Abstract
Gamma interferon (IFN-γ) is an important mediator of endotoxin (lipopolysaccharide [LPS])-induced immune responses. However, the specific cell types that produce IFN-γ in response to LPS and the cellular factors that regulate LPS-induced IFN-γ production have not been fully determined. The present studies were undertaken to characterize the cell populations that produce IFN-γ after LPS challenge in the spleens of mice and to determine the regulatory factors that modulate LPS-induced production of IFN-γ. Our studies show that the levels of splenic IFN-γ mRNA and protein production peak at 6 and 8 h, respectively, after systemic LPS challenge. Approximately 60% of IFN-γ-producing cells are natural killer (NK) cells (CD3−DX5+) and 25% are NKT cells (CD3+DX5+). Most of the remaining IFN-γ-producing cells are T cells (CD3+DX5−), macrophages, and dendritic cells. Functionally, interleukin-12 (IL-12) is the major IFN-γ-stimulating factor after LPS challenge, with costimulation provided by IL-15, IL-18, and B7 proteins. IL-10 is a major inhibitor of LPS-induced IFN-γ production. Unlike intact heat-killed gram-negative and gram-positive bacteria, the class II major histocompatibility complex did not play a functional role in LPS-induced IFN-γ production. LPS is a potent stimulus for splenic IL-10, IL-12 p40, and IL-15 mRNA expression, whereas IL-12 p35 and IL-18 mRNAs, as well as B7 proteins, are constitutively expressed in the mouse spleen. Of the factors studied, IL-18 serves as the most potent costimulus with IL-12 for IFN-γ production, followed by IL-15 and B7 proteins. These data demonstrate that NK cells and NKT cells are the most abundant IFN-γ-producing cells in the mouse spleen after LPS challenge and that IL-10 and IL-12 are key functional regulators of LPS-induced IFN-γ production.
Innate immunity is important for the initiation of antimicrobial defense mechanisms. Macrophages and dendritic cells recognize microbial products such as exotoxins, endotoxins, and bacterial cell wall components and initiate responses that ultimately mobilize nonspecific and organism-specific acquired immunity. Bacterial endotoxin (lipopolysaccharide [LPS]), an intrinsic component of the cell wall of gram-negative bacteria, is a potent activator of innate immunity (20, 34). LPS interacts with host immune cells either by binding to surface CD14 molecules or by forming a complex with soluble CD14 and binding to the cognate sites on macrophages and dendritic cells (5, 36). LPS binding stimulates the production of a variety of cytokines, chemokines, and noncytokine regulatory factors (20, 34). The secreted cytokines activate and amplify antimicrobial responses and usually coordinate effective clearance of the invading pathogen. However, excessive or uncontrolled activation of the innate immune system can cause local or systemic tissue injury.
The most severe example of LPS-induced inflammatory injury is the sepsis syndrome (2). Sepsis is caused by systemic dissemination of gram-negative organisms or their components, particularly LPS. Characteristic features of sepsis include fever, tachycardia, tachypnea, and leukocytosis (2). If severe, sepsis can result in cardiovascular collapse, acute lung injury, organ hypoperfusion, and death (3). Interestingly, prior exposure to sublethal doses of LPS induces a state of immunoparalysis known as LPS tolerance (16, 38). The LPS-tolerant host exhibits suppressed secretion of proinflammatory cytokines such as tumor necrosis factor alpha, interleukin-1β (IL-1β), IL-12, and gamma interferon (IFN-γ) (19). LPS tolerance is thought to be an adaptive response aimed at protecting the host from inflammatory injury. However, whether or not this state of immunosuppression renders the host more susceptible to subsequent infection is unclear.
IFN-γ is a type 1 cytokine that plays a key role in the regulation of both innate and acquired antimicrobial immunity. The expression of IFN-γ is regulated by a set of complex interactions between accessory cells, such as macrophages and dendritic cells, and T lymphocytes and natural killer (NK) cells (32). IFN-γ amplifies antimicrobial immune responses by stimulating macrophage functions such as phagocytosis, respiratory burst activity, antigen presentation, and cytokine secretion (1). In addition, secretion of opsonizing immunoglobulin (Ig) G2a (IgG2a) antibodies by B cells, induction of Th1 cell differentiation, maturation of cytotoxic T cells, and activation of neutrophils are promoted by IFN-γ (1, 15). The functional significance of IFN-γ in antimicrobial defense is demonstrated by the increased susceptibilities of IFN-γ−/− and IFN-γR−/− mice to a variety of infections, particularly to intracellular organisms such as listeriae and mycobacteria (32). IFN-γ also appears to be an important mediator in the pathogenesis of septic shock. Specifically, IFN-γ-deficient mice are more resistant than wild-type mice to the development of inflammatory injury (23). Finally, IFN-γ production is suppressed in LPS-tolerant mice, an effect that appears to be secondary to decreased levels of IL-12 production (35).
The production of IFN-γ is regulated by a variety of factors, with the macrophage-derived cytokines IL-12, IL-15, and IL-18 being the most-characterized positive regulators (30, 37). These cytokines act synergistically to induce IFN-γ production by T lymphocytes and NK cells. Induction of IFN-γ expression is also mediated through the activation of the T-cell receptor (TCR) complex and the interaction of CD28 with B7 proteins (37). However, the roles of these factors in LPS-induced IFN-γ production remains uncharacterized. This study was designed to establish the identities of IFN-γ-producing cells in the mouse spleen after systemic administration of LPS and to determine the functional roles of key known IFN-γ-regulating factors in LPS-induced IFN-γ production. Our results demonstrate that approximately 60% of IFN-γ-producing cells in the mouse spleen after LPS challenge are NK cells and that an additional 20 to 25% of cells are NKT lymphocytes. Macrophages and dendritic cells make up less than 5% of the IFN-γ-producing cells after LPS challenge. IL-12 and costimulatory signals from IL-15 and IL-18 as well as B7 proteins are important positive mediators of LPS-induced IFN-γ expression. IL-10 is a major negative regulator of IFN-γ production after LPS challenge. In contrast to heat-killed bacteria, IFN-γ production in response to LPS is independent of the class II major histocompatibility complex (MHC). Our results better define specific cellular sources of IFN-γ after LPS challenge and identify key regulatory factors that are important in LPS-induced IFN-γ production.
MATERIALS AND METHODS
Animals.
All studies were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and met the guidelines of the National Institutes of Health for the use of experimental animals in research. Female, 6- to 8-week-old BALB/c mice (Harlan Sprague-Dawley, Indianapolis, Ind.) were used in all studies. The mice were housed in a monitored, light-dark-cycled environment and were provided standard laboratory chow and water ad libitum. Their spleens were harvested after LPS challenge for assessment of cytokine expression.
Reagents.
