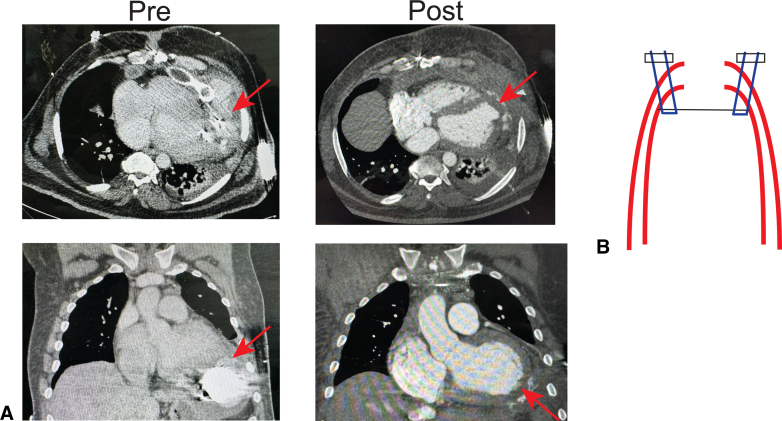
Left ventricular apical pseudoaneurysm with contrast extravasation.
Central Message.
We report a case of infection-related left ventricular pseudoaneurysm and its successful operative treatment, with extirpation of the entire left ventricular assist device system.
Durable mechanical circulatory support (MCS) via continuous-flow (CF) left ventricular (LV) assist device (LVAD) implantation is widely applied to treat end-stage heart failure (HF). However, important drawbacks and limitations exist. Hemocompatibility- and infection-related complications remain, which are (1) contributors to long-term mortality and (2) reasons to urgently undertake heart transplantation (HT).1 Yet, although HT is the “gold standard” therapy for end-stage HF, it is not without drawbacks/limitations. Consequently, increased attention has been focused upon achieving left ventricular (LV) functional recovery with device explantation. This case report highlights the morbidity of infectious LVAD complications, with a satisfactory outcome achieved via LVAD explantation. This is a single patient-based case report. The patient provided informed consent for publication; institutional review board approval was not required.
Case Report
A 40-year-old man with end-stage HF who was not a HT candidate as the result of morbid obesity and medical therapy compliance underwent HeartMate 3 CF LVAD implantation at an outside hospital. He did well, and with adjunctive medical therapy, achieved LV functional recovery sufficient to provide completely circulatory support, albeit with moderate LV systolic dysfunction (left ventricular ejection fraction [LVEF] ∼30%-40%). LVAD “decommission” was performed ∼4 years post-LVAD implantation: the outflow graft was ligated, and the driveline was transected subcutaneously. Although he did well initially, ∼11 months post-LVAD decommissioning, infection with Corynebacterium striatum of the retained driveline developed. This became refractory to debridement, inclusive of bacteremia.
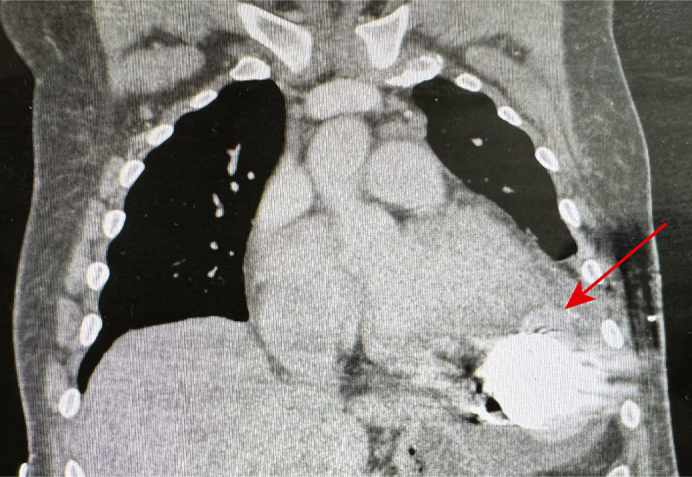
The patient was transferred for consideration of HT, but he was deemed unsuitable because of ongoing medical compliance concerns and morbid obesity. Imaging incidentally demonstrated dehiscence of the LV apical sewing ring from the LV apex and resultant pseudoaneurysm, with contrast extravasation and a left hemothorax (Figure 1, A). Despite this, the patient was asymptomatic, with satisfactory invasive hemodynamic data. Transthoracic echocardiography on separate occasions revealed LVEF varying between 30% and 40%.
