Abstract
The reliability of an in vitro pyrogen test system based on proinflammatory cytokine release from human monocytic cells was assessed by comparison with a test system based on a human whole blood culture as well as with the conventional rabbit pyrogen test. The human cells used as the pyrogen indicator cells were newly selected by subcloning of a human monocytic cell line, Mono-Mac-6. The selected cells, named MM6-CA8, responded to various pyrogens, including endotoxin, peptidoglycan (PG), Staphylococcus aureus Cowan 1 (SAC), and poly(I · C), with a high sensitivity and produced proinflammatory cytokines, such as interleukin 1 (IL-1), IL-6, and tumor necrosis factor alpha. Among these cytokines, IL-6 was produced most sensitively in response to traces of the pyrogens and detected in the largest quantities in the culture medium. The cytokine-producing responses of MM6-CA8 cells correlated significantly with the responses of cultured human whole blood, which represents an ex vivo culture test system reproducing pyrogen-induced cytokine production in the human body. In terms of cytokine inducibility, the pyrogens were ranked in the order endotoxin > PG > poly (I · C) > SAC in both culture systems, a ranking which almost agreed with the ranking of their pyrogenicity as assessed by the rabbit pyrogen test. These results suggest that the in vitro responsiveness of MM6-CA8 cells to various pyrogens is highly relevant for human pyrogenic reactions. Therefore, the in vitro test system is useful and reliable for detecting the presence of materials that are pyrogenic for humans.
Parenteral pharmaceuticals have usually been tested for pyrogenic contamination by the rabbit pyrogen test. This in vivo test is able to detect various kinds of pyrogens but is costly and requires the use of laboratory animals. In addition, it is somewhat doubtful whether rabbits have sensitivities to various kinds of pyrogens comparable to those of humans. In recent years, the Limulus amoebocyte lysate (LAL) test has been adopted as an official test to replace the rabbit pyrogen test. The LAL test is now used widely as a simple and highly sensitive in vitro method for the detection of endotoxin, which is the most typical and the most powerful pyrogen present in the natural world. The in vitro test is based on a clotting reaction elicited in lysates of amoebocytes from the horseshoe crab, Limulus polyphemus, by a small amount of endotoxin. This test, however, has some inherent limitations; it cannot detect any pyrogens except for endotoxin, and there is phylogenic distance between the horseshoe crab and higher vertebrates. Therefore, the development of a novel in vitro alternative to the rabbit pyrogen test is needed to make up for the limitations of the LAL test. It was reported that the parenteral pharmaceutical preparation of methionyl human growth hormone evoked fever when it was injected into healthy volunteers in a phase I trial (8). However, no significant level of exogenous pyrogens or endotoxin had been detected in the preparation by the rabbit pyrogen test or the LAL test. Considering these findings, the new test must be able to detect all kinds of pyrogens and evaluate their pyrogenicity in humans accurately.
When pyrogens enter the circulation, the stimulation of peripheral blood monocytes and macrophages determines the release of proinflammatory cytokines (endogenous pyrogens), such as interleukin 1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α) (35, 39). These cytokines transmit signals to increase the thermostatic set point at the level of the preoptic area of the hypothalamus through prostaglandin E2. These signals are further transmitted to the brain, where complex thermoregulatory mechanisms are triggered to increase the body temperature (7, 18, 24, 31). The novel pyrogen test should be based on in vitro reactions that are highly relevant to the above fever-inducing mechanisms in humans. As representative of such a relevant in vitro reaction, pyrogen-induced proinflammatory cytokine production by human monocytes or human monocytic cell lines has been proposed. The proposed methods include test systems with human whole blood (11, 26), isolated primary cells from human blood (13, 28), and cell lines of the human monocyte/macrophage lineage (10, 21, 25, 38). Such test systems are required to satisfy the practical demands of high sensitivity, simplicity, and low variability. Test systems with human whole blood or isolated primary cells have greater relevance to the in vivo situation than test systems with monocytic cell lines. However, the use of blood or primary cells will not be accepted widely, since there are many difficulties related to their preparation and the individual variances of different donors.
In the present study, we chose a human monocytic cell line, Mono-Mac-6 (MM6) (42), for use as indicator cells in an in vitro pyrogen test system because of their ease of processing and low variability. We first subcloned the MM6 cells and obtained a clone, MM6-CA8, which responds to various pyrogens with a high sensitivity; we then set up the in vitro pyrogen test system with the MM6-CA8 cells. It is possible, however, that the MM6-CA8 cells cloned from human monocytoma-derived MM6 cells have mutations causing some alterations in their responsiveness to pyrogens. In order to evaluate the MM6-CA8 cells for their suitability and reliability as pyrogen indicator cells, we compared their cytokine-producing responses to various pyrogens with those of normal human whole blood as well as with the pyrogenic responses of rabbits. The pyrogens examined in this study included peptidoglycan (PG), Staphylococcus aureus Cowan 1 (SAC), and poly(I · C) in addition to endotoxin. To the best of our knowledge, this is the first report to investigate the cytokine inducibility and pyrogenicity of a variety of pyrogens systematically.
MATERIALS AND METHODS
Pyrogens.
Endotoxin (lipopolysaccharide) from Escherichia coli O55:B5 was purchased from Sigma Chemical Co. (St. Louis, Mo.). Insoluble PG was prepared from formalin-fixed S. aureus (Sigma) by the method of Schleifer and Kandler (32). Briefly, the bacteria were suspended in trichloroacetic acid (10%). The suspension was placed in a boiling water bath for 20 min and then centrifuged. The pellet was washed with distilled water and resuspended in trypsin-phosphate buffer (0.2 mg of trypsin/ml of 0.1 M phosphate buffer [pH 7.9]). The suspension was incubated at 37°C on a shaker for 2 h and centrifuged. The pellet was washed two times with distilled water, lyophilized, and stored at 4°C. Lyophilized PG was resuspended in distilled water and sonicated at 38,000 Hz on ice for 20 min before experiments. SAC (Pansorbin) was purchased from Calbiochem-Novabiochem Co. (San Diego, Calif.). Carboxymethyl-β-(1,3)-d-glucan (CM-curdlan) was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Synthetic poly(I · C) was purchased from Amersham Pharmacia Biotech UK Ltd. (Buckinghamshire, United Kingdom). PG, SAC, and CM-curdlan contained less than 20 pg of endotoxin/mg and poly(I · C) contained 3,000 pg of endotoxin/mg, as determined by the LAL assay (Endospecy; Seikagaku Co., Tokyo, Japan).
Selection of a human monocytic cell line suitable for use as indicator cells.
THP-1 cells were purchased from the American Type Culture Collection (Rockville, Md.). U937 and HL-60 cells were obtained from Japanese Cancer Research Resources Bank-Cell (Osaka, Japan). MM6 cells were purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). The cells were primed with 10 or 100 ng of calcitriol (1α,25-dihydroxyvitamin D3; Wako)/ml. After incubation for 48 or 72 h with calcitriol, the cells were plated in a 24-well plate at 106 cells/0.9 ml/well, and 0.1 ml of endotoxin at various concentrations was added to each well. The cell suspensions were incubated for 17 h at 37°C, and then the supernatants were assayed for TNF-α by a cytotoxicity assay with L929 cells (obtained from Japanese Cancer Research Resources Bank-Cell) as previously described (22).
