Abstract
A total of 258 human sera positive for measles antibodies were divided into four different groups: group 1 contained 54 sera from children after natural measles infection (immunoglobulin M [IgM] positive, early infection phase), group 2 contained 28 sera from children after measles vaccination (IgM positive, early infection phase), group 3 contained 100 sera from healthy adults (natural long-lasting immunity), and group 4 contained 76 sera from healthy children (postvaccinal long-lasting immunity). In the early phase of infection, the percent distributions of measles virus-specific IgG isotypes were similar between natural and postvaccinal immune responses. IgG1 and IgG4 were the dominant isotypes, with mean levels of detection of 100% (natural infection) and 100% (postvaccinal) for IgG1 and 96% (natural infection) and 92% (postvaccinal) for IgG4. In comparison, the IgG4 geometric mean titer (GMT) in the early phase of natural infection was significantly higher than the IgG4 GMT detected in the postvaccinal immune response (80 versus 13; 95% confidence interval). In the memory phase, IgG2 and IgG3 responses decreased significantly in both natural infection and postvaccinal groups, while IgG1 levels were maintained. In contrast, the IgG4 postvaccinal immune response decreased strongly in the memory phase, whereas IgG4 natural long-lasting immunity remained unchanged (9 versus 86%; P < 0.05). The results obtained suggest that IgG4 isotype could be used in the early phase of infection as a quantitative marker and in long-lasting immunity as a qualitative marker to differentiate between natural and postvaccinal immune responses.
Measles has been targeted for global eradication by the World Health Organization's Expanded Programme of Immunization (4). For the effective control and eventual eradication of measles, it is necessary to impair measles transmission by establishing population immunity (5). In addition, laboratory and epidemiological studies should be conducted to address genetic and antigenic measles virus variability as well as measles virus-specific immune responses. Such studies should examine (i) genetic diversity between measles virus vaccine and wild-type strains to ensure that existing vaccines continue to provide a high degree of protection, (ii) the response to measles vaccine provided at different schedules of vaccination (ages and intervals), and (iii) serological markers at different stages of measles infection to globally understand antibody responses to the infection (12, 20, 21). Recently, a subclass-restricted response to antigens was demonstrated; however, limited data are available on measles virus-specific immunoglobulin G (IgG) subclass responses (11, 14, 16, 24). We have defined two highly different measles immune IgG isotypic response patterns which make it possible to differentiate convalescence phase and memory phase immune responses during natural measles infection. (13). The data reported support the hypothesis that the IgG isotypic immune response could be highly useful for the diagnosis and analysis of antibody responses to measles infection.
IgM antibody detection currently is effectively used to diagnose a primary measles infection. In addition, the detection of total measles virus antibodies is an indicator of long-lasting immunity. These serological markers do not differ between natural and postvaccinal responses. The present study was undertaken to compare the specific anti-measles IgG1, IgG2, IgG3, and IgG4 subclass response patterns elicited during natural and postvaccinal responses.
MATERIALS AND METHODS
Serum specimens.
A total of 258 human serum samples positive for measles virus antibodies were used in this study. Serum specimens were classified into four groups according to the source of infection (natural measles infection and vaccination) and the phase of infection (recent or long-lasting immune response).
(i) Group 1.
Group 1 consisted of 54 individuals (2 months to 44 years old; median age, 17 years old) from whom a single serum sample was obtained. The samples were obtained after natural measles infection during a measles virus outbreak in Argentina in 1998. All the samples showed the presence of measles virus-specific IgM; 32 of these (group 1a) were acute-phase serum samples obtained within 1 week after the onset of rash (median, 3 days; range, 1 to 7 days), and 22 (group 1b) were convalescent-phase serum samples obtained between days 8 and 26 after the onset of rash (median, 17 days).
(ii) Group 2.
