Abstract
NK cells are instrumental in innate immune responses, in particular for the early production of gamma interferon (IFN-γ) and other cytokines necessary to control certain bacterial, parasitic, and viral infections. NK cell-mediated effector functions are controlled by a fine balance between distinct receptors mediating activating and inhibitory signals; however, little is known about activating receptors on NK cells and their corresponding ligands. Several studies have shown that commensal lactobacilli isolated from the human gastrointestinal tract activate human mononuclear cells and are potent inducers of IFN-γ and monocyte-derived interleukin 12 (IL-12). NK cell activation was shown for Lactobacillus johnsonii La1. In this study the cellular mechanisms of in vitro NK cell activation by gram-positive bacteria were analyzed. Staphylococcus aureus- and L. johnsonii La1-mediated activation of CD3− CD16+ CD56+ human peripheral blood NK cells, including expression of the activation antigen CD69 and secretion of IFN-γ, required cell contact-dependent costimulation by autologous monocytes. S. aureus- and L. johnsonii-preactivated monocytes retained their capacity to induce NK cell activation. In contrast, cytokine-primed monocytes completely failed to induce NK cell activation unless bacteria were present. This suggests that phagocytosis of bacteria provided additional coactivation signals on accessory cells that may differ from those induced by tumor necrosis factor and IFN-γ. Blocking of costimulatory molecules by B7.1, B7.2, and IL-12 but not CD14 monoclonal antibodies inhibited S. aureus- and L. johnsonii-induced effector function of NK cells. Our data suggest an important role for accessory cell-derived signals in the process of NK cell activation by gram-positive bacteria.
Natural killer (NK) cells are instrumental in innate immune responses, in particular for inducing the early production of gamma interferon (IFN-γ) without prior sensitization, necessary to control bacterial, parasitic, and viral infections. Cytokines such as interleukin 12 (IL-12) stimulate NK cells to release IFN-γ and express natural cytotoxicity. The rapid activation of NK cells is characteristic of innate immunity and constitutes the first line of defense against invading pathogens. Signals generated during the early host response have an additional role in the nature of downstream adaptive immune responses. Production of IFN-γ ensures activation of phagocytosis by monocytes and T helper (Th) differentiation to the Th1 phenotype, thus directing the adaptive immune response towards the appropriate effector pathway to clear the infection. In many studies it has been shown that a rapid IFN-γ response is essential for host survival (36).
It became apparent that NK effector functions, including cytokine release, cytotoxicity, and migration, are regulated by a balance between opposite signals delivered by major histocompatibility complex (MHC) class I-specific inhibitory and activating receptors (11, 53, 56, 67). In human NK cells, both HLA-specific and non-HLA-specific inhibitory receptors have been identified. The killer cell immunoglobulin (Ig)-like receptors and the killer cell lectin-like receptors constitute a structurally distinct family of HLA-specific receptors. The C-lectin-like receptor CD94, which forms a heterodimer with the members of the NKG2 family, recognizes HLA-E and allows monitoring of MHC class I expression on tissues (34, 43). CD94 was also shown to be implicated in the costimulation of human NK cells (31, 64). Inhibitory receptors prevent killing of normal cells and limit the production of NK-mediated proinflammatory cytokines, such as IFN-γ, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor (TNF) (43).
Little is known about activating receptors on NK cells and their corresponding ligands (42). The best-characterized receptor is FcRγIII (CD16), through which NK cells mediate antibody-dependent cellular cytotoxicity (24, 58). NKG2D, which is expressed on NK cells, γδ T cells, and CD8+ αβ T cells, binds several ligands that are structurally related to MHC class I. In association with DAP12, an intracellular adapter molecule, engagement of NKG2D triggers cytotoxicity (39, 47). Two recently identified receptors, the human NKp44 and NKp46 molecules, were also shown to trigger NK cell-mediated cytotoxicity (4, 48, 55). Resting NK cells constitutively express a number of cytokine receptors and can secrete immunoregulatory cytokines following stimulation with monokines. IL-12 produced by monocytes and dendritic cells early during infection sends a powerful signal to activate NK cells and seems particularly potent to induce IFN-γ (10, 37, 62). Other factors responsible for the activation of NK cells during infection have remained elusive but involve IFN-α/β, IL-15, IL-18, TNF, or chemokines (36).
