Abstract
Intestinal intraepithelial lymphocytes (i-IEL) expressing CD8α are located in the intestine and may confer protection against invasion of intestinal microflora. We found that mice rendered deficient in CD8α molecules by homologous recombination were susceptible to 5-fluorouracil (5-FU)-induced lethality accompanied by translocation of members of the enterobacteria. The number of i-IEL was greatly reduced on day 6 after 5-FU administration in both CD8α+/− mice and CD8α−/− mice, whereas the recovery of the level of i-IEL thereafter was significantly impaired in CD8α−/− mice compared with that in CD8α+/− mice. The ability of i-IEL to produce gamma interferon in response to immobilized T-cell receptor (TCR) αβ or TCR γδ monoclonal antibodies was significantly lower in CD8α−/− mice than in CD8α+/− mice. Transfer of CD8+ i-IEL conferred significant protection against 5-FU-induced lethality in CD8α−/− mice. The results suggest that CD8+ i-IEL play an important role in protection against 5-FU-induced lethality with translocation of Enterobacteriaceae.
Intestinal intraepithelial lymphocytes (i-IEL) are localized to the basolateral surface of intestinal epithelial cells (i-EC), which are continuously exposed to numerous environmental antigens via the intestinal epithelium (18, 24). Murine i-IEL consist of approximately equal amounts of T-cell receptor (TCR) αβ and γδ i-IEL and unique populations bearing CD8 homodimeric α chains in addition to those bearing CD8 heterodimeric α and β chains (6, 15, 17, 27). A significant fraction of i-IEL are thought to differentiate extrathymically presumably at a local site of the intestine such as the crypt patch (29, 36, 37, 39).
i-IEL produce a variety of cytokines, including Th1-type cytokines, Th2-type cytokines, and transforming growth factor beta (TGF-β), and have a helper function for local immunoglobulin A (IgA) response (10). i-IEL are also thought to play important roles in homeostasis of intestinal epithelial cells through production of cytokines such as TGF-β and keratinocyte growth factor (5, 25). i-IEL also exhibit non-major histocompatibility complex (MHC)-restricted cytotoxicity via serine esterase- and Fas/Fas-L-dependent mechanisms that provide surveillance against infected cells, premalignant cells, and effete cells (23, 27, 38).
At least a significant fraction of i-IEL represent a first line of host defense against infections with diverse pathogens in nature. We and others have reported that i-IEL produced gamma interferon (IFN-γ) by per os infection with Listeria monocytogenes, suggesting that i-IEL play a role in host defense against oral bacterial infection (20, 30, 43). On the other hand, TCR-δ-deficient mice showed exaggerated intestinal damage after oral infection with Eimeria verformis (35), suggesting that a significant fraction of i-IEL regulate inflammation caused by infection. Taken together, the findings in previous studies suggest that i-IEL play important roles in mucosal immunity via various functions, including surveillance, maintenance of homeostasis, and differentiation.
5-Fluorouracil (5-FU) is an antimetabolic chemotherapeutic agent with multiple mechanisms of action, including inhibition of the synthesis of thymidine nucleotides and incorporation into RNA (14, 32, 33). Although 5-FU is a widely used antineoplastic agent, the cytotoxicity of 5-FU is not limited to tumor tissue. Hematopoietic cells and normal epithelial cells of the gastrointestinal tract are susceptible to 5-FU-induced cytotoxicity, which produces severe leukopenia and intestinal toxicity, leading to lethal translocation of intestinal microflora (21, 26, 31, 41). We have previously reported that the number of CD8+ i-IEL was severely reduced after 5-FU administration and that a nonapaeide thymic hormone, facteur thymique serique (FTS), accelerated recovery in a number of CD8+ i-IEL following 5-FU administration and protected mice from 5-FU-induced bacterial translocation (22). These results suggest that CD8+ i-IEL play an important role in protection against bacterial translocation.
In this study, to elucidate the protective roles of CD8+ i-IEL in bacterial location, we examined the susceptibility of mice genetically deficient in CD8α molecules to 5-FU-induced mortality. We found that CD8α-deficient mice were highly susceptible to 5-FU-induced lethality and that adoptive transfer of CD8+ i-IEL conferred significant protection against 5-FU-induced mortality in CD8α-deficient mice. The implications of the present findings are discussed in terms of the protective roles of CD8+ i-IEL in bacterial translocation.
MATERIALS AND METHODS
Mice.
CD4-deficient mice, CD8α-deficient mice, and their heterozygous controls of the C57BL/6 background were provided by T. W. Mak (University of Toronto, Toronto, Canada) (12, 34). B6-Ly5.1 mice (H-2b) were kindly provided by Kenji Kishihara (Department of Immunology, Medical Institute of Bioregulation, Kyusyu University, Fukuoka, Japan). Mice were used in experiments at 8 weeks of age. Sterile food and water were given ad libitum. All of the mice were bred under specific-pathogen-free conditions.
Treatment of mice with 5-FU.
Mice were injected intraperitoneally (i.p.) with 600 or 800 mg of 5-FU (Kyowa Hakkou Kogyo, Tokyo, Japan) per kg.
Counting of endogenous bacterial colonies.
