Abstract
Clinically visceral leishmaniasis is suspected in only a fraction of infected persons, as the majority of these may not have clinical manifestations and remain asymptomatic. There is scanty information on diagnosing latent infections and predicting disease in asymptomatic persons. We therefore carried out a study on asymptomatic contacts of patients with visceral leishmaniasis and post-kala-azar dermal leishmaniasis by using methods for detection of antibody to recombinant K39 (rK39) antigen. A total of 240 patients with leishmaniasis and 150 asymptomatic contacts were tested for anti-rK39 immunoglobulin G (IgG) and IgA antibodies. Fifty-five asymptomatic persons were found to be seropositive. These individuals were monitored every 3 months for 1 year. On follow-up, 43.9% of the asymptomatic seropositive contacts developed kala-azar within the first 3 months, and a cumulative total of 69% developed kala-azar within 1 year. The rest remained asymptomatic and self-healed the infection. The sensitivity and specificity of rK39 enzyme-linked immunosorbent assay (ELISA) and dipstick tests were 100%, while an in-house-developed latex agglutination test had 80% sensitivity. The antibody profile showed that the IgG anti-rK39 antibodies reached a titer of up to 10−6 within 6 months of infection, started declining thereafter, and completely disappeared in 2 to 3 years in successfully treated cases. Significant titers of IgA antibodies were detectable a little earlier than those of IgG antibodies and were undetectable after 6 months. The study showed that mass screening of family members and contacts by using anti-rK39 ELISA could be a highly reliable tool for early diagnosis and to plan prophylactic treatment of latently infected asymptomatic carriers to eradicate kala-azar.
Visceral leishmaniasis (VL), or kala-azar, is a parasitic disease caused by the flagellated hemoparasite Leishmania donovani in India. The infection is transmitted from infected to uninfected persons through the bites of a tiny insect, the sandfly (11). Occasional reports of transmission through unscreened blood transfusion and of transplacental transmission are also on record (11, 12, 16). The disease is endemic in northeastern parts of India, along the Ganges River, mainly in the states of West Bengal, Bihar, and Uttar Pradesh (11, 12, 16). Recently, new foci in sub-Himalayan parts of north India have also been reported (13).
The infection causes the loss of thousands of lives and prolonged morbidity, with severe economic consequences. Kala-azar is characterized by irregular fever, malaise, loss of weight, splenomegaly, sometimes hepatomegaly, and anemia with or without lymphadenopathy. If untreated, the mortality is nearly 100%. A peculiar feature of Indian kala-azar is darkening of the patient's skin, from which the name kala (blackening)-azar (fever) was derived (11).
A common estimate of the worldwide annual incidence is 600,000 newly reported clinical cases. The overall prevalence of leishmaniasis is 12 million cases, and the estimated population at risk is about 350 million (1). However, it is difficult to provide realistic estimates of the numbers of those infected compared to those at risk. There is an even greater difference between the number of cases actually occurring and the number reported due to several local factors (1). The number of people infected but asymptomatic is much higher than the number infected and presenting with clinical illness. Therefore, it is important to know how many infected persons will develop disease and how they can be diagnosed before they show clinical manifestations. Here, we report our findings on the host immune response against an L. donovani specific antigen (recombinant K39 [rK39]) and its value in predicting the development of clinical disease.
MATERIALS AND METHODS
Patients.
The subjects under investigation were from the state of Bihar, India, where kala-azar is endemic, and were chosen from those who presented with clinical symptoms suggestive of leishmaniasis, i.e., fever, splenomegaly, and wasting. After informed consent was obtained, routine investigations were carried out to rule out other etiologies of fever with hepatosplenomegaly, and finally the diagnosis of VL was established by demonstration of parasites in the bone marrow or splenic aspirates at local health centers or laboratories. In suspected cases of post-kala-azar dermal leishmaniasis (PKDL) with patchy depigmentation of skin, the diagnosis was established by examining a skin biopsy for L. donovani bodies. After the final diagnosis of leishmaniasis (VL or PKDL) was made, asymptomatic family members and neighbors of index case patients were counseled and their consent for antileishmanial antibody estimation was obtained. Approximately 5 to 6 ml of blood was withdrawn from each individual in plain sterile vials. The serum was separated and stored at −20°C until further use. The patients and their asymptomatic contacts who were positive for leishmaniasis by serology were monitored every 3 months for 12 months for clinical outcome and immune profile.
rK39 antigen.