Phenol-extracted LPS (Escherichia coli serotype O111:B4) and normal goat IgG were purchased from Sigma Chemical Co. (St. Louis, Mo.). Recombinant mouse IL-12 and IL-18, polyclonal antibodies against mouse IL-12, CD80 and CD86, and the cytotoxic T lymphocyte-associated (CTLA) protein 4 (CTLA4)-Ig fusion protein were purchased from R&D Systems (Minneapolis, Minn.). Recombinant mouse IL-15 and polyclonal antibodies against mouse IL-15 and IL-18 were purchased from eBioscience (San Diego, Calif.). Anti-CD28 was purchased from Caltag Laboratories (Burlingame, Calif.). CD80-Ig and CD86-Ig fusion proteins were purchased from Ancell Corporation (Bayport, Minn.). Anti-class II MHC was purchased from Leinco Technologies (St. Louis, Mo.). Clinical isolates of Pseudomonas aeruginosa, E. coli, and Staphylococcus aureus were obtained from the Clinical Microbiology Laboratory at the Shriner's Hospital for Children-Galveston Burns Unit.
Isolation of splenocytes and peritoneal macrophages.
Spleens were aseptically harvested from mice and transferred to six-well culture plates containing RPMI 1640 medium supplemented with 10% fetal bovine serum and penicillin (10 U/ml)-streptomycin (10 μg/ml). This medium preparation was used in all experiments unless specified otherwise. The spleens were minced and passed over a sterile mesh, and the erythrocytes were lysed. The remaining cell pellet was resuspended in medium and represented the total splenic mononuclear cell population. Splenocytes, devoid of accessory cells, were prepared by incubating the whole splenic mononuclear cell preparation (107 cells/ml) in 75-cm2 culture flasks for 16 to 18 h (37°C, 5% CO2). The nonadherent lymphocyte population was harvested, washed, and resuspended in fresh medium. For isolation of T cells and NK cells, splenocytes were passed through T-cell-enrichment columns (R&D Systems). These columns contain anti-IgG-coated beads for binding of B lymphocytes and IgG-coated beads for the removal of cells such as macrophages that express Fc receptors on their surfaces. Analysis of the separated cells by flow cytometry showed approximately 90% CD3+/DX5− T cells, 6% CD3−/DX5+ NK cells, and 2% CD3+/DX5+ NKT cells in our isolates, as described previously (35). These ratios of NK cells to T cells are characteristic of those found for intact spleens. The viability of isolated T and NK cells was greater than 95%, as determined by trypan blue exclusion. Resident peritoneal macrophages were harvested by peritoneal lavage with 10 ml of phosphate-buffered saline (PBS). The cells were washed three times, resuspended in medium, and used in isolated culture or coculture experiments with nonadherent splenic T and NK cells.
ELISA for murine IFN-γ.
IFN-γ levels in conditioned media were determined by enzyme-linked immunosorbent assay (ELISA) according to the protocol of the manufacturer (R&D Systems). Briefly, standards or experimental samples were added to microtiter plates coated with monoclonal antibody to the cytokine of interest and the plates were incubated for 2 h. After washing of the plates, horseradish peroxidase-conjugated, cytokine-specific antibody was added to each well, and the plates were incubated for 2 h and washed. Substrate solution was added, the plates were incubated for 30 min, and the reaction was terminated by the addition of stop solution. Cytokine levels were determined by measuring the optical density at 450 nm with a microtiter plate reader (Dynatech Laboratories, Chantilly, Va.).
Flow cytometry.
Fluorescein isothiocyanate (FITC)-conjugated anti-DX5, phycoerythin (PE)-conjugated anti-IFN-γ, and allophycocyanin-conjugated IFN-γ were purchased from B-D Pharmingen (San Diego, Calif.). FITC-conjugated antibodies against CD14, CD11b, CD11c, and CD19 as well as PE-conjugated antibodies against CD80, CD86, and CD28 were purchased from Caltag Laboratories. Isotype controls included FITC-conjugated rat IgG2a, rat IgM, and hamster IgG as well as PE-conjugated rat IgG2a. Isolated splenocytes (106 in 0.1 ml of PBS) were incubated on ice with marker-specific antibodies or isotype controls (0.5 μg of antibody/106 cells) in polystyrene tubes for 30 min. For intracellular staining with anti-IFN-γ, the cells were fixed with Cytofix/Cytoperm buffer and were incubated with anti-IFN-γ diluted in Permwash buffer (B-D Pharmingen). The cells were then washed with 2 ml of PBS and fixed in 1% paraformaldehyde. All analyses were performed on a FACSort flow cytometer (Becton Dickinson, Mountain View, Calif.). Specific staining was determined by comparing the results obtained with experimental samples with the results obtained with samples treated with nonspecific isotype control antibodies.
RPA.
Total RNA was isolated from intact mouse spleens with Tri-Reagent (Molecular Research Center, Cincinnati, Ohio). The RNase protection assay RPA was performed with the Riboquant system (B-D Pharmingen), according to the instructions of the manufacturer. Briefly, radiolabeled RNA probes were synthesized from DNA template sets with T7 RNA polymerase, [32P]UTP, and pooled nonradiolabeled nucleotides. Isolated total RNAs (20 μg/sample) were hybridized with the purified riboprobes and subjected to RNase digestion. DNA template sets included probes for the L32 and GADPH housekeeping genes that served as internal controls. Protected RNA species were separated on 5% polyacrylamide sequencing gels with 0.5× Tris-borate-EDTA running buffer. The gels were run at 50 W of constant power for 70 min and dried under vaccum, and the protected fragments were visualized by autoradiography.
Data analysis.
For comparisons of data from multiple groups, two-way analysis of variance was performed, followed by Tukey's test. A P value of <0.05 was considered significant.
RESULTS
NK and NKT cells are the most predominant IFN-γ-producing cells in the mouse spleen after LPS challenge.
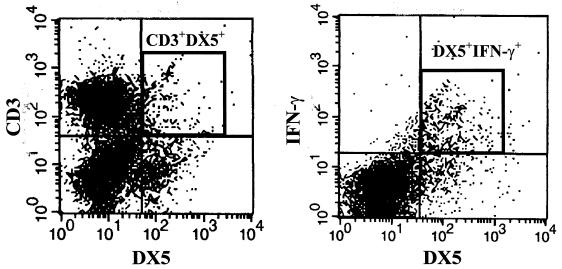
Initially, we sought to characterize the time course of IFN-γ mRNA expression and identify IFN-γ-producing cells in the spleens of mice after LPS challenge. We observed induction of IFN-γ mRNA within 2 h of LPS challenge, with the peak levels of expression occurring at 6 h, followed by a gradual decline (Fig. 1). Using antibodies specific for NK cells (DX5) and T cells (CD3), we showed that approximately 85% of splenic IFN-γ was derived from these two cell types. The level of IFN-γ production peaked at 8 h after LPS challenge in both the NK- and T-cell populations, although the respective amounts of IFN-γ produced were different (Fig. 2). The CD11b+- and CD11c+-cell populations accounted for most of the remaining IFN-γ-producing cells in the spleen. The relative proportions of each cell population producing IFN-γ are shown in Table 1. Looking at the individual cell populations, we showed that the proportion of DX5+ cells producing IFN-γ increased from 1.6 to 72.4% after LPS challenge (a 45-fold increase), whereas the proportion of CD3+ T cells producing IFN-γ increased from 0.4 to 2.0% (Table 1). DX5+ cells accounted for 60% of all IFN-γ-producing cells in the spleen, whereas CD3+ cells accounted for 25%. Further analysis of the CD3+ cells showed that the majority of the IFN-γ-producing cells in this population are NKT cells (CD3+DX5+). Specifically, approximately 6% of CD3+ cells also expressed DX5 on their surfaces, and the CD3+DX5+ population accounted for more than 75% of the IFN-γ-producing CD3+ cells (Fig. 3). Approximately, 9 and 18% of CD11b+ and CD11c+ cells, respectively, produced IFN-γ in response to LPS, accounting for approximately 3 and 5% of all IFN-γ-producing cells in the mouse spleen, respectively. Unlike the other cell types (DX5+, CD3+, and CD11b+), for which the level of IFN-γ production peaked by 8 h after LPS challenge, the level of IFN-γ production by the CD11c+-cell population continued to increase up to 16 h after LPS challenge. CD19+ B cells did not contribute significantly to splenic IFN-γ production (Table 1).