Subcloning of MM6 cells and induction of proinflammatory cytokine release from MM6 cells by pyrogens.
MM6 cells were subcloned by limiting dilution, and MM6-CA8 cells were selected for their superior response to low concentrations of endotoxin and PG in producing large amounts of proinflammatory cytokines with a high sensitivity. The cells were maintained in RPMI 1640 medium (Gibco Laboratories, Grand Island, N.Y.) containing fetal bovine serum (10%), glutamine (2 mM), nonessential amino acids (0.1 mM), sodium pyruvate (1 mM), and bovine insulin (9 μg/ml). MM6-CA8 cells were primed with 10 ng of calcitriol/ml. After incubation for 72 h with calcitriol, the cells were plated in a 24-well plate at 106 cells/0.9 ml/well, and 0.1 ml of pyrogens at various concentrations was added to each well. The cell suspensions were incubated for 17 h (unless otherwise indicated) at 37°C, and then the supernatants were assayed for IL-1, IL-6, and TNF-α with commercial enzyme-linked immunosorbent assay (ELISA) kits (Genzyme, Cambridge, Mass.). The kinetics of endotoxin- or PG-induced IL-1, IL-6, and TNF-α production from MM6-CA8 cells were examined. After incubation for 0.5, 1, 2, 4, 8, 12, and 24 h, cytokine release in the supernatants of cell cultures was measured. The cytokine production induced by both pyrogens increased rapidly during the first 2 to 4 h and then reached a plateau (IL-1 and IL-6) or decreased slightly (TNF-α) (data not shown). These results indicated that IL-1, IL-6, and TNF-α production was almost constant from 4 to 24 h after incubation.
Pyrogen-induced proinflammatory cytokine release from cultured human whole blood cells.
Blood samples were collected from healthy volunteers. Freshly drawn heparinized human peripheral blood (225 μl) was incubated for 4 h at 37°C with 25 μl of saline containing various concentrations of pyrogens in a sterile polypropylene tube (Falcon; Becton Dickinson, San Jose, Calif.). After incubation, the blood samples were diluted with 750 μl of saline and centrifuged for 5 min at 15,000 × g. The supernatants were assayed for IL-1, IL-6, and TNF-α with commercial ELISA kits.
Rabbit pyrogen test.
Male Japanese White rabbits weighing about 3 kg were used throughout this study. Rabbits were given an intravenous dose (1 ml/kg of body weight) of various concentrations of pyrogens. Rectal temperatures were measured with indwelling rectal thermistors and recorded for 5 h after pyrogen administration.
Statistical analysis.
Data were expressed as the mean and standard error of the mean (SEM). The detection limit for each pyrogen was calculated as the minimum concentration of that pyrogen inducing a significant amount of cytokine production (greater than 2 standard deviations above that obtained with 0 ng of pyrogen/ml).
RESULTS
Selection of a human monocytic cell line suitable for use as indicator cells.
We first evaluated four kinds of human myelomonocytic cell lines, MM6, THP-1, U937, and HL-60, for their suitability as indicator cells for the in vitro pyrogen test system by examining their responsiveness to endotoxin (0.1 to 10,000 ng/ml). Among these cell lines, only MM6 cells produced a large amount of TNF-α in response to endotoxin; however, this response occurred only when the cells were primed with calcitriol. Other cell lines produced little TNF-α in response to endotoxin regardless of the treatment with calcitriol (data not shown). We therefore chose MM6 cells and evaluated their usefulness and reliability as pyrogen indicator cells in detail.
Subcloning of MM6 cells.
It is possible that the bulk culture of the MM6 cell line consists of cells heterogeneous in their reactivity to pyrogens. Therefore, we subcloned MM6 cells by limiting dilution to select a cell clone which responds to pyrogens with a high sensitivity. As reference pyrogens, we chose endotoxin and PG, since both are typical bacterial pyrogens and bind individually to their own receptors on the cell surface of monocytes/macrophages, i.e., Toll-like receptor 4 and Toll-like receptor 2, respectively (37).Via cloning, we obtained a subclone, MM6-CA8, that shows superior reactivity to both endotoxin and PG. Table 1 shows the comparison of the responsiveness of MM6-CA8 and MM6 cells. In response to endotoxin and PG, both cells produced IL-6, TNF-α, and IL-1 dose dependently, but the amounts of cytokines produced were much larger in MM6-CA8 cells than in MM6 cells. A marked difference was also found in their sensitivity to PG. Cytokine production increased significantly with 100 ng of PG/ml in MM6-CA8 cells, compared to 1,000 ng/ml in MM6 cells. Thus, MM6-CA8 cells were 10 times as sensitive as MM6 cells to PG. On the other hand, the sensitivities of the cells to endotoxin were almost the same, although MM6-CA8 cells produced very large amounts of cytokines in response to endotoxin, as mentioned above. The other characteristics of MM6-CA8 cells were ease of processing and phenotypic stability. The cells grow in suspension with a doubling time of 36 h, and we have confirmed that the high sensitivity to endotoxin and PG is retained for at least 200 passages (data not shown).
TABLE 1.
Comparison of proinflammatory cytokine release from MM6 cells and MM6-CA8 cells in response to endotoxin and PGa
| Pyrogen and dose | Amt (pg/ml) of the following cytokine produced by the indicated cells:
|
|||||
|---|---|---|---|---|---|---|
| IL-6
|
TNF-α
|
IL-1
|
||||
| MM6 | MM6-CA8 | MM6 | MM6-CA8 | MM6 | MM6-CA8 | |
| Endotoxin (pg/ml) | ||||||
| 0 | 2.6 ± 1.4 | 6.8 ± 1.4 | 1.4 ± 1.0 | 1.6 ± 0.8 | 2.7 ± 1.1 | 7.2 ± 0.5 |
| 1 | 3.4 ± 0.1 | 6.8 ± 1.0 | 1.3 ± 0.7 | 1.5 ± 0.3 | 1.8 ± 0.7 | 8.8 ± 0.6 |
| 5 | 5.7 ± 0.4 | 10.2 ± 2.0 | 1.1 ± 0.3 | 1.8 ± 0.6 | 1.6 ± 0.4 | 9.0 ± 0.7 |
| 10 | 7.2 ± 2.9 | 18.8 ± 4.0 | 1.5 ± 0.2 | 1.8 ± 0.2 | 3.5 ± 1.6 | 13.1 ± 1.0 |
| 100 | 219 ± 73 | 827 ± 128 | 41.8 ± 23.4 | 129 ± 10 | 19.1 ± 6.0 | 82.0 ± 4.0 |
| 1,000 | 1,607 ± 617 | 6,603 ± 883 | 365 ± 223 | 603 ± 27 | 95.5 ± 48.9 | 345 ± 28 |
| PG (ng/ml) | ||||||
| 0 | 3.0 ± 2.1 | 4.8 ± 0.8 | 2.8 ± 1.2 | 2.3 ± 0.6 | 2.8 ± 2.1 | 8.1 ± 2.0 |
| 1 | 3.2 ± 2.0 | 8.0 ± 2.3 | 3.8 ± 1.2 | 3.6 ± 0.2 | 3.7 ± 2.0 | 11.2 ± 2.7 |
| 10 | 3.7 ± 1.7 | 8.0 ± 1.4 | 2.9 ± 0.9 | 3.1 ± 1.0 | 4.0 ± 1.7 | 8.6 ± 2.5 |
| 100 | 3.8 ± 1.8 | 52.5 ± 20.0 | 2.8 ± 0.4 | 5.1 ± 1.4 | 3.3 ± 1.8 | 13.8 ± 1.3 |
| 1,000 | 25.8 ± 12.1 | 284 ± 91 | 4.0 ± 0.4 | 34.2 ± 15.7 | 11.0 ± 4.1 | 78.4 ± 16.8 |
Cells were incubated for 72 h with 10 ng of calcitriol/ml before stimulation with either endotoxin or PG. Data are expressed as the mean and SEM (n = 3).