The livers and spleens were removed and placed separately in homogenizers containing 5 ml of cold phosphate-buffered saline (PBS). The organs were homogenized thoroughly, and the homogenates were serially diluted with PBS. Samples were spread on agar medium plates to detect enterobacteria (MacConkey; Nissui Pharmaceutical, Tokyo, Japan), and colonies were counted after incubation for 24 h at 37°C.
Cell preparation.
i-IEL were prepared according to the previously described procedure with some modifications (22). Briefly, the small intestine from the mice was cut into 5-mm pieces and stirred at room temperature for 30 min in medium 199 (Gibco, Grand Island, N.Y.) containing 10% inactivated fetal calf serum and 1 mM dithiothreitol. After shaking, the cells were passed through gauze to remove debris. The passaged cells were centrifuged through a 25%-40%-75% discontinuous Percoll (Pharmacia, Uppsala, Sweden) gradient at 600 × g at 20°C for 20 min. The i-IEL were obtained at the 40%-75% interface. Splenocytes were obtained by gently crushing the spleens between two slides. The number of viable cells was counted by trypan blue staining.
Flow cytometry.
All cell preparations suspended in Hanks' balanced salt solution (HBSS) containing 2.5% Nu-serum (Becton Dickinson) and 0.1% NaN3 were stained with appropriate monoclonal antibodies (MAbs) at 4°C for 30 min. The MAbs used in these experiments were as follows: fluorescein isothiocyanate (FITC)-conjugated anti-CD3ɛ (145-2C11) MAb, Cy-chrome-conjugated anti-CD4 (L3T4) MAb and phycoerythrin (PE)-conjugated or biotin-conjugated anti-CD8α (Lyt2) MAb, Cy-chrome-conjugated anti-TCRαβ MAb, PE-conjugated anti-TCRγδ MAb, and purified anti-Ly5.1 MAb. The MAbs were all purchased from Pharmingen (San Diego, Calif.). Cy-chrome-conjugated anti-mouse immunoglobulin G (IgG; heavy and light chains) were obtained from Amersham Pharmacia Biotech (Uppsala, Sweden). Red613-conjugated streptavidin was purchased from Gibco-BRL (Gaithersburg, Md.). Two- or three-color analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, Calif.). The live lymphocytes were carefully gated by forward and side scattering. The data were analyzed using CellQuest software (Becton Dickinson).
Cytokine production.
i-IEL were cultured in 200 μl of a complete culture RPMI medium in 96-well flat-bottomed plates (Falcon; Becton Dickinson Ltd., Oxford, England) at a density of 105 cells/well with anti-TCRαβ (H57-597, 10 μg/ml) or anti-TCRγδ MAb (UC7-13D5, 10 μg/ml) that had been immobilized on the plates by prior incubation at 4°C overnight. The cells were cultured for 72 h at 37°C under 5% CO2 in air and pulsed with 1 μCi of [3H]thymidine deoxyribose (TdR) 6 h before harvest. [3H]TdR incorporation was determined by liquid scintillation counting. The supernatant was collected to estimate cytokine production after culturing for 72 h. The cell-free culture supernatants were collected from the 72-h culture of i-IEL, and the cytokine activity in the culture supernatant was assayed by enzyme-linked immunosorbent assay (ELISA).
IgA in fecal samples.
Fecal sample (0.1 g) was incubated with 1 ml of PBS at room temperature for 60 min, vortexed, left for 15 min, revortexed until all materials were suspended, and centrifuged at 2,000 × g for 10 min. The supernatants were removed and tested for total IgA by ELISA.
Cell transfer.
i-IEL (107) from Ly5.1 congenic mice were adoptively transferred into recipient mice via tail vein inoculation. At 3 days after the adoptive transfer of these cells, mice were challenged with 5-FU.
Statistical analysis.
Student's t test was used to determine the significance of differences in cell number. A P value of less than 0.05 was taken as significant. The statistical significance of the survival rate was determined by the generalized Wilcoxon's test.
RESULTS
Increased susceptibility of CD8α-deficient mice to lethality caused by 5-FU administration.
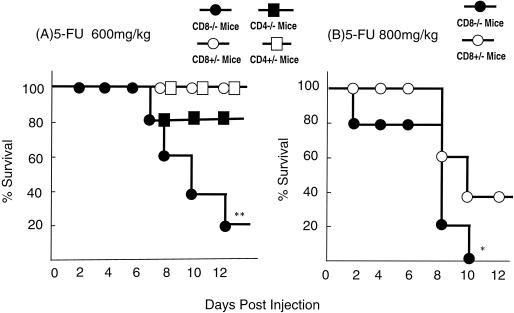
We first compared the susceptibilities of CD8α- and CD4-deficient mice to 5-FU-induced lethality. All of the CD4+/− mice and CD8α+/− mice (n = 20 in each group) survived for more than 18 days after administration of 600 mg of 5-FU per kg. Eighty percent of the CD4−/− mice survived after 5-FU administration, whereas 80% of the CD8α−/− mice died within 12 days after 5-FU administration (P < 0.01, Fig. 1). Similarly, 40% of the CD8α+/− mice (n = 10 in each group) survived following administration of 800 mg of 5-FU per kg, whereas all of the CD8α−/− mice died within 10 days after administration (P < 0.05). Thus, CD8α−/− mice are more susceptible to 5-FU-induced lethality than are CD8α+/− mice.