Group 2 consisted of 28 serum samples selected during a prospective study of adverse reactions to measles vaccine conducted during measles interepidemic periods in Argentina. These samples were obtained from 28 previously unvaccinated children (8 to 24 months old; mean age, 13 months old) who received the combined measles-mumps-rubella viral vaccine (MMR) or monovalent measles vaccine according to the vaccine available at the time and who had an adverse reaction to the vaccine (mean fever, 37.6°C; mild rash occurring 7 to 18 days after measles vaccination). None of these children had an exanthematous disease consistent with measles infection prior to the measles vaccination. The conditions mentioned above allowed us to confirm that the sera assayed were true postvaccination sera and to classify the samples obtained as acute- and convalescent-phase samples according to the day postrash on which they were collected. Fifteen out of 28 (group 2a) were postvaccinal acute-phase serum samples obtained within 1 week after the onset of rash (median, 3 days; range, 1 to 7 days), and 13 (group 2b) were convalescent-phase serum samples obtained between days 8 and 30 (median, 19 days). Measles infection was confirmed in group 1 and group 2 serum samples by the detection of measles virus-specific IgM antibodies by an immunofluorescence assay (IFA) as a screening method and subsequently confirmed by a capture enzyme immunoassay (method described below).
(iii) Group 3.
Group 3 consisted of 100 serum samples obtained from healthy adults (40 to 85 years old; median age, 63 years old) who reported a history of long-past natural measles infection at least 10 years earlier. The sera were collected at random from healthy patients undergoing routine physical examinations.
(iv) Group 4.
Group 4 consisted of 76 serum samples selected from a large serum collection from a measles vaccine efficacy study conducted in a northern area of Cordoba Province, Argentina (San Alberto, San Javier, and Pocho; Sanitary Region 2) in 1994. These samples were from vaccinated healthy children who had received measles vaccine 8 to 12 years earlier (children vaccinated at between 11 and 15 months of age). All these children had documented measles vaccination (combined viral vaccine [MMR] or monovalent measles vaccine, according to the vaccine available at the time), and none of them had an exanthematous disease consistent with measles infection between the time of measles vaccine application and the day on which the serum samples were obtained. When the serum samples were obtained, the children ranged in age from 9 to 14 years old (median age, 10.8 years old). Only one serum sample was obtained from each child, and serum samples were stored at −20°C until they were processed. Past measles infection was confirmed in group 3 and 4 serum samples by the detection of measles virus neutralizing antibody titers of ≥1:32 milli-international feference units (mIRU)/ml.
Antisera.
Mouse monoclonal antibodies to human IgG subclasses were obtained from Sigma Chemical Co., St. Louis, Mo. These antibodies were used at dilutions of 1:100 (IgG1), 1:32 (IgG2), 1:32 (IgG3), and 1:32 (IgG4) according to the manufacturer's instructions. Rabbit monoclonal antibodies to human IgM and total IgG were obtained from Cappel and used at dilutions of 1:100 and 1:150, respectively. The optimal dilutions of monoclonal antibodies were determined by titrations against reference positive sera diluted 1:20 (initial dilution of sera for the IFA).
Preparation of antigen slides.
A suspension of Vero cells (105 cells/ml) was seeded in a 25-cm2 bottle and infected with the Edmonston-Schwartz strain of measles virus at a multiplicity of infection of 0.1. Infected cells (50 μl) and uninfected cells (50 μl) were placed on each well slide. They were incubated for 48 h at 37°C in a humidified incubator with 5% CO2. The monolayers were subsequently washed twice with phosphate-buffered saline (PBS [pH 7.2]) and once with distilled water. The fixation of cells was done with acetone at 4°C for 10 min. Finally, the slides were stored at −20°C for later use.
IFA.
For the IFA, in brief, twofold dilutions of serum samples were incubated with fixed cells for 30 and 90 min for IgG and IgM antibody detection, respectively, at 37°C in a humidified chamber. The samples were then washed three times with PBS for 10 min per wash and incubated for 30 min at 37°C with fluorescein isothiocyanate-conjugated anti-human IgM, total IgG, and IgG subclasses. After two washes with PBS for 10 min per wash, the slides were mounted with glycerol buffer on coverslips and then examined under a fluorescence microscope at a ×40 objective magnification (20).