Responsiveness of MM6-CA8 cells to various pyrogens.
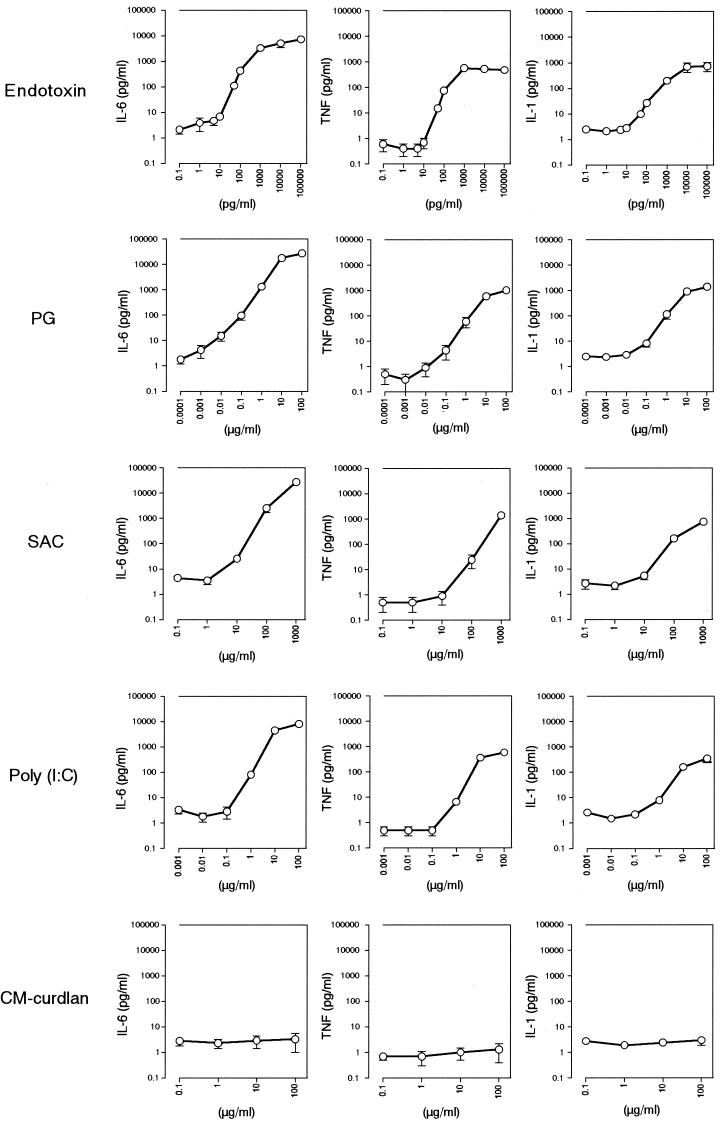
In order to evaluate MM6-CA8 cells for their usefulness and reliability as pyrogen indicator cells, we next examined the responsiveness of the cells to various kinds of pyrogens and checked how the activity of the pyrogens was assessed by the MM6-CA8 culture test system. The pyrogens examined were as follows: (i) endotoxin (from E. coli O55:B5), (ii) PG (from S. aureus), (iii) SAC, (iv) poly(I · C) (synthetic analogue of viral double-stranded RNA), and (v) CM-curdlan (nonpyrogenic but LAL test positive; used as a negative control). As shown in Fig. 1, MM6-CA8 cells responded to all of the pyrogens except for CM-curdlan and produced IL-6, TNF-α, and IL-1 in a dose-dependent manner. Among these cytokines, IL-6 was produced most sensitively in response to traces of the pyrogens and detected in the largest quantities in the culture medium. However, the cytokine-inducing effects of the pyrogens differed considerably, and the differences were expected to reflect the differences in pyrogenic potencies.
FIG. 1.
Induction of release of proinflammatory cytokines from MM6-CA8 cells by various pyrogens. The cells were primed with 10 ng of calcitriol/ml. After incubation for 72 h at 37°C with calcitriol, the cells were plated in a 24-well plate at 106 cells/0.9 ml/well, and 0.1 ml of pyrogen at various concentrations was added to each well. After incubation for 17 h at 37°C, the release of IL-6, TNF-α, or IL-1 in culture supernatants was evaluated by an ELISA. The amounts of cytokines released from MM6-CA8 cells cultured in a medium containing 0 μg of pyrogen/ml were as follows: IL-6, 2.1 ± 0.7 pg/ml; TNF-α, 0.6 ± 0.3 pg/ml; and IL-1, 2.5 ± 0.6 pg/ml. Data are expressed as the mean and SEM (n = 6 to 12).
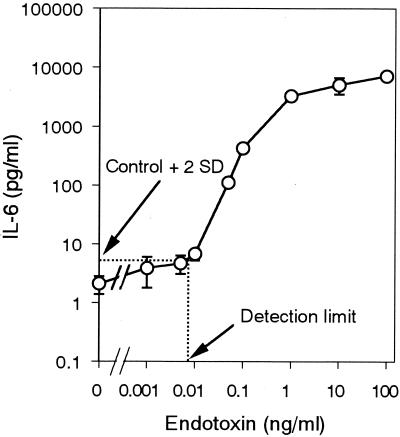
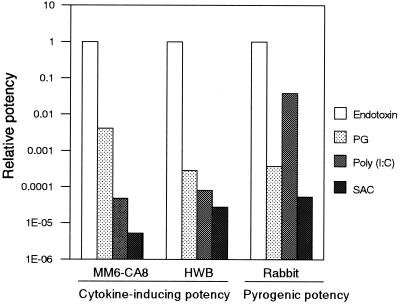
To estimate the cytokine-inducing potency of each pyrogen, we determined the minimum concentration of each pyrogen that induced the production of a significant amount of cytokine (greater than 2 standard deviations above the value obtained with 0 ng of pyrogen/ml) (Fig. 2). The obtained value was equal to the detection limit for the pyrogen in the MM6-CA8 culture test system. The results are summarized in Table 2. Among the pyrogens examined, endotoxin was the most potent and induced the production of IL-6 at a concentration of as low as 9.6 pg/ml. This concentration was almost equivalent to the detection limit of the LAL assay (5 to 50 pg/ml), recognized as the most sensitive assay for endotoxin. With regard to cytokine-inducing potency, the pyrogens were ranked as follows with the MM6-CA8 culture test system: endotoxin > PG > poly(I · C) > SAC (see Fig. 7). Endotoxin was approximately 10,000 times as potent as PG; thus, the cytokine-inducing effect varied widely depending on the pyrogen.