FIG. 1.
Survival rate of CD8α-deficient mice after 5-FU administration. CD8α+/− and CD8α−/− mice were treated i.p. with 600 or 800 mg of 5-FU per kg, and CD4+/− and CD4−/− mice were treated i.p. with 600 mg of 5-FU per kg. Twenty (600 mg/kg) or 10 mice (800 mg/kg) were used in each group. ∗, P < 0.05, and ∗∗, P < 0.01 compared with control litermates by the generalized Wilcoxon's test.
Bacterial translocation in CD8α-deficient mice after 5-FU administration.
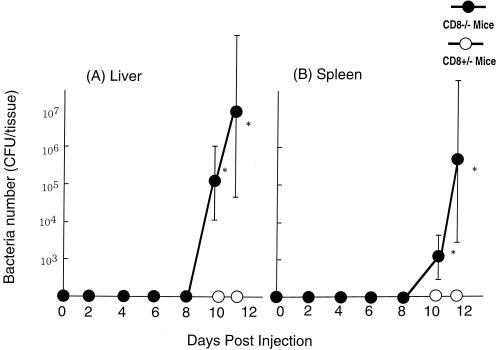
It has been reported that a high dose of 5-FU often induces cytotoxicity in intestinal tissue, including i-EC and i-IEL, resulting in ulceration, diarrhea, and bacterial translocation (8). To determine whether bacterial translocation occurs in CD8α−/− mice following 5-FU administration, we examined the number of enterobacteria in the liver and spleen after administration of 5-FU (600 mg/kg). Large numbers of bacteria were found in the liver and spleen of CD8α−/− mice on day 10 after 5-FU administration, whereas few if any bacteria were detected in CD8α+/− mice on days 10 and 11 after 5-FU administration (P < 0.05, Fig. 2). These results suggest that CD8α-deficient mice are susceptible to 5-FU-induced mortality accompanied by bacterial translocation from the intestine.
FIG. 2.
Growth of endogenous bacteria in CD8α-deficient mice administered 5-FU. Numbers of bacteria in the liver and spleen of CD8α+/− and CD8α−/− mice were determined on the indicated days after intraperitoneal administration of 600 mg of 5-FU per kg. Each point and vertical bar represent the mean ± standard deviation (SD) of six animals. ∗, P < 0.05, significant difference compared with CD8α+/− mice.
Impaired recovery of i-IEL in CD8α-deficient mice after 5-FU administration.
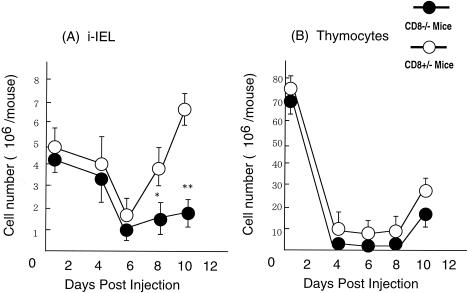
We previously reported that the numbers of i-IEL dramatically decreased in the first 6 days after a single injection of 5-FU and thereafter recovered to the initial level by day 10 (22). In the present study, we next compared the kinetics of i-IEL and thymocytes following 5-FU administration in CD8α−/− mice and CD8α+/− mice.
The numbers of i-IEL in CD8α−/− mice and CD8α+/− mice on day 6 were almost the same (Fig. 3A). The level of i-IEL recovered rapidly to the initial level by day 8 in CD8α+/− mice, whereas the recovery was severely impaired in CD8α−/− mice on day 8 (P < 0.05) and on day 10 (P < 0.01) after 5-FU administration. There was no significant difference between cell numbers in the thymus (Fig. 3B) or the spleen (data not shown) in CD8α−/− and CD8α+/− mice. These results suggest that CD8α molecules play an important role in i-IEL differentiation.
FIG. 3.
Kinetics of i-IEL and thymus of CD8α-deficient mice after 5-FU administration. Cell numbers in (A) i-IEL and (B) thymus in CD8α+/− and CD8α−/− mice were determined on the days indicated after intraperitoneal administration of 5-FU (600 mg/kg). Each point and vertical bar represent the mean ± SD of six animals. ∗, P < 0.05; ∗∗, P < 0.01, significant difference compared with the value in CD8α+/− mice.