In vitro studies have shown that upon stimulation of lymphocytes with bacteria, including species of Staphylococcus, Salmonella, and Corynebacterium, primarily NK cells become activated and produced IFN-γ (14, 49, 61). Also, lactobacilli, which are commensal gram-positive bacteria, are described as potent inducers of monocyte-derived IL-12 (25, 26, 41). Recently, it was shown that the stimulation of peripheral blood mononuclear cells (PBMC) with Lactobacillus johnsonii La1, an intestinal isolate with documented probiotic properties (1, 20, 52), resulted in the activation of CD3− CD56+ NK cells to secrete IL-12 and IFN-γ. This was not observed when PBMC were stimulated with nonpathogenic Escherichia coli or lipopolysaccharide (22). Thus, activation of human lymphocytes by whole gram-positive bacteria differs from that by gram-negative organisms or lipopolysaccharide, as CD14-independent pathways may be implicated (59).
The aim of this study was to further characterize molecular requirements for NK cell activation by gram-positive bacteria in vitro. We provide evidence that IFN-γ production by CD3− CD16+ CD56+ NK cells after stimulation with Staphylococcus aureus or L. johnsonii La1 was dependent on cell contact-dependent costimulation by activated monocytes. Our data support the importance of accessory cell-derived signals in the process of NK cell activation by gram-positive bacteria.
MATERIALS AND METHODS
Bacteria.
S. aureus (Nestlé culture collection, human fecal isolate) was grown aerobically in BHI broth at 37°C. L. johnsonii La 1 (Nestlé culture collection), of human intestinal origin, was cultivated in MRS broth at 37°C. All bacteria were harvested by centrifugation (1,500 × g, 15 min) at stationary growth phase (18 h). Bacteria were washed three times with phosphate-buffered saline (1× PBS, pH 7.2; Gibco BRL) and diluted to a final concentration of 103 to 107 CFU/ml in RPMI 1640 (Gibco BRL). Where indicated, bacteria were heat killed (100°C, 30 min), washed twice with PBS and twice with acetone, and dried at 37°C. Bacterial powders were diluted to final concentrations of 1 ng/ml to 10 μg/ml in RPMI 1640.
Isolation of bacterial CW, PG, and LTA.
Cell wall (CW) and peptidoglycan (PG) of S. aureus and L. johnsonii La1 were purified and prepared by modifications of the method described by Rosenthal and Dziarski (50). Bacteria from a 3-liter culture were harvested at stationary growth phase (18 h) and exposed to 100°C for 30 min. After centrifugation (1,500 × g, 4°C, 15 min) the cells were washed three times with cold PBS, resuspended in PBS containing glass beads (106 μm; Sigma), and broken immediately with a cell disrupter at 4°C. Beads were removed by filtration. From the filtrate, unbroken cells were sedimented by a 10-min centrifugation at 1,500 × g at 4°C. From the supernatant, CWs were sedimented by centrifugation at 6,500 × g at 4°C for 30 min and washed twice with PBS. The absence of whole bacterial cells was controlled by Gram staining. An aliquot of the crude CW suspension was washed twice with deionized, sterile water and twice with acetone and dried at 37°C. PG was purified from isolated crude CWs by enzymatic treatment with RNase A (100 μg/ml; Sigma), DNase I (50 μg/ml; Sigma), and trypsin (200 μg/ml; Sigma) for 18 h at 37°C in PBS (1×, pH 7.2). Toluene was added to prevent bacterial contamination. Enzymatic CW purification was controlled at an optical density at 480 nm. To remove CW carbohydrate structures such as teichoic acid, covalently bound to PG, the enzyme-treated CWs were exposed to 5% trichloroacetic acid at 37°C for 12 h. The purified insoluble PG was sedimented by centrifugation at 6,500 × g at 4°C for 30 min, washed twice with PBS, twice with deionized, sterile water, and twice with acetone, and dried at 37°C. Lipoteichoic acid (LTA) from L. johnsonii La1 was isolated as previously described (18). LTA from S. aureus was purchased from Sigma. For experimental use, powders of bacterial CW, PG, and LTA were diluted in RPMI 1640 to final concentrations of 1 ng/ml to 10 μg/ml.
Isolation of human CD3− CD16+ CD56+ peripheral blood NK cells and CD14+ monocytes.