FIG. 2.
Determination of the detection limit for endotoxin in the MM6-CA8 culture test system. The cells were incubated for 17 h at 37°C with endotoxin after being primed for 72 h with 10 ng/ml of calcitriol. The release of IL-6 in the culture supernatants was evaluated by an ELISA. The detection limit for endotoxin was defined as the minimum concentration inducing a significant amount of cytokine production (greater than 2 standard deviations above that obtained with 0 ng/ml). The arrow indicates the detection limit for IL-6. Data are expressed as the mean and SEM (n = 6).
TABLE 2.
Detection limits and relative potencies of various pyrogens in the MM6-CA8 culture test system
| Pyrogen | Detection limita (relative potencyb) for the following cytokine:
|
||
|---|---|---|---|
| IL-6 | TNF-α | IL-1 | |
| Endotoxin | 0.00956 (1) | 0.0160 (1) | 0.0199 (1) |
| PG | 2.34 (0.0041) | 30.0 (0.00053) | 61.0 (0.00033) |
| SAC | 1,790 (0.0000053) | 14,400 (0.0000011) | 11,400 (0.0000017) |
| Poly(I · C) | 200 (0.000048) | 266 (0.00006) | 727 (0.000027) |
The detection limit was defined as the minimum concentration of each pyrogen inducing a significant amount of cytokine production (greater than 2 standard deviations above that obtained with 0 ng of pyrogen/ml). Values are reported in nanograms per milliliter.
The cytokine-inducing potency of each pyrogen is expressed relative to that of endotoxin.
FIG. 7.
Relative potencies of pyrogens determined by the MM6-CA8 culture system, the human whole blood (HWB) culture system, and the rabbit pyrogen test. The potency of each pyrogen is expressed relative to that of endotoxin.
Comparison with the human whole blood culture test system.
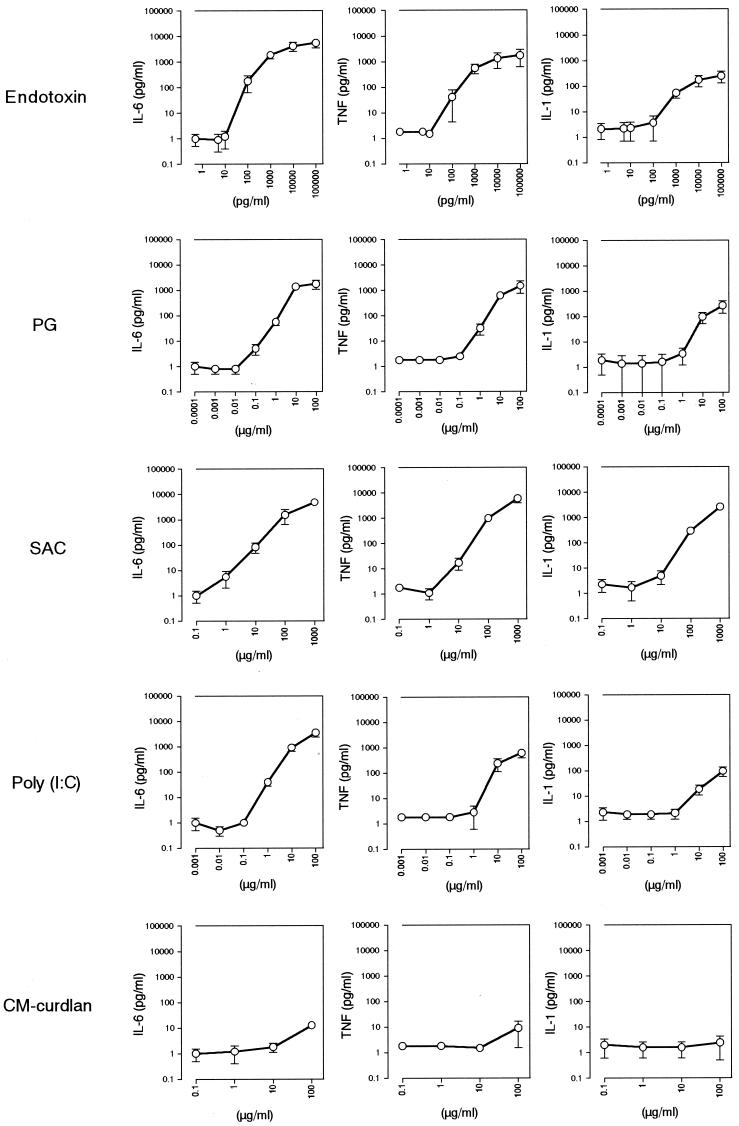
Since peripheral blood monocytes are the major secretory cells for proinflammatory cytokines, it was expected that the pyrogen-induced cytokine production seen in the human body could be reproduced in vitro with the MM6-CA8 culture system. However, we could not exclude the possibility that MM6-CA8 cells have mutations causing alterations in the responsiveness to pyrogens. Furthermore, it is also possible that the single culture of monocytic cells could not completely reproduce reactions in the body to pyrogens, since interactions between different kinds of blood cells, e.g., monocytes and lymphocytes, and some stimulatory and inhibitory serum factors seem to be involved in pyrogen-induced cytokine production in the circulating blood. To investigate these possibilities, we examined the responsiveness (cytokine production) of human whole blood to various pyrogens and compared it with that of MM6-CA8 cells.
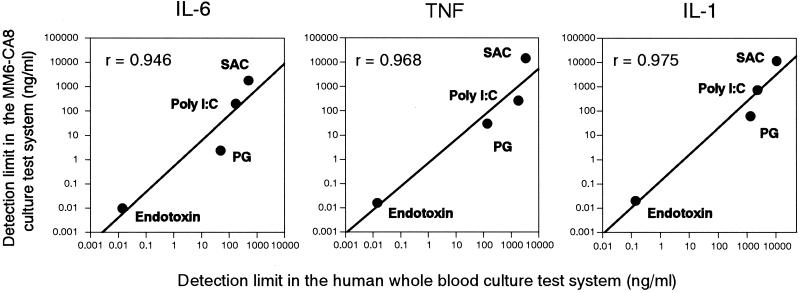
As shown in Fig. 3, the responsiveness of human blood cells to pyrogens was very similar to that of MM6-CA8 cells (Fig. 1). Human blood cells responded to all of the pyrogens examined except for CM-curdlan and produced IL-6, TNF α, and IL-1; among these, IL-6 was produced most sensitively and in the largest quantity in response to each pyrogen. Table 3 shows the cytokine-inducing potency (shown as the detection limit) of each pyrogen estimated with the human whole blood culture test system. The data in Table 3 are comparable to those obtained with the MM6-CA8 culture test system (Table 2); according to both culture systems, the pyrogen ranking for cytokine inducibility was in the order endotoxin > PG > poly(I · C) > SAC (see Fig. 7). Furthermore, as shown in Fig. 4, adequate correlations were confirmed between the pyrogen detection limits in the MM6-CA8 culture system and those in the human whole blood culture system for every cytokine. These results indicate that the responsiveness of MM6-CA8 cells and human whole blood to various kinds of pyrogens is almost the same and suggest that the MM6-CA8 test system can reproduce the pyrogen-induced cytokine production seen in the human body.
