Abstract
We have attempted to establish a gnotobiotic mouse model monoassociated with Mycoplasma pneumoniae following single or repeated infection to examine the mechanism of pathogenesis following M. pneumoniae infection. M. pneumoniae inoculated into germfree mice colonized equally well at 105 CFU/lung in both single infection and repeated infection. In histopathological observation, repeatedly infected mice showed pneumonia with mild infiltration of mononuclear cells and macrophages. Antibody titers against M. pneumoniae rose in the repeatedly infected mice but not in the singly infected mice. The percentage of CD4-positive, CD8-positive, and CD25-positive lymphocytes infiltrated in the lung was increased in the repeatedly infected mice. In contrast, the lymphocyte subset in the spleen was not significantly different among mock-, singly, and repeatedly infected mice. In the study of cytokine productivity of spleen cells, production of interleukin (IL)-4 and IL-10 was significantly increased and that of gamma interferon was remarkably increased in the mice following repeated infection. These results indicate that a gnotobiotic mouse model monoassociated with M. pneumoniae was established and that immune mechanisms might be involved in the pathogenesis in pneumonia following M. pneumoniae infection.
Mycoplasma pneumoniae is a pathogenic agent responsible for numerous outbreaks of acute respiratory infection (7). However, the mechanism by which primary atypical pneumonia is caused by M. pneumoniae has not been clarified. M. pneumoniae pneumonia is known as one of the respiratory infectious diseases found mainly in children to young adults. Histopathologically, the bronchial and bronchiolar lumina are characteristically filled with polymorphonuclear leukocytes, and their walls have a mononuclear infiltrate with plasma cells (26). Therefore, it seems that some other factors of the host may be involved.
The following findings have been clinically known: (i) although the infection in the infant is inapparent, it becomes apparent pneumonia in young adults (11), (ii) severe pathological change of lung is not shown in immunodeficient patients (12), and (iii) transient tuberculin anergy has been observed during its early stage (2, 28). Indirect injury through immune responses besides the direct pathogenic factors of M. pneumoniae seems to be important in M. pneumoniae infection (9, 10, 19).
Experimental infections using the hamster and mouse to examine the role of the cell-mediated immunity in the mycoplasmal pneumonia were carried out (6, 8, 17). In the hamster, pneumonia was induced, but the evidence of the cell-mediated immunity to M. pneumoniae has not been revealed. Infection of the mouse with M. pulmonis was established, but M. pulmonis is not a pathogenic agent for humans. Up to the present time, mycoplasmal pneumonia is not established in experimental infection with M. pneumoniae (3).
In the present study, we have established the gnotobiotic mouse model monoassociated with M. pneumoniae, and bacteriological, histopathological, and immunological studies to reveal a mechanism of the mycoplasmal pneumonia were performed.
MATERIALS AND METHODS
Bacterial strains.
Two strains of M. pneumoniae were used in this experiment. Clinically isolated No. 4 strain and type strain FH, which were stocked at the Department of Infectious Diseases, Kyorin University School of Medicine, were used. Cultivation of M. pneumoniae was carried out at 37°C for 5 to 7 days under an atmosphere of 5% CO2 using PPLO agar plates (Oxoid, Hampshire, United Kingdom) with mycoplasma selective supplement-G (Oxoid). In addition, each M. pneumoniae strain was inoculated in PPLO broth (Oxoid) with mycoplasma selective supplement-G.
PCR.
For extraction of M. pneumoniae DNA, 100 μl was taken from each M. pneumoniae culture in Gene Amp thin-walled reaction tubes with flat cups (Perkin-Elmer), and 400 μl of TE buffer (pH 8.0) containing 10 mM Tris-HCl and 1 mM EDTA was added. After centrifugation at 1,000 × g for 5 min, the top clear layer was decanted and sterilized distilled water was added to a final volume of 500 μl. DNA was extracted by boiling at 100°C for 10 min, and then 10 μl was used for the PCR.
Primer pairs (sense and antisense, respectively) were used for the experiment as follows: 16S-rRNA, 5′-GAATCAAAGTTGAAAGGACCTGC-3′ and 5′-CTCTAGCCATTACCTGCTAAAGTC-3′ (product size, 266 bp) (29); P1-adhesin, 5′-AGGCTCAGGTCAATCTGGCGTGGA-3′ and 5′-GGATCAAACAGATCGGTGACTGGGT-3′ (product size, 345 bp) (5).
Ten microliters of template DNA, 1 μl of primer F, 1 μl of each primer set, and sterilized distilled water were added to Ready to Go 0.2-ml tubes containing Taq polymerase, deoxynucleoside triphosphate (dNTP) mixture, and 10× PE buffer (Amersham Pharmacia Biotech Inc.). Then mineral oil was dropped, and the PCR was performed using a thermal cycler (Perkin-Elmer DNA Thermal Cycler 480; Perkin-Elmer, Norwalk, Conn.). Thermal cycle was enforced as follows: initial denaturation at 96°C, 3 min, 1 cycle; denaturation at 96°C, 1 min, 35 cycles; annealing at 57°C, 1 min, 35 cycles; extension at 72°C, 1 min, 35 cycles; and additional extension, 72°C, 10 min, 1 cycle. For electrophoresis, 10 μl was taken from each sample of M. pneumoniae after PCR, and it was mixed with 3 μl of ethylene bromide. The electrophoresis was done using a 2% agarose gel, and the product DNA was estimated according to molecular weight markers.
Adherence activity of M. pneumoniae strains.
