice diverged with time, were significant at 12 h (P < 0.0003), and remained elevated at up to 24 h after irradiation (P < 0.004) (irradiated versus control mice).
FIG. 4.
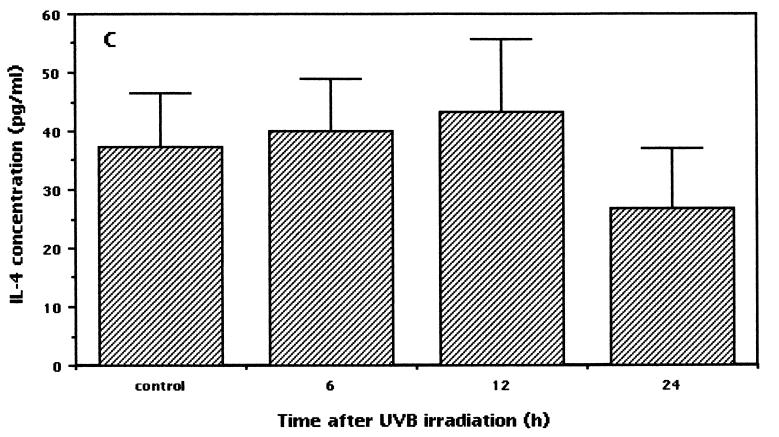
Expression of serum cytokine levels in low-dose UVB-irradiated and control mice. After the last exposure to low-dose irradiation, blood was obtained at various times. Serum samples obtained by centrifugation at 2,000 × g for 20 min at 4°C were analyzed with commercially available ELISA kits to measure IFN-γ, TNF-α, and IL-4 protein concentrations. The data shown represent the time-dependent induction of IFN-γ (A) (asterisk, P < 0.02; double asterisks, P < 0.004; and triple asterisks, P < 0.0003), TNF-α (B) (asterisk, P < 0.03; and double asterisks, P < 0.005), and IL-4 (C) after low-dose UVB irradiation. Each sample was analyzed in duplicate, and data are expressed as means and standard deviations from a representative experiment repeated three times with the same results. Statistically, the significance of the difference between the groups was analyzed by Student's unpaired t test.
It has been reported that TNF-α mediates host resistance against leishmaniasis in a murine model (44). Thus, we assume that TNF-α may also be induced after low-dose UVB irradiation. The present results disclosed that along with the IFN-γ level, the TNF-α level was also increased in low-dose UVB-irradiated mice. Upregulated levels of TNF-α were observed at 6 h (not significant), peaked at 12 h (P < 0.005), and remained elevated at up to 24 h after the last irradiation (P < 0.03) (irradiated versus control mice) (Fig. 4B). In contrast to the upregulated levels of IFN-γ and TNF α expression after low-dose UVB irradiation, there was no significant change in the serum IL-4 level (Fig. 4C). A slight downregulation of the level of IL-4 was observed at 24 h after irradiation, but statistically the difference was not significant (P < 0.3).
Immunohistochemical localization of IFN-γ and TNF-α cytokines in the skin after low-dose UVB irradiation.
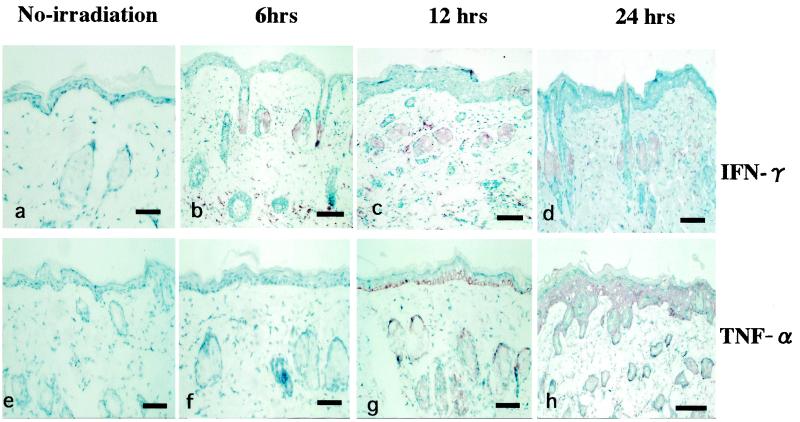
Skin samples from nonirradiated mice showed no immunopositivity for IFN-γ (Fig. 5a). IFN-γ was found to appear first in dermal spindle-like cells and the infundibulum of the hair follicle at 6 h after the last UVB irradiation (Fig. 5b). The intensity and number of cells staining positive for IFN-γ then increased by 12 h (Fig. 5c) and 24 h (Fig. 5d) after the last UVB exposure. Skin samples from control mice and from mice at 6 h after the last UVB exposure showed negative staining for TNF-α (Fig. 5e and f). Positive staining was observed in dermal spindle-like cells and the epidermis at 12 h after the last UVB irradiation (Fig. 5g). TNF-α staining became stronger and was maximal at 24 h (Fig. 5h). TNF-α immunoreactivity was found to localize predominantly in dermal spindle-like cells and the epidermis, whereas IFN-γ-positive staining was restricted to the dermal hair infundibulum and spindle-like cells (Fig. 5c and h).