FIG. 3.
Induction of the release of proinflammatory cytokines from human whole blood cells by various pyrogens. Freshly drawn heparinized human peripheral blood (225 μl) was incubated for 4 h at 37°C with 25 μl of saline containing various concentrations of pyrogens. After incubation, the blood samples were diluted with 750 μl of saline and centrifuged for 5 min at 15,000 × g. The release of IL-6, TNF-α, or IL-1 in the supernatants was evaluated by an ELISA. The amounts of cytokines released from human whole blood cells cultured in blood containing 0 μg of pyrogen/ml were as follows: IL-6, 1.0 ± 0.5 pg/ml; TNF-α, 1.8 ± 0.2 pg/ml; and IL-1, 2.1 ± 1.3 pg/ml. Data are expressed as the mean and SEM for three donors.
TABLE 3.
Detection limits and relative potencies of various pyrogens in the human whole blood culture test system
| Pyrogen | Detection limita (relative potencyb) for the following cytokine:
|
||
|---|---|---|---|
| IL-6 | TNF-α | IL-1 | |
| Endotoxin | 0.0140 (1) | 0.0143 (1) | 0.142 (1) |
| PG | 50.0 (0.00028) | 140 (0.00010) | 1,350 (0.00011) |
| SAC | 497 (0.000028) | 3,420 (0.0000042) | 10,600 (0.000013) |
| Poly(I · C) | 173 (0.000081) | 1,830 (0.0000078) | 2,350 (0.000063) |
The detection limit was defined as the minimum concentration of each pyrogen inducing a significant amount of cytokine production (greater than 2 standard deviations above that obtained with 0 ng of pyrogen/ml). Values are reported in nanograms per milliliter.
The cytokine-inducing potency of each pyrogen is expressed relative to that of endotoxin.
FIG. 4.
Correlation between the detection limit in the MM6-CA8 culture test system and that in the human whole blood culture test system. The detection limit for each pyrogen was defined as the minimum concentration of that pyrogen inducing a significant amount of cytokine production (greater than 2 standard deviations above that obtained with 0 ng of pyrogen/ml). The correlation is expressed for every cytokine; r, linear correlation coefficient.
Comparison with the rabbit pyrogen test.
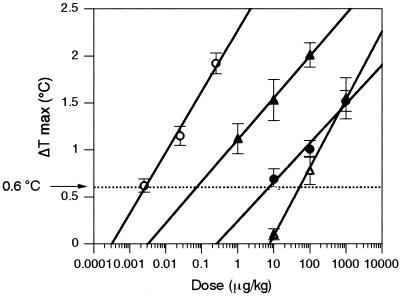
Since fever of microbial or immune origin is mediated by circulating proinflammatory cytokines produced by peripheral blood monocytes, the cytokine-inducing potency of a pyrogen should be correlated with its pyrogenic potency. To verify this point, we examined pyrogenic potency by using the rabbit pyrogen test. As shown in Fig. 5, all of the pyrogens except for CM-curdlan evoked fever dose dependently in rabbits. To determine the pyrogenic potency, we obtained the minimum pyrogenic dose (MPD), i.e., the minimum dose needed to cause a rise in body temperature of ≥0.6°C, from the dose-response curve for each pyrogen (Fig. 6 and Table 4). Based on the MPD, the pyrogens were ranked for potency in the order endotoxin > poly(I · C) > PG > SAC (Fig. 7). When we compared this ranking with that obtained with the culture test systems, we found that the pyrogenic potency of poly(I · C) was estimated to be higher than its cytokine-inducing potency (Fig. 7). The pyrogenic potency of poly(I · C) relative to that of endotoxin was 0.038 (Table 4), while its IL-6-inducing potencies relative to those of endotoxin were 0.00005 (MM6-CA8) (Table 2) and 0.00008 (human whole blood) (Table 3). On the other hand, the pyrogenic potencies of PG and SAC relative to that of endotoxin almost agreed with their cytokine-inducing potencies relative to that of endotoxin (Fig. 7).
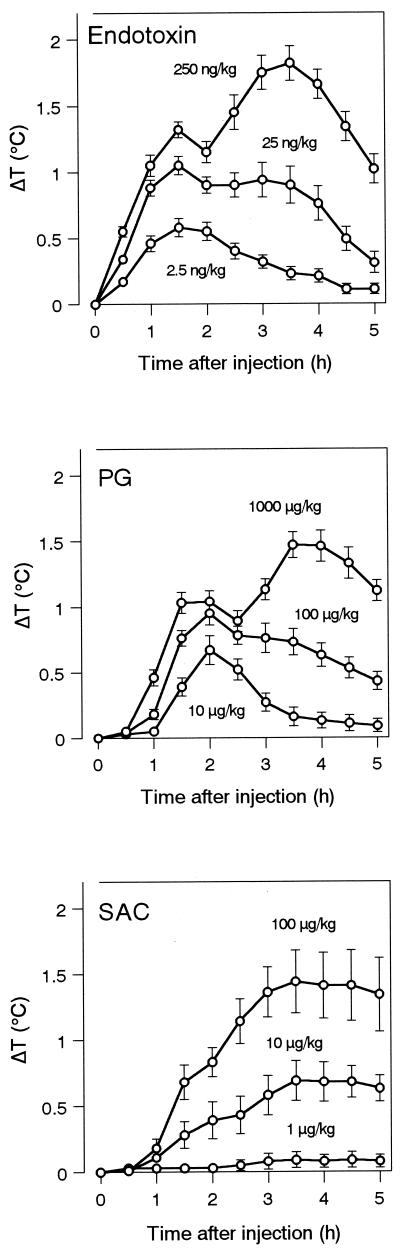
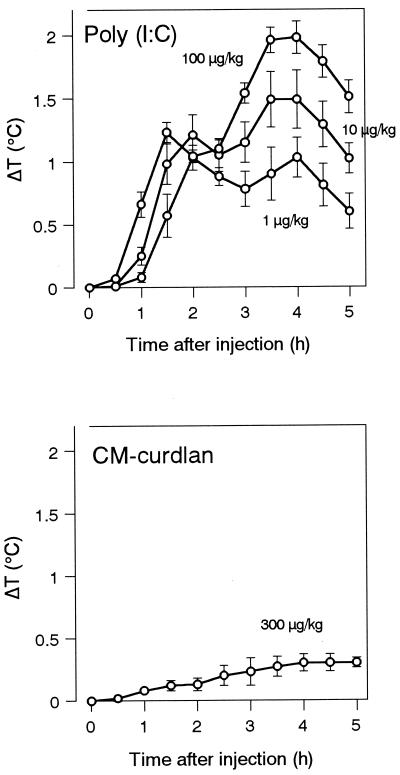
FIG. 5.