Adhesion of each M. pneumoniae strain was examined prior to the experimental infection. Human pharyngeal tumor (HEp-2) cells were cultivated in RPMI 1640 (Nikken Medical Laboratory, Kyoto, Japan) supplemented with 5% fetal bovine serum (FBS). When HEp-2 cells became confluent after 3 days of culture, the adhesion test was carried out according to the method of Taguchi et al. (27). Briefly, after incubation on PPLO agar plates for 4 days, M. pneumoniae strains were washed three times with phosphate-buffered saline (PBS), and the suspension was reacted with lipophilic dye PKH-2 (Zynasis Cell Science, Phoenixville, Pa.) at room temperature for 10 min. The reaction was immediately stopped by adding 5 ml of PBS containing 0.5% bovine serum albumin (PBS-BSA). The labeled M. pneumoniae was washed once in PBS-BSA and suspended in 1 ml of Hanks' balanced salts solution containing 0.1% gelatin (HGS). In addition, these labeled M. pneumoniae were incubated with HEp-2 cells grown in six-well culture plates at 37°C for 1 h under an atmosphere of 5% CO2. Then the monolayer cells were washed four times with HGS. The fluorescence intensity of the stained cells was measured by flow cytometry (FACSCalibur; Dickinson Immunocytometry Systems), and adherence activity of M. pneumoniae was evaluated.
Germ-free mice and breeding conditions.
Germ-free mice (IQI/Jic, 8 weeks old, female) were purchased from Clea Japan (Tokyo, Japan) and bred in vinyl isolators which were sterilized by Expor (Alcide). The mice were fed a cobalt 60-irradiated diet and sterilized water. Prior to the experimental infection, by aerobic and anaerobic culture of the cecum contents using Columbia agar supplemented with 5% sheep blood, asepsis was confirmed. In addition, the lung tissue was homogenized before the infection, and it was inoculated onto PPLO agar plate to ascertain that there was no M. pneumoniae infection.
Infection of germfree mice with M. pneumoniae.
The experimental group was composed of three groups, single infection (n = 6), repeated infection (n = 5), and mock infection (n = 3). IQI germfree mice (8 weeks old) were infected intranasally under anesthesia by intraperitoneal injection of pentobarbital (Dainihon Seiyaku, Osaka, Japan). The inoculum (30 μl ) of the No. 4 and FH strains for single infection was 1.5 × 107 and 5.0 × 107 CFU/mouse, respectively. PPLO broth (30 μl) was inoculated for mock infection. Repeat infection was carried out 4 weeks after the primary infection by the same method. The inoculum (30 μl ) of the No. 4 and FH strains for repeated infection was 1.3 × 106 and 1.6 × 106 CFU/mouse, respectively. In addition, at 2 weeks after the second infection, a third infection was carried out. The inoculum (30 μl ) of the No. 4 and FH strains for the third infection was 2.4 × 106 and 3.7 × 106 CFU/mouse, respectively.
Two weeks after the last infection, the mice were sacrificed under ether anesthesia. The blood sample was taken by exsanguination for serum separation, and the serum was used for the measurement of the antibody titer to M. pneumoniae. The mouse was incised in the middle from the hypogastrium over the thorax, and spleen and lung were extracted. The right upper lobe of the lung was used for the bacteriological examination and the harvest of the lymphocytes, and the right lower lobe was used for the pathological examination. All of the left lung was used for harvest of the lymphocytes.
Bacteriological examination of the infected mouse.
Three milliliters of RPMI 1640 was added to the collected lung, and it was emulsified using the sterilized glass homogenizer. The emulsified specimen was serially diluted in PPLO broth and inoculated onto PPLO agar plates at 37°C under the atmosphere of 5% CO2. CFU counts of M. pneumoniae in the lung were estimated from the number of colonies formed. An M. pneumoniae colony showing typical nipple-like morphology was observed by an inverted microscope.
Pathological examination of the infected mouse.
The lung excised from the mouse was expanded sufficiently for negative pressure by the syringe pump in which the 10% formalin solution came, and these samples were fixed by immersion in it. After the fixation, the dehydrating operation was conducted, and the slice was made after the paraffin embedding followed by hematoxylin and eosin staining.
Immunological examination of the infected mouse. (i) Detection of M. pneumoniae antibody.
Antibody titers to M. pneumoniae in serum of M. pneumoniae-infected mice were measured by particle agglutination (PA) test, using Serodia MycoII (Fujirebio, Tokyo, Japan) in order to confirm whether the immune response to M. pneumoniae was induced. A specimen showing agglutination of sensitized particles (1:40 final dilution) was interpreted as positive.
(ii) Detection of lymphocyte subsets in the lung.
To evaluate the intrapulmonary immune response, the lymphocyte subsets in the lung were detected. Lungs were removed and minced in 5 ml of RPMI 1640 with 5% FBS containing collagenase type I (Worthington Biochemical Corp.), 50 U/ml (18). Then the solution was incubated at 37°C for 30 min and passed through a nylon mesh. The cell suspension was pelleted by centrifugation at 300 × g for 10 min, followed by washing three times in RPMI 1640 with 5% FBS. The cells were overlaid on Lympholyte-M (d = 1.0875) (Cedarlane, Ontario, Canada) and centrifuged at 500 × g for 20 min for separation of lymphocytes.
