Abstract
Infection with Mycobacterium tuberculosis continues to be one of the major global health threats. Strong mycobacterium-specific Th1 immune responses correlate with protection, and decreased Th1 responses correlate with disease progression. In contrast, the impact of Th2 responses on the development of protective immune responses to mycobacteria remains unclear. To analyze whether ongoing Th2 responses present in the lung influence the development of a protective Th1 immune response to mycobacteria, we coinfected mice with the helminth Nippostrongylus brasiliensis and Mycobacterium bovis BCG. We found that the T cells from the lymph nodes of coinfected mice secreted significantly less gamma interferon than did the T cells from mice infected with M. bovis BCG after in vitro stimulation with purified protein from M. tuberculosis when 108 CFU of M. bovis BCG were used for the infection. This result indicates that the helminth infection reduced the Th1 immune response to the mycobacteria in the lung. However, mycobacterial clearance was not delayed in the coinfected animals. Importantly, the infection with BCG after the helminth infection did not reduce the helminth-induced Th2 response in the lung, ruling out the possibility that the lack of a reduction in bacterial clearance in the coinfected mice was due to a downmodulation of the helminth-induced Th2 response. Taken together, our results suggest that ongoing Th2 responses in the lung do not necessarily lead to increased susceptibility to mycobacterial infection.
Mycobacterium tuberculosis and Mycobacterium bovis are facultative intracellular parasites which tend to reside within macrophages. Infections with these bacteria cause tuberculosis in humans and livestock. Macrophages infected with mycobacteria interact with both CD4+ and CD8+ T cells, inducing the release of cytokines by both macrophages and T cells, which in turn activate antimicrobial macrophage functions, usually leading to the control of the mycobacterial infection (5, 24). This effect is mostly mediated by gamma interferon (IFN-γ) and tumor necrosis factor alpha (5, 6, 14, 15, 18, 24). However, the cause of individual variations in susceptibility to mycobacterial disease is only partly understood. It is believed that protection is associated with strong Th1 cell-mediated immune responses, whereas Th2 immune responses with high interleukin-4 (IL-4), IL-5, and IL-10 levels promote disease (5, 24). Supporting this view are the findings that both IL-4 and IL-10 can downmodulate macrophage functions in vitro, which explains how the presence of IL-4 and/or IL-10 could interfere with the effective elimination of mycobacteria in vivo (2, 8, 9, 25). Furthermore, there is also evidence suggesting that the presence of IL-10 delays mycobacterial clearance (1, 7, 16, 17, 21, 22). However, other studies have failed to observe this effect (11, 23). Production of IL-4 and IL-5 during mycobacterial infection does not seem to play a major role in the efficient elimination of mycobacteria in immunocompetent mice (11, 23). However, eosinophils (dependent upon the production of IL-5 [4, 20]) may at least potentially play both positive and negative roles in the elimination of mycobacteria, since it has been reported that eosinophils can take up and kill mycobacteria but also contribute to a more rapid growth of the bacteria in IFN-γ receptor-deficient mice (3, 19). Taken together, the impact of Th2 responses on the efficient elimination of mycobacteria in vivo is unclear. This may also be an important issue in the design of new vaccines or therapies that aim to protect humans from tuberculosis. Furthermore, humans exposed to mycobacteria or harboring a dormant mycobacterial infection may develop allergen- or helminth-induced Th2 responses in their lifetime, possibly leading to increased susceptibility to a primary infection with mycobacteria or to a reactivation of a previously controlled infection. In particular, infections with helminths may play a role in the pathogenesis of tuberculosis in the developing countries of the world, since exposure to mycobacteria and simultaneous infection with helminths are very common.