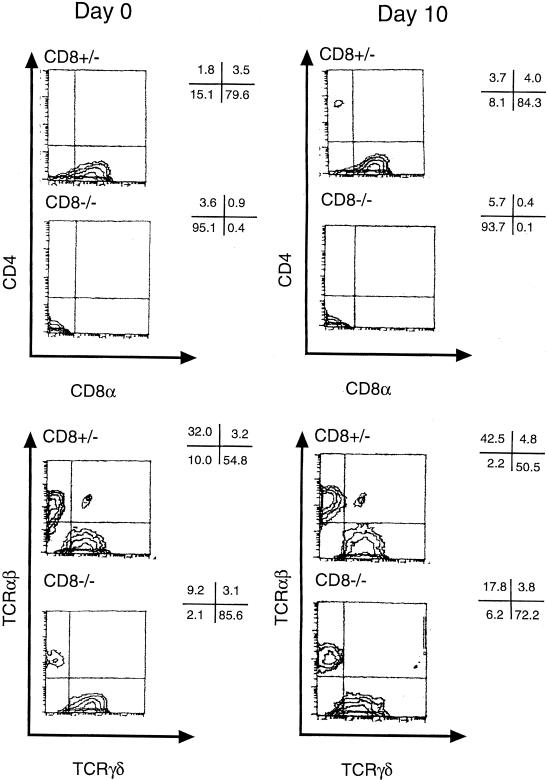
i-IEL consist of unique T-cell subpopulations bearing CD8αα and CD8αβ or TCR αβ and γδ (18, 24). The changes in i-IEL subpopulations were analyzed by flow cytometer before (day 0) and on day 10 after 5-FU administration. Representative results are shown in Fig. 4. The i-IEL in naive CD8α+/− mice consisted of 80% CD4− CD8α+ cells and a few CD4− CD8− T cells and CD4+ CD8+ T cells, whereas most of the i-IEL in naive CD8α−/− mice were of the CD4− CD8− phenotype. Approximately half of the i-IEL in CD8α+/− mice were TCR γδ cells, whereas more than 80% of i-IEL in CD8α−/− mice were TCR γδ cells. In both groups, the composition of i-IEL on day 10 after 5-FU administration was much the same as before 5-FU administration.
FIG. 4.
Flow cytometric analysis of i-IEL for expression of CD4, CD8α, αβ TCR, and γδ TCR in CD8α-deficient mice before and after 5-FU administration. The i-IEL CD8α+/− and CD8α−/− mice were recovered before (day 0) and 10 days after intraperitoneal administration of 5-FU (600 mg/kg). The i-IEL were stained with PE-anti-CD8α MAb or -anti-TCRγδ MAb and Cy-chrome-anti-CD4 MAb or anti-TCR αβ MAb for FACS analysis. Values represent percentages of subpopulations in selected areas. The FACS analysis results shown are representative of three separate experiments.
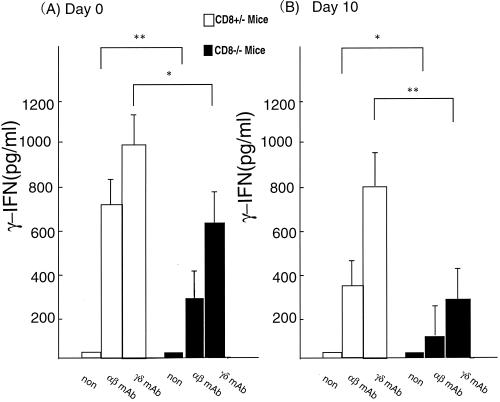
Impaired IFN-γ production by i-IEL in CD8α-deficient mice after 5-FU administration.
i-IEL produce a variety of cytokines, including Th1-type cytokines, Th2-type cytokines, and TGF-β (9). We next examined cytokine production by i-IEL from CD8α−/− mice and CD8α+/− mice before and after 5-FU administration. i-IEL were incubated with immobilized anti-TCR αβ MAb or anti-TCR γδ MAb for 48 h, and cytokine activity was examined in the culture supernatant by ELISA.
As shown in Fig. 5, i-IEL from naive CD8α−/− mice produced significantly less IFN-γ than those from CD8α+/− mice in response to immobilized anti-TCR αβ MAb or anti-TCRγδ MAb. Similarly, the ability of the i-IEL to produce IFN-γ was impaired in CD8α−/− mice on day 8 after 5-FU administration compared with those from CD8α+/− mice. The level of IL-4 in the culture supernatants of i-IEL stimulated with anti-TCR MAbs was marginal in both CD8α−/− and CD8α+/− mice, and conclusive results on TGF-β production could not be obtained because the measurements resulted in high medium background (data not shown).
FIG. 5.
IFN-γ production by i-IEL in CD8α-deficient mice upon triggering of TCR. i-IEL cells were obtained from CD8α+/− and CD8α−/− mice before (day 0) and 10 days after intraperitoneal administration of 5-FU (600 mg/kg). The i-IEL were cultured in the presence or the absence of immobilized anti-TCRαβ or γδ MAb (100 μg/ml). Cytokine levels in the supernatants were determined by ELISA. Data are means ± SD for five mice in each group. ∗, P < 0.05 and ∗∗, P < 0.01 by Student's t test: statistically significant differences from the value for CD8α+/− mice. Representative data from three independent experiments are shown.