Human PBMC were purified from buffy coats (Blood Transfusion Centre, Lausanne, Switzerland) by Ficoll-Hypaque (1077; Pharmacia) gradient centrifugation. PBMC were harvested from the interface, washed five times with RPMI 1640, and incubated in RPMI-10% human AB serum (Sigma) for 2 h at 37°C and 5% CO2 on 225-cm2 tissue culture plates (Costar) to allow adherence. Nonadherent peripheral blood lymphocytes were separated from adherent cells by aspiration. Where indicated, adherent cells were gently washed three times with prewarmed culture medium and harvested by using a rubber policeman (Costar). CD14+ monocytes were purified from peripheral blood by a magnetic cell sorting positive-selection technique (Miltenyi Biotec). CD3− CD16+ CD56+ NK cells were enriched from peripheral blood lymphocytes from the same donor by depletion of T cells, B cells, and myeloid cells using indirect labeling with hapten-conjugated CD3, CD14, CD19, CD36, and anti-IgE monoclonal antibodies (MAb) and magnetic cell sorting Microbeads coupled to an antihapten MAb (NK cell isolation kit; Miltenyi Biotec). Purity was controlled by fluorescence-activated cell sorting analysis using fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-labeled anti-CD4/CD8 MAb, FITC-labeled anti-CD16, FITC-labeled anti-CD19 MAb, FITC-labeled anti-CD56 MAb, FITC-labeled anti-CD14 MAb, and PE-labeled anti-CD3 MAb (all from Becton Dickinson). The purity of the isolated human peripheral blood CD3− CD16+ CD56+ NK and CD14+ monocytes ranged between 92 and 95%. Cell viability was controlled by trypan blue exclusion and was generally >90%. Purified NK cells were diluted in RPMI 1640 containing 10% native human AB serum, gentamicin (100 μg/ml; Gibco BRL), and polymyxin B (100 μg/ml; Gibco BRL) to a final concentration of 2 × 106/ml.
Phagocytosis of BCECF-AM-labeled bacteria by CD14+ monocytes.
Stationary-phase bacteria were washed three times with PBS, diluted to 108 CFU/ml in PBS, and incubated with 10 μl of 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein-acetoxy-methyl ester (1 mg/ml, BCECF-AM; Sigma) for 30 min at 37°C. The potential of CD14+ monocytes to phagocytose BCECF-AM-labeled bacteria was studied by using Phagotest (Orpegen Pharma). Briefly, 20 μl of BCECF-AM-labeled bacteria (108 to 105 CFU/ml) were incubated with monocytes (4 × 105 cells/100 μl) in RPMI 1640 supplemented with 10% native human AB serum and gentamicin (100 μg/ml) for 20 min at 37°C. Control monocytes were incubated at 4°C. To distinguish between adherent and phagocytosed bacteria, 100 μl of quenching solution (Orpegen Pharma) was added to the each sample. Finally, monocytes were washed twice with cell wash (Becton Dickinson), incubated with DNA staining solution (Orpegen Pharma) for 10 min at 4°C, and analyzed by flow cytometry (FACScan; Becton Dickinson). Bacterial uptake was confirmed by confocal microscopy (Zeiss).
Stimulation of CD3− CD16+ CD56+ NK cells.
Freshly isolated CD3− CD16+ CD56+ NK cells were seeded at 2 × 106/ml (500 μl) into 24-well tissue culture plates. Where indicated, a total of 2.5 × 105 monocytes (50 μl) were added to each well. For the stimulation of NK cells, live bacteria (103 to 107 CFU/ml), heat-killed bacteria, bacterial CW, purified PG, LTA (1 ng/ml to 10 μg/ml), or recombinant human IL-12 (rhIL-12; R&D Systems) were added (500 μl). The final ratio between NK cells and bacteria (live/heat killed) or bacterial components (CW, PG, and LTA) was 1:10, 1:1, 10:1, 100:1, or 1,000:1. Where indicated, NK cells were separated from monocytes and bacteria by cell culture inserts (0.4-μm pore size; Nunc). In the presence or absence of monocytes, NK cells were incubated with bacteria or bacterial components for 6, 18, 28, 48, and 72 h at 37°C and 5% CO2. Culture medium alone was used as a control. NK cell viability (>90%) was controlled by trypan blue exclusion. Subsequently, the cells were collected, washed twice with PBS, and used for flow cytometry analysis. Cell culture supernatants were collected separately and kept at −20°C for cytokine analysis by enzyme-linked immunosorbent assay (ELISA).
Antibodies.
NK cell activation was determined in the presence of anti-CD80 (36790D, IgG1 clone L307.4)/CD86 (33430D, IgG2b clone IT2.2) MAb, anti-CD14 MAb, and anti-IL-12 MAb (554659, IgG1 clone C8.6). Mouse anti-IgG1 (33811A, clone MOPC-21) and -IgG2b (33801A, clone 27-35) MAb were used as isotype control antibodies. All antibody preparations were azide free and diluted to a final concentration of 50 μg/ml (Pharmingen).
Prestimulation of CD14+ monocytes with bacteria or proinflammatory cytokines.