To address the question of whether the Th2 response initiated by a helminth infection influences susceptibility to mycobacteria, C57BL/6 mice were infected intraperitoneally (i.p.) with 1,000 L3 larvae of the helminth Nippostrongylus brasiliensis and intranasally (i.n.) 4 days later with either 2 × 104 or 1 × 108 CFU of M. bovis bacillus Calmette-Guérin (BCG) (strain Copenhagen; generously provided by Jürgen Hess) as described previously (10). Helminths initiate a strong Th2 response characterized by eosinophilia and the secretion of IL-4, IL-5, and IL-10 by T cells, first in the lung and then in the gut (12). The experimental protocol we used was aimed at ensuring that the Th1 immune response to the mycobacteria occurred at the same time and site as the Th2 response induced by the helminths migrating through the lung on their way to the gut. We then analyzed whether this Th2 response reduced the development of a protective Th1 immune response to M. bovis BCG. One, two, or four weeks after infection, the mice were sacrificed, their lungs were removed, and the number of bacteria present in the lungs was determined as described previously (10, 11). The number of M. bovis BCG bacteria present in the lungs of coinfected mice was compared with the number detected in the lungs of mice infected only with M. bovis BCG at the same time points. Furthermore, the antimycobacterial Th1 response induced in the lungs was analyzed for the different groups of mice. For this purpose, mediastinal lymph node (MLN) cells from the different groups of mice were stimulated in vitro with purified protein derivative from M. tuberculosis (PPD) (20 μg/ml; Statens Serum Institute, Copenhagen, Denmark), and the amount of IFN-γ present in the culture supernatant was determined as described previously (10, 11). Since it has previously been reported that an infection with M. bovis BCG prior to an infection with N. brasiliensis decreased the Th2 response to helminths in the lung (10), we also analyzed whether the M. bovis BCG infection 4 days after the helminth infection could produce a similar effect. For this purpose, mice were infected with N. brasiliensis only or with N. brasiliensis and, 4 days later, 108 CFU of M. bovis BCG (see above). Eleven days later, the mice were sacrificed, bronchoalveolar lavages (BAL) were performed, and single-cell suspensions of MLN cells were prepared. BAL fluid cells were counted and stained with hematoxylin and eosin, and the number of eosinophils was identified microscopically. The MLN cells were stimulated in vitro for 48 h on anti-CD3-bound plates in the presence of IL-2. The levels of IL-4, IL-5, and IL-10 present in the supernatant were determined by enzyme-linked immunosorbent assay (ELISA) as described previously (11).
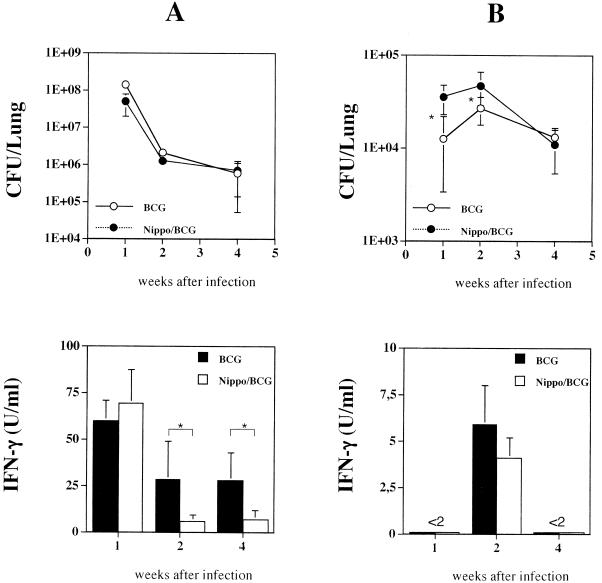
Our results showed that bacterial clearance was not delayed in the coinfected mice compared to that in animals infected only with 108 CFU of M. bovis BCG (Fig. 1A). A repetition of the experiment also showed that the helminth infection did not result in a delay of bacterial clearance (data not shown). In contrast, mice coinfected with the helminths and 2 × 104 CFU of M. bovis BCG eliminated the mycobacteria significantly more slowly at 1 and 2 weeks but not 4 weeks after infection than mice infected only with the mycobacteria (Fig. 1B). However, a repetition of this experiment showed no delay in bacterial clearance in mice coinfected with N. brasiliensis and M. bovis BCG (1 week, 30.9 × 103 ± 14.0 × 103 CFU in BCG-only mice versus 32.9 × 103 ± 14.2 × 103 CFU in coinfected mice; 2 weeks, 84.4 × 103 ± 32.3 × 103 CFU in BCG-only mice versus 58.5 × 103 ± 16.6 × 103 CFU in coinfected mice; 4 weeks, 27.5 × 103 ± 12.2 × 103 CFU in BCG-only mice versus 13.6 × 103 ± 8.0 × 103 CFU in coinfected mice [mean values with standard deviations of seven mice per group]). Although the results of one of the two experiments suggest that an infection