Abstract
In animal studies, vitamin A deficiency induces a shift from type 2 (humoral) to type 1 (cellular) cytokines; there are no similar data for humans. Control of human immunodeficiency virus (HIV) and Mycobacterium tuberculosis infections requires type 1 cytokine (cellular) immunity. These infections and vitamin A deficiency are highly prevalent in Africa. We therefore examined the interactions among serum vitamin A levels, immune parameters, HIV infection status, Mycobacterium bovis BCG vaccine scarring (as an indicator of a type 1 cytokine profile), and clinical findings for 70 hospitalized children in Malawi, Africa. Directly conjugated monoclonal antibodies and flow cytometry were used to assess cell-specific cytokine production by peripheral blood monocytes and lymphocyte subpopulations. The statistical techniques employed included nonparametric statistics and logistic regression analyses. Thirty percent of the participants had severe vitamin A deficiency (<10 μg/dl), 34% had moderate deficiency (10 to <20 μg/dl), and 36% had normal levels (≥20 μg/dl). Vitamin A levels were lower for HIV-positive than for HIV-negative children (median, 10 and 17 μg/dl, respectively). Vitamin A-deficient children (<20 μg/dl) were more likely than non-vitamin A-deficient children to have higher proportions of natural killer (NK) cells (median, 8.3 and 5.2%, respectively) and lower ratios of interleukin-10-producing monocytes to tumor necrosis factor alpha-producing monocytes after induction (median, 1.0 and 2.3, respectively). Vitamin A-deficient children were also more likely than non-vitamin A-deficient children to exhibit respiratory symptoms (47% versus 12%) and visible BCG vaccine scars (83% versus 48%), which are indicative of a type 1 response to vaccination. Vitamin A status did not vary with gender, age, incidence of malaria parasitemia, blood culture positivity, or rates of mortality (6% of vitamin A-deficient children died versus 20% of non-vitamin A-deficient children). Lower vitamin A levels were associated with a relative type 1 cytokine dominance and proportionately more NK cells, both of which may be somewhat beneficial to persons who are exposed to HIV, M. tuberculosis, or other type 1 pathogens.
Vitamin A deficiency is associated with severe cases of measles, pneumonia, and diarrhea (3, 14, 21, 25, 28). Vitamin A supplementation may reduce measles-associated, diarrhea-associated, and overall infant mortalities (8, 12, 14, 21, 32, 33). In developing countries, where human immunodeficiency virus (HIV) is now endemic, vitamin A supplementation is provided to all children but does not protect against, and might even be associated with an increase in, pneumonia-specific mortality in 0.5- to 5-year-old children, acute diarrhea in normally nourished children, or respiratory symptoms in HIV-seronegative children (8, 12, 14, 32, 33). Thus, the risks and benefits of vitamin A supplementation need clarification, including better delineation of the effects of vitamin A on immune response in the context of HIV infection and associated coinfections, e.g., with Mycobacterium tuberculosis (13).
Vitamin A is key in the development and function of virtually all cells, not just those of epithelium and endothelium. It can inhibit cellular potassium currents (27) and protein kinase C-associated signal transduction (15, 23), costimulates T cells via CD3 (1), and is involved in T-cell maturation, differentiation, and proliferation (11, 25). In animal models, vitamin A deficiency is associated with a shift from type 2 cytokines (generating humoral immunity, antibody production, and immunoglobulin maturation) to predominantly type 1 cytokines (necessary for cellular immunity and cytotoxicity) (4). Retinoic acid (vitamin A) downregulates the gene expression of gamma interferon (IFN-γ), a type 1 cytokine; retinoic acid deficiency is associated with increased IFN-γ production, followed by decreased production of interleukin-5 (IL-5; a type 2 cytokine acting on eosinophil progenitors) and IL-10 (a type 2 cytokine that also regulates a general shift toward a type 2 profile) (4, 6). In a murine model, vitamin A deficiency resulted in constitutive production of type 1 cytokine transcripts, including those of IFN-γ and IL-12 (an immunoregulatory cytokine inducing a shift toward a type 1 profile), but not of type 2 transcripts, including that of IL-4 (a type 2 cytokine associated with IgE production) or IL-10; subsequent stimulation through the T-cell antigen receptor led to excessive production of IFN-γ but not of IL-4 or IL-10 (5). Conversely, in murine and rat systems, vitamin A supplementation led to decreased IFN-γ but increased IL-4, IL-5, and IL-10 production (20). Little related data for humans are available (30). Since an effective immune response to measles requires type 2 cytokine function, the beneficial effect of vitamin A supplementation on measles-related pneumonia is consistent with the results of these animal studies. In one recent study of immunodeficient patients, many of whom were vitamin A deficient, vitamin A supplementation led to a shift toward a type 2 profile, with increased production of IL-10 and immunoglobulin A (IgA) in plasma and IgG in vitro and decreased production of tumor necrosis factor alpha (TNF-α), a proinflammatory, type 1 cytokine, in plasma (2).