Figure 1.
A, Computed tomography scan demonstrating left ventricular assist device inflow dehiscence with pseudoaneurysm (Pre) and after repair (Post). B, Schematic illustration of left ventricular apical patch underlay repair. The arrows denote the site of dehiscence for A, and the site of repair for B.
With a nonfunctioning LVAD serving only as a nidus of infection, a decision was made to urgently extirpate the entire LVAD system, because this would be the only means by which eradication of infection could be ensured. The patient was taken to the operating room. After induction of general anesthesia with a dual-lumen endotracheal tube and standard monitoring inclusive of transesophageal echocardiography (LVEF ∼40% under general anesthesia), the right axillary artery was cannulated using a sidegraft (modifying an aortic arch graft such that an intra-aortic balloon pump/endovascular LVAD could be placed through one graft limb and through the right axillary artery while on right axillary arterial outflow-based cardiopulmonary bypass), and the right common femoral vein was percutaneously cannulated. A reoperative sternotomy was performed, with lysis of adhesions and dissection of the outflow graft.
The patient was anticoagulated with heparin, and cardiopulmonary bypass was initiated, with maintenance of systemic normothermia and right lung ventilation. The ascending thoracic aorta was clamped using a partial occlusion clamp, encompassing the site of the outflow graft anastomosis. The anastomotic suture line was excised, detaching the graft from the ascending thoracic aorta. The resultant ascending thoracic aortotomy was closed with a bovine pericardial patch using polypropylene suture; a primary simple continuous suture line was created, subsequently empirically reinforced circumferentially using pledgeted horizontal mattress sutures. Next, a left anterolateral thoracotomy was created; even on cardiopulmonary bypass, adequate visualization of the LV apex was not feasible through the sternotomy alone. There was an organized hematoma around the left lung, adherent to the LVAD; this was completely removed. A half-circumference dehiscence of the sewing ring from the LV was identified. This infectious left ventriculotomy was extended circumferentially along with a <5-mm rim of LV wall encompassing the inflow anastomotic suture line, thus removing the entire LVAD system as a single specimen. The LVAD clearly had been implanted using a “core-then-sew” technique, with an appreciable thickness of LV wall between an interrupted pledgeted suture line and the sewing ring; dense adhesions in this area were observed. The LV apical ventriculotomy was closed using a bovine pericardial patch. An oversized patch was used (2 cm greater diameter than the ventriculotomy), with an underlay technique (using interrupted pledgeted horizontal mattress polypropylene sutures) being employed, such that LV intracavitary pressure would seal the patch against the LV endocardial surface (Figure 1, B). This was similarly reinforced empirically. Topical hemostatic agents were applied to both patch repair sites. The patient was transitioned from cardiopulmonary bypass to venoarterial extracorporeal membrane oxygenation (V-A ECMO) using the established cannulation strategy in a planned fashion as the result of antecedent moderate LV systolic dysfunction plus the requirement of small volume LV apical resection, in the presence of chronic renal insufficiency (such that high-dose inotropic/vasoconstrictor support could be avoided with V-A ECMO use). Native LV systolic function was sufficient to maintain robust aortic valve opening on only modest inotropic support, and additional direct left-sided MCS through the second arm of the right axillary arterial sidegraft was not required. The chest was kept open as the result of coagulopathy and closed on postoperative day 2.