FIG. 5.
Immunohistochemical detection of IFN-γ and TNF-α cytokine expression at different times after low-dose UVB irradiation. (a and e) Samples from nonirradiated mice used as controls. (b and f, c and g, and d and h) Samples from mice at 6, 12, and 24 h after the last UVB exposure, respectively.
Effects of low-dose UVB irradiation on cytokine mRNA expression.
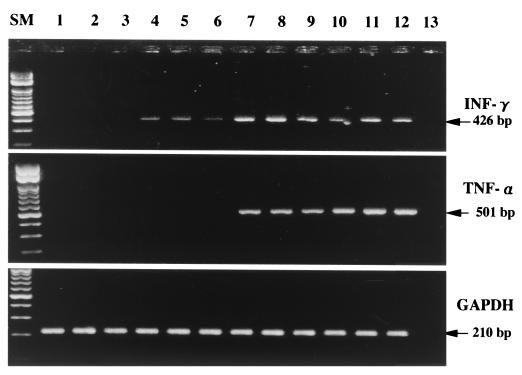
To assess the local induction of cytokines after low-dose UVB irradiation, mRNA levels for IFN-γ, TNF-α, and IL-4 were examined by RT-PCR. Figure 6 shows IFN-γ and TNF-α mRNA expression in tissue samples taken from low-dose UVB-irradiated and control mice. A weak positive signal of 426 bp for IFN-γ was obtained at 6 h, and a strong positive signal was obtained at 12 and 24 h after the last irradiation, while control mice showed negative results. A positive signal of 501 bp for TNF-α was obtained at 12 and 24 h after the last irradiation. In contrast, at 6 h after the last irradiation and in control mice, negative results were seen for TNF-α. The same method of PCR repeatedly failed to yield a signal for IL-4, despite an increase in the number of cycles to 45.
FIG. 6.
Detection by RT-PCR assays of IFN-γ, TNF-α, and GAPDH mRNAs in tissues from nonirradiated control mice and low-dose UVB-irradiated mice at different times. A total of six mice per group were used. Results for representative samples taken from three mice in each group are shown. Lanes 1 to 3, nonirradiated mice; lanes 4 to 6, 7 to 9, and 10 to 12, mice at 6, 12, and 24 h after the last UVB irradiation, respectively. Lane 13, no DNA added (negative control). Lane SM, size markers.
DISCUSSION
Different studies have demonstrated that high-dose or chronic UVB irradiation suppresses host immunity to several infectious diseases (7, 10, 18, 20, 21, 33, 48) and is one of the major causes of skin carcinogenesis. UVB irradiation influences immunosuppression, not only locally but also systemically. As evidence of this immunosuppression, it has been reported that the numbers of T lymphocytes and natural killer, OKT6+, and epidermal Langerhans cells are reduced after UVB irradiation in humans and mice (17, 27, 41). UVB irradiation also impairs the cell-mediated immune response; i.e., it induces predominantly Th2 cytokines and reduces the levels of Th1 cytokines (11). On the basis of these facts, the present study was designed to investigate whether low-dose UVB irradiation at the inoculation site prior to Leishmania infection would influence the subsequent development of clinical disease in L. amazonensis infection. Surprisingly, mice irradiated with low-dose UVB and challenged with L. amazonensis promastigotes showed a significant delay in and suppression of the apparent onset and development of cutaneous lesions compared to control mice.