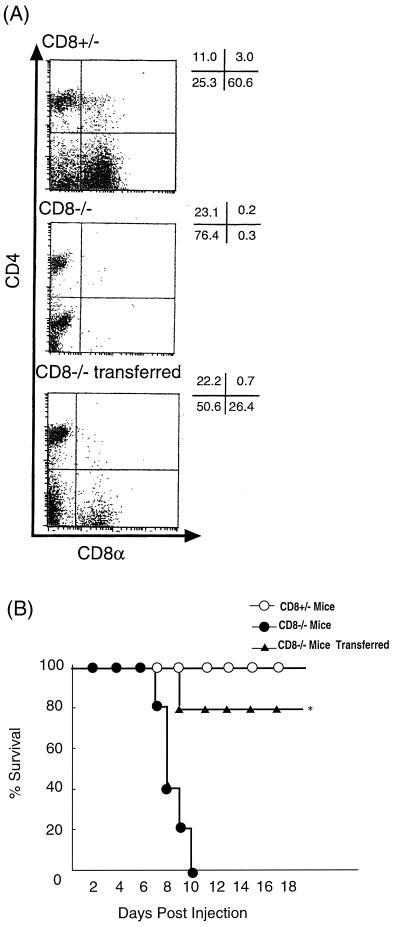
Effect of adoptive transfer of i-IEL from normal mice on protection of CD8α−/− mice from 5-FU-induced lethality.
To try to elucidate the role of i-IEL in protection against 5-FU-induced lethality, adoptive transfer experiments were conducted in Ly5.2+ CD8α−/− mice using i-IEL from naive Ly5.1 congeneic mice. We confirmed that adoptive transfer with Ly5.1+ i-IEL reconstituted the i-IEL population in Ly5.2+ CD8α−/− mice 3 days after transfer (Fig. 6A). As shown in Fig. 6B, the adoptive transfer with i-IEL conferred protection against 5-FU-induced lethality in CD8α−/− mice. No bacteria were detected in surviving CD8−/− mice receiving i-IEL on day 10 after 5-FU administration.
FIG. 6.
Survival rate of CD8α-deficient mice receiving i-IEL transfer following 5-FU administration. i-IEL (107) from Ly5.1 congeneic mice were adoptively transferred into Ly5.2 recipient mice via tail vein inoculation. (A) FACS analysis of i-IEL from CD8α−/− mice into which i-IEL had been transferred. i-IEL were collected on day 3 after transfer and stained with Cy-chrome-anti-CD4 MAb and PE-anti-CD8α MAb or PE-anti-TCR γδ MAb and anti-Ly5.1 MAb plus Cy-chrome anti-mouse IgG. Dot plot analysis is presented as typical two-dimensional profiles. Numbers represent the percentage of total cells found in each quadrant. (B) At 3 days after the adoptive transfer of these cells, mice were challenged with 5-FU (600 mg/kg) and monitored for survival. Representative data from three independent experiments are shown. ∗, P < 0.01 by the generalized Wilcoxon's test; statistically significant difference from CD8α−/− mice.
DISCUSSION
In the present study, we demonstrated that CD8α-deficient mice were highly susceptible to 5-FU-induced lethality accompanied by translocation of enterobacteria. Transfer of i-IEL conferred resistance to the 5-FU-induced lethality in CD8α-deficient mice. These results suggest that CD8+ i-IEL play an important role in protection against 5-FU-induced lethality with bacterial translocation.
A high dose of 5-FU would destroy the first line of defense, such as intestinal epithelial cells and immunocompetent cells derived from hematopoetic cells, -against intestinal microflora, leading to lethal translocation of intestinal microflora (21, 26, 31, 41). CD8+ i-IEL are located at the basolateral surfaces of i-EC, which are continuously exposed to numerous environmental antigens via the intestinal epithelium (18, 24). We showed that the number of i-IEL was severely reduced on day 5 after 5-FU administration in both CD8α+/− mice and CD8α−/− mice, whereas the recovery of the level of i-IEL thereafter was significantly impaired in CD8α−/− mice compared with CD8α+/− mice.
The IEL from CD8α−/− mice before and after 5-FU administration showed an impaired ability to produce IFN-γ, which is important for protection against bacteria. Yamamoto et al. reported that i-IEL produce IFN-γ after per os infection with Listeria monocytogenes (43). We have also shown that i-IEL produced IFN-γ at the early stage during oral infection with L. monocytogenes (20, 30). Taken together, the finding suggests that IFN-γ produced by CD8+ i-IEL function in host defense against microbial translocation and that the absence of CD8α i-IEL in CD8α-deficient mice hampers the control of intestinal microflora, leading to lethal translocation of intestinal microflora.
i-IEL are thought to produce a variety of cytokines, including not only Th1-type cytokines but also Th2-type cytokines and TGF-β, and have a helper function for local IgA response (5, 25). IgA against intestinal microflora is important for protection against invasion of microbial flora (1, 7). Therefore, it is also possible that IgA production is impaired in CD8α-deficient mice, leading to lethal bacterial translocation. However, there are several lines of evidence that T-cell-mediated immunity is more important for protection against bacterial translocation than IgA (3, 44).
Our preliminary data showed that total IgA in feces did not differ between CD8α−/− mice and CD8α+/− mice (6.74 ± 2.21 versus 5.85 ± 0.86 μg/mg). The difference between IgA specific for intestinal microflora in CD8α−/− mice and that in CD8α+/− mice may not be responsible for the difference in susceptibilities to 5-FU-induced bacterial translocation. A significant fraction of i-IEL, such as γδ i-IEL, have been reported to play important roles in homeostasis of intestinal epithelial cells through production of cytokines such as keratinocyte growth factor (5, 25). Therefore, it is also possible that turnover of i-EC may be impaired in CD8α−/− mice administered 5-FU, resulting in increased bacterial translocation from the intestine. Furthermore, maturation of the i-EC in CD8α−/− mice may be impaired, making them highly susceptible to 5-FU-induced cytotoxicity. Further analysis of the mode of action by which CD8α i-IEL protect mice from 5-FU-induced lethality is needed.
