Abstract
Levels of soluble CD30 (sCD30) in serum were elevated in patients with Plasmodium falciparum malaria but showed decline following treatment. The levels of sCD30 in serum were correlated significantly with the expression of gamma interferon by peripheral T cells. These data suggest that CD30+ cells are upregulated during a malaria attack and that they may play a regulating role at the site of inflammation.
Malaria is associated with the activation and redistribution of T cells (9), but the role of these cells during infection still remains unclear.
CD30 is a member of the tumor necrosis factor-nerve growth factor superfamily (2, 16) and is expressed mostly on a subset of activated CD4+ CD45RO+ cells (4) but also on activated γδ T cells (1). In vitro CD30+-T-cell clones express T-helper 2 or 0 cytokines, although CD30 cannot be used as a marker for the identification of Th2 cells (7, 13).
Recently, CD30+ T cells have been suggested to have a counterregulatory activity as part of a homeostatic response that attempts to control inflammation in Th1-driven diseases (5), probably as a consequence of the production of anti-inflammatory cytokines.
To determine whether CD30 could be involved in the pathogenesis of malaria, we measured levels of soluble CD30 (sCD30) in plasma samples from Ghanaian children with malaria.
All the children (n = 21) were admitted as inpatients to the Department of Child Health, Korle-Bu Teaching Hospital, Accra, Ghana, with a diagnosis of Plasmodium falciparum malaria. Only patients that could be categorized as having cerebral malaria, severe anemia, or uncomplicated malaria, according to a set of strict inclusion and exclusion criteria described elsewhere, were admitted (12), and all had elevated levels of plasma gamma interferon (IFN-γ) (data not shown). All patients were treated with standard regimens of chloroquine or artesunate, and all recovered fully from their infections. In addition to the malaria patients, we also included a group of age-matched, healthy children from a nearby community (n = 21).
This study was approved by the Ethical and Protocol Review Committee, University of Ghana Medical School, and by the Ghanaian Ministry of Health.
Peripheral blood samples were obtained on days 0 (admission), 2, 3, 7, 14, and 30 (for investigation of levels of sCD30 in plasma) or on day 0 (for investigation of expression of IFN-γ by T cells). From each of the healthy control donors, only a single blood sample was obtained.
Plasma was separated from the heparinized blood and stored at −20°C until analysis. sCD30 was measured by enzyme-linked immunosorbent assay (CD30 [Ki-1 antigen]; DAKO, Glostrup, Denmark).
Peripheral blood mononuclear cells (PBMC) were isolated by gradient centrifugation and cryopreserved at −196°C until time of assay, as previously described (8).
For intracellular staining of IFN-γ, PBMC were resuspended in RPMI 1640, supplemented with 15% heat-inactivated pooled human serum-20 IU of penicillin/ml-20 μg of streptomycin (Gibco, Paisley, United Kingdom)/ml, and seeded into 24-well multidish plates (Nunc, Roskilde, Denmark). Each well contained 106 PBMC in 1 ml of medium. The cells were cultured at 37°C in a humidified atmosphere with 5% CO2 and incubated with a solution containing 1.5 μM monensin (Sigma, St. Louis, Mo.), 1 μM ionomycin, and 50 ng of phorbol myristate acetate/ml for 4 h. Intracellular cytokine staining was based on the method of another study (10). In brief, cells were harvested, washed in phosphate-buffered saline (PBS), resuspended in PBS containing 0.5% bovine serum albumin and 0.01% NaN3 (staining buffer), and labeled with antibody directed against CD3 (UCHT1; DAKO). The cells were then washed twice in PBS-bovine serum albumin-NaN3, fixed with 2% formaldehyde (Sigma) in staining buffer, washed twice in staining buffer and twice in a freshly made saponin buffer (staining buffer containing 0.1% [wt/vol] saponin [Sigma]), and finally incubated with anti-IFN-γ (Pharmingen, San Diego, Calif.). Following cytokine labeling, the cells were washed twice in saponin buffer and twice in staining buffer, resuspended in the staining buffer, and analyzed on an EPICS XL-multi carousel loader flow cytometer (Coulter, Miami, Fla.). Analysis of the data was done with Winlist software (Verity, Topsham, Maine).
Different groups were compared by analysis of variance by using the Ranks-Dunn test; P values of <0.05 were considered significant. Correlations between parameters were tested by Pearson's product moment correlation; P values of <0.05 were considered significant.