We measured the serum vitamin A levels of members of a cohort of children hospitalized at Lilongwe Central Hospital in Malawi, Africa, where HIV is endemic and mycobacterial infections are prevalent. We sought evidence of an association between vitamin A levels and a type 1 or type 2 cytokine dominance. Further, we evaluated the relationships between these levels and both laboratory and clinical immune findings, including the presence (versus absence) of Mycobacterium bovis bacillus Calmette-Guerin (BCG) vaccine scarring, as an indicator of a type 1 response to immunization. Finally, we examined the levels in relation to clinical findings, including HIV infection status, symptoms, and mortality.
MATERIALS AND METHODS
Study population.
We enrolled all 149 children (all <13 years old) who had been admitted to the Lilongwe Central Hospital in Lilongwe, Malawi, Africa, between 7 and 15 April 1998 and between 28 July and 22 August 1998 into a study of the immune determinants of bloodstream infections (16, 17, 19). Sera from 66 of these 149 children were available for assessment of vitamin A levels. The children who underwent vitamin A testing were older than those whose sera were unavailable (median age of 2.6 years [range, 0.3 to 12 years] versus 1.3 years [range, 0.1 to 12 years], P < 0.001), which was as expected since older, larger children can safely donate larger blood volumes. Sera from an additional three of the four persons who were 13 years old and one of the six persons who were 15 years old were also available and were evaluated for vitamin A levels. For those children ≥0.3 year, the rates of BCG vaccine scarring did not differ between those with and those without vitamin A level assessment, nor did these two groups differ in gender distributions, survival rates, HIV serostatuses, transferrin receptor levels, or the percentages of lymphocytes expressing CD4 (data not shown). The results for the four patients older than 12 years who had vitamin A level assessments were included in our study; excluding them would not have altered our conclusions. Further, vitamin A levels did not vary with the patients' ages.
Blood samples, epidemiologic data, and medical histories were obtained upon patient admission, and physical examinations were performed by one of the investigators. The study protocol was approved by the institutional review boards of the Centers for Disease Control and Prevention (CDC) and the Malawi Health Sciences Research Committee; informed consent was obtained from all participants and/or their guardians.
In Malawi, it is required that all children be vaccinated within the first 3 days of life with 0.05 ml of Pasteur Mérieux Connaught BCG vaccine, which is derived from M. bovis strain 1077 and contains between 0.4 × 106 and 1.6 × 106 culturable particles per newborn dose. Vitamin A supplementation is provided at health clinics every 6 months to all children younger than 5 years old.
Definitions.
Respiratory findings included a respiratory rate of ≥60 breaths/min, lung crackles, or auscultatory findings suggesting pulmonary consolidation. A diagnosis of pneumonia was made based on the presence of either all three of the respiratory symptoms or two of these and a total peripheral blood white cell count of <3,000 or >12,000 cells/mm3 and/or a temperature of <36 or >38°C. Symptoms of sepsis were based on the presence of at least two of the following symptoms with or without a positive blood culture: a temperature of <36 or >38°C, a respiratory rate of ≥60 breaths/min, and a heart rate of ≥160 beats/min.
Serum vitamin A levels were assessed with reversed-phase high-performance liquid chromatography (29). Blood cultures were performed (22); thick and thin malarial smears were done at admission and were considered positive if any (Plasmodium) asexual parasites were seen. HIV antibody testing was done at the time of study enrollment with enzyme-linked immunosorbent assay test kits (Murex Diagnostics Inc., Norcross, Ga.). Three of the 21 children who were <1.3 years old were HIV antibody positive; all three had detectable plasma HIV RNA levels (AMPLICOR HIV-1 MONITOR, version 1.5; Roche Diagnostics, Indianapolis, Ind.). Heparinized whole blood was stimulated for 5 h at 37°C with phorbol 12-myristate 13-acetate (200 ng/ml; Sigma Chemical Co., St. Louis, Mo.) and ionomycin (4 μg/ml; Sigma) in the presence of brefeldin-A (40 μg/ml; Sigma) and RPMI 1640 with l-glutamine (induced cytokine expression), or it was retained in the identical medium without phorbol 12-myristate 13-acetate and ionomycin but with brefeldin-A (spontaneous cytokine expression) (16, 17, 19). Lymphocytes were permeabilized and fixed with Permeafix (Ortho Diagnostic Systems, Inc., Raritan, N.J.), and shipped at 4 to 8°C to the CDC, stained, and processed for four-color flow cytometric assessment with a FACSort or FACSCalibur flow cytometer and CellQuest software (Becton Dickinson Immunochemistry Systems, San Jose, Calif.) (16, 17, 19). Cell gating and identification of T cells, CD8+ T cells, CD8− T cells, B lymphocytes, CD3− CD16/56+ lymphocytes (natural killer [NK] cells), CD3+ CD16/56+ lymphocytes (natural T cells), and monocytes were done as previously described (16, 17, 19). The four-color tube panel and monoclonal antibodies used have been described previously (17). Between 50,000 and 80,000 ungated events were collected from each tube in the panel.
Statistical analysis.