Purified CD14+ monocytes (106/ml) were stimulated with live bacteria (106 CFU/ml), a mixture of TNF-α (5 μg/ml) and IFN-γ (50 U/ml), or medium alone. After 12 h of incubation, monocytes were washed three times with PBS and finally transferred to NK cell cultures (NK cell/monocyte ratio, 4:1). Viability of prestimulated monocytes was >90%, as assessed by trypan blue exclusion.
Expression of the activation antigen CD69 on CD3− CD16+ CD56+ NK cells.
The activation of CD3− CD16+ CD56+ NK cells was determined by the expression of the surface antigen CD69 after 8, 18, 28, 48, and 72 h. Therefore, cells were washed (cell wash; Becton Dickinson), double stained with direct conjugated MAb CD69-FITC and CD56-PE (Becton Dickinson) for 30 min on ice, washed twice, centrifuged, and resuspended in cell wash. The percent CD69+ cells in the CD3− CD56+ NK cell population was compared to that in nontreated cells by flow cytometry (FACScan; Becton Dickinson).
ELISA.
The concentrations of IFN-γ, IL-12 p70, IL-15, IL-10, TNF-α, and IL-1β in cell culture supernatants were determined by ELISA (Eli-pair; Diaclone).
Statistics.
Values are given as the means of duplicates or triplicates ± standard deviations. Results were confirmed for at least three different blood donors in independent experiments. Significance was tested by applying the Mann-Whitney U test.
RESULTS
Activation of human CD3− CD16+ CD56+ NK cells after stimulation with S. aureus or L. johnsonii La1 required cellular interaction with monocytes.
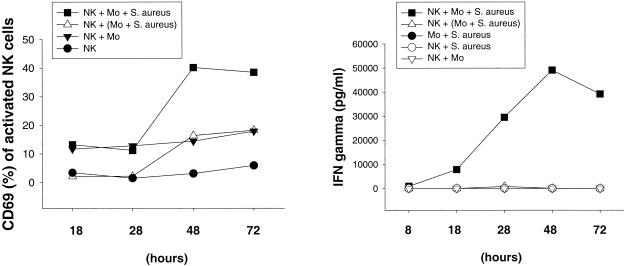
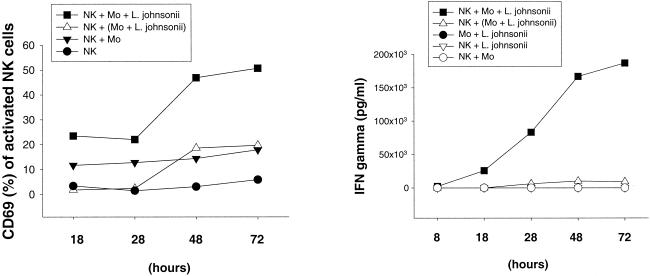
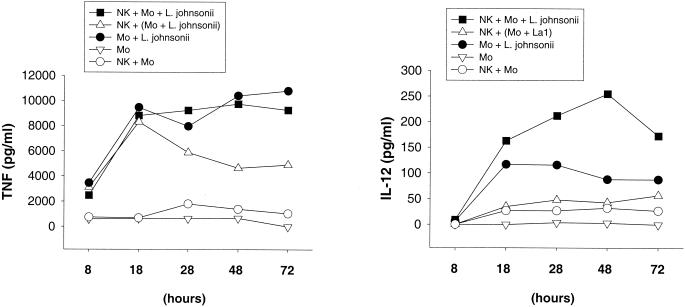
Human peripheral blood CD3− CD16+ CD56+ NK cells were stimulated with live or heat-killed S. aureus or L. johnsonii La1 for 72 h in the presence or absence of purified CD14+ autologous monocytes. NK cell activation was assessed by the expression of the leukocyte activation antigen CD69 and IFN-γ secretion, demonstrating that the activation of NK cells in response to both S. aureus and L. johnsonii La1 required the presence of autologous monocytes. Bacterium-mediated NK cell activation resulted in the upregulation of CD69 and the secretion of IFN-γ in a dose-dependent manner (data not shown). To underline the importance of cellular interactions between effector and accessory cells, monocytes were stimulated with S. aureus or L. johnsonii La1 (106 CFU/ml) on cell culture inserts (0.4-μm pore size) separated from NK cells in the lower compartment of a Transwell culture system. Induction of CD69 and IFN-γ by S. aureus (Fig. 1) - or L. johnsonii La1 (Fig. 2) -activated NK cells occurred after 28 h of stimulation if monocytes were present and reached maximal values between 48 and 72 h of incubation. By contrast, NK cells which had been separated from bacterium-stimulated monocytes revealed significantly reduced CD69 expression and IL-12 and IFN-γ secretion. Coincubation of NK cells, monocytes, and L. johnsonii enhanced the secretion of IL-12 but not TNF compared to that obtained with bacterium-stimulated monocytes alone (Fig. 3). Of importance, IL-12 concentrations remained relatively low in all experiments (∼100 to 300 pg/ml). These results suggest that interactions between NK and accessory cells are mandatory for NK cell activation by gram-positive bacteria. CD69 expression in unstimulated NK cells and NK cell-monocyte cocultures remained at low levels.