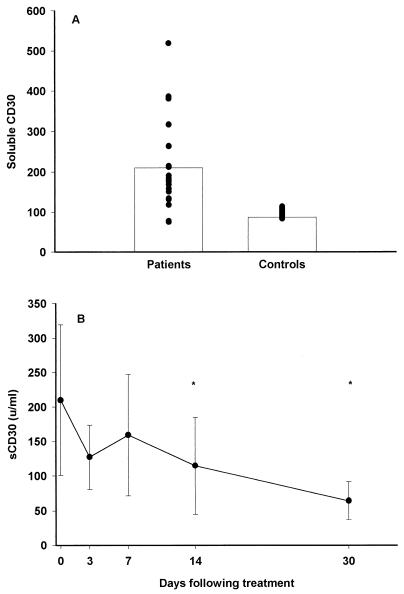
In healthy adults, levels of sCD30 in serum were low (6, 14), but high, sustained levels were found in newborns and infants (11). Elevated levels of sCD30 in plasma concomitant with increased numbers of peripheral CD30+ T cells have been reported for some infections such as measles (17), but for others such as human immunodeficiency virus (15), increased levels of sCD30 are not associated with a rise in the CD30+-T-cell count. Our study showed significantly increased levels of sCD30 in the plasma samples of malaria patients compared to levels in samples from a group of healthy age-matched children (Fig. 1A). We did not observe any differences between the clinical categories or a relationship to parasitemia (data not shown). Following treatment, levels of sCD30 remained elevated for a period but decreased significantly on days 14 and 30. (Fig. 1B).
FIG. 1.
(A) Levels of sCD30 (means are indicated with bars) in 21 patients with acute malaria (before treatment) in comparison to those in 21 age-matched healthy controls; (B) mean levels of sCD30 in sera from 21 patients following treatment. Error bars indicate standard deviations. An ∗ indicates a significant difference from the value at day 0.
The origin of the detected CD30 was not clear since the increased levels of sCD30 were concomitant with the absence of CD30+ T cells from blood in circulation (data not shown). This suggests that CD30+ T cells probably play a role at the site of inflammation and are redistributed away from the periphery during the infection, as previously suggested with malaria-antigen-reactive T cells (3).
Recently, a study of rheumatoid arthritis (5) has suggested a regulatory role for CD30+ T cells where CD30+ T cells are distributed to sites of inflammation to subdue damage.
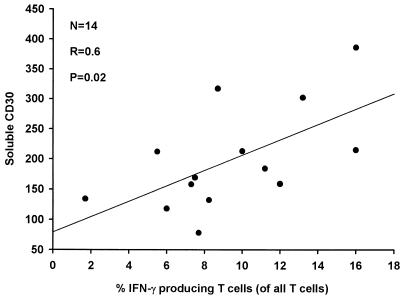
Increased IFN-γ production in cells by peripheral T cells may reflect increased inflammation, and in this study, increased levels of sCD30 were correlated to high frequencies of IFN-γ-producing T cells (Fig 2). This correlation was not found between sCD30 and peripheral cells expressing interleukin-4 (data not shown).
FIG. 2.
Correlation between levels of sCD30 in serum and peripheral T cells expressing IFN-γ after stimulation with phorbol myristate acetate and ionomycin for 14 patients with acute malaria before treatment.
The data in this study suggest that a subset of CD30+ cells is upregulated during malaria and that these cells may act as counterregulating cells in response to increased inflammation.
Acknowledgments
This work was supported by grants from the Danish International Development Agency (RUF and the ENRECA programs), the Danish Biotechnology Program, and the Novo Nordisk Foundation.
