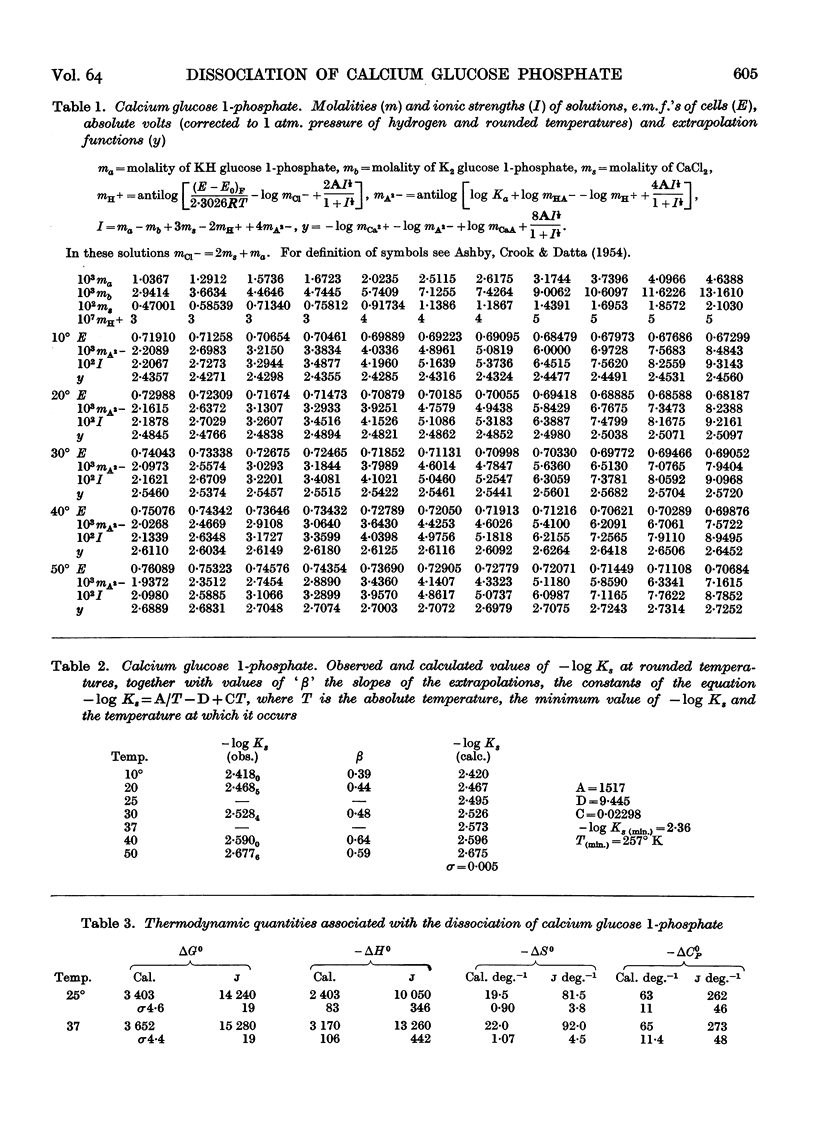
Full text
PDF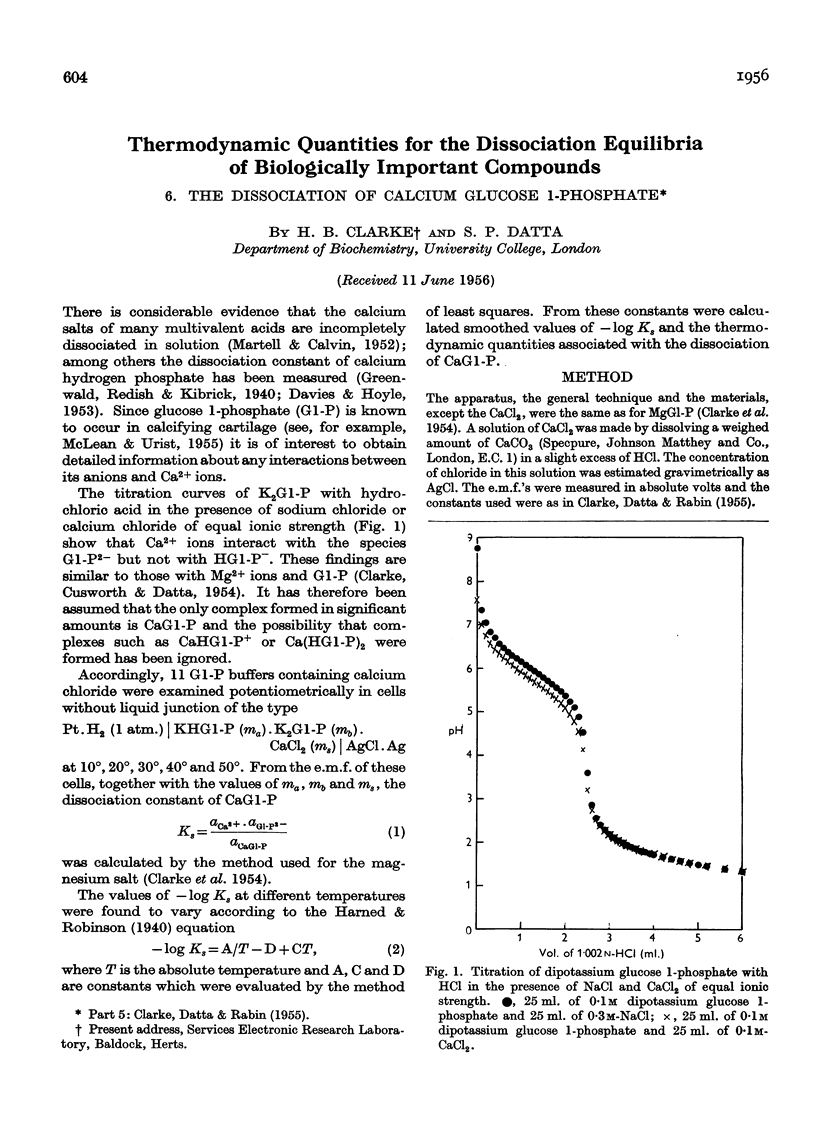

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHBY J. H., CROOK E. M., DATTA S. P. Thermodynamic quantities for the dissociation equilibria of biologically important compounds. I. Theory and experimental method. Biochem J. 1954 Feb;56(2):190–196. doi: 10.1042/bj0560190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE H. B., CUSWORTH D. C., DATTA S. P. Thermodynamic quantities for the dissociation equilibria of biologically important compounds. 3. The dissociations of the magnesium salts of phosphoric acid, glucose 1-phosphoric acid and glycerol 2-phosphoric acid. Biochem J. 1954 Sep;58(1):146–154. doi: 10.1042/bj0580146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE H. B., DATTA S. P., RABIN B. R. Thermodynamic quantities for the dissociation equilibria of biologically important compounds. 5. The second and dissociation of 2-aminoethanol 1-phosphoric acid. Biochem J. 1955 Feb;59(2):209–218. doi: 10.1042/bj0590209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLEASE N. W. Estimation of the variance of the data used in the calculation of dissociation constants. Biochem J. 1954 Feb;56(2):196–198. doi: 10.1042/bj0560196. [DOI] [PMC free article] [PubMed] [Google Scholar]