CD8α-deficient mice had a large number of CD4− CD8− i-IEL and a small number of CD4+ CD8− i-IEL. However, CD8α-deficient mice were found to be more susceptible to 5-FU-induced lethality than normal mice. Thus, CD8+ i-IEL but not CD4+ CD8− or CD4− CD8− i-IEL may be essential for protection against 5-FU-induced lethality.
Mouse CD8+ i-IEL include unique populations bearing CD8 homodimeric α chains besides i-IEL bearing CD8 heterodimeric α and β chains (18, 24). Most of the CD8αβ i-IEL are thought to recognize antigens from intestinal flora presented by MHC class Ia molecules, composed of an α chain associated with β2 microglobulin (β2m), whereas CD8+ i-IEL recognize TAP-independent peptides in the context of MHC class Ib molecules, such as Tla, encoded by the T3 and T18 genes, and CD1 molecules, both of which are expressed by i-EC in association with β2m (4, 11, 13, 19, 40, 42). Beagley et al. reported that the reactivity of i-IEL to syngeneic spleen cells is enhanced by bacterial antigens such as PPD, HSP70, and HSP60 (2). It has also been reported that human γδ i-IEL recognize stress-induced MHC class I-like molecules MICA and -B (16). We speculate that CD8αβ and CD8αα i-IEL recognize bacterial antigen or stress-induced proteins in the context of MHC class Ia or class Ib, respectively, and confer protection against bacterial translocation. On the other hand, CD4− CD8− i-IEL may not be able to efficiently recognize such antigens in the absence of CD8α molecules and thus not confer protection against 5-FU-induced lethality. At present, it is not known which population of CD8αβ and CD8αα i-IEL is mainly involved in protection against 5-FU-induced lethality. Further experiments using mice deficient in the CD8β molecule and transfer experiments with each population are needed to clarify this.
In conclusion, we demonstrated that CD8α-deficient mice were highly susceptible to 5-FU-induced lethality accompanied by bacterial translocation of enterobacteria. Transfer of i-IEL conferred resistance to 5-FU-induced lethality in CD8α-deficient mice. These results indicate that CD8+ i-IEL play an important role in protection against 5-FU-induced lethality. Transfer of CD8+ i-IEL precursor cells or cytokines derived from CD8+ i-IEL may be useful for prevention of 5-FU-induced bacterial translocation.
Acknowledgments
We thank T. W. Mak (Toronto University) and K. Kishihara (Kyushu University) for providing the mice.
This work was supported in part by a Grant-in Aid for Scientific Research, Yamada Science Foundation and the Yakult Bioscience Foundation.
REFERENCES
- 1.Albanese, C. T., S. D. Smith, S. Watkins, A. Kurkchubasche, R. L. Simmons, and M. I. Rowe. 1994. Effect of secretory IgA on transepithelial passage of bacteria across the intact ileum in vitro. J. Am. Coll. Surg. 179:679-688. [PubMed] [Google Scholar]
- 2.Beagley, K. W., K. Fujihashi, C. A. Black, A. S. Lagoo, M. Yamamoto, J. R. McGhee, and H. Kiyono. 1993. The mycobacterium tuberculosis 71kDa heat-shock protein induces proliferation and cytokine secretion by murine gut intraepithelial lymphocytes. Eur. J. Immunol. 23:2049-2052. [DOI] [PubMed] [Google Scholar]
- 3.Berg, R. D., and A. W. Garlington. 1980. Translocation of Escherichia coli from the gastrointestinal tract to the mesenteric lymph nodes in gnotobiotic mice receiving Escherichia coli vaccines before colonization. Infect. Immun. 30:894-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleicher, P. A., S. P. Balk, S. J. Hagen, R. S. Blumberg, T. Flotte, and J. C. Terhorst. 1990. Expression of murine CD1 on gastrointestinal epithelium. Science 250:679-683. [DOI] [PubMed] [Google Scholar]
- 5.Boismenu, R., and W. L. Havran. 1994. Modulation of epithelial cell growth by intraepithelial cell growth byintraepithelialgd cell. Science 266:1253-1255. [DOI] [PubMed] [Google Scholar]
- 6.Bonneville, M., C. A. Janeway, K. Ito, W. Haser, I. Ishida, N. Nakanishi, and S. Tonegawa. 1988. Intestinal intraepithelial lymphocytes are a distinct set of γδ T cells. Nature 336:479-481. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson, E. C., J. C. Gorga, M. Garrett, R. Tuncer, P. Boyle, S. C. Watkins, S. M. Alber, M. Parizhskaya, M. Trucco, M. I. Rowe, and H. R. Ford. 1998. Immunoglobulin A supplementation abrogates bacterial translocation and preserves the architecture of the intestinal epithelium. Surgery 124:284-290. [PubMed] [Google Scholar]
- 8.Erlichman, C., S. Fine, A. Wong, and T. A. Elhakim. 1988. A randomized trial of fluorouracil and folinicacid in patients with metastatic colorectal cancer. J. Clin. Oncol. 6:469-475. [DOI] [PubMed] [Google Scholar]
- 9.Fujihashi, K., M. Yamamoto, J. R. McGhee, K. W. Beagley, and H. Kiyono. 1993. Function of αβ TCR+ intestinal intraepithelial lymphocytes: Th1- and Th2-type cytokine production by CD4+CD8− and CD4+CD8+ T cells for helper activity. Int. Immunol. 5:1473-1481. [DOI] [PubMed] [Google Scholar]
- 10.Fujihashi, K., M. Yamamoto, and J. R. McGhee. 1993. Mucosal immunology: intraepithelial lymphocytes p. 89. In H. Kyono and J. R. McGhee (ed.), Immunoregulatory function and cytokine production by αβTCR+ and γδTCR+ T cells for mucosal immune responses. Raven Press, Ltd., New York, N.Y.