For fluorescence-activated cell sorting (FACS) analysis, the lymphocytes were washed and adjusted to 106 cells/ml in PBS without calcium chloride and magnesium chloride [PBS(−)]. Aliquots (500 μl) of the cell suspensions were incubated with fluorescein isothiocyanate (FITC)-conjugated or phycoerythrin (PE)-conjugated monoclonal antibodies to various CD antigens described below for 60 min on ice in the dark. The samples were centrifuged, washed, and resuspended in PBS. The labeled cells were analyzed by flow cytometry (FACS Vantage, Becton Dickinson Immunocytometry Systems). The following monoclonal antibodies were used in this study: rat anti-mouse CD4 (T helper cell marker; FITC labeled), rat anti-mouse CD8 (cytotoxic and suppressor T-cell marker; PE labeled), and rat anti-mouse CD25 (interleukin-2 [IL-2] receptor and activated T-cell marker; PE labeled) (PharMingen).
(iii) Detection of lymphocyte subsets in the spleen.
To evaluate systemic immune response, the lymphocyte subsets in the spleens were examined. The spleens were minced in RPMI 1640 with 5% FBS passed together through a nylon mesh. The lymphocytes in the spleens were obtained and analyzed by flow cytometry. The following monoclonal antibodies were used in this study: rat anti-mouse CD4, rat anti-mouse CD8, rat anti-mouse CD25, and rat anti-mouse CD19 (matured B-cell marker; FITC labeled) (PharMingen).
(iv) Cytokine productivity of spleen cells.
Cytokine productivity of the splenic lymphocytes of the infected mouse was examined. The lymphocytes of the spleen were adjusted to 105 cells/ml in RPMI 1640 with 5% FBS and seeded in 200 μl of complete RPMI 1640 with 5% FBS in flat-bottomed microtiter plates (Iwaki, Tokyo, Japan) followed by incubation at 37°C for 24 h under 5% CO2. Then 10 μl of M. pneumoniae No. 4 suspension (4.4 × 108 CFU/ml ) or FH suspension (1.7 × 108 CFU/ml ) was added to each well. The lymphocytes were incubated at 37°C under 5% CO2 for 3 days. Supernatants from these cultures were collected by centrifugation at 15,000 × g for 10 min and stored at −80°C until use.
The quantitative analysis of IL-2, IL-4, IL-10, and gamma interferon (IFN-γ) in the supernatants was done by the method of enzyme-linked immunosorbent assay (ELISA). The following cytokine kits were used in this study: AN′ALYZA mouse IL-2 immunoassay, AN′ALYZA mouse IL-4 immunoassay, AN′ALYZA mouse IL-10 immunoassay, and mouse IFN-γ ELISA kit (all these kits were purchased from Genzyme, Cambridge, Mass.).
Statistical analysis.
Results obtained were expressed as mean ± standard deviation (SD). Statistical significance was analyzed with Student's t test, and results were considered to be significant if P was less than 0.05.
RESULTS
Characterization of M. pneumoniae No. 4 and FH strains.
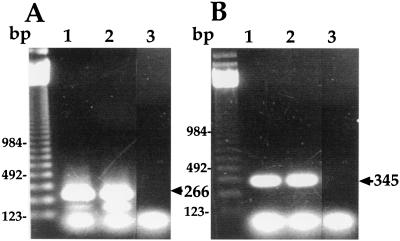
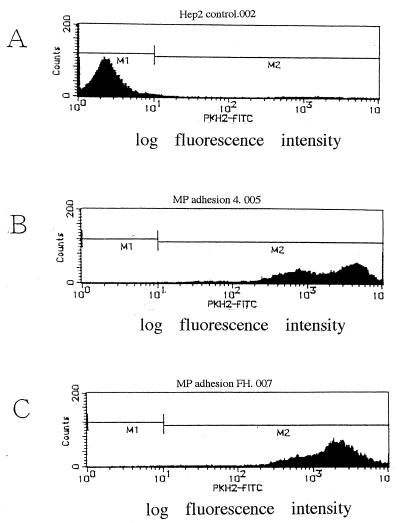
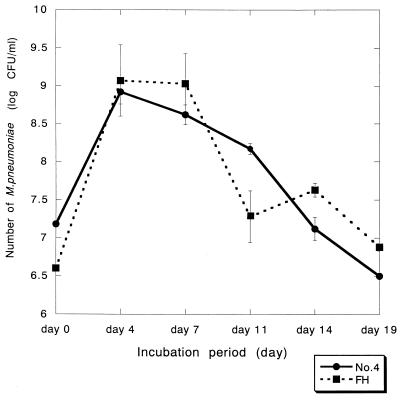
Characterization of the No. 4 and FH strains was performed. A DNA band with 266 bp was amplified for both strains after PCR using 16S rRNA primers, confirming that both strains are M. pneumoniae species (Fig. 1A). In addition, a DNA band with 345 bp was amplified for both strains after PCR using P-1 adhesin primers (Fig. 1B). Adhesion activity of these strains to HEp-2 cells was examined by flow cytometric analysis (Fig. 2). The mean fluorescence intensity of control HEp-2 cells was 34.5. In contrast, the mean fluorescence intensity of HEp-2 cells incubated with PKH-2-labeled M. pneumoniae No. 4 and FH strains was 2,676.7 and 2,078.4, respectively, indicating that these strains adhered effectively to HEp-2 cells. The growth rate of these strains in PPLO broth was compared (Fig. 3). The maximum growth of these strains was observed at 4 days after infection, and there was no significant difference in the growth curve between the two strains.
FIG. 1.