The patient was decannulated from V-A ECMO on postoperative day 3. He was liberated from mechanical ventilation on postoperative day 5 and subsequently weaned from inotropic support by postoperative day 12. Intraoperative cultures from tissues surrounding the LVAD pump mechanism demonstrated Staphylococcus epidermidis and C striatum. The patient was discharged on intravenous antibiotics to a rehabilitation facility on guideline-directed medical therapy, on room air, without having sustained perioperative renal failure. He eventually went home; antibiotics were discontinued ∼2 months postoperation. He is currently alive and doing well at home, ∼8 months postoperation. In the setting of upwardly titrated guideline-directed medical therapy, LVEF is ∼25%.
Discussion
End-stage HF treatment requires either HT or durable MCS. Although HT continues to have superior long-term survival to CF LVAD implantation, MCS permits possible cardiac recovery and MCS discontinuation. However, radial-type (“centrifugal”) LVADs, such as the HeartMate 3, are ill-suited to explantation in comparison with the axial HeartMate II LVAD. Consequently, decommissioning has been employed.2 Yet, retention even of a small driveline remnant poses infectious risks, as illustrated in this case. Furthermore, whether patients ought to be maintained on anticoagulation is unknown. This case illustrates (1) the morbidity of ascending driveline infection with infectious LV pseudoaneurysm, (2) consequent drawbacks to LVAD decommissioning, albeit with the recognition of (3) poor suitability of the HeartMate 3 LVAD to explantation, with a more technically challenging operation required.
Decision-making in situations such as the one illustrated is complex. The vast majority of CF LVAD recipients do not experience meaningful LV recovery; for these patients, total system extirpation may be accomplished in the context of HT. However, HT lacks clear indications in the setting of substantial LV recovery. Although this case highlights the infectious risks of LVAD decommissioning, there may be thromboembolic risks associated with LVAD retention. Yet, these risks are long-term rather than short-term, and short-term risks disproportionately adversely impact mortality and morbidity. Conversely, total HeartMate 3 LVAD system extirpation (described for bridging to HT and bridging to reoperative LVAD implantation, respectively, in Shaw and colleagues3 and Mueller and colleagues4) should carry greater perioperative risks than decommissioning but lacks its long-term risks. Decision-making ought to be individualized. In patients for whom perioperative risks may be high as the result of previous operations, a greater anticipated likelihood of recurrent HF, or comorbid conditions inclusive of increased age, decommissioning may be more appropriate. In contrast, in patients with less-complex operative histories, lower anticipated likelihood of recurrent HF, and fewer/less severe comorbid conditions, total system explantation may be more appropriate.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Maitra N.S., Dugger S.J., Balachandran I.C., Civitello A.B., Khazanie P., Rogers J.G. Impact of the 2018 UNOS heart transplant policy changes on patient outcomes. JACC Heart Fail. 2023;11(5):491–503. doi: 10.1016/j.jchf.2023.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Shannon S., Ghorpade N., Schaffer S.A. Decommission of a HeartMate 3 LVAD in a patient with left ventricular recovery. J Card Surg. 2022;37(12):5528–5530. doi: 10.1111/jocs.17155. [DOI] [PubMed] [Google Scholar]
- 3.Shaw T.B., Morton J., Deschner W.P., Mohammed A., Copeland H. Impella 5.0: an intermediate strategy for bridging a patient from infected durable LVAD to cardiac transplant. J Card Surg. 2022;37(3):685–687. doi: 10.1111/jocs.16186. [DOI] [PubMed] [Google Scholar]
- 4.Mueller M., Potapov E., Krabatsch T. Usefulness of a temporary endovascular left ventricular assist system as a bridge to facilitate treatment of mediastinitis associated with a permanent device. J Heart Lung Transplant. 2019;38(4):476–478. doi: 10.1016/j.healun.2018.10.001. [DOI] [PubMed] [Google Scholar]