Our results disclosed that exposure to low-dose UVB prior to L. amazonensis infection makes BALB/c mice resistant to Leishmania parasites. It is noteworthy that the protective dose of UVB irradiation used in this study (250 J/m2) against L. amazonensis is well below the minimum erythematous dose of 400 J/m2 (35). Protection persisted even up to 12 weeks of infection, when the animals were sacrificed. Our data are consistent with those of Giannini (13), who first reported that cutaneous lesion development was significantly suppressed by low-dose UVB irradiation in mice infected with L. major.
Previous studies demonstrated that lesion histological patterns reflect the host immune status in leishmaniasis (2, 36). Therefore, to corraborate the results obtained above, we examined histological changes in low-dose UVB-irradiated mice and nonirradiated control mice at 12 weeks after infection. Necrotic areas were fewer in number and smaller in size in UVB-irradiated mice. In addition, in UVB-irradiated mice, areas of mixed-cell infilammatory reaction and areas of granulomatous reaction were also noted. Only few parasitized macrophages could be found among these cells. This type of tissue reaction correlates with resistance in leishmaniasis (3). Veress et al. (46) reported that PV were the result of the inability of macrophages to kill parasites and hypothesized that they were the morphological signs of intercellular parasite survival and multiplication by binary fission. Our ultrastructural observations disclosed that PV were of a smaller size and degenerated Leishmania parasites were seen in low-dose UVB-irradiated mice compared to control mice. Interestingly, Leishmania parasites were phagocytized by eosinophils in low-dose UVB-irradiated mice. These results suggest that the leishmanicidal activity of the macrophages and eosinophils might have been induced by low-dose UVB irradiation. A major question raised by these results is related to the mechanism of protection against Leishmania infection observed in mice exposed to low-dose UVB.
Different hypotheses can be postulated to explain the mechanism by which low-dose UVB irradiation causes the suppression of cutaneous lesions, as seen in this study. It may be hypothesized that increased activity of T lymphocytes through the expression of the Th1 response inhibits lesion development. In leishmaniasis, host resistance depends on cell-mediated immunity; the Th1 (IFN-γ, TNF-α, and IL-12) response is considered to be the most important element (5), while the Th2 (IL-4 and IL-10) response is disease exacerbating. Leishmania infection in BALB/c mice usually leads to a dominant Th2 (IL-4) response, and uncontrolled lesions develop; however, other strains of mice, such as C3H/HeN and C57BL/6, develop a Th1 (IFN-γ) response, control parasite multiplication, heal spontaneously, and develop resistance to infection (16).
In our previous study we reported that preirradiation with low-dose UVA significantly suppressed lesion development in mice infected with L. amazonensis, and it was demonstrated that this phenomenon appeared as significantly upregulated levels of IFN-γ, TNF-α, and IL-12 and downregulated IL-4 and IL-10 cytokine expression (22). The present results showed that IFN-γ synthesis was profoundly upregulated by low-dose UVB irradiation, as indicated by the increased level of this cytokine in serum. Interestingly, however, considerable time-dependent expression of IFN-γ mRNA was also detected in skin tissues of low-dose UVB-irradiated mice by RT-PCR and immunohistochemical analysis. Our data are consistent with those of Shen et al. (43), who observed increased serum IFN-γ expression immediately after UVB irradiation. In contrast, Araneo et al. (4) and Gensler et al. (12) irradiated mice with UVB at 3,500 and 6 × 105 J/m2, respectively, and showed a 75 to 100% reduction in serum IFN-γ expression. The difference between the previous studies and the present one is the dose of UVB irradiation. In the present study, we used a 10-fold smaller UVB dose (250 J/m2). The exact dose of UVB exposure that will contribute to a Th1 response, however, still remains unknown.