FIG. 1.
NK cell activation by whole S. aureus requires cell contact-dependent costimulation by autologous monocytes. Expression of CD69 and IFN-γ secretion by NK cells were determined in duplicate after 18, 28, 48, and 72 h. CD3− CD16+ CD56+ NK cells were stimulated with 106 CFU of live S. aureus per ml in the presence of CD14+ monocytes. Where indicated (triangles), NK cells were separated from bacterium-stimulated CD14+ monocytes by using Transwell cell culture inserts (0.4-μm pore size). Results are representative of three experiments.
FIG. 2.
NK cell activation by whole L. johnsonii cells requires cell contact-dependent costimulation by autologous monocytes. Expression of CD69 and IFN-γ secretion by NK cells were determined in duplicate after 18, 28, 48, and 72 h. CD3− CD16+ CD56+ NK cells were stimulated with 106 CFU of live S. aureus per ml in the presence of CD14+ monocytes. Where indicated (triangles), NK cells were separated from bacterium-stimulated CD14+ monocytes by using Transwell cell culture inserts (0.4-μm pore size). Results are representative of three experiments.
FIG. 3.
TNF and IL-12 secretion by NK cells and monocytes after stimulation with whole L. johnsonii. TNF and IL-12 secretion by NK cells, NK cell-monocyte cultures, and monocytes alone was determined in duplicate after 8, 18, 28, 48, and 72 h. Where indicated (triangles), NK cells were separated from bacterium-stimulated CD14+ monocytes by using Transwell cell culture inserts (0.4-μm pore size). Results are representative of two experiments.
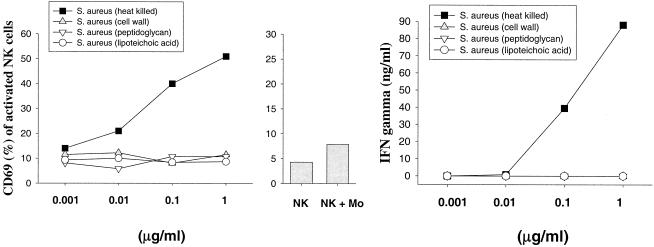
Whole gram-positive bacteria are necessary to induce IFN-γ secretion by activated NK cells.
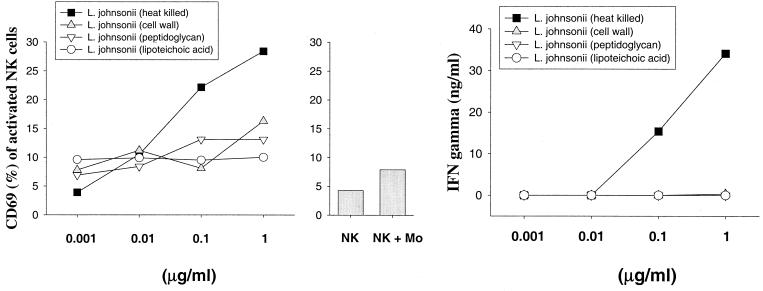
To investigate whether NK cell activation is mediated by heat-stable bacterial components of gram-positive bacteria, CW, PG, and LTA were isolated from heat-killed S. aureus (Fig. 4) and L. johnsonii La1 (Fig. 5) and used for stimulation of NK cell-monocyte cocultures. Notably, administration of either purified CW, PG, or LTA from both bacteria completely failed to activate NK cells, as determined by the expression of CD69 and IFN-γ secretion. These results suggest that disintegration of the bacterial CW significantly reduced the potential of gram-positive bacteria to activate NK cells.
FIG. 4.
Expression of CD69 and IFN-γ secretion by NK cells upon stimulation by S. aureus cell components. CD3− CD16+ CD56+ NK cells were stimulated in duplicate for 72 h with heat-killed bacteria, purified CWs, PG, and LTA in the presence of CD14+ monocytes. The bar chart shows the expression of CD69 on control NK cells. Results are representative of three experiments.