with N. brasiliensis leads to a delay in bacterial clearance when 2 × 104 CFU of M. bovis BCG are used for the infections, a repetition of the experiment (with 5 × 104 CFU of M. bovis BCG) revealed no difference in the rates of bacterial clearance between coinfected mice and mice infected only with M. bovis BCG (data not shown). Taken together, these data suggest that infection with helminths does not significantly interfere with the efficient elimination of M. bovis BCG from the lungs of mice. One week after infection with 108 CFU of M. bovis BCG, the T cells from the draining lymph nodes of the lungs of coinfected mice secreted similar amounts of IFN-γ after mycobacterium-specific restimulation in vitro. However, 2 and 4 weeks after infection, significantly less IFN-γ was produced by the T cells from the MLN of the coinfected mice after restimulation with PPD (Fig. 1A). When 2 × 104 CFU of M. bovis BCG were used for the i.n. infection, PPD-induced IFN-γ secretion could be observed only at 2 weeks after infection. At this time point, the T cells from the MLN of coinfected mice produced amounts of IFN-γ similar to those produced by the T cells from the MLN of mice infected only with M. bovis BCG (Fig. 1B).
FIG. 1.
Mice coinfected with the helminth N. brasiliensis (Nippo) and M. bovis BCG show no delay in bacterial clearance in comparison to mice infected only with M. bovis BCG. Mice were coinfected with 1,000 L3 larvae of the helminth N. brasiliensis (i.p., day 0) and then with 1 × 108 or 2 × 104 CFU of M. bovis BCG (i.n., day 4). In parallel, mice were also infected with similar amounts of M. bovis BCG only. One, two, and four weeks after the mycobacterial infection, the numbers of CFU per lung were determined for the different groups of mice. In parallel to the preparation of the lungs, single-cell suspensions (2 × 105/well) of total MLN cells from mice at 1, 2, and 4 weeks postinfection were stimulated in vitro for 48 h with PPD (20 μg/ml). The level of IFN-γ present in the supernatants was determined by ELISA. Shown are the mean values with standard deviations (error bars) obtained when 1 × 108 CFU (A) or 2 × 104 CFU (B) of M. bovis BCG were used for the i.n. infection of five to eight mice per group. Statistical analysis was performed by using the unpaired Student t test. Asterisks indicate P values of <0.05.
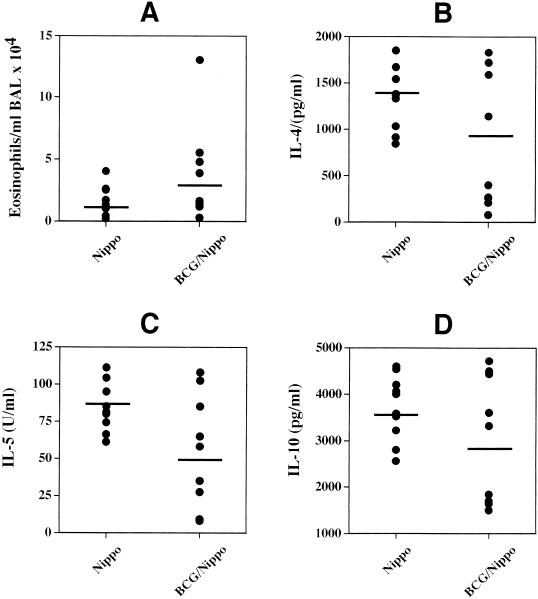
Taken together, these results suggest that the development of a Th1 immune response after infection with M. bovis BCG is reduced by the helminth infection when 1 × 108 CFU (at 2 and 4 weeks but not 1 week after mycobacterial infection) but not 2 × 104 CFU of M. bovis BCG are used. However, the elimination of M. bovis BCG from the lungs of mice was not delayed by the helminth infection. This result was surprising since it is well established that infections with the helminth N. brasiliensis induce strong Th2 responses in the lung, leading to the secretion of IL-4 and IL-10, which in turn have been associated with the deactivation of macrophages and the inefficient elimination of intracellular parasites and bacteria (2, 5, 24). A possible explanation for this finding is that the Th1 immune response that was initiated after the development of the helminth-induced Th2 response shut down the Th2 response, so no effect on bacterial clearance could be detected in the helminth-infected mice. This effect was reported to occur when mice infected with N. brasiliensis were treated with recombinant IL-12 a few days after the infection (13). However, the levels of Th2-associated cytokines and the numbers of eosinophils in the lungs of coinfected mice at 1 week after infection with 108 CFU of M. bovis BCG were similar to those in mice infected only with N. brasiliensis (Fig. 2). This clearly indicates that the M. bovis BCG infection 4 days after the helminth infection did not inhibit the generation of an N. brasiliensis-induced Th2 response in the lung.