TNF-α has been evaluated as an essential cytokine in host resistance to a variety of infectious diseases, including Listeria monocytogenes infection (31), Trypanosoma cruzi infection (47), and Plasmodium chabaudi adami infection (8) in mice. Liew et al. (29) reported that TNF-α plays an important role in host resistance against cutaneous leishmaniasis in mice. Our results disclosed that TNF-α synthesis was upregulated by low-dose UVB irradiation, as indicated by the increased level of this cytokine in serum. Interestingly, however, considerable time-dependent levels of TNF-α mRNA were also detected at 12 and 24 h after the last UVB exposure in mouse skin tissues by RT-PCR and immunohistochemical analysis. Our results are in agreement with those of previous studies reporting that serum TNF-α and TNF-α mRNA in human and mouse keratinocytes and in dermal fibroblasts were induced by UVB irradiation at doses of 2, 10 to 100, and 1,400 J/m2, respectively (9, 24, 25). Considering these findings, it is highly possible that UVB at a dose of 250 J/m2 will exert a protective effect on the course of L. amazonensis infection, i.e., delaying and significantly suppressing lesion development in irradiated mice compared to nonirradiated control mice.
Leishmania infection in BALB/c mice induces a Th2 response, characterized by elevated levels of serum IL-4 and IL-4 mRNA (16, 42). Afonso and Scott (1) detected IL-4 only at the early stage of L. amazonensis infection; later, when lesions had developed, no production of IL-4 was observed. Our data showed that serum IL-4 expression remained unchanged and that IL-4 mRNA was not detected in low-dose UVB-irradiated mice. Previous studies indicated that IL-4 does not appear to be sufficient to make C57BL/6 mice susceptible to infection with L. major (40) and that neutralizing monoclonal antibodies against IL-4 protect BALB/c mice from infection with L. major only when given in the first week of infection (28, 39). Therefore, our results suggest that, at least at the level of Th-cell differentiation, the major mechanism of action operating during low-dose UVB irradiation and possibly responsible for protection against the immunopathological response elicited by L. amazonensis is the inhibition of Th precursor cells from developing into Th2 cells.
The presence of IFN-γ and TNF-α after low-dose UVB irradiation, during the initial interactions of parasites and host cells, is critical for Leishmania disease outcome. TNF-α can mediate host resistance against Leishmania infection (27, 43) and most likely acts in synergy with IFN-γ to activate macrophages to synthesize nitric oxide, which kills the intracellular parasites (14). At present, however, the results presented here demonstrate that, like preirradiation with low-dose UVB, stimulated production of IFN-γ and TNF-α provides a potent leishmanicidal pathway against L. amazonensis. The major conclusion that can be drawn from our study is that the IFN-γ and TNF-α cytokines are responsible for the suppression of the lesion development of L. amazonensis infection. On the basis of our previous (22) and present results, we speculate that low-dose UV irradiation enhances host immunity through the upregulation of Th1 cytokines. We also hypothesize that UV irradiation is not always immunosuppressive and may be beneficial if used in low doses. An evaluation of the range of low-dose UV irradiation which enhances the host immune response through the upregulation of Th1 cytokines is under way.
Acknowledgments
This study was supported in part by grant-in-aid 13770458 for science research to M.M. from the Ministry of Education, Science, Sports and Culture.
REFERENCES
- 1.Afonso, L. C., and P. Scott. 1993. Immune responses associated with susceptibility of C57BL/10 mice to Leishmanaia amazonensis. Infect. Immun. 61:2952-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida, R. P., M. Barral-Netto, A. Ribeiro de Jesus, L. A. R. Freitas, E. M. Carvalho, and A. Barral. 1996. Biological behavior of Leishmania amazonensis isolated from humans with cutaneous, mucosal or visceral leishmaniasis in BALB/c mice. Am. J. Trop. Med. Hyg. 54:178-184. [DOI] [PubMed] [Google Scholar]