Detection of 16S rRNA and P-1 adhesin genes in M. pneumoniae No. 4 and FH strains by PCR. (A) PCR using 16S RNA primers. Lane 1, No. 4 strain; lane 2, FH strain; lane 3, control (distilled water). (B) PCR using P-1 adhesin primers. Lane 1, No. 4 strain; lane 2, FH strain; lane 3, control (distilled water).
FIG. 2.
Adherence of M pneumoniae No. 4 and FH strains to HEp-2 cells evaluated by flow cytometric analysis. (A) Control HEp-2 cells. (B) HEp-2 cells adhered with PKH-2-labeled M. pneumoniae No. 4 strain. (C) HEp-2 cells adhered with PKH-2-labeled M. pneumoniae FH strain. Adherence of M. pneumoniae was evaluated according to its mean fluorescence intensity.
FIG. 3.
Growth curve of M. pneumoniae No. 4 and FH strains in PPLO broth. The quantification of M. pneumoniae was performed under the atmosphere of 5% CO2 by using PPLO agar plates with mycoplasma selective supplement-G.
Persistent infection of M. pneumoniae in the lung.
Fourteen days after the intranasal inoculation, persistent infection of M. pneumoniae in the lung of singly infected and repeatedly infected mice was observed (Table 1). In the single-infection group, the mean CFU per lung was 3.0 × 105 and 4.3 × 105 for the mice infected with the No. 4 and FH strains, respectively. In the repeated-infection group, the mean CFU per lung was 2.3 × 105 and 5.1 × 105 for the No. 4 and FH strains, respectively. There was no significant difference in the colonization of M. pneumoniae between the single- and repeated-infection groups.
TABLE 1.
Persistent infection of M. pneumoniae in the lunga
| Mouse | CFU/lung | |
|---|---|---|
| Single infection | ||
| No. 4 | ||
| 1 | 4.8 × 105 | |
| 2 | 2.5 × 105 | |
| 3 | 1.8 × 105 | |
| FH | ||
| 1 | 2.3 × 105 | |
| 2 | 7.4 × 105 | |
| 3 | 3.1 × 105 | |
| Repeated infection | ||
| No. 4 | ||
| 1 | 3.0 × 104 | |
| 2 | 5.9 × 105 | |
| 3 | 7.8 × 104 | |
| FH | ||
| 1 | 4.1 × 105 | |
| 2 | 6.0 × 105 |
The lung was isolated from gnotobiotic mice monoassociated with M. pneumoniae and emulsified and inoculated onto PPLO agar plates. Viable M. pneumoniae in the lung were estimated from the number of colonies formed.
Histopathological examination of mouse lung following single and repeated infection with M. pneumoniae No. 4 and FH strains.
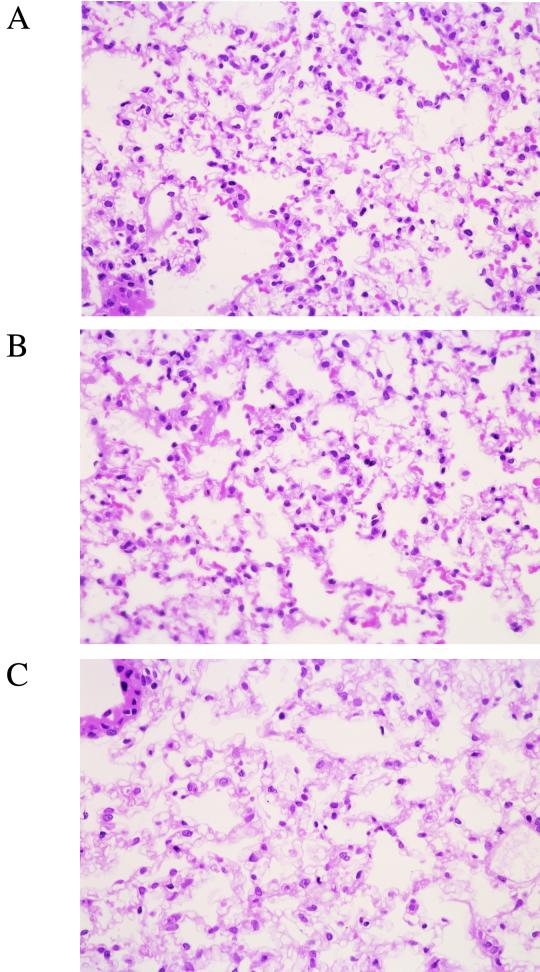
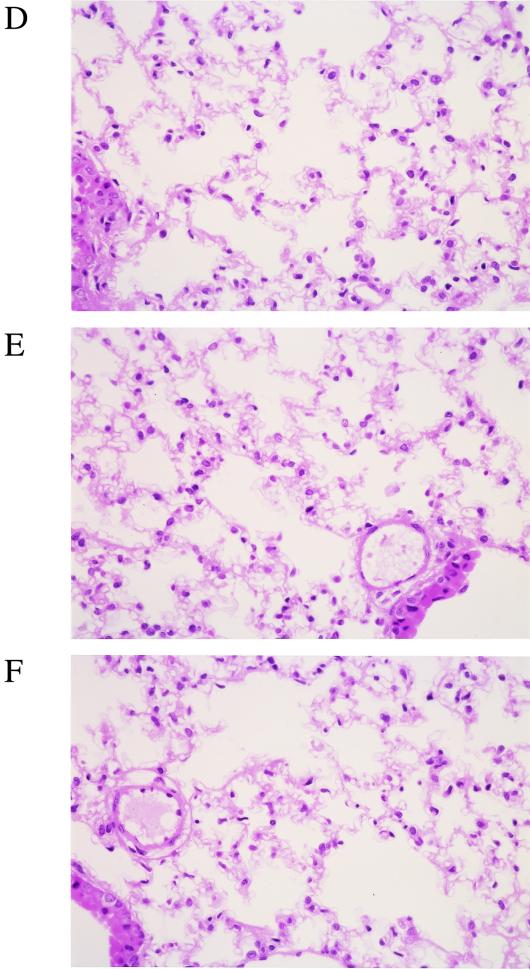
In the lung tissue of gnotobiotic mice following repeated infection with M. pneumoniae, mild infiltration of lymphocytes and macrophages was detected, showing that pneumonitis change was induced by 14 days after the last intranasal inoculation (Fig. 4A and B). There was no difference in the intensity of the infiltration between No. 4 strain- and FH strain-infected lung tissue. In contrast, no infiltration of inflammatory cells was observed in the lung tissues of gnotobiotic mice following single infection with M. pneumoniae (Fig. 4C and D) and mice mock infected with PPLO broth (Fig. 4E and F).