Pyrogenicities of various pyrogens in rabbits. Rabbits were injected intravenously with the indicated doses of pyrogens. Rectal temperatures were measured for 5 h after injection. ΔT, change in temperature. Data are expressed as the mean and SEM (n = 6 to 12).
FIG. 6.
Determination of the MPD of each pyrogen in the rabbit pyrogen test. ΔT max, maximum change in body temperature after pyrogen injection. Data are expressed as the mean and SEM (n = 6 to 12). Symbols: ○, endotoxin; •, PG; ▵, SAC; ▴, poly(I · C).
TABLE 4.
MPDs and relative pyrogenic potencies of various pyrogens
| Pyrogen | MPDa (μg/kg) | Relative potencyb |
|---|---|---|
| Endotoxin | 0.0027 | 1 |
| PG | 7.3 | 0.00037 |
| SAC | 51 | 0.000053 |
| Poly(I · C) | 0.072 | 0.038 |
The MPD was defined as the minimum dose needed to cause a rise in body temperature of ≥0.6°C, as determined from the dose-response curve for each pyrogen (Fig. 2).
The pyrogenic potency is expressed relative to that of endotoxin.
DISCUSSION
Several investigators have proposed the use of cell lines of the human monocyte/macrophage lineage as test systems for detecting pyrogenic contamination in pharmaceutical products (10, 21, 25, 38). Although cell culture-based test systems have an advantage over the established LAL test in that they can detect pyrogens other than endotoxin, most of these investigations have focused on endotoxin as the pyrogen to be detected. Furthermore, such studies have not explicitly addressed whether the responses of the cell lines to pyrogens are relevant to the responses of normal human monocytes/macrophages and, more importantly, to the pyrogenic response in humans. With these issues in mind, here we evaluated the reliability of a cell culture-based pyrogen test system by comparing it with the human whole blood culture test system and with the rabbit pyrogen test.
As pyrogen indicator cells, we selected the MM6 cell line, established from the peripheral blood of a patient with monoblastic leukemia, because of its high sensitivity to endotoxin compared with those of the human monocytic cell linesTHP-1, U937, and HL-60. We further subcloned MM6 and obtained MM6-CA8, which shows superior responsiveness to endotoxin and also to PG. MM6 appears to be the only cell line to constitutively express the phenotypic and functional features of mature monocytes (42). The advantages of using MM6 as an endotoxin indicator have already been reported. Taktak et al. (38) showed that MM6 cells respond to lower doses of endotoxin with a steeper dose-response curve than THP-1 cells and that the sensitivity to endotoxin of MM6 cells (2.5 pg/ml) is similar to that of freshly prepared human monocytes and even to that in the LAL test. More recently, Moesby et al. (21) reported a detection limit of 3.1 pg/ml with MM6 cells. In another study, Eperon and Jungi (10) obtained a detection limit of 15 pg/ml with calcitriol-primed MM6 subclones, a value comparable to that for MM6-CA8 in this study (9.6 pg/ml). For comparison, the sensitivity of the rabbit pyrogen test is approximately 1 ng/ml (27). Eperon et al. (9) proved that MM6 cells are sensitive to lipopolysaccharide (endotoxin) of various origins. Furthermore, several studies have shown that the MM6 cell assay correlates well with the LAL test but is superior when samples containing high concentrations of protein are used (9, 25, 28, 38). Thus, studies investigating the suitability of the MM6 cell assay seem to have provided evidence for its superiority over the LAL test and the rabbit pyrogen test when endotoxin is the pyrogen to be detected. However, the responsiveness of MM6 cells to pyrogens other than endotoxin or the relevance of their response to the human pyrogenic response has not been evaluated precisely.
To investigate how the response of MM6-CA8 cells is relevant to the human pyrogenic response, we compared the responsiveness of MM6-CA8 cells with that of cultured human whole blood. The human whole blood culture system has been proposed as a superior system for reproducing the natural environment found in the human body, since the culture includes circulating stimulatory and inhibitory mediators, e.g., cytokines and chemokines, and natural cell-to-cell contacts, e.g., monocytes to lymphocytes (6). Thus, the human whole blood culture system is able to reproduce pyrogen-induced in vivo production of proinflammatory cytokines ex vivo. Our data showed that the responsiveness of MM6-CA8 cells to endotoxin, PG, SAC, poly(I · C), and CM-curdlan was very similar to that of cultured human whole blood. Both cultures responded to all of the pyrogens examined except for CM-curdlan and produced IL-6, TNF-α, and IL-1. The rankings of pyrogens for cytokine inducibility estimated by the two culture test systems matched exactly. Furthermore, good correlations were confirmed between the pyrogen detection limits in the MM6-CA8 culture test system and the human whole blood culture test system. These findings suggest that MM6-CA8 cells are almost equivalent to normal human monocytes with regard to their responsiveness to pyrogens and, further, that the MM6-CA8 culture test system can reproduce the pyrogen-induced proinflammatory cytokine production seen in the human body. Thus, the MM6-CA8 culture system can be expected to estimate accurately the pyrogenicity not only of endotoxin but also of other pyrogens in humans.
Since proinflammatory cytokines produced by peripheral blood monocytes mediate the fever response as endogenous pyrogens (7, 18, 24), the cytokine-inducing activity of a pyrogen should be correlated with its pyrogenic potency. On the other hand, it is generally accepted that rabbits and humans are almost equal in their sensitivities to endotoxin (12, 40). We further examined whether the cytokine-inducing activity of the pyrogens assessed with the MM6-CA8 culture system was correlated with their pyrogenic potency assessed by the rabbit pyrogen test. We found that the relative potencies of the pyrogens estimated with the two different test systems were almost the same; the exception was poly(I · C), whose pyrogenic activity was estimated to be considerably higher than its cytokine-inducing activity. One possible explanation for this result is that the sensitivity of humans to poly(I · C) differs from that of rabbits. In fact, the MPD of poly(I · C) in humans was reported to be 1 mg/kg after systematic administration (5), compared with 72 ng/kg in rabbits, suggesting that rabbits are about 10,000 times more sensitive to poly(I · C) than humans. Although verification is needed, the present results suggest that the pyrogenic responses of humans differ from those of rabbits, depending on the pyrogen, and that the MM6-CA8 culture system could be an effective test system for evaluating pyrogenicity in humans.