The recombinant antigen is a 39-amino-acid protein (rK39), cloned and expressed in Escherichia coli, from the C terminus of the kinesin protein of Leishmania major (4). This antigen has been reported to be highly specific for diagnosing kala-azar (3, 4, 14). The rK39 antigen and immunochromatographic dipsticks for anti-rK39 antibody detection were kind gifts from S. G. Reed of Corixa Corp., Seattle, Wash., and from InBios Inc., Seattle, Wash.
ELISA for anti-rK39 antibody detection.
Serum samples were tested for immunoglobulin G (IgG) or IgA anti-rK39 antibody detection by enzyme-linked immunosorbent assay (ELISA) according to the standard method described earlier, with minor modifications (14). In brief, Dynatech polystyrene microtiter plates were coated overnight with 10 ng of rK39 in 50 μl of 0.1 M bicarbonate buffer, pH 9.3. The wells were then blocked with 200 μl of 1% bovine serum albumin (Sigma manual, Sigma Chemical Co., St. Louis, Mo.) for 1 h, washed three times with phosphate-buffered saline (PBS) with Tween 20, and incubated for 2 h with 50 μl of patient serum diluted from 10−2 to the end point in PBS-Tween 20. The wells were again washed three times with the same buffer and incubated with 50 μl of goat anti-human IgG or α-chain-specific IgA conjugated with alkaline phosphatase (Boehringer, Mannheim, Germany) at a 1:10 dilution for another 2 h, followed by rinsing three times with PBS-Tween 20. After incubation for 30 min at 37°C with 50 μl of para-nitrophenylphosphate (Sigma Chemical Co.) in diethylamine buffer (pH 9), the reaction was stopped with 50 μl of 3 N NaOH. The optical density of each well was measured at 405 nm with an Anthos-2 plate reader. Each sample was assayed at least in triplicate together with appropriate negative and positive serum controls. The ELISA reader was set to subtract the reading of the blank control from those of the test samples. An optical density reading of ≥0.5 U was taken as positive on the basis of values from negative controls (from areas of nonendemicity).
Dipstick ELISA.
VL assay dipsticks were prepared by Corixa Corp. and InBios Inc. The test is based on immunochromatographic principles. The purified recombinant 39-amino-acid antigen (rK39) expressed in E. coli is immobilized on a nitrocellulose membrane in a band form alongside an anti-human antibody (total) band, which serves as a test control. All instructions of the manufacturer were followed. In brief, 5 to 10 μl of serum or whole blood (whole blood was used in this study) is placed on the middle of the absorbent pad below the indicator. Following this, 2 to 3 drops of wash buffer (provided) is applied below the blood spot so that the antibodies (if present in the blood) are carried up by the siphoning action of the membrane along with the buffer. The results are read after 5 min but within 10 min. The appearance of two pink lines (one control and one test) indicates a positive result, while the appearance of only one line (control) indicates that the test methodology is correct but the sample is negative for antibodies. The test has successfully been evaluated with kala-azar patients from the Indian subcontinent (3).