FIG. 4.
Histopathological examination of lung tissue. Lung tissues were fixed and stained with hematoxylin and eosin at 14 days after the last intranasal inoculation with M. pneumoniae No. 4 or FH strain (magnification, ×75). (A) Repeated infection with No. 4 strain. (B) Repeated infection with FH strain. (C) Single infection with No. 4 strain. (D) Single infection with FH strain. (E) Mock infection with PPLO broth. (F) Untreated control.
Detection of PA antibody to M. pneumoniae in sera of mice following repeated infection.
The titer of PA antibody to M. pneumoniae in the sera of the mice was determined (Table 2). No PA antibody was detected in the sera of the gnotobiotic mice following single infection with either the No. 4 or FH strain. On the other hand, the titer of 32 to 256 of PA antibody to M. pneumoniae was detected in the sera of the gnotobiotic mice following repeated infection with either the No. 4 or FH strain, indicating that the rise of antibody to M. pneumoniae was induced by repeated infection. PA antibody titer did not rise in the control mice mock infected with PPLO broth.
TABLE 2.
PA antibody to M. pneumoniae in the serum of infected micea
| Mouse | PA titer |
|---|---|
| Mock infection | |
| 1 | <4 |
| 2 | <4 |
| 3 | <4 |
| Single infection | |
| No. 4 | |
| 1 | <4 |
| 2 | <4 |
| 3 | <4 |
| FH | |
| 1 | <4 |
| 2 | <4 |
| 3 | <4 |
| Repeated infection | |
| No. 4 | |
| 1 | 32 |
| 2 | 64 |
| 3 | 64 |
| FH | |
| 1 | 64 |
| 2 | 256 |
Gelatin particles sensitized with the limited membrane of M. pneumoniae were used for the measurement of antibody titers of the infected mice.
Lymphocyte subsets in the lung of infected mice.
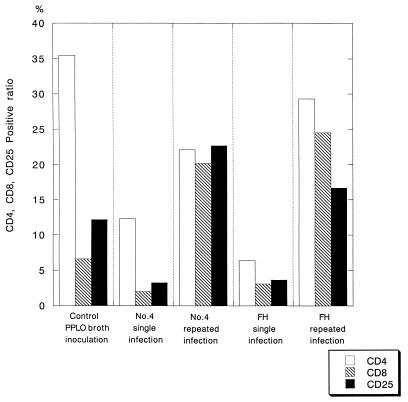
The lymphocytes in the lung were identified and gated via their volume and side scatter properties by FACS analysis. The lymphocyte subsets were determined using appropriate FITC- or PE-conjugated antibodies (Fig. 5). The percentage of helper T cells (CD4) in the lung of the gnotobiotic mice following repeated infection with either the No. 4 or FH strain was higher than that of the mice following single infection (Fig. 5), although the percentage (35.5%) of CD4-positive T cells was remarkably high in the control mice mock infected with PPLO broth. Similarly, the percentage of cytotoxic and suppressor T cells (CD8) in the lung of the gnotobiotic mice repeatedly infected with M. pneumoniae strains was higher than that of the singly infected mice (Fig. 5). The percentage of activated or IL-2 receptor-bearing T cells (CD25) was also increased in the gnotobiotic mice following repeated infection (Fig. 5).
FIG. 5.
Lymphocyte subsets in the lung tissue of gnotobiotic mice singly or repeatedly infected with M.pneumoniae No. 4 or FH strain. The percentages of CD4-positive (□), CD8-positive (▧), and CD25-positive (▪) T cells in the lungs of the infected mice are shown. For analysis of lymphocyte subsets, 2 × 103 cells were scored.
Lymphocyte subsets in the spleen of infected mice.
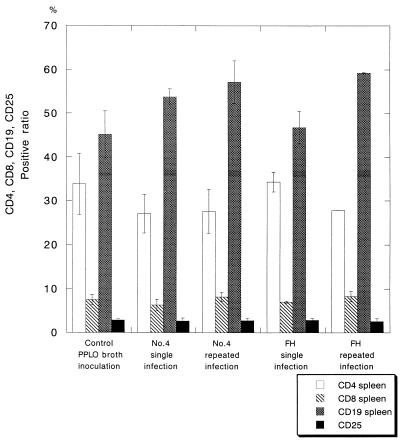
The lymphocytes of the spleens were identified, and the lymphocyte subset was determined by FACS analysis (Fig. 6). The percentage of CD4-positive T lymphocytes in the spleen of the singly and repeatedly infected mice was 27.1% ± 4.4% and 27.6% ± 5.0% for infection with No. 4 strain and 34.3% ± 2.3% and 27.8% ± 0.01% for infection with FH strain, respectively. There was no significant difference in the percentage of CD4-positive T lymphocytes between the single-infection and repeated-infection groups. The percentages of CD8-positive, CD19-positive, and CD25-positive T lymphocytes in the spleen of the infected and mock-infected mice were determined. No significant difference in these percentages was observed among singly infected, repeatedly infected, and mock-infected mice.