Comparisons of continuous data between nominal categories were made by use of nonparametric techniques, including the Wilcoxon rank sum for dichotomized data, Kruskal-Wallis tests for trichotomized data, and logistic regression analyses (LRA). Vitamin A levels were trichotomized (<10 μg/dl, 10 to <20 μg/dl, and ≥20 μg/dl) or dichotomized in two ways (<10 versus ≥10 μg/dl [severe vitamin A deficiency] and <20 versus ≥20 μg/dl [vitamin A deficiency]). Medians, ranges, and interquartile (IQ) ranges (25th to 75th percentile) were determined for continuous data. Proportions were compared by Fisher's exact test. For assessments in which vitamin A levels were the dichotomized dependent variable, initial LRA included as independent variables the variable of interest, age, gender, HIV serostatus, BCG vaccine scarring status, and acute infection status. The last of these was defined in four different ways, with separate LRA run with each of the four variables included individually as an independent variable. The four variables were the following: a four-value variable, with values representing Salmonella sp. bacteremia, bacteremia with another gram-negative organism, malaria parasitemia, and blood culture- and smear-negative results, and three dichotomous variables representing the presence or absence of a history of acute respiratory symptoms, pulmonary symptoms, and a diagnosis of possible pneumonia. The significance level was set at 0.05; data not presented in this paper did not reach this level of significance on any type of analysis.
RESULTS
Vitamin A levels.
Of the 70 children whose vitamin A levels were assessed, 21 (30%) had severe vitamin A deficiency, with levels of <10 μg/dl (mean, 6.2 μg/dl; median, 6 μg/dl; range, 2 to 9 μg/dl; IQ range, 5 to 8 μg/dl); 24 (34%) had moderate vitamin A deficiency, with levels of 10 to <20 μg/dl (mean, 14.0 μg/dl; median, 14 μg/dl; range, 10 to 19 μg/dl; IQ range, 12 to 17 μg/dl); and 25 (36%) had normal vitamin A levels, i.e., ≥20 μg/dl (mean, 29.3 μg/dl; median, 25 μg/dl; range, 20 to 72 μg/dl; IQ range, 22 to 33 μg/dl). The median vitamin A level was lower for HIV-positive children (10 μg/dl; range, 2 to 72 μg/dl; IQ range, 6 to 12 μg/dl; n = 17) than for HIV-negative children (17 μg/dl; range, 3 to 45 μg/dl; IQ range, 9 to 23 μg/dl; n = 51) (P = 0.016). For HIV-positive and HIV-negative children analyzed separately, the percentages of lymphocytes expressing CD4 did not differ significantly by vitamin A status (Table 1).
TABLE 1.
Characteristics of children tested for serum vitamin A levels
| Characteristica | Value for children with vitamin A level of:
|
||
|---|---|---|---|
| <10 μg/dl (n = 21) | 10 to <20 μg/dl (n = 24) | >20 μg/dl (n = 25) | |
| Male (% of group) | 43 | 54 | 52 |
| Age (yr) | |||
| Mean | 4.2 | 2.9 | 4.2 |
| Median | 3.0 | 2.5 | 3.0 |
| Range | 0.6-13 | 0.3-12 | 0.3-15 |
| IQ rangeb | 1.3-5 | 1.0-4 | 1.0-5 |
| Died (% of group) | 13 | 0 | 20 |
| Malaria positive (no.) | 1 | 2 | 4 |
| Bacteremic with Salmonella sp. (no.) | 1 | 2 | 2 |
| Bacteremic with other gram- negative organisms (no.) | 0 | 1 | 1 |
| HIV positive (% of group) | 38 | 26 | 13 |
| Lymphocytes (median %) expressing CD4 in: | |||
| All patients | 30 | 32 | 38 |
| HIV-positive patients | 19 | 19 | 17 |
| BCG vaccine scar (% of group) | 83 | 82 | 48 |
| Respiratory findings (% of group) | 43 | 50 | 12 |
| Suspected pneumonia (% of group) | 38 | 25 | 4 |
| Symptoms of sepsisc (% of group) | 61 | 59 | 22 |
The following data were incomplete for various individuals: clinical history and family history data, including the presence or absence of a family history of cough (absent, n = 7 each); malaria status (n = 1); HIV status (n = 2); and clinical findings, including the presence or absence of a BCG vaccine scar, sepsis status (n = 7 each), and mortality status (n = 13).
IQ range, 25th to 75th percentile.
Sepsis symptoms were defined as follows: (i) a temperature of <36 or >38°C that was associated with a heart rate of ≥160 beats/min or a respiratory rate of ≥60 breaths/min or (ii) a heart rate of ≥160 breaths/min that was associated with a respiratory rate of ≥60 breaths/min in cases where the temperature was either normal or not taken.
Laboratory immune response findings (Table 2).
TABLE 2.