FIG. 1.
IFN-γ mRNA expression after endotoxin (LPS) challenge. Mice were challenged with LPS (4 mg/kg of body weight, intraperitoneally), spleens were harvested at the specified times, and total RNA was isolated. IFN-γ mRNA levels were determined by RPA. L32, a housekeeping gene, served both as a loading standard and as an internal control.
FIG. 2.
Characterization of IFN-γ-producing cells in the splenocytes after endotoxin (LPS) challenge. Mice were challenged with LPS (4 mg/kg of body weight, intraperitoneally), and their spleens were harvested at the indicated time points. The amounts of IFN-γ produced by DX5+ (NK) and CD3+ (T) cells were determined by flow cytometry. The DX5+IFN-γ+ and CD3+IFN-γ+ populations are circumscribed within the box at the 8-h time point, at which the level of IFN-γ production was maximal.
TABLE 1.
Characterization of IFN-γ-producing splenic cell populations after LPS challengea
| Cell type | % of cells producing IFN-γ at the following times (h) after LPS challenge:
|
% of all cells producing IFN-γ at peak | |||
|---|---|---|---|---|---|
| 0 | 4 | 8 | 16 | ||
| DX5+ | 1.6 | 15.3 | 72.4 | 30.9 | 59.8 |
| CD3+ | 0.4 | 1.1 | 2.0 | 1.8 | 24.1 |
| CD11b+ | 0.8 | 5.8 | 9.4 | 6.0 | 4.9 |
| CD11c+ | 5.8 | 7.5 | 12.7 | 17.5 | 3.2 |
| CD19+ | 0.5 | 0.6 | 0.4 | 0.3 | 0.1 |
Splenocytes were isolated and stained for the individual surface marker followed by intracellular staining with anti-IFN-γ. Data are expressed as the percentage of each cell population that was IFN-γ positive and the percentage of each cell type that contributed to total IFN-γ production.
FIG. 3.
The majority of CD3+ cells producing IFN-γ following LPS challenge are NKT cells. (A) The percentage of splenocytes expressing CD3 and DX5 was determined by flow cytometry after staining with FITC-DX5 and PE-CD3. The CD3+DX5+ NKT-cell population is designated. (B) Splenocytes were harvested from mice 8 h after LPS challenge. The percentage of CD3+ cells that coexpress DX5 and IFN-γ was determined after staining with FITC-DX5, PE-CD3, and allophycocyanin-IFN-γ. The CD3+ population was electronically gated and analyzed for DX5 and IFN-γ expression by flow cytometry. The CD3+DX5+ and the DX5+IFN-γ+ populations are designated.
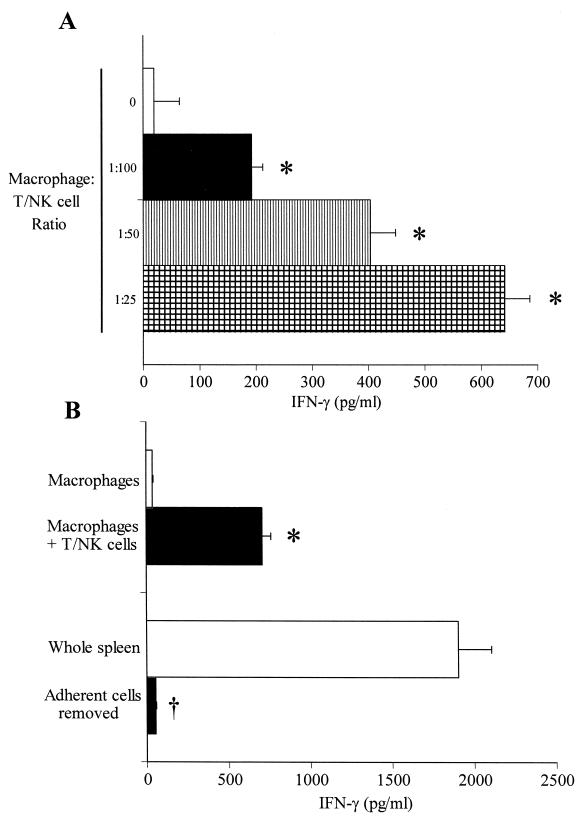
Additional studies were undertaken to characterize the role of accessory cells in the induction of IFN-γ production by T and NK cells (Fig. 4). Nonadherent T and NK cells cultured in the absence of peritoneal macrophages produced low levels of IFN-γ in response to LPS (Fig. 4A), whereas addition of resident peritoneal macrophages to T- and NK-cell cultures at ratios of 1:100 to 1:25 caused a concentration-dependent increase in the level of LPS-induced IFN-γ production. We also observed that cultured peritoneal macrophages, by themselves, produced little IFN-γ (Fig. 4B). However, the addition of isolated T and NK cells significantly increased the level of IFN-γ production. We extended this observation to splenocytes by showing that the removal of adherent cells resulted in a marked decrease in the level of IFN-γ production compared to total splenocytes (Fig. 4B).
FIG. 4.
Roles of accessory cells in endotoxin (LPS)-induced IFN-γ production. (A) Isolated splenic T and NK cells were incubated with resident peritoneal macrophages at the indicated ratios for 24 h in the presence of LPS (1 μg/ml). The levels of IFN-γ secreted in conditioned medium were determined by ELISA (n = 4 to 6 wells/group). ∗, significantly (P < 0.05) greater than the levels produced by T and NK cells incubated without peritoneal macrophages. (B) Resident peritoneal macrophages were incubated alone or with isolated splenic T and NK cells (1:20 ratio) for 24 h in the presence of LPS (1 μg/ml). In additional studies, whole splenocyte populations or splenocytes depleted of adherent cells were coincubated with LPS (1 μg/ml) for 24 h. The levels of IFN-γ secreted in the conditioned medium were determined by ELISA (n = 6 to 8 wells/group). ∗, significantly (P < 0.05) greater than the levels produced by macrophages cultured alone; †, significantly (P < 0.05) less than the levels produced by whole spleen.
LPS-induced IFN-γ production is class II MHC independent but is dependent on IL-12 and accessory signals provided by B7 proteins, IL-15, and IL-18.