Development of a latex agglutination test using rK39 antigen.
rK39 antigen was used to develop an in-house latex agglutination methodology according to the standard method of Limet et al. (10) with minor modifications. In this method, the rK39 antigen at a concentration of 1 mg/ml in glycine-buffered saline (GBS) (glycine, 0.17 mol/liter; NaCl, 0.1 mol/liter; NaN3, 0.1 g/liter [pH 8.2]) was resuspended in equal volume of latex beads (0.9-μm diameter) (Sigma manual, Sigma Chemical Co.). The suspension was incubated for 2 h at room temperature, followed by addition of an equal volume of GBS-0.1% bovine serum albumin solution and further incubation for 1 h at room temperature to stabilize the coupling. After 1 h, the suspension was centrifuged and the latex bead pellet was resuspended in a volume of GBS equal to that of the original GBS-BSA. This formed the working reagent, which was stored at 4°C for further use in clinical diagnosis of kala-azar. For the diagnosis of leishmaniasis, one drop of this latex-rK39 preparation was mixed with a drop of serum or plasma on a glass slide with gentle rocking for 4 to 5 min. After 5 min, the slide was visually inspected for clearly visible agglutination of latex particles, which would occur if the serum sample contained any isotype of anti-rK39 antibodies.
Diagnosis of kala-azar or PKDL in the field.
For the study, field camps were organized under the medical supervision of S.S. The local village leaders were informed about the rapid method of kala-azar diagnosis. After that, cases of kala-azar and PKDL were identified with the help of local village leaders and representatives of the local primary health center. The treatment records of the patients were checked. Those with parasitological diagnosis from government hospitals and/or private laboratories were confirmed with dipstick ELISA and the latex agglutination test, and those positive by all methods were considered index cases.
For the known cases of kala-azar, the retrospective period of illness and date of confirmed diagnosis of VL were noted, and these patients were further followed up prospectively to understand the kinetics of IgG and IgA anti-rK39 antibodies. The total period of follow-up was determined on the basis of retrospective data from the date of first diagnosis of leishmaniasis made by others and prospective follow-up by us for up to 2 years, where the total period was 4 years.
Antibody titers in family members of index patients.
All volunteering asymptomatic family members and villagemates of index case patients from the Samastipur, Vaishali, and Shahibganj districts of Bihar (areas of endemicity) with no enlargement of the spleen or liver constituted the contact group. They were not subjected to bone marrow or splenic aspirate examinations for ethical reasons. However, after informed consent their blood samples were collected and sera were first tested on the spot using latex agglutination and dipstick ELISA tests and then transported to Delhi for further testing. At the All India Institute of Medical Sciences, New Delhi, their serum samples were tested for presence of IgG and IgA classes of antibodies against rK39 antigen by using microwell ELISA as described above. All asymptomatic persons who were anti-rK39 antibody positive were followed up every 3 months for 12 months, and their blood samples were retested for anti-rK39 IgG antibodies. All asymptomatic persons who were found to be antibody positive were first given posttest counseling (by S.S.). The limitations of the tests were explained, and they were told that the tests indicated that they had been infected by the same germ that had caused disease in their family and that they might get similar disease over time but that there was also an equal probability that their body's immune mechanism might overcome the infection. During the follow-up period the asymptomatic persons were advised to maintain good health and have the best possible diet. They were also given a prescription advising local practitioners not to prescribe them steroids for minor ailments unless it was extremely necessary, a common practice in India that could trigger the flaring of latent infection (15).
For comparative evaluation of the specificities and sensitivities of the three test methods, all parasitologically proven index case patients (as described above) and 80 negative controls from an area where kala-azar is not endemic (Delhi) were tested by using the same methodology.
RESULTS
Sensitivities and specificities of the serological tests.