The data presented here should not only help to establish a new in vitro pyrogen test system but also to provide valuable information on the mode of action of pyrogens in humans. It is interesting that the amount of IL-6 induced by each pyrogen was larger than that of TNF-α or IL-1. Furthermore, IL-6 was induced by lower concentrations of each pyrogen than TNF-α or IL-1. Consistent with these findings, it has been reported that the amount of IL-6 released into peripheral blood is larger than that of TNF-α and IL-1 after the administration of endotoxin in humans (2, 18, 35). This report supports our view that the MM6-CA8 culture system can reproduce the pyrogen-induced proinflammatory cytokine production seen in the human body. Although it is recognized that circulating IL-6, TNF-α, and IL-1 mediate fever of microbial or immune origin, the relative importance of each cytokine as an endogenous pyrogen is not yet clear. Since cytokines can interact at the level of production and in their effects (23), their functional hierarchy is difficult to determine. In recent studies, the majority of evidence points to IL-6 as a likely candidate for a major circulating endogenous pyrogen. For instance, IL-6 is found in the circulation following injury and correlates significantly with an increase in body temperature (17, 29, 30). It has been suggested that TNF-α-induced or IL-1-induced fever depends on the release of IL-6 as a mediator downstream of TNF-α or IL-1 (3, 18, 36, 41). These recent findings, together with our data showing that IL-6 is produced more sensitively in response to traces of the various pyrogens than TNF-α or IL-1, indicate that IL-6 is the most suitable measuring index in the MM6-CA8 culture system for detecting and quantifying the activities of various pyrogens in humans.
This is the first study in which the activities of several kinds of pyrogens have been investigated systematically using in vitro and in vivo test systems. In the past several years, the incidence of septic shock caused by gram-positive bacteria has risen markedly (4, 34). Today, gram-positive bacteria account for almost half of the incidents of septicemia (4), and the physiological activities of gram-positive bacteria and their components have attracted the attention of many researchers. There is general agreement that gram-negative septic shock involves an excessive activation of host monocytes/macrophages by endotoxin, resulting in the overproduction of proinflammatory cytokines. It has been suggested that gram-positive septic shock similarly involves excessive activation of cells. However, the mechanisms involved are still poorly defined (14, 20). In the present study, we examined the activities of endotoxin from gram-negative E. coli, gram-positive SAC, and the major cell wall component PG, which may cause a variety of signs associated with gram-positive septic shock. In addition to the pyrogens derived from bacteria, we took note of pyrogenic substances associated with viruses. As a representative of such a substance, we examined the activity of poly(I · C), which is a synthetic double-stranded RNA commonly used in fever research as a model of virus infection (16, 19). Virus-associated double-stranded RNA is known to be a good cytokine inducer and to be associated with the majority of viral infections; it induces the production of proinflammatory cytokines and substantial amounts of alpha interferon (15). However, the direct action (such as fever) of these cytokines in the influenza virus syndrome is less clear. We further examined the activity of a synthetic compound, CM-curdlan, as a negative control. β-(1,3)-d-Glucans purified from fungi and yeasts have been shown to bind to receptors on the surface of monocytes/macrophages, and some of them stimulate a number of immune responses, such as cytokine release (1, 33). Nevertheless, no pyrogenicity has been reported for any kind of β-(1,3)-d-glucan preparation.
In summary, our findings demonstrate that the in vitro pyrogen test system with human monocytic MM6-CA8 cells and with IL-6 as a measuring index can detect various kinds of pyrogens with a high sensitivity and that responsiveness to the pyrogens is highly relevant for human pyrogenic reactions. Based on these results, we conclude that the in vitro pyrogen test system is useful and reliable for detecting and quantifying various pyrogens.
Acknowledgments
This work was supported by grants-in aid for scientific research from the Japan Health Science Foundation.
REFERENCES
- 1.Abel, G., and J. K. Czop. 1992. Stimulation of human monocyte beta-glucan receptors by glucan induces production of TNF-alpha and IL-1 beta. Int. J. Immunopharmacol. 14:1363-1373. [DOI] [PubMed] [Google Scholar]
- 2.Bruunsgaard, H., A. N. Pedersen, M. Schroll, P. Skinhøj, and B. K. Pedersen. 1999. Impaired production of proinflammatory cytokines in response to lipopolysaccharide (LPS) stimulation in elderly humans. Clin. Exp. Immunol. 118:235-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chai, Z., S. Gatti, C. Toniatti, and T. Bartfai. 1996. Interleukin (IL)-6 gene expression in the central nervous system is necessary for fever response to lipopolysaccharide or IL-1 beta: a study on IL-6-deficient mice. J. Exp. Med. 183:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, J., and E. Abraham. 1999. Microbiological findings and correlations with serum tumor necrosis factor-α in patients with severe sepsis and septic shock. J. Infect. Dis. 180:116-121. [DOI] [PubMed] [Google Scholar]
- 5.Cornell, J. C. J., K. A. Smith, G. G. Cornwell, I. I. I., G. P. Burke, and O. R. Mclntyre. 1976. Systemic effects of intravenous polyriboinsinic-polyribocytidylic acid in man. J. Natl. Cancer Inst. 57:1211-1216. [DOI] [PubMed] [Google Scholar]
- 6.De Groote, D., P. F. Zangerle, Y. Gevaert, M. F. Fassotte, Y. Beguin, F. Noizat-Pirenne, J. Pirenne, R. Gathy, M. Lopez, I. Dehart, D. Igot, M. Baudrihaye, D. Delacroix, and P. Franchimont. 1992. Direct stimulation of cytokines (IL-1β, TNFα, IL-6, IL-2, IFN-γ and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine 4:239-248. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello, C. A. 1999. Cytokines as endogenous pyrogens. J. Infect. Dis. 179(Suppl. 2):S294-S304. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello, C. A., J. V. O'Connor, G. Lopreste, and R. L. Swift. 1984. Human leukocytic pyrogen test for detection of pyrogenic material in growth hormone produced by recombinant Escherichia coli. J. Clin. Microbiol. 20:323-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eperon, S., D. D. Groote, G. Werner-Felmayer, and T. W. Jungi. 1997. Human monocytoid cell lines as indicators of endotoxin: comparison with rabbit pyrogen and Limulus amoebocyte lysate assay. J. Immunol. Methods 207:135-145. [DOI] [PubMed] [Google Scholar]