Fluorescence intensity may be considered semiquantitative on the basis of the guidelines established by the Centers for Disease Control and Prevention, Atlanta, Ga.: 4+, maximal fluorescence, brilliant yellow-green; 3+, less brilliant yellow-green fluorescence; 2+, definite but dull yellow-green fluorescence; and 1+, very dim and subdued fluorescence.
A serum dilution was considered positive for measles virus IgM and IgG antibodies if, in the presence of a fluorescence intensity of 1+ or more, there was well-defined staining of cytoplasmatic granules in cells coalescing to form multinucleated giant cells.
To define the IFA cutoff, a panel of true measles virus-negative IgG serum samples (reference technique, neutralization test) was assayed. Some negative serum samples at dilutions of <1:20 (1:5 and 1:10) showed nonpecific reactions. Therefore, a dilution of 1:20 was defined as the starting working serum dilution. A serum dilution was considered negative for measles virus IgM or IgG antibodies if the cells exhibited less than 1+ fluorescence and displayed the reddish orange counterstain or if the fluorescence observed was not the specific staining pattern for measles.
IgM antibody capture enzyme immunoassay.
The IgM antibody capture enzyme immunoassay for measles confirmation was carried out at the National Reference Laboratory (Santa Fe, Argentina) as described by Erdman et al. (7). Briefly, goat anti human IgM antibodies diluted in PBS were used to coat microtiter plates for 1 h at 37°C. After the plates were washed, serum diluted 1:100 in PBS was added to four consecutive wells, and the plates were incubated for 1 h at 37°C. After the plates were washed, either baculovirus-measles virus nucleoprotein or uninfected S9 cell control lysate diluted in PBS-GT (0.5% gelatin and 0.15% Tween 20) with 4% normal goat serum was added to duplicate wells. The plates were then incubated for 2 h at 37°C and washed, biotinylated antibody to measles virus was added to each specimen, and the plates were incubated for 1 h at 37°C. The plates were washed three times, streptavidin-peroxidase was added, and the plates were incubated for 20 min at 37°C. Then, a solution containing tetramethylbenzidine and hydrogen peroxide was added, and the mixture was incubated for 15 min at room temperature. Color development was stopped by the addition of 1 N sulfuric acid solution, and the absorbance was read at 450 nm. For each sample, we calculated the difference between the mean optical density for the antigen-positive wells and the mean optical density for the negative control wells (difference, P − N values).
Seroneutralization assay.
A seroneutralization assay was performed as described by Nates et al. (17). The highest dilution of serum that completely inhibited the cytopathic effect was regarded as the endpoint of antibody titration. Titers equal to or higher than 1:2 were considered positive. A measles virus control and an international reference preparation of measles virus-specific antibody were used in every 96-well microculture plate and diluted in the same manner as the test samples. Results were converted to mIRU per milliliter of serum based on parallel assay results. Titers of ≥32 mIRU/ml were defined as seropositive based on the test sensitivity at the first serum dilution of 1:2.
Data analysis.
Results are expressed with a 95% confidence interval (CI), and the chi-square distribution test was used to analyze data.