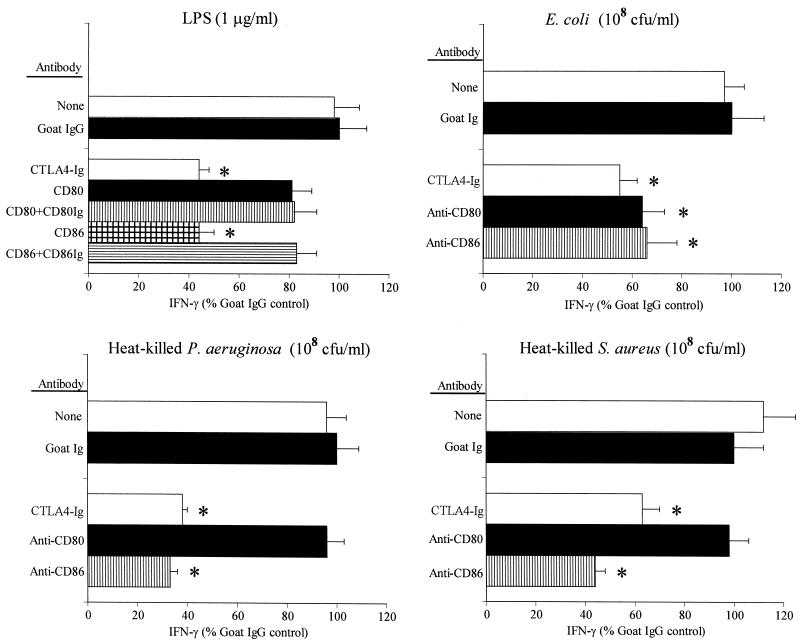
Based on our observation that adherent cells are critical for LPS-induced production of IFN-γ, we evaluated the functional roles of several adherent cell factors in the regulation of LPS-induced IFN-γ production. The class II MHC plays a key role in antigen presentation and activation of antigen-specific T cells. Our data show that LPS-induced production of IFN-γ is independent of the class II MHC (Fig. 5). The addition of antibody to the mouse class II MHC did not change the level of LPS-induced IFN-γ production compared to the amount produced after the addition of nonspecific goat IgG. However, class II MHC-specific antibodies significantly decreased the level of IFN-γ production in response to heat-killed E. coli, P. aeruginosa, and S. aureus.
FIG. 5.
LPS-induced IFN-γ production is class II MHC independent. Isolated splenocytes were cultured with LPS (1 μg/ml) or 108 CFU of heat-killed E. coli, P. auruginosa, or S. aureus per ml for 24 h in the presence of nonspecific goat IgG (10 μg/ml) or anti-MHC class II (10 ng/ml). The IFN-γ levels in the conditioned media were determined by ELISA (n = 4 to 8 wells/group). ∗, significantly (P < 0.05) less than the levels produced in the goat IgG group.
There are several cytokines that modulate innate immunity by regulating cellular IFN-γ production after exposure to microbes. We sought to identify individual cytokines that play a functional role in the regulation of LPS-induced IFN-γ production (Fig. 6). We observed that when isolated splenocytes were cultured with IL-10-specific antibodies 30 min prior to exposure to LPS (1 μg/ml) or heat-killed bacteria, the level of LPS-induced production of IFN-γ was increased by 1- to 1.5-fold. The inhibitory role of IL-10 in regulating IFN-γ production was also seen after stimulation with all three strains of heat-killed bacteria. Neutralization of IL-12 resulted in 90% inhibition of LPS-induced IFN-γ production. IL-12 antibodies also decreased the level of IFN-γ production by 80 to 90% in response to heat-killed bacteria. Antibodies against IL-15 significantly decreased the level of LPS-induced IFN-γ production (by 31%). In response to heat-killed E. coli, antibodies against IL-15 decreased the level of IFN-γ production by approximately 10% compared to the amount produced by controls, but this difference was not statistically significant. The level of IFN-γ production in response to heat-killed P. aeruginosa or S. aureus was significantly decreased (by approximately 20%) (Fig. 6). Antibodies against IL-18 decreased the level of LPS-induced IFN-γ production by approximately 40% and was also effective in significantly inhibiting IFN-γ production in response to all three strains of heat-killed bacteria (Fig. 6). Antibodies against IL-1β, IL-4, and IL-6 did not significantly alter the level of production of IFN-γ following LPS challenge (data not shown).
FIG. 6.
Effects of cytokine-specific antibodies on LPS-induced IFN-γ production. Isolated splenocytes were incubated with LPS (1 μg/ml) or 108 CFU of heat-killed bacteria per ml for 24 h in the presence of nonspecific goat IgG (10 μg/ml), anti-IL-10 (10 μg/ml), anti-IL-12 (1 μg/ml), anti-IL-15 (10 μg/ml), or anti-IL-18 (10 μg/ml). The IFN-γ levels in the conditioned media were determined by ELISA (n = 8 to 12 wells/group). †, significantly (P < 0.05) greater than the levels produced after the addition of goat IgG; ∗, significantly (P < 0.05) less than the levels produced in the goat IgG group.
B7 proteins represent a group of costimulatory molecules that play a crucial role in mediating interactions between antigen-presenting cells and T lymphocytes (29). The B7 proteins (B7.1 [CD80] and B7.2 [CD86]) bind to their cognate receptors, CD28 and CTLA4, and modulate T-cell proliferation, differentiation, and cytokine production in conjunction with TCR activation (13, 29). We sought to determine the roles of the B7 proteins in LPS-induced IFN-γ production. Preincubation of splenocytes with the CTLA4-Ig fusion protein, a potent inhibitor of the B7-CD28 interaction, significantly decreased the level of IFN-γ production (by approximately 40%) compared to the amount produced by preincubation of splenocytes with control goat IgG (Fig. 7). The CTLA4-Ig fusion protein also significantly inhibited IFN-γ production in response to all three strains of heat-killed bacteria. Antibody against CD80 did not decrease the level of IFN-γ production after LPS challenge or stimulation with heat-killed P. aeruginosa or S. aureus but did cause a significant (38%) decrease in the level of IFN-γ production after E. coli challenge. Antibodies against CD86 inhibited LPS-induced production by approximately 60%, and preincubation of CD86-specific antibodies with the CD86-Ig fusion protein neutralized this inhibitory effect (Fig. 6). Furthermore, antibodies against CD86 significantly inhibited production of IFN-γ in response to all three strains of heat-killed bacteria (Fig. 7).
FIG. 7.
Effects of B7 inhibitors on LPS-induced IFN-γ production. Isolated splenocytes were incubated with LPS (1 μg/ml) or 108 CFU of heat-killed bacteria per ml in the presence of nonspecific goat IgG (10 μg/ml), CTLA4-Ig (1 μg/ml), anti-CD80 (10 μg/ml), or anti-CD86 (10 μg/ml) for 24 h. The IFN-γ levels in the conditioned media were determined by ELISA (n = 4 to 8 wells/group). In some experiments, anti-CD80 and anti-CD86 were preabsorbed with CD80-Ig or CD86-Ig to assess specificity. ∗, significantly (P < 0.05) less than the levels produced in the goat IgG group.