Of the 228 parasitologically proven index cases, all were correctly diagnosed by rK39 microwell IgG ELISA as well as by the rK39 rapid dipstick test. Neither of the two tests was positive on samples from an area where the disease is not endemic (Delhi). Thus, both of these tests had 100% sensitivity and specificity. The end point titers in microwell ELISA showed mean anti-rK39 IgG antibody titers in the range of 10−4 in active kala-azar or PKDL cases when the patient presented with typical signs and symptoms. These titers further continued to increase and reached a peak of 10−6 between 6 to 9 months; after that they started to decline irrespective of medication or no medication. Overall, the IgG anti-rK39 titers in clinically diagnosed cases of VL and PKDL ranged from 10−3 to as high as >10−6 despite use of a high cutoff optical density value and 10 ng of antigen per well. The end point titer in healthy controls from an area of nonendemicity was less than or equal to 10−2. However, the sensitivity of the latex agglutination test was only 80% compared to ELISA. This was due to a low power of antibody detection by this test. The test could detect the antibodies only if the antibody titers were 10−4 or more in ELISAs. Since in the normal population from the area of nonendemicity the end point titers were 10−2 or less, no false positive was detected by any of the three tests. The specificity and interassay concordance of the latex agglutination test, dipstick ELISA, and microwell (IgG) ELISA were 100% compared to parasitological diagnosis. The latex agglutination was stable at 4°C for 9 months and at room temperature in the field for 3 months.
The ELISA for anti-rK39 IgA antibodies was positive for only 23% of VL patients and 33% of PKDL patients. Ten percent of the asymptomatic family members were also found to be IgA reactive, but these patients were also reactive for IgG antibodies. Other patients, even those positive for IgG antibodies, were negative for IgA antibodies. No patient was IgA positive and IgG negative (Table 1).
TABLE 1.
Antibody response in clinically symptomatic patients and asymptomatic persons infected with L. donovani
| Group | No. tested | No. (%) positive for:
|
|
|---|---|---|---|
| IgG | IgA | ||
| Kala-azar patients | 228 | 228 (100) | 52 (22.8) |
| PKDL patients | 12 | 12 (100) | 4 (33.3) |
| Asymptomatic persons | |||
| Total | 150 | 55 (36.6) | 15 (10) |
| Family members | 55 | 24 (43.6) | 10 (18.2) |
| Neighbors | 95 | 31 (32.6) | 5 (5.2) |
| Controlsa | 80 | 0 | 0 |
| Total | 470 | 295 | 71 |
From an area of nonendemicity.
Antibody response in asymptomatic persons and prediction of disease development.
Of 150 asymptomatic contacts tested for IgG anti-rK39 antibodies, 55 (36.6%) were found to be positive by microwell ELISA as well as the dipstick test. Fifteen (10% of the total number tested) were found to be positive for IgA anti-rK39 antibodies in microwell ELISA. Of the IgG-positive contacts, 27% also had IgA antibodies; these consisted of 41% (10 of 24) of the family members and 16.1% (5 of 31) of the neighbors. The difference in IgA positivity between family members and neighbors was statistically significant (P < 0.05). This is probably due to accessibility and testing of the family members at an earlier stage of infection than neighbors. All IgA-positive persons also had specific IgG antibodies, and no subject was found to be IgG negative and IgA positive.
Spleen size in 20% (30 of 150) of the asymptomatic persons was increased to a palpable size. All those with splenomegaly developed kala-azar disease manifestations within the first 3 months (Table 2). However, spleen size was not an independent disease predictor, as no splenomegaly was observed in 5 (2.25%) of the parasitologically confirmed symptomatic patients with kala-azar.
TABLE 2.
Anti-rK39 antibody detection as a predictor of kala-azar disease manifestationa
| Index case of VL/PKDL | No. of contactsb positive for anti-rK39 | No. of persons with manifestations of kala-azar at:
|
Totalc | ||
|---|---|---|---|---|---|
| 3 mo | 6 mo | 9 mo | |||
| CK/11 | 4 | 2 | 2 | 0 | 4 |
| VK/15 | 2 | 0 | 0 | 0 | 0 |
| DK/16 | 2 | 0 | 0 | 0 | 0 |
| SK/18 | 16 | 6 | 3 | 2 | 11 |
| SK/44 | 2 | 1 | 0 | 0 | 1 |
| VR/46 | 2 | 0 | 0 | 0 | 0 |
| PD/26 | 4 | 3 | 0 | 1 | 4 |
| CK/77 | 3 | 2 | 0 | 1 | 3 |
| GD/82 | 8 | 4 | 1 | 1 | 6 |
| NR/59B | 12 | 6 | 1 | 2 | 9 |
| Total | 55 | 24 (43.9%) | 7 (12.7%) | 7 (12.7%) | 38 (69%) |
Asymptomatic family members found positive for IgG and IgA antibodies were followed up every 3 months for 12 months.