G. Grauert is thanked for excellent technical support.
REFERENCES
- 1.Biswas, P., P. Rovere, C. D. Filippi, S. Heltai, C. Smith, L. Dagna, G. Poli, A. A. Manfredi, and M. Ferrarini. 2000. Engagement of CD30 shapes the secretion of cytokines by human gamma delta T cells. Eur. J. Immunol. 30:2172-2180. [DOI] [PubMed] [Google Scholar]
- 2.Durkop, H., U. Latza, M. Hummel, F. Eitelbach, B. Seed, and H. Stein. 1992. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell 68:421-427. [DOI] [PubMed] [Google Scholar]
- 3.Elhassan, I. M., L. Hviid, G. Satti, B. Akerström, P. H. Jakobsen, J. B. Jensen, and T. G. Theander. 1994. Evidence of endothelial inflammation, T cell activation, and T cell reallocation in uncomplicated Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 51:372-379. [DOI] [PubMed] [Google Scholar]
- 4.Ellis, T. M., P. E. Simms, D. J. Slivnick, H. M. Jack, and R. I. Fisher. 1993. CD30 is a signal-transducing molecule that defines a subset of human activated CD45RO+ T cells. J. Immunol. 151:2380-2389. [PubMed] [Google Scholar]
- 5.Gerli, R., C. Lunardi, F. Vinante, O. Bistoni, G. Pizzolo, and C. Pitzalis. 2001. Role of CD30+ T cells in rheumatoid arthritis: a counter-regulatory paradigm for Th1-driven diseases. Trends Immunol. 22:72-77. [DOI] [PubMed] [Google Scholar]
- 6.Gerli, R., D. Monti, O. Bistoni, A. M. Mazzone, G. Peri, A. Cossarizza, M. Di Gioacchino, M. E. Cesarotti, A. Doni, A. Mantovani, C. Franceschi, and R. Paganelli. 2000. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech. Ageing Dev. 121:37-46. [DOI] [PubMed] [Google Scholar]
- 7.Gilfillan, M. C., P. J. Noel, E. R. Podack, S. L. Reiner, and C. B. Thompson. 1998. Expression of the costimulatory receptor CD30 is regulated by both CD28 and cytokines. J. Immunol. 160:2180-2187. [PubMed] [Google Scholar]
- 8.Hviid, L., G. Albeck, B. Hansen, T. G. Theander, and A. Talbot. 1993. A new portable device for automatic controlled-gradient cryopreservation of blood mononuclear cells. J. Immunol. Methods 157:135-142. [DOI] [PubMed] [Google Scholar]
- 9.Kemp, K., B. D. Akanmori, V. Adabayeri, B. Q. Goka, J. A. L. Kurtzhals, C. Behr, and L. Hviid. 2002. Cytokine production and apoptosis among T cells from patients under treatment for Plasmodium falciparum malaria. Clin. Exp. Immunol. 127:151-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kemp, K., and H. Bruunsgaard. 2001. Identification of IFN-gamma-producing CD4+ T cells following PMA stimulation. J. Interferon Cytokine Res. 21:503-506. [DOI] [PubMed] [Google Scholar]
- 11.Krampera, M., F. Vinante, L. Tavecchia, L. Morosato, M. Chilosi, S. Romagnani, M. E. Zanolin, and G. Pizzolo. 1999. Progressive polarization towards a T helper/cytotoxic type-1 cytokine pattern during age-dependent maturation of the immune response inversely correlates with CD30 cell expression and serum concentration. Clin. Exp. Immunol. 117:291-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurtzhals, J. A., V. Adabayeri, B. Q. Goka, B. D. Akanmori, J. O. Oliver-Commey, F. K. Nkrumah, C. Behr, and L. Hviid. 1998. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 351:1768-1772. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura, T., R. K. Lee, S. Y. Nam, B. K. Al Ramadi, P. A. Koni, K. Bottomly, E. R. Podack, and R. A. Flavell. 1997. Reciprocal regulation of CD30 expression on CD4+ T cells by IL-4 and IFN-gamma. J. Immunol. 158:2090-2098. [PubMed] [Google Scholar]
- 14.Pizzolo, G., and S. Romagnani. 1995. CD30 molecule (Ki-1 Ag): more than just a marker of CD30+ lymphoma. Haematologica 80: 357-366. [PubMed] [Google Scholar]
- 15.Pizzolo, G., F. Vinante, L. Morosato, G. Nadali, M. Chilosi, G. Gandini, A. Sinicco, R. Raiteri, G. Semenzato, H. Stein, et al. 1994. High serum level of the soluble form of CD30 molecule in the early phase of HIV-1 infection as an independent predictor of progression to AIDS. AIDS 8:741-745. [DOI] [PubMed] [Google Scholar]
- 16.Smith, C. A., H. J. Gruss, T. Davis, D. Anderson, T. Farrah, E. Baker, G. R. Sutherland, C. I. Brannan, N. G. Copeland, N. A. Jenkins, et al. 1993. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell 73:1349-1360. [DOI] [PubMed] [Google Scholar]
- 17.Vinante, F., M. Krampera, L. Morosato, A. Rigo, S. Romagnani, and G. Pizzolo. 1999. Peripheral T lymphocyte cytokine profile (IFNgamma, IL-2, IL-4) and CD30 expression/release during measles infection. Haematologica 84:683-689. [PubMed] [Google Scholar]