FIG. 5.
Expression of CD69 and IFN-γ secretion by NK cells upon stimulation by L. johnsonii cell components. CD3− CD16+ CD56+ NK cells were stimulated in duplicates for 72 h with heat-killed bacteria, purified CWs, PG, and LTA in the presence of CD14+ monocytes. The bar chart shows the expression of CD69 on control NK cells. Results are representative of three experiments.
Bacteria but not cytokine-activated monocytes induce NK cell activation.
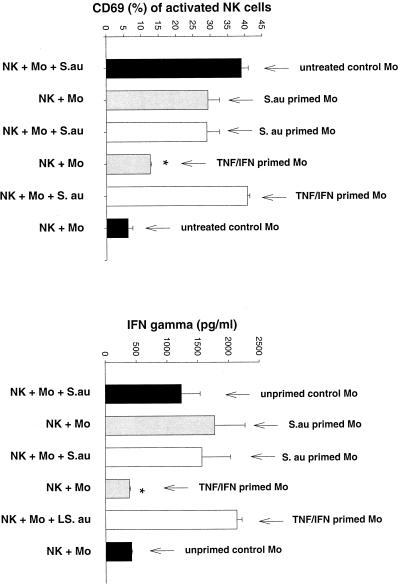
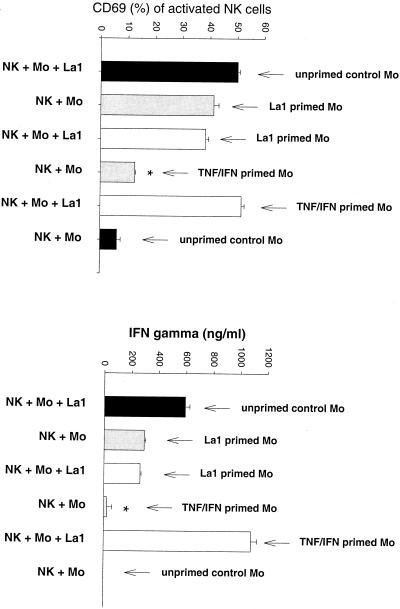
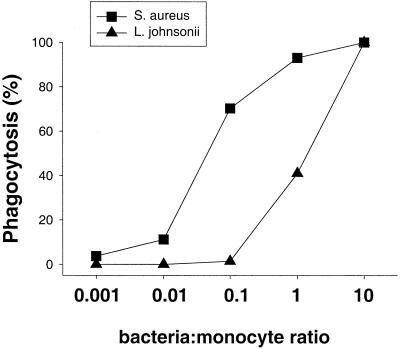
It appeared that monocytes play a critical role in the process of NK cell activation by gram-positive bacteria. To investigate whether NK cells specifically require bacterium-activated monocytes, peripheral blood CD14+ monocytes were preincubated with S. aureus or L. johnsonii La1 (106 CFU/ml), a mixture of TNF and IFN-γ, or medium alone for 16 h. Monocyte activation was controlled by IL-1β secretion in the supernatant (data not shown). Preactivated and unstimulated monocytes were washed prior to transfer to NK cells in order to remove nonphagocytosed/nonattached bacteria. As demonstrated in Fig. 6 (S. aureus) and Fig. 7 (L. johnsonii), gram-positive bacteria but not cytokine-primed monocytes had a residual capacity to induce CD69 expression and IFN-γ secretion by NK cells. However, NK cell activation in the presence of TNF-IFN-γ-primed monocytes was strongly reinforced after addition of live S. aureus or L. johnsonii. Analysis of phagocytosis of BCECF-AM-labeled bacteria revealed that S. aureus was 10 times more efficiently phagocytosed than L. johnsonii La1 (Fig. 8). However, IL-1β secretion by activated monocytes was similar for both strains.
FIG. 6.
S. aureus but not cytokine-activated monocytes induces NK cell activation. CD14+ monocytes (106/ml) were preincubated for 16 h with 106 live CFU of S. aureus per ml, a mixture of TNF (5 μg/ml) and IFN-γ (50 U/ml), or medium alone. Monocyte activation was controlled by measuring IL-1β in the supernatants. NK cells were then incubated for 72 h with pretreated monocytes as follows: (i) monocytes and bacteria, (ii) bacterium-activated monocytes, (iii) bacterium-activated monocytes and bacteria, (iv) cytokine-activated monocytes, and (v) cytokine-activated monocytes and bacteria. Unstimulated NK cell-monocyte cultures were used as a control. The expression of CD69 and the secretion of IFN-γ were determined in triplicate. Results are representative of two experiments. ∗, P < 0.05.