Immune findings significantly associated with vitamin A level
| Parameter | Vitamin A level (μg/dl) | Value for patients
|
Wilcoxon P value | |
|---|---|---|---|---|
| Vitamin A deficient | Non-vitamin A deficient | |||
| % of lymphocytes that were CD3− CD16/56+ (44, 25)a: | <20 | |||
| Median | 8.3 | 5.2 | 0.008 | |
| Range | 1.5-35.8 | 1.7-22.2 | ||
| IQ range | 5.1-12.8 | 3.5-8.2 | ||
| IL-10-producing monocytes/TNF-α-producing monocytes, after induction (42, 23): | <20 | |||
| Median | 1.0 | 2.3 | 0.018 | |
| Range | 0-10 | 0.1-100 | ||
| IQ range | 0.3-2.3 | 1.0-7.5 | ||
| % of T cells spontaneously producing TNF-α and IFN-γ in the same cell (21, 49): | <10 | |||
| Median | 0.1 | 0.1 | 0.002 | |
| Range | 0-0.7 | 0-0.2 | ||
| IQ range | 0.1-0.3 | 0-0.1 | ||
Numbers in parentheses refer to the number of patients tested for the indicated parameter who were deficient and not deficient, respectively, in vitamin A.
Vitamin A levels of <20 μg/dl were associated with a higher percentage of NK cells and a lower ratio of the percentage of IL-10-producing monocytes to the percentage of TNF-α-producing monocytes after induction. LRA that included each of the acute infection-related variables (see Materials and Methods) showed that these parameters remained significantly related to vitamin A deficiency (for NK cells, 0.017 ≤ P ≤ 0.033; for the ratio variable, 0.004 ≤ P ≤ 0.040). Vitamin A levels of <10 μg/dl were associated with slightly higher proportions of CD3+ (T) lymphocytes spontaneously producing both TNF-α and IFN-γ in the same cells. LRA showed that the percentages of these doubly positive cells remained related to severe vitamin A deficiency (P values of <0.001).
Relation to BCG vaccine scarring.
A higher proportion of children with vitamin A deficiency had a visible BCG vaccine scar than did those with vitamin A levels of ≥20 μg/dl (P = 0.009) (Tables 1 and 3) Scarring was not related to age (median age of children with scars, 2.8 years [range, 0.3 to 13 years; IQ range, 0.9 to 4.3 years], versus median age of children without scars, 2.0 years [range, 0.3 to 15 years; IQ range, 1.0 to 5 years]; P = 0.99), HIV serostatus (P = 0.12), or symptoms of sepsis (P = 0.42), but it was related to pulmonary symptoms (45% of those with scars had symptoms versus 10% of those without scars; P = 0.009) and suspected pneumonia (32% of those with scars versus 5% of those without; P = 0.026).
TABLE 3.
Vital signs and vitamin A levels
| Parameter | Value for patients with vitamin A levels of:
|
Wilcoxon P value | |
|---|---|---|---|
| <20 μg/dl | ≥20 μg/dl | ||
| Pulse (beats/min) (40, 23)a: | |||
| Median | 160 | 128 | 0.005 |
| Range | 80-240 | 76-184 | |
| IQ range | 134-180 | 108-144 | |
| Respiratory rate (breaths/min) (40, 23): | |||
| Median | 60 | 36 | 0.003 |
| Range | 16-99 | 16-90 | |
| IQ range | 38-70 | 28-52 | |
| Temp (°C) (36, 20): | |||
| Median | 38.4 | 37.1 | 0.003 |
| Range | 35.1-40.8 | 36.1-39.9 | |
| IQ range | 37.2-39.6 | 36.7-37.6 | |
Numbers in parentheses refer to the number of patients evaluated for the indicated parameter who had vitamin A levels of <20 and ≥20 μg/dl, respectively. Various data were incomplete for various individuals.
Clinical findings.
The gender distributions, ages, rates of mortality, incidences of malaria parasitemia (positive smear; n = 8), degrees of malaria parasitemia, and numbers of positive blood cultures (n = 7) did not differ significantly among the three groups of children (defined by their vitamin A levels) (Table 1). Mycobacteria were not isolated from the blood of any child. For the children with an organism identified in their blood, the type of organism did not vary by vitamin A level (Table 1). Those with any level of vitamin A deficiency had higher pulses, respiratory rates, and temperatures than did those not deficient in vitamin A (Table 3). Lower vitamin A levels were associated with respiratory symptoms, suspected pneumonia, and signs of sepsis (Tables 1 and 4). Vitamin A level was not significantly related to the presence or absence of diarrhea, although the difference approached significance in those with severe diarrhea (Table 4). Of the 70 children whose vitamin A levels were assessed, 42 were discharged, 5 died, 7 were still in the hospital, and 3 had been signed out against medical advice; outcome data was missing for 13 children. Of those known to have died, two had severe vitamin A deficiency and three had normal vitamin A levels; all five had negative blood cultures and malarial smears.
TABLE 4.
Clinical findings and vitamin A levels
| Clinical finding | Vitamin A level (μg/dl) for patients with finding
|
Wilcoxon P value | |
|---|---|---|---|
| Present | Absent | ||
| BCG vaccine scar (44, 19)a: | |||
| Median | 13 | 21 | 0.007 |
| Range | 2-42 | 3-72 | |
| IQ range | 8-20 | 13-32 | |
| History of acute respiratory problems (43, 27): | |||
| Median | 13 | 21 | 0.038 |
| Range | 2-72 | 3-45 | |
| IQ range | 7-20 | 10-27 | |
| Diagnosis of possible pneu- monia (15, 55): | |||
| Median | 8 | 17 | 0.002 |
| Range | 2-21 | 3-72 | |
| IQ range | 5-14 | 10-24 | |
| History of mild diarrhea (8, 56): | |||
| Median | 24 | 15 | 0.153 |
| Range | 9-45 | 2-72 | |
| IQ range | 12-26 | 9-22 | |
| History of severe diarrhea (8, 56): | |||
| Median | 9 | 15 | 0.215 |
| Range | 5-26 | 2-72 | |
| IQ range | 6-18 | 9-22 | |
Numbers in parentheses refer to the number of patients for whom the indicated parameter was present and absent, respectively. Various data were incomplete for various individuals.