FIG. 6.
Lymphocyte subsets in the spleen of gnotobiotic mice singly or repeatedly infected with M. pneumoniae No. 4 or FH strain. The percentages of CD4-positive (□), CD8-positive (▧), CD19-positive (▩), and CD25-positive (▪) T cells in the spleens of the infected mice are shown. For analysis of lymphocyte subsets, 104 cells were scored.
Cytokine productivity of T lymphocytes in the spleen of gnotobiotic mice infected with M. pneumoniae.
Production of cytokines such as IL-2, IL-4, IL-10, and IFN-γ by T lymphocytes in the spleen of the gnotobiotic mice infected with M. pneumoniae was examined (Table 3). IL-2 production by splenic T lymphocytes was increased in the mice repeatedly infected with M. pneumoniae No. 4 or FH strain in comparison to that of the mice singly infected with these strains. Similarly, IL-4 production was detected in the repeatedly infected mice, but no IL-4 was detected in the singly infected and mock-infected mice. IL-10 concentration produced by splenic T lymphocytes of the repeatedly infected mice was significantly higher than that of the singly or mock-infected mice. With respect to the production of IFN-γ, a remarkable increase was detected in the repeatedly infected mice with No. 4 strain (828.8 ± 171.4 pg/ml ) or FH strain (1,271.0 ± 244.6 pg/ml). FH strain induced much more secretion of IL-4, IL-10, and IFN-γ cytokines from the splenic T lymphocytes following repeated infection than the No. 4 strain.
TABLE 3.
Cytokine productivity of T lymphocytes in the spleen of M. pneumoniae-infected micea
| Stimulating strain | Infection | Mean concn (pg/ml) |
|||
|---|---|---|---|---|---|
| IL-2 | IL-4 | IL-10 | IFN-γ | ||
| No. 4 | Mock | 3.4 ± 1.5 | ND | 3.0 ± 0.2 | 5.6 ± 0.3 |
| Single | 7.3 ± 0.4 | ND | 22.3 ± 1.2∗∗ | 1.3 ± 0.1 | |
| Repeated | 21.6 ± 2.5∗ | 8.6 ± 1.8∗† | 86.1 ± 1.9∗∗ | 828.0 ± 171.4∗† | |
| FH | Mock | 4.8 ± 2.9 | ND | 2.7 ± 0.1 | ND |
| Single | 7.9 ± 1.1 | ND | 34.6 ± 1.7∗ | 4.1 ± 0.2 | |
| Repeated | 14.6 ± 4.2∗ | 14.5 ± 0.4∗∗ | 203.8 ± 30.3∗† | 1271.0 ± 244.6∗† | |
Each cytokine was analyzed by ELISA. Values are means ± standard deviation of the mean. ND, not detected. ∗, P <0.05 versus mock infection; †, P < 0.05 versus single infection. ∗∗, P <0.005 versus mock or single infection.
DISCUSSION
Gnotobiotic animals are an excellent tool for investigating bacterial interactions, because the effects of bacterial flora can be ruled out in such animals (4, 16). However, such a gnotobiological study has not been performed for M. pneumoniae infection. In this study, we have established an animal model for M. pneumoniae infection using germfree mice. Further, pathological changes and interaction of M. pneumoniae infection with host immune response were investigated without any influence by normal bacterial flora. By single or repeated infection of germfree mice with M. pneumoniae No. 4 and FH strains, all the mice showed colonization of M. pneumoniae in the lung tissue, and there was no difference in the number of M. pneumoniae colonized between singly and repeatedly infected mice. However, the infiltration of lymphocytes and macrophages to the interstitial tissue of the lung was observed only in repeatedly infected mice, suggesting that single infection with M. pneumoniae might not induce pathological damage in the lung, and the antibody to M. pneumoniae was detected only in the sera of the repeatedly infected mice. This is in part due to the decreased level of immunoglobulin G (IgG) and IgA in germfree mice compared with conventional mice (13, 14). However, it was reported that single infection of germfree mice with Vibrio cholerae or Shigella flexneri resulted in IgA response (23, 24). Therefore, the antibody response only raised by repeated infection might be characteristic of M. pneumoniae infection.
We have examined the lymphocyte subsets in lung and spleen. The percentages of the CD4-positive lymphocytes were decreased in the lungs of the mice following single infection, but they were recovered to the same level as in mock-infected mice in the lungs of the mice following repeated infection. In contrast, in the spleen, there was no significant difference in the ratio of CD4-, CD8-, CD25-, and CD19-positive cells among mock, single, and repeated infection in the gnotobiotic mice monoassociated with M. pneumoniae. Sugawara et al. (25) showed that the CD4-positive cells predominated in the lung with the progress of the pneumonia and the CD4/CD8 ratio was increased in the experimental infection of LEW rats with Mycoplasma pulmonis. Hayashi et al. (15) reported that the increase of CD4-positive T lymphocytes and the decrease of CD8-positive T lymphocytes with increased CD4/CD8 ratio were detected in the bronchoalveolar lavage fluid of patients with M. pneumoniae infection. Our results might be partially due to the characteristics of germfree mice. It was considered that further investigation on the percentages of lymphocytes in the lung was needed.