- 11.Fujiura, Y., M. Kawaguchi, Y. Kondo, S. Obana, H. Yamamoto, M. Nanno, and H. Ishikawa. 1996. Development of CD8 alpha alpha+ intestinal intraepithelial T cells in beta 2-microglobulin- and/or TAP1-deficient mice. J. Immunol. 156:2710-2715. [PubMed] [Google Scholar]
- 12.Fung-Leung, W. P., M. W. Schilham, A. Rahemtulla, T. M. Kundig, M. Vollenweider, J. Potter, W. van Ewijk, and T. W. Mak. 1991. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell 65:443-452. [DOI] [PubMed] [Google Scholar]
- 13.Gapin, L., H. Cheroute, and M. Kronenberg. 1999. TCRαβ+ CD8αα+ T cells are found in intestinal intraepithelial lymphocytes of mice that lack class I molecules. J. Immunol. 163:4100-4104. [PubMed] [Google Scholar]
- 14.Geoffroy, F. J., C. J. Allegra, B. Sinha, and J. L. Grem. 1994. Enhanced cytotoxicity with interleukin-1 alpha and 5-fluorouracil in HCT116 colon cancer cells. Oncol. Res. 6:581-591. [PubMed] [Google Scholar]
- 15.Goodman, T., and L. Lefrançois. 1988. Expression of the γ-δ T-cell receptor on intestinal CD8+ intraepithelial lymphocyes. Nature 333:855-858. [DOI] [PubMed] [Google Scholar]
- 16.Groh, V., A. Steinle, S. Bauer, and T. Spies. 1999. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science 279:1737-1740. [DOI] [PubMed] [Google Scholar]
- 17.Guy-Grand, D., N. Cerf-Bensussan, B. Malissen, M. Malassis-Seris, C. Briottet, P. Vassalli. 1991. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J. Exp. Med. 173:471-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guy-Grand, D., and P. Vassalli. 1993. Gut intraepithelial T lymphocytes. Curr. Opin. Immunol. 5:247-252. [DOI] [PubMed] [Google Scholar]
- 19.Hershberg, R., P. Eghtesady, B. Sydora, K. Brorson, H. Cheroutre, R. R. Modlin, and M. M. Kronenberg. 1990. Expression of the thymus leukemia antigen in mouse intestinal epithelium. Proc. Natl. Acad. Sci. USA 87:9727-9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirose, K., H. Suzuki, H. Nishimura, A. Mitani, J. Washizu, T. Matsuguchi, and Y. Yoshikai. 1998. Interleukin-15 might be responsible for early activation of intestinal intraepithelial lymphocytes after oral infection with Listeria monocytogenes in rats. Infect. Immun. 66:5677-5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ijiri, K., and C. S. Potten. 1987. Further studies on the response of intestinal crypt cells of different hierarchical status to eighteen different cytotoxic agents. Br. J. Cancer 55:113-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inagaki-Ohara, K., N. Kobayashi, H. Nishimura, T. Sakai, Y. Matumoto, K. Hiromatsu, A. Awaya, and Y. Yoshikai. 1996. Effects of a nonapeptide thymic hormone on intestinal intraepithelial lymphocytes in mice following administration of 5-fluorouracil. Cell. Immunol. 171:30-40. [DOI] [PubMed] [Google Scholar]
- 23.Inagaki-Ohara, K., H. Nishimura, H. Inagaki, T. Sakai, M. Takano, D. H. Lynch, and Y. Yoshikai. 1997. Involvement of fas antigen/fas ligand interaction in apoptosis of epithelial cells by intraepithelial lymphocytes in murine small intestine. Lab. Investig. 77:421-429. [PubMed] [Google Scholar]
- 24.Klein, J. R. 1995. Advances in intestinal T-cell development and function. Immunol. Today 16:322-324. [DOI] [PubMed] [Google Scholar]
- 25.Komano, J. R., Y. Fujiura, M. Kawaguchi, S. Matsumoto, Y. Hashimoto, S. Obana, P. Mombaerts, S. Tonegawa, H. Yamamoto, S. Itohara, M. Nanno, and H. Ishikawa. 1995. Homeostatic regulation of intestinal epithelia by intraepithelial γδ T cells. Proc. Natl. Acad. Sci. USA 92:6147-6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreger, E. B., E. D. Craven, C. P. Catliong, and R. W. McMabe. 1980. Gram-negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients. Am. J. Med. 68:332-343. [DOI] [PubMed] [Google Scholar]
- 27.Lefrançois, L., and T. Goodman. 1989. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-gamma delta+ intraepithelial lymphocytes. Science 243:1716-1718. [DOI] [PubMed] [Google Scholar]
- 28.Lefrançois, L. 1991. Phenotypic complexity of intraepithelial lymphocytes of the small intestine. J. Immunol. 147:1746-1751. [PubMed] [Google Scholar]