FIG. 2.
Infection with M. bovis BCG does not inhibit the development of a Th2 response in the lungs of mice previously infected with N. brasiliensis (Nippo). In an experiment separate from the one described in the legend to Fig. 1, mice were either infected with 1,000 L3 larvae of the helminth N. brasiliensis only (i.p., day 0) or coinfected with N. brasiliensis (i.p., day 0) and 108 CFU of M. bovis BCG (i.n., day 4). Eleven days after the initial infection with N. brasiliensis, BAL were performed and single-cell suspensions of MLN cells were prepared. BAL fluid cells were counted and stained with hematoxylin and eosin, and the different cell types were identified microscopically. The MLN cells were stimulated in vitro for 48 h on anti-CD3-bound plates in the presence of IL-2. The amounts of IL-4, IL-5, and IL-10 in the supernatants were determined by ELISA. Shown are the numbers of eosinophils present in the BAL fluid (A) and the amounts of IL-4 (B), IL-5 (C), and IL-10 (D) secreted by the MLN cells after in vitro stimulation of individual mice in each group. The mean numbers of eosinophils and levels of cytokines detected in the BAL fluid and supernatants from the MLN cells of the different groups of mice are indicated. The experiment was repeated once with similar results.
In conclusion, our results demonstrate that the Th1 response mounted against M. bovis BCG leads to the efficient elimination of the bacteria from the lungs of mice, irrespective of an ongoing Th2 response at the same time and in the same organ. However, it remains to be elucidated if the same effect will be observed when more-virulent mycobacteria such as M. avium or M. tuberculosis are used for the coinfection studies. It is also possible that the Th2 response induced by N. brasiliensis has a stronger inhibitory effect on bacterial clearance when a poorly replicating strain of, for example, M. avium is used. The rationale behind this view is that the weak Th1 response induced by slow-growing relatively avirulent mycobacteria may not be sufficient to overcome the potential negative effects of IL-4 and/or IL-10 on macrophage activation. Furthermore, due to the short-lived Th2 response induced in the lungs by infection with N. brasiliensis, the use of multiple infections with N. brasiliensis or Schistosoma eggs for coinfection studies may yield different results.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, the Bundesministerium für Bildung und Forschung, and the Bayerisches Staatsministerium für Wissenschaft, Forschung und Kunst.
We thank Susanne Stumpf and Ursula Tatsch for their excellent technical assistance.