It has been reported that various cytokines play an important role in mycoplasmal pneumonia. Arai et al. (1) showed that the tumor necrosis factor alpha (TNF-α) production was induced by M. pneumoniae adherence to the lymphocytes. Similarly, Nishimoto et al. (20) reported that the production of TNF-α and IFN-γ by mouse lymphocytes was detected in early stages of pneumonia following M. pulmonis infection. Pietsch and Jacobs (22) examined cytokine gene expression in the lung and spleen of M. pneumoniae-infected BALB/c mice by qualitative and semiquantitative PCR-mediated mRNA amplification. They tested expression of genes for various cytokines such as TNF-α, IFN-γ, IL-1β, IL-2, IL-6, and IL-10 in the lungs of the mice after primary and secondary infection and showed a significantly higher expression of TNF-α, IFN-γ, and IL-6 in secondary infection. In addition, TNF-α, IFN-γ, and IL-1β mRNAs were detected in the spleen in the secondarily infected mice. Similarly, Opitz et al. (21) reported that mRNAs for TNF-α, IFN-γ, IL-1β, IL-2, and IL-6 were promptly detected in the lung of C57BL/6 mice reinfected with M. pneumoniae. They also showed that mRNA of these cytokines, particularly IL-2 and IFN-γ, was significantly reduced in the mice depleted of CD4-positive T cells, suggesting that specific CD4-positive T cells were crucially involved in the pathogenesis of M. pneumoniae infection, as these T cells accelerated inflammatory cell recruitment in the site of infection and initiated the prolonged release of proinflammatory cytokines.
In the present study, by using germfree mice, we confirmed that the production of IFN-γ, IL-4, and IL-10 by splenic lymphocytes was significantly increased in the repeatedly infected mice. In particular, the concentration of IFN-γ was remarkably high. Our results did not contradict the results by Pietsch and Jacobs (22) and Opitz et al. (21), but we could not examine the productivity of cytokines by pulmonary lymphocytes due to the harvest of a small number of lymphocytes from lung tissue. Further investigation on expression of cytokine genes by PCR-mediated mRNA amplification in the lung of gnotobiotic mice following repeated infection is needed.
We have succeeded in establishing mycoplasmal pneumonia in germfree mice by repeated infection with M. pneumoniae and shown the possibility that the immune responses play a significant role in the development of mycoplasmal pneumonia.
Acknowledgments
We thank M. Kai (National Institute of Infectious Diseases, Tokyo, Japan) for excellent assistance in PCR.
REFERENCES
- 1.Arai, S., M. Furukawa, T. Munakata, K. Kuwano, H. Inoue, and T. Miyazaki. 1990. Enhancement of cytotoxicity of active macrophages by mycoplasma: role of mycoplasma-associated induction of tumor necrosis factor-α (TNF-α) in macrophages. Microbiol. Immunol. 34:231-243. [DOI] [PubMed] [Google Scholar]
- 2.Biberfeld, G., and G. Sterner. 1976. Tuberculin anergy in patients with Mycoplasma pneumoniae infection. Scand. J. Infect. Dis. 8:71-73. [DOI] [PubMed] [Google Scholar]
- 3.Brunner, H. 1997. Models of mycoplasma respiratory and genital tract infections. Wien Klin. Wochenschr. 109:569-573. [PubMed] [Google Scholar]
- 4.Butterton, R. J., T. E. Ryan, A. R. Shahin, and B. S. Calderwood. 1996. Development of a germfree mouse model of Vibrio cholerae infection. Infect. Immun. 64:4373-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chadieux, N., P. Label, and R. Brousseau. 1993. Use of a triplex polymerase chain reaction for the detection and differentiation of Mycoplasma pneumoniae and Mycoplasma genitalium in the presence of human DNA. J. Gen. Microbiol. 139:2431-2437. [DOI] [PubMed] [Google Scholar]
- 6.Dajani, A. S., W. A. Clyde, and F. W. Denny. 1965. Experimental infection with Mycoplasma pneumoniae (Eaton's agent). J. Exp. Med. 121:1071-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denny, F. W., D. Taylor-Robinson, and A. C. Allison. 1972. The role of thymus-dependent immunity in Mycoplasma pulmonis infections of mice. J. Med. Microbiol. 5:327-336. [DOI] [PubMed] [Google Scholar]
- 8.Fernald, G. W. 1969. Immunologic aspect of experimental Mycoplasma pneumoniae infection. J. Infect. Dis. 119:255-266. [DOI] [PubMed] [Google Scholar]
- 9.Fernald, G. W., and W. A. Clyde. 1976. Pulmonary immune mechanisms in Mycoplasma pneumoniae diseases, p. 101-130. In C. H. Kirkpatrick and H. Y. Renolds (ed.), Immunologic and infectious reactions in the lung. Marcel Dekker, New York, N.Y.
- 10.Fernald, G. W., W. A. Clyde, and F. W. Denny. 1981. Immunology of mycoplasma infection, p. 415-439. In N. Aj and R. J. O'Reilly (ed.), Immunology of human infection. Plenum Medical Book Company, New York, N.Y.