RESULTS
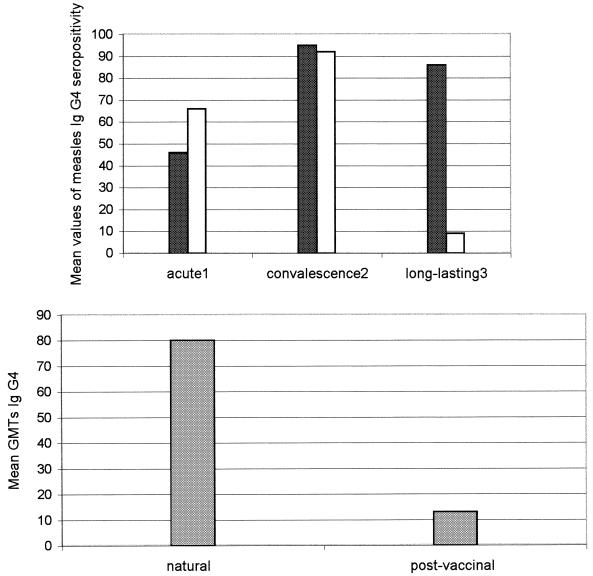
A total of 258 human serum samples were studied by IFA for the detection of IgG1, IgG2, IgG3, and IgG4 measles virus-specific antibodies. The means and standard deviations (95% CI) for the seropositive measles virus-specific antibody isotypes from groups 1, 2, 3, and 4 are shown in Table 1. The measles virus-specific IgG1 subclass was detected at the acute phase in both naturally infected and vaccinated individuals (93% group 1a and 100% group 2a, respectively) (P > 0.05) and remained unmodified in long-lasting immunity. Similary, no significant differences were found between IgG2 and IgG3 natural and postvaccinal immune responses (P > 0.05). Thus, the daily percentages of IgG2 and IgG3 seropositivity gradually increased, reaching means of 64% (group 1b) and 54% (group 2b) and of 72% (group 1b) and 70% (group 2b), respectively, in the convalescence phase. The percentages of IgG2 and IgG3 seropositivity dropped significantly in the memory phase: 2% (group 3) and 2% (group 4) for IgG2 and 3% (group 3) and 7% (group 4) for IgG3. No significant differences between natural and postvaccinal responses (P > 0.05) were observed in the percent distributions of the measles virus-specific IgG4 subclass in the acute and convalescence phases. In contrast, the IgG4 postvaccinal immune response dropped strongly in the memory phase, but IgG4 natural long-lasting immunity remained unchanged (9 versus 86%) (P < 0.05) (Fig. 1). Thus, the postvaccinal memory immune response was represented only by the IgG1 immune response. In addition, the IgG4 GMTs in natural measles infection were significantly higher than those detected in the postvaccinal response (80 versus 13) (P < 0.05) in both the acute and the convalescence phases (early phase of infection) (Fig. 1). In contrast, the IgG1 GMTs were high in both natural and postvaccinal immune responses (≥320) (P > 0.05).
TABLE 1.
Percent distributions of measles virus-specific IgG isotypes in the population studied
| Study group (source, phase of measles infection) | Time after onset of rash | Total no. of samples | Mean % (range) of isolates seropositive fora:
|
|||
|---|---|---|---|---|---|---|
| IgG1 | IgG2 | IgG3 | IgG4 | |||
| 1a (natural, acute) | 0-7 days | 32 | 94 (87-100) | 34 (27-41) | 44 (37-51) | 50 (40-54) |
| 2a (postvaccinal, acute) | 0-7 days | 15 | 100 (89-100) | 13 (2-24) | 40 (29-51) | 68 (56-78) |
| 1b (natural, convalescence) | 8-26 days | 22 | 100 (91-100) | 64 (55-73) | 72 (63-81) | 96 (87-100) |
| 2b (postvaccinal, convalescence) | 8-30 days | 13 | 100 (88-100) | 54 (42-66) | 70 (58-82) | 92 (80-100) |
| 3 (natural, long-lasting immunity) | 10-80 years | 100 | 100 (94-100) | 2 (0-8) | 3 (0-9) | 86 (80-92) |
| 4 (postvaccinal, long-lasting immunity) | 8-12 years | 76 | 100 (93-100) | 2 (0-9) | 7 (0-13) | 9 (2-16) |
95% CI.
FIG. 1.
Qualitative profile of measles virus-specific IgG4 in the population studied. (Top) Serum samples were obtained 1 to 7 days after the onset of measles rash (acute 1), 8 to 30 days after the onset of measles rash (convalescence 2), and 8 to 30 years after measles infection (long-lasting 3). Immune responses shown are natural (black bars) and postvaccinal (stippled bars). (Bottom) Quantitative profile of measles IgG4 in the populations studied. Serum samples were obtained 6 to 10 days after the onset of rash.