Multivariable assessments of clinical data.
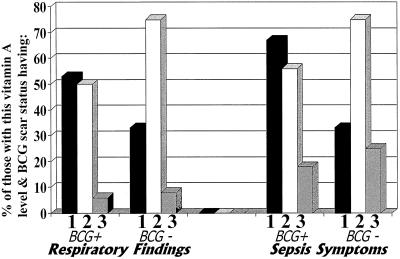
Because vitamin A deficiency and the presence of a BCG vaccine scar were related both to one another and to clinical indices, we examined their interaction through both stratification (Fig. 1) and LRA. Vitamin A levels tended to be lower in those with BCG vaccine scarring whether or not pulmonary symptoms were present (i.e., when analysis was stratified by the presence or absence of respiratory findings) (Fig. 1) and whether or not a diagnosis of suspected pneumonia had been made. Among those diagnosed with suspected pneumonia, those with BCG vaccine scars had a median vitamin A level of 7 μg/dl (range, 2 to 18 μg/dl; IQ range, 5 to12 μg/dl). Only one person without a scar received this diagnosis; he had a serum vitamin A level of 21 μg/dl. Among those not diagnosed with suspected pneumonia, those with BCG vaccine scars had a median vitamin A level of 16 μg/dl (range, 5 to 42 μg/dl; IQ range, 10 to 22 μg/dl) and those without scars had a median vitamin A level of 22 μg/dl (range, 3 to 72 μg/dl; IQ range, 13 to 32 μg/dl).
FIG. 1.
Clinical findings, grouped by vitamin A level and the presence or absence of a BCG vaccine scar. Bars: 1, severe vitamin A deficiency (serum vitamin A levels of <10 μg/dl); 2, moderate vitamin A deficiency (serum vitamin A levels of ≥10 but <20 μg/dl); 3, no vitamin A deficiency (serum vitamin A levels of ≥20 μg/dl). Of those with severe vitamin A deficiency, 15 had BCG vaccine scars and 3 did not; of those with moderate vitamin A deficiency, 18 had scars and 4 did not; and of those with no vitamin A deficiency, 11 had scars and 12 did not. Sepsis symptoms were defined as follows: (i) a temperature of <36 or >38°C that was associated with a heart rate of ≥160 beats/min or a respiratory rate of ≥60 breaths/min or (ii) a heart rate of ≥160 beats/min that was associated with a respiratory rate of ≥60 breaths/min in cases where the temperature was either normal or not taken.
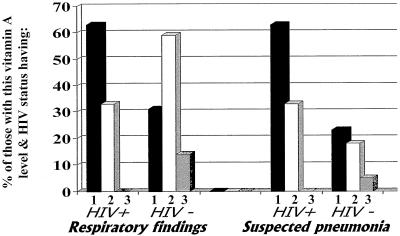
HIV seropositivity was not significantly related to any clinical parameter but did tend to be related to a finding of suspected pneumonia (Fig. 2) or a history of a family member with a cough (53% of HIV-positive children had a family member with a cough versus 24% of HIV-negative children; P = 0.065), so HIV serostatus was included in the initial LRA of clinical data. Specific clinical findings were analyzed as dichotomized (present or absent) dependent variables. In the initial LRA, the independent variables included age, gender, type of infection (stratified into positive blood culture, positive malarial smear, or negative blood culture), the presence or absence of BCG vaccine scarring, vitamin A level (three strata), HIV infection status, and terms for the interactions between BCG vaccine scarring and vitamin A stratum and between HIV infection status and vitamin A deficiency group. In these models, vitamin A level was the only variable related to the presence of respiratory findings (vitamin A b = −0.743, P = 0.025) or sepsis (b = −0.872, P = 0.011) (values for reduced models). The interaction between HIV infection status and vitamin A level was the strongest and only significant variable associated with a finding of suspected pneumonia, and thus this LRA was uninterpretable. All of these findings are consistent with the patterns shown in Fig. 1 and 2.
FIG. 2.
Clinical findings, grouped by vitamin A level and HIV infection status. Vitamin A levels are indicated by numbered bars as described in the legend to Fig. 1. Of those with severe vitamin A deficiency, 8 were HIV positive and 13 were HIV negative; of those with moderate vitamin A deficiency, 6 were HIV positive and 17 were HIV negative; and of those with no vitamin A deficiency, 3 were HIV positive and 21 were HIV negative.
Results of LRA showed that BCG vaccine scarring remained related to vitamin A levels (<20 versus ≥20 μg/dl) even when the immune response findings noted above and the acute clinical status (as defined in Materials and Methods) were included in the analyses (0.003 ≤ P ≤ 0.020) (the analyses were not done with data concerning suspected pneumonia because of the strong interaction noted above).