- 11.Foy, H. M., G. E. Kenny, R. McMahan, G. Kaiser, and J. T. Grayson. 1971. Mycoplasma pneumoniae in the community. Am. J. Epidemiol. 93:55-62. [DOI] [PubMed] [Google Scholar]
- 12.Foy, H. M., H. Ochs, S. D. Davis, G. E. Kenny, and R. R. Luce. 1975. Mycoplasma pneumoniae infection in patients with immunodeficiency syndromes: report of four cases. J. Infect. Dis. 127:388-393. [DOI] [PubMed] [Google Scholar]
- 13.Gordon, H. A. 1972. Gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol. Rev. 35:390-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanna, M. G., Jr., P. Nettesheim, and H. E. Walburg, Jr. 1969. A comparative study of the immune reaction in germfree and conventional mice. Adv. Exp. Med. Biol. 3:237-248. [Google Scholar]
- 15.Hayashi, S., Y. Ichikawa, K. Fujino, K. Motomura, M. Kaji, K. Yasuda, and M. Yokoyama. 1986. Analysis of lymphocyte subsets in peripheral blood and bronchoalveolar lavage fluid in patient with pneumonia due to Mycoplasma pneumoniae. Nihon Kyobu Shikkan Gakkai Zasshi 24:162-167. (In Japanese.) [PubMed] [Google Scholar]
- 16.Kamiya, S., H. Taguchi, H. Yamaguchi, T. Osaki, M. Takahashi, and S. Nakamura. 1997. Bacterioprophylaxis using Clostridium butyricum for lethal caecitis by Clostridium difficile in gnotobiotic mice. Rev. Med. Microbiol. 8(Suppl. 1):S57-S59. [Google Scholar]
- 17.Krause, D. C., and D. Taylor-Robinson. 1992. Mycoplasmas which infect humans, p. 417-444. In J. Maniloff, R. N. McElhaney, L. R. Finch, and J. B. Baseman (ed.), Mycoplasmas: molecular biology and pathogenesis. American Society for Microbiology, Washington. D.C.
- 18.Liu, M. C. L., K. Ishizaka, and M. Plaut. 1982. T lymphocyte responses of murine lung: immunization with alloantigen induces accumulation of cytotoxic and other T lymphocytes in the lung. J. Immunol. 129:2653-2661. [PubMed] [Google Scholar]
- 19.Mizutani, H., H. Mizutani, T. Kitayama, A. Hayakawa, E. Nagayama, J. Kato, K. Nakamura, E. Tamura, and T. Izuchi. 1971. Delayed hypersensitivity in Mycoplasma pneumoniae infections. Lancet i:186-187. [DOI] [PubMed] [Google Scholar]
- 20.Nishimoto, M., A. Akashi, K. Kuwano, C. C. Tseng, K. Ohizumi, and S. Arai. 1994. Gene expression of tumor necrosis factor α and interferon γ in the lungs of Mycoplasma pulmonis-infected mice. Microbiol. Immunol. 38:345-352. [DOI] [PubMed] [Google Scholar]
- 21.Opitz, O., K. Pietsch, S. Ehlers, and E. Jacobs. 1996. Cytokine gene expression in immune mice reinfected with Mycoplasma pneumoniae: the role of T cell subsets in aggravating the inflammatory response. Immunobiology 196:575-587. [DOI] [PubMed] [Google Scholar]
- 22.Pietsch, K., and E. Jacobs. 1993. Characterization of the cellular response of spleen cells in BALB/C mice inoculated with Mycoplasma pneumoniae or the P1 protein. Med. Microbiol. Immunol. 182:77-85. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki, S., N. Ohnishi, T. Shimamura, R. Maeda, K. Mizuno, and T. Takahashi. 1973. Significance of intestinal bacterial flora to infection, p. 389-393. In J. B. Heneghan (ed.), Germ-free research. Academic Press, New York, N.Y.
- 24.Shimamura, T. 1972. Immune response in germfree mice orally immunized with Vibrio cholerae. Keio J. Med. 21:113-126. [DOI] [PubMed] [Google Scholar]
- 25.Sugawara, H., H. Tamura, and T. Uede. 1988. The effect of cell-mediated immune response in the development of lung lesion in rats with Mycoplasma pulmonis infection. Sapporo Igakkaishi 57:453-467. (In Japanese.) [Google Scholar]
- 26.Susan, R., C. Thomas, and C. Frederic. 1986. Open lung biopsy in Mycoplasma pneumoniae pneumonia. Arch. Pathol. Med. 110:34-41. [PubMed] [Google Scholar]
- 27.Taguchi, H., T. Osaki, H. Yamguchi, and S. Kamiya. 1995. Flow cytometric analysis using lipophilic dye PKH-2 for adhesion of Vibrio cholerae to intestine 407 cells. Microbiol. Immunol. 39:891-894. [DOI] [PubMed] [Google Scholar]
- 28.Tsunekawa, H., E. Takagi, H. Kishimoto, and K. Shimokawa. 1987. Depressed cellular immunity in Mycoplasma pneumoniae pneumonia. Eur. J. Respir. Dis. 70:293-299. [PubMed] [Google Scholar]
- 29.Weisbergh, W. G., J. G. Tully, D. L. Rose, J. P. Petzel, H. Oyaizu, D. Yang, L. Mandelco, J. Sechrest, T. G. Lawrence, J. Van Etten, et al. 1989. A phylogenetic analysis of the mycoplasmas: basis for their classification. J. Bacteriol. 171:6455-6467. [DOI] [PMC free article] [PubMed] [Google Scholar]