DISCUSSION
The response to measles virus at the IgG subclass level had not been previously characterized for vaccinated children; therefore, no measles data exist with which our results may be matched. Thus, our findings could be compared only to earlier investigations of antibody responses following varicella-zoster virus and hepatitis B virus infections. Our results indicated that in the early phase of infection, the percent distributions of measles virus-specific IgG isotypes are similar between natural and postvaccinal immune responses. Both responses showed the same isotype profile: in the acute phase, the IgG1 response was seen first, followed by IgG2, IgG3, and IgG4 responses, which increased gradually during convalescence. IgG1 and IgG4 were the dominant isotypes, reaching a high percent detection of 100 and 94%, respectively. Gregorek et al. (9) reported that for convalescent-phase sera from naturally infected children, anti-HBs antibodies were highly restricted to IgG1 and IgG3, while IgG2 and IgG4 subclasses did not play significant roles. Similarly, in children immunized with recombinant HbsAg, IgG1 and IgG3 isotypes were predominant until 1 month after the vaccination schedule was completed. Therefore, in agreement with our results, the profile of anti-HBs antibodies in vaccinated children was very similar to that found after natural seroconversion. However, by comparison, our results showed that the measles virus IgG4 GMT in natural infection was significantly higher than the IgG4 GMT detected in the postvaccinal immune response (80 [95% CI, 33 to 191] versus 13 [95% CI, 7 to 26]). Thus, it is possible to establish a significant association between IgG4 antibody titers and probable exposure to measles vaccine or wild-type measles virus so that IgG4 titers could be used as a quantitative marker to differentiate the source of measles infection. In other words, IgG4 antibody titers of ≤30 determined by an IFA could be designated to represent a vaccinal source of immunity and those of >30 could be designated to represent a natural infection. This study was conducted with vaccinated children who displayed an adverse reaction to the vaccine (group 2) because during epidemics of measles, it is necessary to distinguish between an adverse vaccine reaction and a natural measles infection.
In the memory phase, IgG2 and IgG3 responses decreased significantly in both natural infection and postvaccinal groups, while the IgG1 levels were maintained thereafter. The most interesting findings in our study were the high frequency and percent contributions of specific IgG4 to the total IgG antimeasles response in natural versus postvaccinal long-lasting immunity (86 versus 8.7%). These findings are supported by the results of Asano et al. (1), who found high levels of IgG1 and IgG4 antibody activity to varicella-zoster virus in the memory phase in the natural infection. These authors also reported that IgG2 and IgG3 isotypes could not be detected 10 years after varicella-zoster virus infection. However, Asano et al. (1) reported that the pattern of subclass-specific antibody response induced by the vaccine was almost equal to that seen after natural infection. In contrast, our findings defined a characteristic subclass restriction pattern to differentiate the source of measles infection in life-long immunity.
The regulation of antibody subclass expression in humans is not well understood. There is evidence that isotype switch recombination is a highly regulated process controlled by soluble cytokines and by T-cell membrane interaction regulation with the CD40 molecule on the B-cell surface (2, 3, 8, 15, 18). On the other hand, several recent studies have shown that antigen structure, inoculum dose, and immunological status of the host seem to be the most important factors affecting the induction of a given profile of cytokines which, in turn, are responsible for specific isotype selection (6, 22). With regard to the measles virus antigens, the vaccine and wild-type viruses share common epitopes. However, the wild-type strain must have at least one unique or sufficiently modified region on the H protein that is perhaps more immunodominant than that present on the vaccine strain (23, 25). This notion could support the significant difference in the IgG4 GMT detected in the early phase of measles infection versus the IgG4 GMT detected in the early phase of the postvaccinal response and could explain, in part, the different isotype patterns detected in the long-lasting responses induced by natural infection versus the vaccine. On the other hand, the age at which the different IgG subclasses reach stable values has not been uniformly established yet. Zegers et al. (26) reported differences in the terminal maturation of subsets of B lymphocytes into plasma cells which, in turn, could reflect differences in the development of the various immunoglobulin isotypes. Similar results were reported by Gregorek et al. (10) and Quiles Dura et al. (19). For this reason, the differences in the IgG4 antibody levels in naturally infected versus vaccinated individuals could be interpreted as a relative inefficiency of the subset of B lymphocytes in the synthesis of the IgG4 isotype, since most measles immunization occurs when children are approximately 1 year old, whereas many cases of natural infection occur at a later age.