We undertook additional studies to determine the importance of B7-CD28 ligation in combination with cytokine-mediated signals for LPS-induced IFN-γ production (Fig. 8). The combination of IL-12 antibodies and CTLA4-Ig did not inhibit IFN-γ production more than IL-12 antibodies alone in response to LPS or heat-killed bacteria. However, the combinations of antibodies specific for IL-15 or IL-18 with CTLA4-Ig caused an additive inhibition of IFN-γ production compared to that caused by either agent alone in response to LPS or heat-killed bacteria. Antibodies against IL-1β, IL-4, or IL-6 in combination with CTLA4-Ig did not inhibit IFN-γ production more than CTLA4-Ig alone did (data not shown).
FIG. 8.
Additive effects of cytokine-specific antibodies and CTLA4-Ig on LPS-induced IFN-γ production. Isolated splenocytes were incubated with LPS (1 μg/ml) or 108 CFU of heat-killed bacteria per ml for 24 h in the presence of nonspecific goat IgG (10 μg/ml), anti-IL-12 (1 μg/ml), anti-IL-15 (10 μg/ml), or anti-IL-18 (10 μg/ml) in combination with CTLA4-Ig (1 μg/ml). The IFN-γ levels in the conditioned media were determined by ELISA (n = 6 to 8 wells/group.). ∗, significantly (P < 0.05) less than the levels produced in the goat IgG group.
LPS challenge temporally modulates expression profiles of IFN-γ-inducing cytokine mRNAs.
We measured the levels of mRNAs for a variety of IFN-γ-regulating cytokines in the spleen after LPS challenge (Fig. 9). IL-12 p35 mRNA, but not IL-12 p40 mRNA, was constitutively expressed in the mouse spleen (Fig. 9A). LPS challenge induced a marked increase in the level of IL-12 p40 mRNA expression within 2 h that regressed during the next 6 h. Like IL-12 p35, IL-18 was constitutively expressed in the mouse spleen, and the expression of these two cytokines was sustained until 16 h after LPS challenge (Fig. 9A). Splenic IL-15 mRNA expression was induced within 2 h of LPS challenge and tapered off over the next few hours, a pattern that closely paralleled that of IL-12 p40 mRNA (Fig. 9B). The inhibitory cytokine IL-10 was also induced within 2 h of LPS challenge, but its level continued to increase during the entire 16-h study period (Fig 9B). As described earlier, IFN-γ mRNA was induced within 2 h of LPS challenge, and its level peaked at 6 h and declined thereafter (Fig. 9B).
FIG. 9.
Splenic cytokine mRNA expression following LPS challenge. Mice were challenged with LPS (4 mg/kg of body weight, intraperitoneally), and their spleens were harvested at the specified time points. Total splenic RNA was isolated, and the level of cytokine mRNA expression was determined by RPA.
We also assessed cytokine receptor mRNA expression in splenocytes after LPS challenge. IL-15Rα mRNA was evident within the first 2 h after LPS challenge, and its levels remained elevated until 16 h after LPS challenge (Fig. 10A). IL-2Rβ mRNA, which serves as the second functional subunit of IL-15R, was constitutively expressed in the mouse spleen and remained elevated throughout the 16-h study period. The level of expression of mRNA for IL-10R, constitutively expressed at the time of challenge, peaked at 2 h and gradually tapered off to the baseline levels by 16 h after LPS challenge (Fig. 10B). Initially, IL-12Rβ1 mRNA exhibited a low level of constitutive expression but was strongly induced within 4 to 8 h after LPS challenge. IL-12Rβ2 mRNA did not exhibit constitutive expression but was induced within 2 h of LPS challenge and tapered off to undetectable levels by 12 h after LPS challenge. Both the IFN-γRα and IFN-γRβ subunits were strongly expressed prior to LPS challenge, and their levels remained elevated throughout the 16-h study period (Fig. 10B).
FIG. 10.
Splenic cytokine mRNA receptor expression following LPS challenge. Mice were challenged with LPS (4 mg/kg of body weight, intraperitoneally), and their spleens were harvested at the specified time points. Total splenic RNA was isolated, and the level of cytokine receptor mRNA expression was determined by RPA.
LPS challenge modulates expression of B7 proteins and CD28 in the mouse spleen.
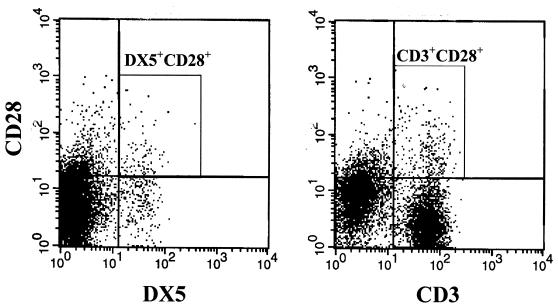
We studied the effect of LPS challenge on CD80 and CD86 expression by CD14+ macrophages, CD19+ B lymphocytes, and CD3+ T lymphocytes (Fig. 11). We found that 66% of CD14+ cells expressed CD80 on their surfaces at the baseline and that 64% expressed CD80 after LPS challenge. The mean fluorescence intensity (MFI) for CD80 was marginally increased after LPS challenge. CD80 was expressed on less than 20% of B cells and T cells at the baseline, and its level of expression was not significantly changed after LPS challenge. CD86 was expressed by greater than 80% of CD14+ cells before LPS challenge, and the percentage of CD14+/CD86+ cells decreased to approximately 53% after LPS challenge. Like CD80, CD86 was expressed on 20% or less of B cells and T cells, with little change in the level of expression after LPS challenge. Expression of CD28, a receptor for B7 molecules, by NK and T cells was also examined by flow cytometry. Approximately 30% of NK cells expressed CD28 on their surfaces after LPS challenge, and these accounted for 9% of all CD28+ cells (Fig. 12). Approximately 6% of CD3+ cells became positive for CD28 after LPS challenge. CD3+ T cells made up more than 70% of all CD28+ cells.
FIG. 11.
Expression of splenic B7 proteins (CD80 and CD86) following LPS challenge. Mice were challenged with LPS (4 mg/kg of body weight, intraperitoneally), and their spleens were harvested 8 h later. Saline-injected mice served as controls. The levels of CD80 and CD86 expression on CD14+, CD19+, and CD3+ splenocytes were determined by flow cytometry.
FIG. 12.
Expression of CD28 on splenocytes after LPS challenge. Mice were challenged with LPS (4 mg/kg of body weight, intraperitoneally), and their spleens were harvested 8 h later. The levels of CD28 expression on DX5+ and CD3+ cells were determined by flow cytometry. The DX5+CD28+and CD3+CD28+ populations are circumscribed within the respective boxes.
IL-15, IL-18, and B7 proteins serve as costimuli with IL-12 for induction of IFN-γ expression.