FIG. 7.
L. johnsonii but not cytokine-activated monocytes induces NK cell activation. CD14+ monocytes (106/ml) were preincubated for 16 h with 106 CFU of live L. johnsonii per ml, a mixture of TNF (5 μg/ml) and IFN-γ (50 U/ml), or medium alone. Monocyte activation was controlled by measuring IL-1β in the supernatants. NK cells were then incubated for 72 h with pretreated monocytes as follows: (i) monocytes and bacteria, (ii) bacterium-activated monocytes, (iii) bacterium-activated monocytes and bacteria, (iv) cytokine-activated monocytes, and (v) cytokine-activated monocytes and bacteria. Unstimulated NK cell-monocyte cultures were used as a control. The expression of CD69 and the secretion of IFN-γ were determined in triplicate. Results are representative of two experiments. ∗, P < 0.05.
FIG. 8.
Phagocytosis of S. aureus and L. johnsonii by monocytes. Bacteria were labeled with BCECF-AM and incubated with CD14+ monocytes for 20 min as described in Materials and Methods. Percent phagocytosis was determined by flow cytometry.
Role of IL-12, costimulatory molecules, and CD14 in NK cell activation by whole gram-positive bacteria.
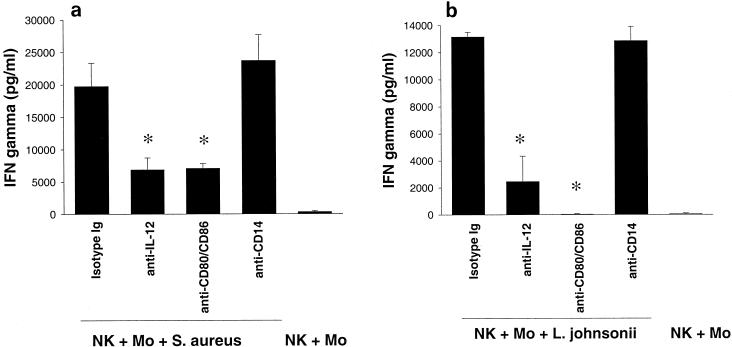
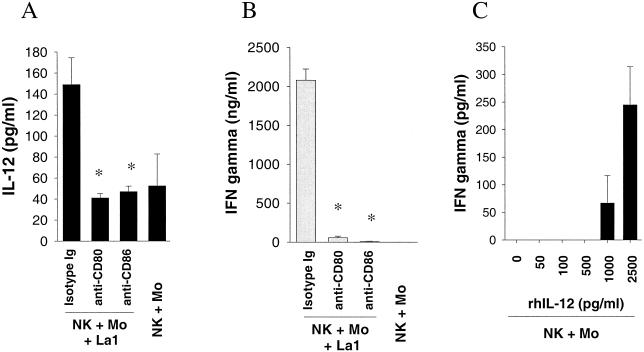
When NK cell-monocyte cocultures were stimulated with S. aureus (Fig. 9a) and L. johnsonii La1 (Fig. 9b) in the presence of anti-IL-12 MAb or anti-CD80/CD86 MAb (50 μg/ml), IFN-γ secretion was significantly reduced (P < 0.05). However, IFN-γ production was not affected in the presence of anti-CD14 MAb (50 μg/ml), indicating that activation of NK cells by gram-positive bacteria was CD14 independent. IgG1 isotype antibodies were used in the controls. To dissect the role of the costimulatory molecules CD80 and CD86, the production of IFN-γ and IL-12 by NK cell-monocyte cultures was measured after 72 h of stimulation with L. johnsonii. The secretion of IL-12 (Fig. 10A) and IFN-γ (Fig. 10B) was reduced to baseline levels in the presence of anti-CD80 MAb (50 μg/ml) and anti-CD86 MAb (50 μg/ml), respectively. Finally, the potential of IL-12 alone to induce IFN-γ secretion by NK cell-monocyte cultures was examined at various concentrations (0, 50, 100, 500, 1,000, and 2,500 pg/ml). As shown in Fig. 10C, the addition of rhIL-12 to NK cell-monocyte cultures for 72 h resulted in weak induction of IFN-γ (∼50 to 300 pg/ml) at higher IL-12 concentrations. This suggests that IL-12 alone is not responsible for the strong IFN-γ production in NK cell-monocyte cultures after stimulation with gram-positive bacteria. We conclude from these results that cell-dependent costimulation of NK cells with monocytes is required to initiate the production of IFN-γ by gram-positive bacteria.