- 3.Andrade, Z. A., S. G. Reed, S. Roters, and M. Sadigursky. 1984. Immuno-patology of experimental cutaneous leishmaniasis. Am. J. Pathol. 114:137-148. [PMC free article] [PubMed] [Google Scholar]
- 4.Araneo, B. A., T. Dowell, H. B. Moon, and R. A. Daynes. 1989. Regulation of murine lymphokine production in vivo: ultraviolet radiation exposure depresses IL-2 and enhances IL-4 production by T cells through an IL-1-dependent mechanism. J. Immunol. 143:1737-1744. [PubMed] [Google Scholar]
- 5.Barrel-Netto, M., J. S. daSilva, A. Barrel, and S. Reed. 1995. Up-regulation of T helper 2 and down-regulation of T helper 1 cytokines durin murine retrovirus-induced immunodeficiency syndrome enhances susceptibility of a resistant mouse strain to Leishmania amazonensis. Am. J. Pathol. 146:635-642. [PMC free article] [PubMed] [Google Scholar]
- 6.Bogdan, C., A. Gessner, W. Solbach, and M. Rollinghoff. 1996. Invasion control and persistance of Leishmania parasites. Curr. Opin. Immunol. 8:517-525. [DOI] [PubMed] [Google Scholar]
- 7.Brown, E. L., J. M. Rivas, S. E. Ullrich, C. R. Young, S. J. Norris, and M. L. Kripke. 1995. Modulation of immunity to Borrelia burgdorferi by ultraviolet irradiation: differential effect on Th1 and Th2 immune responses. Eur. J. Immunol. 25:3017-3022. [DOI] [PubMed] [Google Scholar]
- 8.Clark, I. A., N. H. Hunt, G. A. Butcher, and W. B. Cowden. 1987. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant IFN-γ or tumor necrosis factor and its enhancement by butylated hydroxyanisole. J. Immunol. 139:3493-3496. [PubMed] [Google Scholar]
- 9.deKossodo, S., D. Jr. Cruz, I. Dougherty, P. Thompson, M. Silva-Valdez, and B. Beutler. 1995. Expression of the tumor necrosis factor gene by dermal fibroblasts in response to ultraviolet irradiation or lipopolysaccharide. J. Investig. Dermatol. 104:318-322. [DOI] [PubMed] [Google Scholar]
- 10.Denkins, Y., I. J. Fidler, and M. L. Kripke. 1989. Exposure of mice to UVB radiation suppresses delayed hypersensitivity to Candida albicans. Photochem. Photobiol. 49:615-619. [DOI] [PubMed] [Google Scholar]
- 11.Garssen, J., R. J. Vandebriel, F. R. deGruijl, D. A. Wolvers, M. van Dijk, A. Fluitman, and H. vanLoveren. 1999. UVB exposure-induced systemic modulation of Th1-and Th2-mediated immune responses. Immunology 97:506-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gensler, H. L., J. Simpson, K. Gerrish, and J. Gilmore. 1995. Reduction of interferon-gamma as a critical mechanism by which ultraviolet radiation prevents tumor rejection. Photochem. Photobiol. 62:862-868. [DOI] [PubMed] [Google Scholar]
- 13.Giannini, M. S. H. 1986. Suppression of pathogenesis in cutaneous leishmaniasis by UV irradiation. Infect. Immun. 51:838-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green, S. J., R. M. Crawford, J. T. Hockmeyer, M. S. Meltzer, and C. A. Nacy. 1990. Leishmania major amastigotes initiate the l-arginine-dependent killing mechanism in IFN-γ-stimulated macrophages by induction of tumor necrosis factor-α. J. Immunol. 145:4290-4297. [PubMed] [Google Scholar]
- 15.Hayashi, S., C. C. Chan, R. T. Gazzinelli, and F. G. Roberge. 1996. Contribution of nitric oxide to host parasite equilibrium in toxoplasmosis. J. Immunol. 156:1476-1481. [PubMed] [Google Scholar]
- 16.Heinzel, F. P., M. D. Sadick, B. J. Holaday, and R. M. Locksley. 1989. Reciprocal expression on IFN-γ or IL-12 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell 4 subsets J. Exp. Med. 169:59-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hersey, P., G. Haran, E. Hasic, and A. Edwards. 1983. Altration of T cell subsets and induction of suppressor T-cell activity in normal subjects after exposure to sunlight. J. Immunol. 31:171-174. [PubMed] [Google Scholar]
- 18.Howie, S., M. Norval, and J. Maingay. 1986. Exposure to low-dose ultraviolet radiation suppresses delayed-type hypersensitivity to herpes simplex virus in mice. J. Investig. Dermatol. 86:125-128. [DOI] [PubMed] [Google Scholar]