DISCUSSION
We evaluated the relationship between vitamin A levels and cell-specific cytokines in hospitalized children in Malawi, where HIV, malaria, and mycobacterial infections are endemic. In these children, vitamin A deficiency was associated with laboratory evidence of a relative type 1 cytokine dominance. Interestingly, vitamin A deficiency also was associated with BCG vaccine scarring, which is indicative of a type 1 immune response to the vaccine (7, 31). The association between vitamin A deficiency and type 1 cytokine dominance has been demonstrated in animals. This association has been only indirectly suggested to exist in humans through data showing that vitamin A supplementation can increase antibody response to tetanus toxoid and diphtheria vaccines (but not necessarily to other vaccines) (30).
We also found vitamin A deficiency to be associated with a relatively greater proportion of peripheral blood NK cells, which are associated with innate immunity. This finding differs from those of several animal studies in which vitamin A deficiency was associated with decreased proportions of splenic NK cells (24); perhaps this difference could be due to the difference in cell source. We do not know of any human studies other than our own that have examined the relationship of NK cells and vitamin A levels.
Vitamin A supplementation has long been provided to children in most African countries to decrease infectious disease-associated morbidity and mortality. Because the HIV epidemic has strikingly altered the pathogens infecting persons in developing countries (19, 22), we examined the relationships between vitamin A levels and acute illness and mortality. Although vitamin A deficiency affects the gut mucosa and can lead to malabsorption (30), vitamin A levels were not significantly associated with the presence or absence of diarrhea in the Malawian children we studied. However, those with more severe diarrhea did tend to have lower vitamin A levels. Lower vitamin A levels were significantly associated with pulmonary and systemic symptoms, but mortality was not greater in those with vitamin A deficiency.
The relationship between vitamin A levels and BCG vaccine scarring was statistically strong and physiologically consistent with our laboratory immune response findings and with the association of vitamin A deficiency with type 1 cytokine reactivity. In a 1989 study done in northern Malawi, only 60% of children of ≤4 years old had an apparent BCG vaccine scar despite being vaccinated in the first year of life (9). BCG vaccination quickly leads to ulceration, usually followed over several weeks by scarring (9) that is associated with type 1 cytokines at the vaccination site (7, 31). Scarring is a stable phenomenon, with positive signs of scarring rarely reverting to negative or developing following an initial period without scarring; scarring evaluations are highly reproducible between individual readers and over time (10). Consequently, the incidences of BCG vaccine scarring did not vary with the ages of the patients in our cohort. The median age of the participants in our study was <3 years, so many of these participants had been vaccinated fairly recently. Thus, the twofold-higher rate of BCG vaccine scarring in the vitamin A-deficient children suggests that, at least in some children, vitamin A deficiency was present at the time of vaccination and was associated with a type 1 cytokine response to vaccination. Obviously, this finding should be interpreted with caution, since vitamin A assessment was not done at the time of BCG vaccination.
Recent studies and a meta-analysis of vitamin A supplementation trials suggest that prophylactic vitamin A supplementation may have no effect or adverse effects on some subgroups of children (8, 26, 32). Moderately low vitamin A levels may, in some situations and in some respects, be useful immunologically in patients like ours. We found an association between lower vitamin A levels and laboratory evidence of a type 1 cytokine dominance (and perhaps clinical evidence in terms of the BCG vaccine scarring data). Both HIV and mycobacteria are intracellular organisms requiring a type 1 immune response (19). Thus, this cytokine pattern could be life sustaining for persons infected with HIV and/or M. tuberculosis (19). Additionally, vitamin A deficiency was associated with a higher percentage of NK cells in peripheral blood. NK cells are associated with innate, rather than acquired, immunity and can respond to mycobacterial antigens. Furthermore, in severe HIV infection, where adaptive immunity is highly compromised, innate immunity may be crucial for survival (18, 19). Consistent with this possibility, few of the vitamin A-deficient children in our study had positive blood cultures, though they had higher rates of respiratory symptoms and pneumonia; thus, their infections may have been localized and partially controlled. Further, mortality was not related to vitamin A levels, suggesting that the positive effects may balance the negative effects over a wide range of vitamin A levels, at least in this population.
In summary, our findings support the hypothesis that lower levels of vitamin A in humans may be associated with a relative type 1 cytokine dominance and a higher proportion of NK cells. Very low vitamin A levels would be undesirable in any setting, given the essential role of vitamin A in epithelial and general cell maturation and function. However, the cytokine shifts associated with moderately low levels of vitamin A may be in some ways beneficial in an environment where HIV infection, M. tuberculosis infection, or other type 1 infections are highly prevalent and/or when acquired immunity is compromised.