We undertook studies to determine the relative potencies of costimulatory factors in the induction of IFN-γ expression (Fig. 13). We found that IL-12, IL-15, IL-18, and anti-CD28 individually induced secretion of less than 25 pg of IFN-γ per ml into conditioned media. Furthermore, combinations of these factors did not induce significant levels of IFN-γ production in the absence of IL-12 (data not shown). The combination of IL-12 and IL-15 significantly enhanced the level of IFN-γ production compared to that induced by either agent alone, and of the factors tested, IL-12 plus IL-18 provided the most potent costimulus. Activation of CD28 with CD28-specific antibodies, CD80-Ig, or CD86-Ig in combination with IL-12 also enhanced the level of IFN-γ production, but not to the levels observed with IL-12 plus IL-15 or IL-12 plus IL-18. The combination of CD28 antibodies with IL-12 and IL-15 or IL-12 and IL-18 did not significantly increase the level of IFN-γ production compared to the levels obtained with these cytokine combinations alone (Fig. 13). Activation of the TCR with anti-CD3 was another potent stimulus for IFN-γ production by splenocytes. Specifically, anti-CD3 was as effective as the combination of IL-12 and IL-18 for stimulation of IFN-γ production by isolated splenic T and NK cells (Fig. 13). The addition of IL-12, IL-15, IL-18, or anti-CD28 did not increase the level of anti-CD3-induced IFN-γ production any further. Thus, our data show that there exist at least two pathways that promote IFN-γ production. However, activation of the TCR does not appear to play a functional role in LPS-induced production of IFN-γ.
FIG. 13.
IFN-γ production by isolated splenic T and NK cells in response to IFN-γ-regulating factors. Isolated splenic T and NK cells were incubated with IL-12 (2 ng/ml) alone or with IL-12 plus IL-15 (20 ng/ml), IL-18 (20 ng/ml), agarose-coupled anti-CD28 (10 μg/ml), CD80-Ig (10 μg/ml), CD86-Ig (10 μg/ml), or anti-CD3 (10 μg/ml) in the indicated combinations for 24 h. The IFN-γ levels in the conditioned media were determined by ELISA (n = 4 to 8 wells/group).
DISCUSSION
Eradication of invading microbes requires rapid recognition of the pathogen, followed by an appropriate immune response. Innate immunity is quickly mobilized by microbial products such as LPS and functions to contain infection while acquired immunity, if present, becomes activated. IFN-γ is a cytokine that plays a major role in the amplification of both innate and acquired immune responses. IFN-γ production is regulated by complex interactions among cytokines, accessory molecules, and TCR (6, 29). However, the specific cell types that produce IFN-γ early after LPS challenge and the mechanisms responsible for regulation of LPS-induced IFN-γ production have yet not been fully characterized. An understanding of these mechanisms is important because alterations in IFN-γ production occur in septic patients and patients with severe trauma or burns (9). A decreased level of IFN-γ production appears to play a functional role in the immunosuppression that occurs after major injury or sepsis (9). IFN-γ is also an important mediator of the sepsis syndrome and the Shwartzman reaction (8, 23). The present studies show that NK cells are the predominant IFN-γ producers after LPS challenge and, in combination with T cells, make up approximately 85% of the IFN-γ-producing cells in the mouse spleen, whereas small amounts of IFN-γ are produced by macrophages and dendritic cells. The production of IFN-γ by NK and T cells requires the presence of accessory cells. IL-12 is the predominant IFN-γ-inducing factor, whereas IL-10 is a potent inhibitor of LPS-induced IFN-γ production. Costimulatory signals provided by IL-15, IL-18, and B7 proteins act in concert with IL-12 to promote IFN-γ production following LPS challenge. Unlike heat-killed microorganism-induced IFN-γ production, LPS-induced IFN-γ production remained independent of the class II MHC.
The ability of NK and T cells to produce IFN-γ after LPS challenge has been demonstrated previously (17, 24). However, the relative contribution of each cell type to total IFN-γ production has been controversial. Some investigators have reported that NK cells comprise 100% of IFN-γ-producing cells in the spleen, whereas others have reported that T cells are the major source of splenic IFN-γ (24, 27). We showed that approximately 60% of IFN-γ-producing cells in the spleens of BALB/c mice are NK cells and that 25% are T lymphocytes. This finding is consistent with other studies that have demonstrated that NK cells are the predominant source of LPS-induced IFN-γ production (17). We also showed that more than 75% of CD3+ cells producing IFN-γ following LPS challenge are NKT (CD3+DX5+) cells. This finding demonstrates that NK and NKT cells are the major IFN-γ-producing cells in the mouse spleen following LPS challenge and that CD3+DX5− T cells are minor contributors. CD11b+ and CD11c+ cells also contribute to IFN-γ production after LPS challenge. However, these cell populations make up less than 5% of IFN-γ-producing cells in the mouse spleen. It is likely that the CD11b+- and CD11c+-cell populations represent macrophages/monocytes and dendritic cells, respectively. Prior studies have reported that macrophages and dendritic cells possess the ability to produce IFN-γ (22, 26). Although we demonstrated that approximately 85% of IFN-γ-producing cells in the mouse spleen are NK and T cells, the functional contribution of each cell type as a source of IFN-γ after LPS challenge remains unclear. Previous studies have shown that depletion of NK cells and NK 1+ T cells will nearly eliminate LPS-induced IFN-γ production and protect mice from the generalized Shwartzman reaction (25). This finding suggests the functional importance of NK cells and T cells as sources of IFN-γ. Other investigators have shown the importance of dendritic cell-derived IFN-γ in the regulation of innate and acquired immune responses (12, 26). Recently, it was reported that Stat4-deficient macrophages and dendritic cells produce decreased levels of IFN-γ, have impaired nitric oxide-mediated bacterial killing mechanisms, and are susceptible to Toxoplasma infection (12). Our study shows that IFN-γ expression by NK and T cells, as well as by macrophages and dendritic cells, is rapidly mobilized after systemic LPS challenge. The selective roles and functional importance of the IFN-γ produced by each cell remains to be fully determined.
IL-12 is a potent modulator of IFN-γ production and has been shown by many investigators to be a major stimulus for IFN-γ production (22, 26, 27, 29, 33). In addition, several costimulatory molecules such as IL-15, IL-18, B7 proteins, and the antigen-laden class II MHC act in combination with IL-12 to facilitate induction of IFN-γ production (7, 15, 22, 26, 27). Our neutralization studies with IL-12 antibodies demonstrate that IL-12 is the primary positive mediator of IFN-γ production after challenge with LPS or heat-killed bacteria. IL-12 p40 mRNA expression is rapidly induced by LPS challenge and peaks approximately 4 h before the peak level of IFN-γ mRNA is reached. IL-12 p35 mRNA is constitutively expressed in the mouse spleen, and its level of expression does not change appreciably during the first 6 h after LPS challenge. mRNAs of both IL-12 receptor subunits (IL-12Rβ1 and IL-12Rβ2) were induced by LPS and peaked at 4 to 8 h after challenge, at about the time of the peak level of LPS-induced IFN-γ mRNA expression.