REFERENCES
- 1.Bermudez, L. E., and J. Champsi. 1993. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance in mice. Infect. Immun. 16:3093-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogdan, C., and C. Nathan. 1993. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann. N. Y. Acad. Sci. 685:713-739. [DOI] [PubMed] [Google Scholar]
- 3.Castro, A. G., N. Esaguy, P. M. Macedo, A. P. Aguas, and M. T. Silva. 1991. Live but not heat-killed mycobacteria cause rapid chemotaxis of large numbers of eosinophils in vivo and are ingested by the attracted granulocytes. Infect. Immun. 59:3009-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffman, R. L., B. W. Seymour, S. Hudak, J. Jackson, and D. Rennick. 1989. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science 245:308-310. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, A. M., and J. L. Flynn. 1995. The protective immune response to Mycobacterium tuberculosis. Curr. Opin. Immunol. 7:512-516. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon-gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denis, M., and E. Ghadirian. 1993. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J. Immunol. 151:5425-5430. [PubMed] [Google Scholar]
- 8.de Waal Malefyt, R., J. Abrams, B. Bennett, C. G. Figdor, and J. E. de Vries. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Waal Malefyt, R., J. Haanen, H. Spits, M. G. Roncarolo, A. te Velde, C. Figdor, K. Johnson, R. Kastelein, H. Yssel, and J. E. de Vries. 1991. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 174:915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erb, K. J., J. W. Holloway, A. Sobeck, H. Moll, and G. Le Gros. 1998. Infection of mice with Mycobacterium bovis-bacillus Calmette-Guerin suppresses allergen-induced airway eosinophilia. J. Exp. Med. 187:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erb, K. J., J. Kirman, B. Delahunt, W. Chen, and G. Le Gros. 1998. IL-4, IL-5 and IL-10 are not required for the control of M. bovis-BCG infection in mice. Immunol. Cell Biol. 76:41-46. [DOI] [PubMed] [Google Scholar]
- 12.Finkelman, F. D., T. Shea-Donohue, J. Goldhill, C. A. Sullivan, S. C. Morris, K. B. Madden, W. C. Gause, and J. F. Urban. 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 15:505-533. [DOI] [PubMed] [Google Scholar]
- 13.Finkelman, F. D., K. B. Madden, A. W. Cheever, I. M. Katona, S. C. Morris, M. K. Gately, B. R. Hubbard, W. C. Gause, and J. F. Urban. 1994. Effects of interleukin 12 on immune responses and host protection in mice infected with intestinal nematode parasites. J. Exp. Med. 179:1563-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 15.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groux, H., F. Cottrez, M. Rouleau, M. G. Roncarolo, and R. L. Coffman. 1998. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J. Immunol. 162:1723-1729. [PubMed] [Google Scholar]
- 17.Jacobs, M., N. Brown, N. Allie, R. Gulert, and B. Ryffel. 2000. Increased resistance to mycobacterial infection in the absence of interleukin-10. Immunology 100:494-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamijo, R., J. Le, D. Shapiro, E. A. Havell, S. Huang, M. Aguet, M. Bosland, and J. Vilcek. 1993. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J. Exp. Med. 178:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirman, J., Z. Zakaria, K. McCoy, B. Delahunt, and G. Le Gros. 2000. Role of eosinophils in the pathogenesis of Mycobacterium bovis BCG infection in gamma interferon receptor-deficient mice. Infect. Immun. 68:2976-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopf, M., F. Brombacher, P. D. Hodgkin, A. J. Ramsay, E. A. Milbourne, W. J. Dai, K. S. Ovington, C. A. Behm, G. Kohler, I. G. Young, and K. I. Matthaei. 1996. IL-5-deficient mice have a developmental defect in CD5(+) B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 4:15-24. [DOI] [PubMed] [Google Scholar]
- 21.Murray, P. J., and R. A. Young. 1999. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect. Immun. 67:3087-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray, P. J., L. Wang, C. Onufryk, R. I. Tepper, and R. A. Young. 1997. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J. Immunol. 158:315-321. [PubMed] [Google Scholar]
- 23.North, R. J. 1998. Mice incapable of making IL-4 or IL-10 display normal resistance to infection with Mycobacterium tuberculosis. Clin. Exp. Immunol. 113:55-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaible, U. E., H. L. Collins, and S. H. Kaufmann. 1999. Confrontation between intracellular bacteria and the immune system. Adv. Immunol. 71:267-377. [DOI] [PubMed] [Google Scholar]
- 25.Sieling, P. A., J. S. Abrams, M. Yamamura, P. Salgame, B. R. Bloom, T. H. Rea, and R. L. Modlin. 1993. Immunosuppressive roles for IL-10 and IL-4 in human infection. J. Immunol. 150:5502-5510. [PubMed] [Google Scholar]