A Contact was defined as a member of the family or neighbor in the village. Since there was no statistical difference between family members and villagemates, they were grouped together (see Table 1).
No new cases were detected after 9 months.
Disappearance of IgG and IgA antibodies.
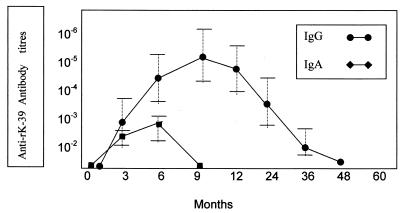
The sera and clinical data of the parasitologically confirmed patients who were treated successfully were also analyzed retrospectively as well as prospectively for a total period of 4 years (see Materials and Methods). Anti-rK39 IgG and IgA antibody detection in these patients was attempted at intervals of 3 to 6 months. The results indicated that IgG antibody titers peaked between 6 and 9 months and that IgA antibodies peaked between 3 and 6 months. The IgA antibody levels became undetectable after 6 months of successful treatment, while IgG antibody titers started declining 3 months after successful treatment and became undetectable after 2 years but always before 4 years (Fig. 1). The presence of persistent detectable levels of IgG anti-rK39 antibodies for more than 4 years indicated failure of the treatment or recurrence.
FIG. 1.
IgG and IgA anti-rK39 antibody titers in patients with clinically and parasitologically diagnosed cases of L. donovani infection and in asymptomatic patients who later manifested with kala-azar. The antibody titers were plotted by analyzing the prospective antibody titers in family members and neighbors who within 1 year developed kala-azar and the antibody titers found in the kala-azar index cases combined, before and after the parasitological diagnosis was made. Retrospective sera were available to us for most of the kala-azar cases. Error bars indicate standard deviations.
DISCUSSION
The promising diagnostic and prognostic significance of rK39 antigen has been shown by us and others previously (3, 4, 12, 14). In a previous study we reported its superiority in diagnosing difficult cases which could have been misdiagnosed by other conventional tests, including bone marrow and splenic aspirates (12). In another study, using end point titer enzyme immunoassay, we reported that anti-rK39 antibody titers correlated well with the parasite load (14). We found that patients with recent infection and having parasite loads of 1+ according to World Health Organization guidelines had antibody titers in the range of 10−2 to 10−4, while those with parasite loads of 4+ had very high antibody titers. These antibody titers also correlated with disease prognosis: those with decreasing titers showed clinical improvement, and those with persistent or increasing antibody titers showed clinical unresponsiveness to the antileishmanial treatment (14). The present study is a further expansion of the potential use of anti-rK39 antibodies in disease prediction for latently infected persons. The antigen showed 100% sensitivity and specificity in either the microwell ELISA or dipstick test protocol. In our microwell test protocol, we used the lowest possible concentration of rK39 antigen and yet the antibody titers were highest, which was not reported by others. Our earlier report also indicated similar results (14). The latex agglutination test developed by us is a single-step, rapid, and highly specific technique that can be very effectively used in the field. No electricity or any aid is required. Preparation of reagents is also easy and does not require sodium dodecyl sulfate-polyacrylamide gel electrophoresis, nylon membrane, or gold conjugate, etc. However, its low sensitivity limited its use in diagnosing and screening subclinical cases but not in diagnosing full-blown kala-azar and PKDL cases.
In the present study we could correctly diagnose exposure to L. donovani in almost 100% of the cases. We could also predict the disease development in family and neighborhood contacts of the patients infected with L. donovani by using anti-rK39 tests, with a 44% predictive value for disease development in the next 3 months and a 56.6% probability for development in the next 6 months. This prediction of disease development could be done at as early as 9 months by using anti-rK39 antibodies. The present study also revealed a prolonged incubation and latent period of L. donovani infection in this area of kala-azar endemicity in India.