Both of the costimulatory cytokines IL-15 and IL-18 appeared to play a functional role in LPS-induced IFN-γ production. Antibodies against either cytokine decreased the level of LPS-induced IFN-γ production by 30 to 40% and effectively inhibited IFN-γ production after challenge with heat-killed bacteria. The patterns of expression of IL-15 and IL-15R mRNAs closely paralleled the patterns observed for IL-12p40 and IL-12R mRNAs. Specifically, the level of IL-15 mRNA expression peaked at 2 to 4 h after LPS challenge, and the level of IL-15Rα expression was also rapidly induced, with maximum expression occurring 4 to 8 h after LPS challenge. IL-2Rβ, the other component of the functional IL-15R, was constitutively expressed in the mouse spleen, and its mRNA was further induced by LPS challenge, with the level of expression peaking after 4 to 8 h. Unlike IL-15 mRNA, IL-18 mRNA was constitutively expressed in the mouse spleen and was further induced by LPS within 2 h of LPS challenge. Our previous study has shown that the IL-18 protein is constitutively produced by mouse splenocytes and is present in mouse sera prior to LPS challenge (35). The abilities of IL-15 and IL-18 to serve as costimuli with IL-12 for the induction of IFN-γ production have been well established (4, 10, 11, 22, 27). In addition, one study showed that CD28 also acts as a costimulus with IL-12 in NK cell-specific IFN-γ production (37). We showed that IL-15 and IL-18 play functional roles in IL-12-dependent IFN-γ production after LPS challenge and that these cytokines, together with B7 proteins, stimulated IFN-γ production in an additive manner. Furthermore, a combination of blocking antibodies against IL-15 or IL-18 with the B7-CD28 inhibitor CTLA4-Ig decreased the level of LPS-induced IFN-γ production more strongly than each factor alone did.
Our study shows that both CD80 and CD86 can serve as costimuli with IL-12 for induction of IFN-γ by splenic T cells and NK cells. Several cell types in the spleen, most prominently, the CD14+ population, express CD80 and CD86. LPS challenge did not significantly change surface B7 protein levels. Subsets of CD3+ and DX5+ cells stained positive for CD28, thus demonstrating that both CD3+ T cells and DX5+ NK cells express CD28. The functional role of B7-CD28 interactions in LPS-induced IFN-γ production was demonstrated by the inhibition of IFN-γ production by the B7 inhibitor CTLA4-Ig after LPS challenge. Expression of CD28 by T cells and NK cells has been demonstrated previously (13, 37). Our study suggests that this pathway has a functional role in LPS-induced IFN-γ production. In addition, we showed that, unlike heat-killed bacteria, LPS-induced IFN-γ production is independent of the class II MHC. Mattern and colleagues (18) have demonstrated that LPS-induced T-cell proliferation is class II MHC unrestricted. However, Piani and coworkers (28) have shown that mice expressing low levels of class II MHC have impaired LPS responsiveness. Our study indicated that although activation of the TCR is a potent stimulus for IFN-γ production, the class II MHC does not function as a direct mediator of IFN-γ production after LPS challenge.
The present study also shows that IL-10 is a functional inhibitor of LPS-induced IFN-γ production. IL-10 mRNA expression is strongly induced by LPS, and blockade of IL-10 during LPS challenge causes a one- to twofold increase in the level of IFN-γ production. IL-10 is produced by macrophages, monocytes, and Th2 cells and negatively regulates numerous immune functions such as cytokine production, antigen presentation, and Ig synthesis (21). Most LPS-induced production of IL-10 is likely to be derived from macrophages and monocytes. Recent studies have shown that macrophages and monocytes have the ability to produce IL-10 in response to LPS, a response that is potentiated by Fcγ receptor ligation (14). The primary function of IL-10 is to inhibit inflammatory responses (21). The functional significance of IL-10 as an anti-inflammatory agent is shown by the increased susceptibility of IL-10−/− mice to inflammatory injury and the ability of IL-10 administration to decrease the level of inflammatory injury during sepsis (29, 31). Thus, our finding that IL-10 serves as a negative regulator of IFN-γ production after LPS challenge, in combination with earlier observations on the stimulatory roles of IL-12, IL-15, IL-18, and B7 proteins, extends our understanding of the key factors regulating IFN-γ production after the activation of innate immunity.
Acknowledgments
This study was supported by research grants K08 GM61243 from NIH and 8650 from the Shriners of North America to E.R.S. T.E.T-K. is the recipient of NIH postdoctoral fellowship 08256.
REFERENCES
- 1.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 2.Bone, R. C., R. A. Balk, F. B. Cerra, R. P. Dellinger, A. M. Fein, W. A. Knaus, R. M. Schein, W. J. Sibbald, et al. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 101:1644-1655. [DOI] [PubMed] [Google Scholar]
- 3.Butt, W. 2001. Septic shock. Pediatr. Clin. N. Am. 48:601-625. [DOI] [PubMed] [Google Scholar]
- 4.Carson, W. E., M. E. Ross, R. A. Baiocchi, M. J. Marien, N. Boiani, K. Grabstein, and M. A. Caligiuri. 1995. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J. Clin. Investig. 96:2578-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cauwels, A., K. Frei, S. Sansano, C. Fearns, R. Ulevitch, W. Zimmerli, and R. Landmann. 1999. The origin and function of soluble CD14 in experimental bacterial meningitis. J. Immunol. 162:4762-4772. [PubMed] [Google Scholar]
- 6.Chang, J. T., B. M. Segal, K. Nakanishi, H. Okamura, and E. M. Shevach. 2000. The costimulatory effect of IL-18 on the induction of antigen-specific IFN-gamma production by resting T cells is IL-12 dependent and is mediated by up-regulation of the IL-12 receptor beta2 subunit. Eur. J. Immunol. 30:1113-1119. [DOI] [PubMed] [Google Scholar]
- 7.Coyle, A. J., and J. C. Gutierrez-Ramos. 2001. The expanding B7 superfamily: increasing complexity in costimulatory signal regulating T cell function. Nat. Immunol. 2:203-209. [DOI] [PubMed] [Google Scholar]
- 8.Dieli, F., G. Sireci, D. Russo, M. Taniguchi, J. Ivanyi, C. Fernandez, M. Troye-Blomberg, G. De Leo, and A. Salerno. 2000. Resistance of natural killer T cell-deficient mice to systemic Shwartzman reaction. J. Exp. Med. 192:1645-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Docke, W. D., F. Randow, U. Syrbe, D. Krausch, K. Asadullah, P. Reinke, H. D. Volk, and W. Kox. 1997. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat. Med. 3:678-681. [DOI] [PubMed] [Google Scholar]
- 10.Doherty, T. M., R. A. Seder, and A. Sher. 1996. Induction and regulation of IL-15 expression in murine macrophages. J. Immunol. 156:735-741. [PubMed] [Google Scholar]