- 19.Inafuku, K., A. Takamiyagi, M. Oshiro, T. Kinjo, Y. Nakashima, and S. Nonaka. 1999. Alterations of mRNA levels of δ-aminolevulinic acid synthase, ferro-chelatase and heme oxygenase-1 in griseofulvin induced protoporphyria mice. J. Dermatol. Sci. 19:189-198. [DOI] [PubMed] [Google Scholar]
- 20.Jeevan, A., and M. L. Kripke. 1989. Effect of a single exposure to ultraviolet radiation on Mycobacterium bovis bacillus Calmette-Guerin infection in mice. J. Immunol. 143:2837-2843. [PubMed] [Google Scholar]
- 21.Jeevan, A., K. Gilliam, H. Heard, and M. L. Kripke. 1992. Effects of ultraviolet radiation on the pathogenesis of Mycobacterium lepraemurin in mice. Exp. Dermatol. 1:152-160. [DOI] [PubMed] [Google Scholar]
- 22.Khaskhely, N. M., M. Maruno, A. Takamiyagi, H. Uezato, M. A. K. Khan, A. Hosokawa, K. Kariya, Y. Hashiguchi, E. A. L. Gomez, and S. Nonaka. 2001. Pre-exposure with low-dose UVA suppresses lesion development and enhances Th1 response in BALB/c mice infected with Leishmania (Leishmania) amazonensis. J. Dermatol. Sci. 26:217-232. [DOI] [PubMed] [Google Scholar]
- 23.Khaskhely, N. M., H. Uezato, T. Kamiyama, M. Maruno, K. Kariya, M. Oshiro, and S. Nonaka. 2000. Association of human papillomavirus type 6 with verruciform xanthoma. Am. J. Dermatopathol. 22:445-452. [DOI] [PubMed] [Google Scholar]
- 24.Kibitel, J., V. Hejmadi, L. Alas, A. O'Connor, B. M. Sutherland, and D. Yarosh. 1998. UV-DNA damage in mouse and human induces the expression of tumor necrosis factor alpha. Photochem. Photobiol. 67:541-546. [PubMed] [Google Scholar]
- 25.Kock, A., T. Schwarz, R. Kirnbauer, A. Urbanski, P. Perry, J. C. Ansel, and T. A. Luger. 1990. Human keratinocytes are source for tumor necrosis factor-α: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J. Exp. Med. 172:1609-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondo, S. 2000. The role of cytokines in photoaging. J. Dermatol. Sci. 23:S30-S36. [DOI] [PubMed] [Google Scholar]
- 27.Koulu, L., C. T. Jansen, and M. Viander. 1985. Effect of UVA and UVB irradiation on human epidermal Langerhans cell membrane markers defined by ATPase activity and monoclonal antibodies (OKT6 and anti-Ia). Photodermatology 2:339-346. [PubMed] [Google Scholar]
- 28.Lezama-Davila, C. M., D. M. Williams, G. Gallagher, and J. Alexander. 1992. Cytokine control of Leishmania infection in the BALB/c mouse: enhancement and inhibition of parasite growth by local administration of IL-2 or IL-4 is species and time dependent. Parasite Immunol. 14:37-48. [DOI] [PubMed] [Google Scholar]
- 29.Liew, F. Y., C. Parkinson, S. Millott, A. Severn, and M. Carrier. 1990. Tumor necrosis factor (TNF-α) in leishmaniasis. 1. TNF-α mediates host protection against cutaneous leishmaniasis. Immunology 69:570-573. [PMC free article] [PubMed] [Google Scholar]
- 30.Miyauchi, H., and T. Horio. 1995. Ultraviolet B-induced local immuno suppression of contact hypersensitivity is modulated by ultraviolet irradiation and hapten application. J. Investig. Dermatol. 104:364-369. [DOI] [PubMed] [Google Scholar]
- 31.Nakane, A., T. Minagawa, and K. Kato. 1988. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect. Immun. 56:2563-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noonan, F. P., C. Bucana, D. N. Sauder, and E. C. DeFabo. 1984. Mechanism of systemic immune suppression by UV irradiation in vitro. II. The UV effects on number and morphology of epidermal Langerhans cells and the UV-induced suppression of contact hypersensitivity have different wavelength dependencies. J. Immunol. 132:2408-2416. [PubMed] [Google Scholar]
- 33.Noonan, F. P., and F. A. Alewis. 1995. UVB-induced immune suppression and infection with Schistosoma mansoni. Photochem. Photobiol. 61:99-105. [DOI] [PubMed] [Google Scholar]
- 34.O'Garra, A., and K. Murphy. 1994. Role of cytokines in determining T-lymphocyte function. Curr. Opin. Immunol. 6:458-466. [DOI] [PubMed] [Google Scholar]
- 35.Parrish, J. A. 1983. Photobiology and immunology, p. 3-20. In J. A. Parrish (ed.), The effect of ultraviolet radiation on the immune system. Johnson & Johnson Baby Products Co., Skillman, N.J.