Acknowledgments
We acknowledge the gracious and thoughtful advice of Elaine Gunter of the Division of Laboratory Sciences (DLS), National Center for Environmental Health (NCEH), CDC, who suggested assessing the vitamin A levels in our patients, and of Mary Serdula, Division of Nutrition and Physical Activity, National Center for Chronic Disease Prevention and Health Promotion, CDC, who suggested we contact E. Gunter. Rosemary Schleicher, HuiPing Chen,, and Mary Xu (DLS, NCEH) all assisted in running the vitamin A assay. As is so often the case in our studies, Timothy A. Green assisted us with the figures and gave clear, concise statistical advice. We thank the nursing and medical staff of Lilongwe Central Hospital and the patients and their parents who so generously cooperated in this study.
REFERENCES
- 1.Allende, L. M., A. Corell, A. Madroño, R. Góngora, C. Rodrígues-Gallego, A. López-Goyanes, M. Rosal, and A. Arnaiz-Villena. 1997. Retinol (vitamin A) is a cofactor in CD3-induced human T-lymphocyte activation. Immunology 90:388-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aukrust, P., F. Müller, T. Ueland, A. M. Svardal, R. K. Berge, and S. S. Frøland. 2000. Decreased vitamin A levels in common variable immunodeficiency: vitamin A supplementation in vivo enhances immunoglobulin production and downregulates inflammatory responses. Eur. J. Clin. Investig. 30:252-259. [DOI] [PubMed] [Google Scholar]
- 3.Bloem, M. W., M. Wedel, R. J. Egger, A. J. Speek, J. Schrijver, S. Saowakontha, and W. H. P. Schreurs. 1990. Mild vitamin A deficiency and risk of respiratory tract diseases and diarrhea in preschool and school children in northeastern Thailand. Am. J. Epidemiol. 31:332-339. [DOI] [PubMed] [Google Scholar]
- 4.Cantorna, M. T., F. E. Nashold, and C. E. Hayes. 1994. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J. Immunol. 152:1515-1522. [PubMed] [Google Scholar]
- 5.Cantorna, M. T., F. E. Nashold, and C. E. Hayes. 1995. Vitamin A deficiency results in a priming environment conducive for Th1 cell development. Eur. J. Immunol. 25:1673-1679. [DOI] [PubMed] [Google Scholar]
- 6.Carmen, J. A., and C. E. Hayes. 1991. Abnormal regulation of IFN-γ secretion in vitamin A deficiency. J. Immunol. 147:1247-1252. [PubMed] [Google Scholar]
- 7.Chu, C. Q., M. Field, E. Andrew, D. Haskard, M. Feldmann, and R. N. Maini. 1992. Detection of cytokines at the site of tuberculin-induced delayed-type hypersensitivity in man. Clin. Exp. Immunol. 90:522-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fawzi, W. W., R. Mbise, D. Spegelman, M. Fataki, E. Hertzmark, and G. Ndossi. 2000. Vitamin A supplements and diarrheal and respiratory tract infections among children in Dar es Salaam, Tanzania. J. Pediatr. 137:660-667. [DOI] [PubMed] [Google Scholar]
- 9.Fine, P. E., J. M. Ponnighaus, and N. Maine. 1989. The distribution and implications of BCG scars in northern Malawi. Bull. W. H. O. 67:35-42. [PMC free article] [PubMed] [Google Scholar]
- 10.Floyd, S., J. M. Ponnighaus, L. Bliss, D. K. Warndorff, A. Kasunga, P. Mogha, and P. E. Fine. 2000. BCG scars in northern Malawi: sensitivity and repeatability of scar reading, and factors affecting scar size. Int. J. Tuberc. Lung Dis. 4:1133-1142. [PubMed] [Google Scholar]
- 11.Garbe, A., B. Jochen, and U. Hämmerling. 1992. Retinoids are important cofactors in T cell activation. J. Exp. Med. 176:109-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghana VAST Study Team. 1993. Vitamin A supplementation in northern Ghana: effects on clinic attendances, hospital admissions, and child mortality. Lancet 342:7-12. [PubMed] [Google Scholar]
- 13.Griffiths, J. K. 2000. The vitamin A paradox. J. Pediatr. 137:604-607. [DOI] [PubMed] [Google Scholar]
- 14.Hussey, G. D., and M. Klein. 1990. A randomized, controlled trial of vitamin A in children with severe measles. N. Engl. J. Med. 323:160-164. [DOI] [PubMed] [Google Scholar]
- 15.Isakov, N. 1988. Regulation of T-cell-derived protein kinase C activity by vitamin A derivatives. Cell. Immunol. 115:288-298. [DOI] [PubMed] [Google Scholar]
- 16.Jason, J., L. Archibald, O. Nwanyanwu, M. Bell, I. Buchanan, J. Larned, P. N. Kazembe, H. Dobie, B. Parekh, M. G. Byrd, A. Eick, A. Han, D. Razsi, and W. R. Jarvis. 2001. Cytokine correlates of malaria parasitemia. Clin. Immunol. 100:208-218. [DOI] [PubMed] [Google Scholar]