- 11.Fehniger, T. A., H. Yu, M. A. Cooper, K. Suzuki, M. H. Shah, and M. A. Caligiuri. 2000. Cutting edge: IL-15 costimulates the generalized Shwartzman reaction and innate immune IFN-gamma production in vivo. J. Immunol. 164:1643-1647. [DOI] [PubMed] [Google Scholar]
- 12.Fukao, T., D. M. Frucht, G. Yap, M. Gadina, J. J. O'Shea, and S. Koyasu. 2001. Inducible expression of Stat4 in dendritic cells and macrophages and its critical role in innate and adaptive immune responses. J. Immunol. 166:4446-4455. [DOI] [PubMed] [Google Scholar]
- 13.Galea-Lauri, J., D. Darling, S. U. Gan, L. Krivochtchapov, M. Kuiper, J. Gaken, B. Souberbielle, and F. Farzaneh. 1999. Expression of a variant of CD28 on a subpopulation of human NK cells: implications for B7-mediated stimulation of NK cells. J. Immunol. 163:62-70. [PubMed] [Google Scholar]
- 14.Gerber, J. S., and D. M. Mosser. 2001. Reversing lipopolysaccharide toxicity by ligating the macrophage Fcγ receptors. J. Immunol. 166:6861-6868. [DOI] [PubMed] [Google Scholar]
- 15.Hasbold, J., J. S. Hong, M. R. Kehry, and P. D. Hodgkin. 1999. Integrating signals from IFN-gamma and IL-4 by B cells: positive and negative effects on CD40 ligand-induced proliferation, survival, and division-linked isotype switching to IgG1, IgE, and IgG2a. J. Immunol. 163:4175-4181. [PubMed] [Google Scholar]
- 16.Kaufmann, A., D. Gemsa, and H. Sprenger. 2000. Differential desensitization of lipopolysaccharide-inducible chemokine gene expression in human monocytes and macrophages. Eur. J. Immunol. 30:1562-1567. [DOI] [PubMed] [Google Scholar]
- 17.Kim, S., K. Iizuka, H. L. Aguila, I. L. Weissman, and W. M. Yokoyama. 2000. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc. Natl. Acad. Sci. USA 97:2731-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattern, T., H. D. Flad, L. Brade, E. T. Rietschel, and A. J. Ulmer. 1998. Stimulation of human T lymphocytes by LPS is MHC unrestricted, but strongly dependent on B7 interactions. J. Immunol. 160:3412-3418. [PubMed] [Google Scholar]
- 19.Medvedev, A. E., K. M. Kopydlowski, and S. N. Vogel. 2000. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J. Immunol. 164:5564-5574. [DOI] [PubMed] [Google Scholar]
- 20.Moore, K. J., L. P. Andersson, R. R. Ingalls, B. G. Monks, R. Li, M. A. Arnaout, D. T. Golenbock, and M. W. Freeman. 2000. Divergent response to LPS and bacteria in CD14-deficient murine macrophages. J. Immunol. 165:4272-8420. [DOI] [PubMed] [Google Scholar]
- 21.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 22.Munder, M., M. Mallo, K. Eichmann, and M. Modolell. 1998. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J. Exp. Med. 187:2103-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura, S., T. Otani, Y. Ijiri, R. Motoda, M. Kurimoto, and K. Orita. 2000. IFN-gamma-dependent and -independent mechanisms in adverse effects caused by concomitant administration of IL-18 and IL-12. J. Immunol. 164:3330-3336. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen, K. B., and C. A. Biron. 1999. Synergism for cytokine-mediated disease during concurrent endotoxin and viral challenges: roles for NK and T cell IFN-gamma production. J. Immunol. 162:5238-5246. [PubMed] [Google Scholar]
- 25.Ogasawara, K., K. Takeda, W. Hashimoto, M. Satoh, R. Okuyama, N. Yanai, M. Obinata, K. Kumagai, H. Takada, H. Hiraide, and S. Seki. 1998. Involvement of NK1+ T cells and their IFN-gamma production in the generalized Shwartzman reaction. J. Immunol. 160:3522-3527. [PubMed] [Google Scholar]
- 26.Ohteki, T., T. Fukao, K. Suzue, C. Maki, M. Ito, M. Nakamura, and S. Koyasu. 1999. Interleukin 12-dependent interferon gamma production by CD8α+ lymphoid dendritic cells. J. Exp. Med. 189:1981-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otani, T., S. Nakamura, M. Toki, R. Motoda, M. Kurimoto, and K. Orita. 1999. Identification of IFN-gamma-producing cells in IL-12/IL-18-treated mice. Cell. Immunol. 198:111-119. [DOI] [PubMed] [Google Scholar]
- 28.Piani, A., J. P. Hossle, T. Birchler, C. A. Seigrist, D. Heimann, G. Daves, S. Loeliger, R. Seger, and R. P. Lauener. 2000. Expression of MHC class II molecules contributes to lipopolysaccharide responsiveness. Eur. J. Immunol. 30:3140-3146. [DOI] [PubMed] [Google Scholar]
- 29.Rogy, M. A., T. Auffenberg, N. J. Espat, R. Philip, D. Remick, G. K. Wollenberg, E. M. Copeland III, and L. L. Moldawer. 1995. Human tumor necrosis factor receptor (p55) and interleukin 10 gene transfer in the mouse reduces mortality to lethal endotoxemia and also attenuates local inflammatory responses. J. Exp. Med. 181:2289-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salkowski, C. A., K. E. Thomas, M. J. Cody, and S. N. Vogel. 2000. Impaired IFN-gamma production in IFN regulatory factor-1 knockout mice during endotoxemia is secondary to a loss of both IL-12 and IL-12 receptor expression. J. Immunol. 165:3970-3977. [DOI] [PubMed] [Google Scholar]
- 31.Sewnath, M. E., D. P. Olszyna, R. Birjmohun, F. J. ten Kate, D. J. Gouma, and T. van Der Poll. 2001. IL-10-deficient mice demonstrate multiple organ failure and increased mortality during Escherichia coli peritonitis despite an accelerated bacterial clearance. J. Immunol. 166:6323-6331. [DOI] [PubMed] [Google Scholar]
- 32.Shtrichman, R., and C. Samuel2001. The role of gamma interferon in antimicrobial immunity. Curr. Opin. Microbiol. 4:251-259. [DOI] [PubMed] [Google Scholar]
- 33.Trinicheri, G. 1998. Interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 16:495-521. [DOI] [PubMed] [Google Scholar]
- 34.Ulevitch, R. J., and P. S. Tobias. 1999. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 11:19-22. [DOI] [PubMed] [Google Scholar]
- 35.Varma, T., T. Toliver-Kinsky, C. Lin, A. Koutrouvelis, J. Nichols, and E. R. Sherwood. 2001. Cellular mechanisms causing suppressed gamma interferon secretion in endotoxin-tolerant mice. Infect. Immun. 69:5249-5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verbon, A., P. E. Dekkers, T. ten Hove, C. E. Hack, J. P. Pribble, T. Turner, S. Souza, T. Axtelle, F. J. Hoek, S. J. van Deventer, and T. van der Poll. 2001. IC14, an anti-CD14 antibody, inhibits endotoxin-mediated symptoms and inflammatory responses in humans. J. Immunol. 166:3599-3605. [DOI] [PubMed] [Google Scholar]
- 37.Walker, W., M. Aste-Amezaga, R. A. Kastelein, G. Trinchieri, and C. A. Hunter1999. IL-18 and CD28 use distinct molecular mechanisms to enhance NK cell production of IL-12-induced IFN-gamma. J. Immunol. 162:5894-5901. [PubMed] [Google Scholar]
- 38.Wysocka, M., S. Robertson, H. Riemann, J. Caamano, C. Hunter, A. Mackiewicz, L. J. Montaner, G. Trinchieri, and C. L. Karp. 2001. IL-12 suppression during experimental endotoxin tolerance: dendritic cell loss and macrophage hyporesponsiveness. J. Immunol. 166:7504-7513. [DOI] [PubMed] [Google Scholar]