- 36.Pompeu, M. M., L. A. R. Freitas, M. L. V. Santos, A. Barral, and M. Barral-Netto. 1992. Leishmania amazonensis infection: a comparison of in vivo leishmaniacidal mechanism between immunized and naive infected BALB/c mice. Exp. Parasitol. 74:169-176. [DOI] [PubMed] [Google Scholar]
- 37.Reed, S. G., and P. Scott. 1993. T-cells and cytokine responses in leishmaniasis. Curr. Opin. Immunol. 5:524-531. [DOI] [PubMed] [Google Scholar]
- 38.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 39.Sadick, M. D., F. P. Heinzel, B. J. Holaday R. T. Pus, R. S. Dawkins, and R. M. Locksley. 1990. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, IFN-γ-independent mechanism. J. Exp. Med. 171:115-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadick, M. D., N. Street, T. R. Mosmann, and R. M. Locksley. 1991. Cytokine regulation of murine leishmanisis: interleukin 4 is not sufficient to mediate progressive disease in resistant C57BL/6 mice. Infect. Immun. 59:4710-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schacter, B., M. M. Lederman, M. J. LeVine, and J. J. Ellner. 1983. Ultraviolet radiation inhibits natural killer cell activity and lymphocyte proliferation. J. Immunol. 130:2484-2487. [PubMed] [Google Scholar]
- 42.Scott, P., E. Pearce, A. W. Cheever, R. L. Coffman, and A. Sher. 1989. Role of cytokines and CD4+ T cell subsets in the regulation of parasite immunity and disease. Immunol. Rev. 112:161-182. [DOI] [PubMed] [Google Scholar]
- 43.Shen, J., S. Bao, and V. E. Reeve. 1999. Modulation of IL-10, IL-12, and IFN-γ in the epidermis of hairless mice by UVA (320-400 nm) and UVB (280-20 nm) radiation. J. Investig. Dermatol. 113:1059-1064. [DOI] [PubMed] [Google Scholar]
- 44.Titus, R. G., B. Sherry, and A. Cerami. 1989. Tumor necrosis factor plays a protective role in experimental cutaneous leishmaniasis. J. Exp. Med. 170:2097-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uezato, H., K. Hagiwara, A. Hosokawa, M. Maruno, S. Nonaka, M. Oshiro, M. Furuya, E. A. L. Gomez, and Y. Hashiguch. 1998. A preliminary study aimed at the detection of Leishmania parasites in subjects with cutaneous Leishmaniasis using polymerase chain reaction. J. Dermatol. 25:290-298. [DOI] [PubMed] [Google Scholar]
- 46.Veress, B., R. E. Abdalla, and A. M. El Hassan. 1981. Electron microscope investigations on the leishmaniasis in the Sudan. II. Ultrastructural morphology of macrophage-parasite interaction in human and hamaster macrophages in vivo. Ann. Trop. Med. Parasitol. 75:607-613. [DOI] [PubMed] [Google Scholar]
- 47.Wirth, J. J., and F. Kierszenbaum.1988. Recombinant tumor necrosis factor enhances macrophage destruction of Trypanosoma cruzi in the presence of bacterial endotoxin. J. Immunol. 141:286-288. [PubMed] [Google Scholar]
- 48.Yasumoto, S., Y. Hayashi, and L. Aurelian. 1987. Immunity to herpes Simplex virus type 2: suppression of virus-induced immune responses in ultraviolet B-irradiated mice. J. Immunol. 139:2788-2793. [PubMed] [Google Scholar]
- 49.Yoshikawa, T., V. Rae, W. Bruins-Slot, W. Vanden Berg, R. J. Taylor, and J. W. Streilein. 1990. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J. Investig. Dermatol. 95:530-536. [DOI] [PubMed] [Google Scholar]