- 17.Jason, J., L. K. Archibald, O. C. Nwanyanwu, M. G. Byrd, P. N. Kazembe, H. Dobbie, and W. R. Jarvis. 2001. Comparison of serum and cell-specific cytokines in humans. Clin. Diagn. Lab. Immunol. 8:1097-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jason, J., L. Archibald, C. McDonald, W. M. Hart, S. Rheanppumikankit, S. Tansuphwaswadikul, M. G. Byrd, J. Larned, A. Han, T. A. Green, and W. R. Jarvis. 1999. Immune determinants of organisms and outcome in febrile hospitalized Thai patients with bloodstream infections. Clin. Diagn. Lab. Immunol. 6:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jason, J., I. Buchanan, L. Archibald, O. C. Nwanyanwu, M. Bell, T. A. Green, A. Eick, A. Han, D. Razsi, P. N. Kazembe, H. Dobbie, M. Midathada, and W. R. Jarvis. 2000. Natural T, γδ, and NK cells in mycobacterial, Salmonella, and human immunodeficiency virus infections. J. Infect. Dis. 182:474-481. [DOI] [PubMed] [Google Scholar]
- 20.Long, K. Z., and J. I. Santos. 1999. Vitamins and the regulation of the immune response. Pediatr. Infect. Dis. J. 18:283-290. [DOI] [PubMed] [Google Scholar]
- 21.Markowitz, L., N. Nzilambi, W. J. Driskell, M. G. Sension, E. Z. Rovira, P. Neiburg, and R. W. Ryder. 1989. Vitamin A levels and mortality among hospitalized measles patients, Kinshasa, Zaire. J. Trop. Pediatr. 35:109-112. [DOI] [PubMed] [Google Scholar]
- 22.McDonald, L. C., L. K. Archibald, S. Rheanpumikankit, S. Tansuphaswadikul, B. Eampokalap, O. Nwanyanawu, P. Kazembe, H. Dobbie, L. B. Reller, and W. R. Jarvis. 1999. Unrecognized Mycobacterium tuberculosis bacteraemia among hospital inpatients in less developed countries. Lancet 354:1159-1163. [DOI] [PubMed] [Google Scholar]
- 23.Ortaldo, J. R., H. A. Young, and L. Varesio. 1989. Modulation of CD3− large granular lymphocyte functions by agonist and antagonists of protein kinase C: effects of NK and lymphokine-activated killer activity and production of IFN-γ. J. Immunol. 143:366-371. [PubMed] [Google Scholar]
- 24.Ross, A. C., and C. B. Stephensen. 1996. Vitamin A and retinoids in antiviral responses. FASEB J. 10:979-985. [PubMed] [Google Scholar]
- 25.Semba, R. D. 1999. Vitamin A and immunity to viral, bacterial, and protozoan infections. Proc. Nutr. Soc. 58:719-727. [DOI] [PubMed] [Google Scholar]
- 26.Sempértegui, F., B. Estrella, V. Camaniero, V. Betancourt, R. Izurieta, W. Ortiz, E. Fiallo, S. Troya, A. Rodriguez, and J. K. Griffiths. 1999. The beneficial effects of weekly low-dose vitamin A supplementation on acute lower respiratory infections and diarrhea in Ecuadorian children. Pediatrics 104. [Online.] http://www.pediatrics.org/cgi/content/full/104/1/e1. [DOI] [PubMed]
- 27.Sidell, N., and L. Schlichter. 1986. Retinoic acid blocks potassium channels in human lymphocytes. Biochem. Biophys. Res. Commun. 138:560-567. [DOI] [PubMed] [Google Scholar]
- 28.Sommer, A., J. Katz, and I. Tarwotjo. 1984. Increased risk of respiratory disease and diarrhea in children with preexisting mild vitamin A deficiency. Am. J. Clin. Nutr. 40:1090-1095. [DOI] [PubMed] [Google Scholar]
- 29.Sowell, A. L., D. L. Huff, P. R. Yeager, S. P. Caudill, and E. W. Gunter. 1994. Retinol, α-tocopherol, lutein/zeaxanthin, β-cryptoxanthin, lycopene, α-carotene, trans-β-carotene, and four retinyl esters in serum determined simultaneously by reversed-phase HPLC with multiwavelength detection. Clin. Chem. 40:411-416. [PubMed] [Google Scholar]
- 30.Stephensen, C. B. 2001. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 21:167-192. [DOI] [PubMed] [Google Scholar]
- 31.Tsicopoulos, A., Q. Hamid, V. Varney, S. Ying, R. Moqbel, S. R. Durham, and A. B. Kay. 1992. Preferential messenger RNA expression of Th1-type cells (IFNγ+, IL-2+) in classical delayed-type (tuberculin) hypersensitivity reactions in human skin. J. Immunol. 148:2058-2061. [PubMed] [Google Scholar]
- 32.The Vitamin A and Pneumonia Working Group. 1995. Potential interventions for the prevention of childhood pneumonia in developing countries: a meta-analysis of data from field trials to assess the impact of vitamin A supplementation on pneumonia morbidity and mortality. Bull. W. H. O. 73:609-619. [PMC free article] [PubMed] [Google Scholar]
- 33.West, K. P., Jr., R. P. Pokhrel, J. Katz, S. C. LeClerq, S. K. Khatry, S. R. Shrestha, E. K. Pradhan, J. M. Tielsch, M. R. Pandey, and A. Sommer. 1991. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet 338:67-71. [DOI] [PubMed] [Google Scholar]