- 29.Lefrançois, L, and L. Puddington. 1995. Extrathymic intestinal T-cell development: virtual reality? Immunol. Today 16:16-21. [DOI] [PubMed] [Google Scholar]
- 30.Mitani, A., H. Nishimura, K. Hirose, J. Washizu, Y. Kimura, S. Tanaka, T. Noguchi, and Y. Yoshikai. 1999. Interleukin-15 might be responsible for early activation of intestinal intraepithelial lymphocytes after oral infection with Listeria monocytogenes in mice. Immunology 97:92-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nomoto, K., T. Yokokura, and K. Nomoto. 1992. Prevention of 5-fluorouracil-induced infection with indigenous Escherichia coli in tumor-bearing mice by nonspecific immunostimulation. Can. J. Microbiol. 38:774-778. [DOI] [PubMed] [Google Scholar]
- 32.Parker, W. B., and Y. C. Cheng. 1990. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol. Ther. 48:381-395. [DOI] [PubMed] [Google Scholar]
- 33.Pritchard, D. M., A. J. M. Watson, C. S. Potten, A. L. Jackman, and J. A. Hickmann. 1997. Inhibition by uridine but not thymidine of p53-dependent intestinal apoptosis initiated by 5-fluorouracil:evidence for the involvement of RNA perturbation. Proc. Natl. Acad. Sci. USA 94:1795-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahemtulla, A., W. P. Fung-Leung, M. W. Schilham, T. M. Kundig, S. R. Sambhara, A. Narendran, A. Arabian, A. Wakeham, C. J. Paige, and R. M.Zinkernagel. 1991. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature 353:180-183. [DOI] [PubMed] [Google Scholar]
- 35.Roberts, S. J., A. L. Smith, A. B. West, L. Wen, R. C. Findly, M. J. Owen, and A. C. Hayday. 1996. T-cell αβ+ and γδ+ deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of theintestinal epithelium. Proc. Natl. Acad. Sci. USA 93:11774-11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rocha, B., P. Vassalli, and D. Guy-Grand. 1994. Thymic and extrathymic origins of gut intraepitherial lymphocyte populations in mice. J. Exp. Med. 180:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito, H., Y. Kanamori, T. Takemori, H. Nariuchi, E. Kubota, H. Takahashi-Iwanaga, T. Iwanaga, and H. Ishikawa. 1998. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science 280:275-278. [DOI] [PubMed] [Google Scholar]
- 38.Sakai, T., K. Kusugami, H. Nishimura, T. Ando, T. Yamaguchi, M. Ohsuga, K. Ina, A. Enomoto, Y. Kimura, and Y. Yoshikai. 1997. Fas-mediated cytotoxicity by host intestinal intraepithelial lymphocytes is involved in the enteropathy during acute graft-vs.-host disease. Gastroenterology 113:168-174. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki, K., T. Oida, H. Hamada, O. Hitotsumatsu, M. Watanabe, T. Hibi, H. Yamamoto, E. Kubota, S. Kaminogawa, and H. Ishikawa. 2000. Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity 5:691-702. [DOI] [PubMed] [Google Scholar]
- 40.Sydora, B. C., L. Brossay, A. Hagenbaugh, M. Kronenberg, and H. Cheroutre. 1996. TAP-independent selection of CD8+ intestinal intraepithelial lymphocytes. J. Immunol. 156:4209-4216. [PubMed] [Google Scholar]
- 41.Tancrede, C. H., and A. O. Andremont. 1985. Bacterial translocation and gram-negative bacteremia in patients with hematological malignancies. J. Infect. Dis. 152:99-103. [DOI] [PubMed] [Google Scholar]
- 42.Wu, M. L., S. van Kaer, S. Itohara, and S. Tonegawa. 1991. Highly restricted expression of the thymus leukemia antigens on intestinal epithelial cells. J. Exp. Med. 174:213-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto, S., F. Russ, H. C. Teixeira, P. Conradt, and S. H. Kaufmann. 1993.Listeria monocytogenes-induced gamma interferon secretion by intestinal intraepithelial γ/δ T lymphocytes. Infect. Immun. 61:2154-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamazaki, S., K. Machii, S. Tsuyuki, H. Momose, T. Kawashima, and K. Ueda. 1985. Immunological responses to monoassociated Bifidobacterium longum and their relation to prevention of bacterial invasion. Immunology 56:43-50. [PMC free article] [PubMed] [Google Scholar]