Although occasional reports of parasite circulation in the peripheral blood of asymptomatic patients with L. donovani and Leishmania tropica infection have been published earlier (5, 8) ours is probably the first study to elucidate the immune response as followed up prospectively and its diagnostic significance in predicting the outcome of latent infection with a fairly large number of subjects.
Previous reports on Leishmania chagasi from Brazil indicate that in areas where VL is endemic, only about 20 to 45% of subjects infected with L. chagasi will develop classical VL (6, 7). The majority of the infected individuals will have a subclinical infection that may remain completely asymptomatic or will have an oligosymptomatic form of the disease. Subjects with oligosymptomatic infection may develop clinical disease months after the seroconversion or may self-heal their infections 1 or 2 years later (2), and that study also indicated that serological diagnosis is the most sensitive and specific tool for diagnosing asymptomatic L. chagasi infection. However, the study of Badaro et al. (2) had several weaknesses. First, the authors analyzed the clinical courses of their seropositive patients retrospectively, while we followed up the patients prospectively, every 3 months. Also, the comparison of the sensitivities of two antigens (the lysate and rK39) done by those authors was not convincing. It is difficult from their table to understand why a range of sensitivity data is given although it was a single study. In their study the number of asymptomatic patients with positive ELISA results was also very low compared to that in our study. Even though in our study the antigen concentration was far less than in theirs, the antibody titers were much higher in the Indian sera than in the Brazilian sera. In an earlier study we hypothesized that this difference was due to high copy numbers of the kinesin gene (14).
As shown in Table 2, several families had a clustering of patients; this could be an indication that genetic factors may predispose one to the development of the disease (7). Alternatively a clustering of VL cases in families may be related to increased exposure, since Leishmania transmission is predominantly peridomiciliary (7). However, those studies were done at only one time point, and the seropositive persons were not followed up for an extended period. On the basis of such single-time-point investigation, the specificity of the rK39 dipstick test has been doubted (9). In fact, the test is extremely sensitive and hence detects latent infection before the infection is proved by prolonged follow-up for seroconversion and clinical evaluation of the seropositive asymptomatic individuals, as was done in the present study.
There was a significant difference in IgA reactivity between family members and neighbors. It is yet to be established why IgA was detected more frequently in family members (18.2%) than in neighbors (5.2%). Whether this was due to genetic heterogeneity or to another reason is being studied.
Limitation of the study.
Our study had a scientific limitation, as we could not rule out reinfection as opposed to reactivation of latent infection in asymptomatic individuals still residing in the area of endemicity. This was not possible in a field study such as ours. It could be confirmed only by following up the seropositive patients after isolating them in an area where the disease is not endemic, which would be a socially and economically difficult option.
Acknowledgments
We thank S. G. Reed of Corixa Corp. (Seattle, Wash.) and Shyamal Raychaudhuri of InBios Inc. (Seattle, Wash.) for a free supply of dipsticks. Thanks are also due to K. P. Chang of Chicago Medical School, Chicago, Ill., for arranging the supply of rK39 antigen from S. G. Reed. Our sincere thanks go to Ram Singh and R. K. Das of the NICD Patna branch for field assistance. We thank Jugesh Kumar and Brijesh Kumar, Department of Laboratory Medicine, All India Institute of Medical Sciences, New Delhi, for collection of the blood samples.
Financial assistance to carry out this work was provided from the Department of Biotechnology, Ministry of Science and Technology, Government of India, New Delhi (grant no. BT/R&D/9/10/95).