In the present study, the general pattern of subclass-specific antibody responses induced by the measles vaccine was similar to that seen after natural infection. Nevertheless, there were two highly salient points: (i) the IgG4 GMT was higher in the early phase of natural measles infection than in the same phase in vaccinated children, and (ii) long-lasting natural immunity was represented by IgG1 and IgG4 while vaccine memory immunity was represented only by IgG1. It is likely that the IgG1 subclass plays a leading role in protection against natural infection because IgG1 has sufficient functions for complement fixation and binding to mononuclear cells. In contrast, whereas IgG4 is present at the lowest concentrations of any IgG subclass in serum, the IgG4 antibody activities were the second highest at 10 years after natural infection. However, such a large contribution of IgG4 antibodies to the natural measles immune response and its long persistence are difficult to understand because IgG4 does not activate the complement system and has a low affinity for Fcγ receptors. Nevertheless, our results suggest that the IgG4 isotype can be used as an early-phase quantitative marker or a long-lasting immunity qualitative marker to differentiate between natural and postvaccinal immune responses.
REFERENCES
- 1.Asano, Y., Y. Hiroishi, N. Itakura, S. Hirose, Y. Kajita, T. Nagai, T. Yazaki, and M. Takahashi. 1987. Immunoglobulin subclass antibodies to varicella-zoster virus. Pediatrics 80:933-936. [PubMed] [Google Scholar]
- 2.Biere, F., C. Servet-Delprat, J. M. Bridon, J. M. Saint-Remy, and J. Banchereau. 1994. Human interleukin 10 induces naive surface immunoglobulin D+ B cells to secrete IgG1 and IgG3. J. Exp. Med. 179:757-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boer, B. A., Y. C. Kruize, and M. Yazdanbaksh. 1998. In vitro production of IgG4 by peripheral blood mononuclear cells (PBMC): the contribution of committed B cells. Clin. Exp. Immunol. 114:252-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunell, P. A. 1990. Measles control in the 1990's. Measles serology. Expanded Programme of Immunization. WHO/EPI/GEN/90.4. World Health Organization, Geneva, Switzerland.
- 5.Cutts, F. T., R. H. Henderson, C. J. Clementes, R. T. Chen, and P. A. Patriarca. 1991. Principles of measles control. Bull. W. H. O. 69:1-7. [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly, J. J., A. Friedman, R. R. Deck, C. M. Dewitt, J. Caulfield, et al. 1997. Adjuvant effects of DNA vaccines, p. 105-111. In F. Brown, D. Burton, P. Doherty, J. Mekalanos, and E. Norrby (ed.), Vaccines 97: molecular approaches to the control of infectious diseases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 7.Erdman, D. D., L. J. Anderson, D. R. Adams, J. A. Stewart, L. Makowitz, and W. Bellini. 1991. Evaluation of monoclonal antibody-based capture enzyme immunoassays for detection of specific antibodies to measles virus. J. Clin. Microbiol. 29:1466-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fjieda, S., A. Saxon, and K. Zhang. 1996. Direct evidence that gamma 1 and gamma 3 switching in human B cells is interleukin-10 dependent. Mol. Immunol. 33:1335-1343. [DOI] [PubMed] [Google Scholar]
- 9.Gregorek, H., K. Madalinski, M. Woynarowski, J. Mikolajewicz, M. Syczewska, and J. Socha. 2000. The IgG subclass profile of anti-HB response in vaccinated children and children seroconverted after natural infection. Vaccine 18:1210-1217. [DOI] [PubMed] [Google Scholar]
- 10.Gregorek, H., D. Imielska, J. Gornicki, J. Mikolajewicz, B. Przeradzka, and K. Madalinski. 1994. Development of IgG subclasses in healthy Polish children. Arch. Immunol. Ther. Exp. 42:377-382. [PubMed] [Google Scholar]