- 10.Eperon, S., and T. W. Jungi. 1996. The use of human monocytoid lines as indicators of endotoxin. J. Immunol. Methods 194:121-129. [DOI] [PubMed] [Google Scholar]
- 11.Fennrich, S., M. Fischer, T. Hartung, P. Lexa, T. Montag-Lessing, H.-G. Sonntag, M. Weigandt, and A. Wendel. 1999. Detection of endotoxins and other pyrogens using human whole blood. Dev. Biol. Stand. 101:131-139. [PubMed] [Google Scholar]
- 12.Greisman, S. E., and R. B. Hornick. 1969. Comparative pyrogenic reactivity of rabbit and man to bacterial endotoxin. Proc. Soc. Exp. Biol. Med. 131:1154-1158. [DOI] [PubMed] [Google Scholar]
- 13.Hansen, E. W., and J. D. Christensen. 1990. Comparison of cultured human mononuclear cells, limulus amebocyte lysate and rabbits in the detection of pyrogen. J. Clin. Pharm. Ther. 15:425-433. [DOI] [PubMed] [Google Scholar]
- 14.Heumann, D., C. Barras, A. Severin, M. P. Glauser, and A. Tomasz. 1994. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect. Immun. 62:2715-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura, M., L. A. Toth, H. Agostini, A. B. Candy, J. A. Majde, and J. M. Krueger. 1994. Comparison of acute phase responses induced in rabbits by lipopolysaccharide and double-stranded RNA. Am. J. Physiol. 267:R1596-R1605. [DOI] [PubMed] [Google Scholar]
- 16.Kimura-Takeuchi, M., J. A. Majde, L. A. Toth, and J. M. Kuruegar. 1992. The role of double-stranded RNA in induction of the acute-phase response in an abortive influenza virus infection model. J. Infect. Dis. 166:1266-1275. [DOI] [PubMed] [Google Scholar]
- 17.Kluger, M. J. 1991. Fever: role of pyrogens and cryogens. Physiol. Rev. 71:93-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luheshi, G., and N. Rothwell. 1996. Cytokines and fever. Int. Arch. Allergy Immunol. 109:301-307. [DOI] [PubMed] [Google Scholar]
- 19.Majde, J. A. 2000. Viral double-stranded RNA, cytokines and the flu. J. Interferon Cytokine Res. 20:259-272. [DOI] [PubMed] [Google Scholar]
- 20.Mattsson, E., L. Verhage, J. Rollof, A. Fleer, J. Verhoef, and H. van Dijk. 1993. Peptidoglycan and teicholic acid from Staphylococcus epidermidis stimulate human monocytes to release tumor necrosis factor-α, interleukin-β and interleukin-6. FEMS Immunol. Med. Microbiol. 7:281-287. [DOI] [PubMed] [Google Scholar]
- 21.Moesby, L., S. Jensen, E. W. Hansen, and J. D. Christensen. 1999. A comparative study of Mono Mac 6 cells, isolated mononuclear cells and Limulus amoebocyte lysate assay in pyrogen testing. Int. J. Pharm. 191:141-149. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura, Y., T. Murai, and Y. Ogawa. 1996. Effect of in vitro and in vivo administration of dexamethasone on rat macrophage functions: comparison between alveolar and peritoneal macrophages. Eur. Respir. J. 9:301-306. [DOI] [PubMed] [Google Scholar]
- 23.Neta, R., T. J. Sayers, and J. J. Oppenheim. 1992. Relationship of TNF to interleukins. Immunol. Ser. 56:499-566. [PubMed] [Google Scholar]
- 24.Netea, M. G., B. J. Kullberg, and J. W. M. Van der Meer. 1999. Do only circulating pyrogenic cytokines act as mediators in the febrile response? A hypothesis. Eur. J. Clin. Investig. 29:351-356. [DOI] [PubMed] [Google Scholar]
- 25.Peterbauer, A., S. Eperon, T. W. Jungi, E. R. Werner, and G. Werner-Felmayer. 2000. Interferon-γ-primed monocytoid cell lines: optimizing their use for in vitro detection of bacterial pyrogens. J. Immunol. Methods 233:67-76. [DOI] [PubMed] [Google Scholar]
- 26.Pool, E. J., G. Johaar, S. James, I. Petersen, and P. Bouic. 1998. The detection of pyrogens in blood products using an ex vivo whole blood culture assay. J. Immunoassay 19:95-111. [DOI] [PubMed] [Google Scholar]
- 27.Poole, S., and M. V. Mussett. 1989. The international standard for endotoxin: evaluation in an international collaborative study. J. Biol. Stand. 17:161-171. [DOI] [PubMed] [Google Scholar]
- 28.Poole, S., R. Thorpe, A. Meager, and A. J. H. Gearing. 1988. Assay of pyrogenic contamination in pharmaceuticals by cytokine release from monocytes. Dev. Biol. Stand. 69:121-123. [PubMed] [Google Scholar]
- 29.Rothwell, N. J. 1990. Mechanism of the pyrogenic actions of cytokines. Eur. Cytokine Netw. 1:211-213. [PubMed] [Google Scholar]
- 30.Rothwell, N. J., and S. J. Hopkins. 1995. Cytokines and nervous system. II. Actions and mechanisms of action. Trends Neurosci. 18:130-136. [DOI] [PubMed] [Google Scholar]
- 31.Saper, C. B., and C. D. Breder. 1994. The neurologic basis of fever. N. Engl. J. Med. 330:1880-1886. [DOI] [PubMed] [Google Scholar]
- 32.Schleifer, K. H., and O. Kandler. 1972. Peptidoglycan types of bacterial cell wall and their taxonomic implications. Bacteriol. Rev. 36:407-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soltys, J., and M. T. Quinn. 1999. Modulation of endotoxin- and enterotoxin-induced cytokine release by in vivo treatment with β-(1,6)-branched β-(1,3)-glucan. Infect. Immun. 37:244-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sriskandan, S., and J. Cohen. 1999. Gram-positive sepsis; mechanisms and differences from gram-negative sepsis. Infect. Dis. Clin. North Am. 13:397-412. [DOI] [PubMed] [Google Scholar]
- 35.Suffredini, A. F., H. D. Hochstein, and F. G. McMahon. 1999. Dose-related inflammatory effects of intravenous endotoxin in humans: evaluation of a new clinical lot of Escherichia coli O:113 endotoxin. J. Infect. Dis. 179:1278-1282. [DOI] [PubMed] [Google Scholar]
- 36.Sundgren-Andersson, A. K., P. Östlund, and T. Bartfai. 1998. IL-6 is essential in TNF-α-induced fever. Am. J. Physiol. 275:R2028-R2034. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 38.Taktak, Y. S., S. Selkirk, A. F. Bistow, A. Carpenter, C. Ball, B. Rafferty, and S. Poole. 1991. Assay of pyrogens by interleukin-6 release from monocytic cell line. J. Pharm. Pharmacol. 43:578.. [DOI] [PubMed] [Google Scholar]
- 39.Van Deventer, S. J. H., H. R. Büller, J. W. ten Cate, L. A. Aarden, C. E. Hack, and A. Sturk. 1990. Experimental endotoxin in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood 76:2520-2526. [PubMed] [Google Scholar]
- 40.Wolff, S. M. 1973. Biological effects of bacterial endotoxins in man. J. Infect. Dis. 128(Suppl.):259.. [DOI] [PubMed] [Google Scholar]
- 41.Zetterstoröm, M., A. K. Sundgren-Andersson, P. Östlund, and T. Bartfai. 1998. Delineation of the proinflammatory cytokine cascade in fever induction. Ann. N. Y. Acad. Sci. 856:48-52. [DOI] [PubMed] [Google Scholar]
- 42.Ziegler-Heitbrock, H. W. L., E. Thiel, A. Fütterer, V. Herzog, A. Wirtz, and G. Riethmmüler. 1988. Establishment of a human cell line (MONO MAC 6) with characteristics of mature monocytes. Int. J. Cancer 41:456-461. [DOI] [PubMed] [Google Scholar]