REFERENCES
- 1.Ashford, R. W., P. Desjeux, and P. de Readt. 1992. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitol. Today 8:104-105. [DOI] [PubMed] [Google Scholar]
- 2.Badaro, R., D. Benson, M. C. Eulalio, M. Freire, S. Cunha, E. M. Netto, D. Pedral-Sampaio, C. Madureira, J. M. Burns, R. L. Houghton, J. R. David, and S. G. Reed. 1996. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J. Infect. Dis. 173:758-761. [DOI] [PubMed] [Google Scholar]
- 3.Bern, C., S. N. Jha, A. B. Joshi, G. D. Thakur, and M. B. Bista. 2000. Use of recombinant K39 dipstick test and the direct agglutination test in a setting endemic for visceral leishmaniasis in Nepal. Am. J. Trop. Med. Hyg. 63:153-157. [DOI] [PubMed] [Google Scholar]
- 4.Burns, J. M., W. G. Shreffler, D. R. Benson, H. W. Ghalib, R. Badaro, and S. G. Reed. 1993. Molecular characterisation of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc. Natl. Acad. Sci. USA 90:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandra, J., R. N. Rai, S. K. Mittal, and D. Sharma. 1991. Kala-azar without hepatosplenomegaly. Indian Paediatr. 28:1185-1186. [PubMed] [Google Scholar]
- 6.D'Oliveira, A., Jr., S. R. M. Costa, A. B. Barbosa, M. L. G. O. Orge, and E. M. Carvalho. 1997. Asymptomatic Leishmania chagasi infection in relatives and neighbors of patients with visceral leishmaniasis. Mem. Inst. Oswaldo Cruz 92:15-20. [DOI] [PubMed] [Google Scholar]
- 7.Evans, T. G., M. J. Teizeira, I. T. Mc Auliffe, I. Vasconceles, A. W. Vasconceles, A. D. Sousa, J. W. Lima, and R. D. Pearson. 1992. Epidemiology of visceral leishmaniasis in northeast Brazil. J. Infect. Dis. 166:1129-1132. [DOI] [PubMed] [Google Scholar]
- 8.Grogle, M., J. L. Daugirda, D. L. Hoover, A. J. Magill, and J. D. Berman. 1993. Survivability and infectivity of viscerotropic Leishmania tropica from Operation Desert Storm participants in human blood products maintained under blood bank conditions. Am. J. Trop. Med. Hyg. 49:308-315. [DOI] [PubMed] [Google Scholar]
- 9.Indian Council of Medical Research. 2000. Report of Indian Council of Medical Research-World Health Organization Joint Symposium on Treatment and Control of Kala-azar. Indian Council of Medical Research, New Delhi.
- 10.Limet, J. N., A. Berbinschi, A. Cloeckaert, C. L. Cambiaso, and P. L. Massom. 1988. Longitudinal study of brucellosis in mice by immunoassay of lipopolysaccharide-related antigens in blood and urine. J. Med. Microbiol. 26:37.. [DOI] [PubMed] [Google Scholar]
- 11.Sanyal, R. K. 1985. Leishmaniasis in the Indian subcontinent, p. 443-467. In K. P. Chang and R. S. Bray (ed.), Leishmaniasis. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 12.Singh, S., V. P. Chaudhary, and J. P. Wali. 1996. Transfusion-transmitted kala-azar in India. Transfusion 36:848-849. [DOI] [PubMed] [Google Scholar]
- 13.Singh, S., A. Biswas, N. Wig, P. Aggarwal, R. Sood, and J. P. Wali. 1999. A new focus of kala-azar cases in Kumaon region of India. J. Commun. Dis. 31:73-77. [PubMed] [Google Scholar]
- 14.Singh, S., S. G. Reed, A. G. Sacks, and K. P. Chang. 1995. Diagnostic and prognostic value of rK39 antigen in Indian leishmaniasis. J. Parasitol. 81:1000-1003. [PubMed] [Google Scholar]
- 15.Singh, S., N. Singh, and K. A. Razavi. 1995. Tuberculosis following immunosuppressive treatment after exposure to toxic smoke. Lancet 345:1379-1380. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization Expert Committee on the Control of Leishmaniasis. 1990. Control of the leishmaniases. Technical Report Series, no. 793. World Health Organization, Geneva, Switzerland. [PubMed]