- 11.Gupta, C. K., J. Leszczynski, R. K. Gupta, and G. R. Siber. 1996. IgG subclass antibodies to human cytomegalovirus in normal human plasma samples and immuneglobulins and their neutralizing activities. Biologicals 24:117-124. [DOI] [PubMed] [Google Scholar]
- 12.Hersh, B., G. Tambini, A. Nogueira, P. Carrasco, and C. de Quadros. 2000. Review of regional measles surveillance data in the Americas, 1996-99. Lancet 355:1943-1948. [DOI] [PubMed] [Google Scholar]
- 13.Isa, M. B., L. Martínez, M. Giordano, M. Zapata, C. Passeggi, M. C. De Wolf, and S. Nates. 2001. Measles virus-specific iImmunoglobulin G isotype immune response in early and late infections. J. Clin. Microbiol. 39:170-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linde, G. A. 1985. Subclass distribution of rubella virus-specific immunoglobulin G. J. Clin. Microbiol. 21:117-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malisan, F., F. Briere, J. M. Bridon, N. Harindranath, F. Molls, E. Max, J. Banchereau, and H. Martinez-Valdez. 1996. Interleukin-10 induces immunoglobulin G isotype switch recombination in human CD40-activated naive B lymphocytes. J. Exp. Med. 183:937-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathiesen, T., C. Brattstrom, J. Anderson, A. Linde, P. Ljungam, and B. Wahren. 1992. Immunoglobulin G subclass and lymphocyte stimulatory responses to cytomegalovirus in transplant patients with primary cytomegalovirus infections. J. Med. Virol. 36:65-69. [DOI] [PubMed] [Google Scholar]
- 17.Nates, S. V., G. Y. Rey, M. O. Giordano, M. Zapata, A. R. Depetris, and J. Boshell. 1994. Modified seroneutralization assay for measles virus antibody detection. Res. Virol. 145:45-49. [DOI] [PubMed] [Google Scholar]
- 18.Punnonen, J., G. Aversa, B. G. Cocks, A. N. McKenzie, S. Menon, G. Zurawski, R. de Waal Malefyt, and J. E. de Vries. 1993. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc. Natl. Acad. Sci. USA 90:3730-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quiles Dura, J. L., M. J. Enguidanos Subira, J. Brines Solanes, and B. Balsalobre Hernández. 1993. The determination of IgG subclasses in healthy children by ELISA with monoclonal antibodies. An. Esp. Pediatr. 39:209-213. [PubMed] [Google Scholar]
- 20.Rossier, E., H. Miller, B. McCulloch, L. Sullivan, and K. Ward. 1991. Comparison of immunofluorescence and enzyme immunoassay for detection of measles virus-specific immunoglobulin M antibody. J. Clin. Microbiol. 29:1069-1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rota, J. S., Z. D. Wang, P. A. Rota, and W. J. Bellini. 1994. Comparison of sequences of the H, F and N coding genes of measles virus vaccine strains. Virus Res. 31:317-370. [DOI] [PubMed] [Google Scholar]
- 22.Rota, J. S., K. B. Hummet, P. A. Rota, and W. J. Bellini. 1992. Genetic variability of the glycoprotein genes of current wild-type measles isolate. Virology 188:135-142. [DOI] [PubMed] [Google Scholar]
- 23.Siegrist, C. A., F. Saddallah, C. H. Tougne, X. Martinez, J. Kovarik, and P. H. Lambert. 1998. Induction of neonatal TH1 and CTL responses by live viral vaccines: a role for replication patterns within antigen presenting cells? Vaccine 16:1473-1478. [DOI] [PubMed] [Google Scholar]
- 24.Sundqvist, V. A., A. Linde, and B. Wahren. 1984. Virus-specific immunoglobulin G subclass in herpes simplex and varicella-zoster virus infections. J. Clin. Microbiol. 20:94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamin, A., P. A. Rota, Z. D. Wang, J. Heath, L. J. Anderson, and W. J. Bellini. 1994. Antigenic analysis of current wild type and vaccine strains of measles virus. J. Infect. Dis. 170:795-801. [DOI] [PubMed] [Google Scholar]
- 26.Zegers, B. J., M. van der Giessen, E. E. Reerink-Brongers, and J. W. Stoop. 1980. The serum IgG subclass levels in healthy infants of 13-62 weeks of age. Clin. Chim. Acta 101:265-269. [DOI] [PubMed] [Google Scholar]