FIG. 9.
Effect of anti-CD80/CD86, anti-CD14, and anti-IL-12 MAb on NK cell activation upon stimulation with live S. aureus (a) and L. johnsonii (b) (106 CFU/ml) in the presence of CD14+ monocytes. IFN-γ secretion was detected in triplicate after 72 h of stimulation. MAb were used at a final concentration of 50 μg/ml. Isotype control antibodies did not affect NK cell activation. Statistical significance was tested by the Mann-Whitney U test. ∗, P < 0.05.
FIG. 10.
Role of CD80, CD86, and rhIL-12 in the production of IFN-γ by NK cell-monocyte cultures. NK cell-monocyte cultures were stimulated with 106 CFU of live L. johnsonii per ml (A and B) and rhIL-12 (C) at various concentrations. IFN-γ and IL-12 secretion was detected in triplicate after 72 h of stimulation. Anti-CD80 and anti-CD86 MAb and isotype control antibodies were used at a final concentration of 50 μg/ml. Statistical significance was tested by the Mann-Whitney U test. *, P < 0.05.
DISCUSSION
In this study it was shown that whole (live or heat-killed) gram-positive S. aureus and L. johnsonii La1 cells induced a dose-dependent IFN-γ response in NK cells in the presence of autologous monocytes. Lactobacilli are nonpathogenic gram-positive bacteria residing in the human gut as a component of the normal microflora. They are also found in fermented foods. Distinct Lactobacillus strains have been postulated to have health-beneficial effects, such as stimulation of the immune system (33, 60). Staphylococci and streptococci are important gram-positive pathogens that cause a wide range of infections, including toxic shock syndrome (13, 57). It seems controversial that lactobacilli, which have been carefully studied regarding safety (23, 27), and pathogenic gram-positive bacteria induce similar immune responses in human leukocytes (21, 22, 25, 26, 40). In fact, Miettinen et al. showed that both the nonpathogenic Lactobacillus paracasei GG (formerly Lactobacillus rhamnosus) and the pathogenic Streptococcus pyogenes induce secretion of the monocyte-derived cytokines (monokines) IL-12 and IL-18 (41) and activate similar signaling pathways in human monocytes, including NF-κB and STAT DNA binding activity (39). Thus, certain CW components common to nonpathogenic and pathogenic gram-positive bacteria seem to exhibit similar stimulation activities for induction of innate immune responses. In fact, the potential of pathogenic staphylococci and streptococci to produce superantigenic exotoxins is thought to primarily cause the massive cytokine induction leading to conditions of septic shock (57).
Our data suggest that monocyte-derived soluble mediators alone could not drive activation of NK cells by gram-positive bacteria. Although TNF, IL-1β, and IL-12 were secreted by bacterium-stimulated monocytes, NK cell activation, as assessed by CD69 and IFN-γ expression, did not occur when cellular interactions between NK cells and monocytes were prevented. Several authors have shown that murine NK cells are activated by target cells expressing the costimulatory molecules B7.1, B7.2, and CD40 (9, 35). We showed that blocking of B7.1, B7.2, and IL-12 by MAb was highly effective in abolishing NK cell activation, suggesting that both costimulatory signals and IL-12 are necessary to trigger IFN-γ secretion by gram-positive bacteria. This observation is also supported by reports on the increased NK cell cytotoxicity after transfection of CD80 and CD86 into target tumor cells (65). Cell contact-dependent costimulation of NK cells does not appear to involve known receptors that can costimulate T cells, including CD28, CD27, and CD29 (29), although a recent report suggested the possibility of a variant CD28 on human NK cells (17). However, the relevance of this finding is still a matter of controversy. By contrast, human NK cells may use other receptors. The ability of CD28 to signal via the phosphatidylinositol 3-kinase activation pathway raises the possibility that DAP10, a recently described transmembrane adapter molecule implicated in NK cell activation, may play a role similar to that of CD28 in T cells (30, 66).
We showed that bacteria but not cytokine-preactivated monocytes retained the capacity (although reduced) to induce IFN-γ release from NK cells. Thus, it appears that phagocytosis of S. aureus or L. johnsonii La1 provided expression of additional coactivation signals on accessory cells that may differ from those induced by TNF and IFN-γ. However, addition of live bacteria to bacterium-primed monocytes did not modulate IFN-γ production by cocultured NK cells. This could be based on downregulation of the monocyte-specific mannose-receptor after ligation and phagocytosis of the gram-positive bacteria (54). In contrast, cytokine-primed monocytes completely failed to induce IFN-γ secretion in NK cells except when live bacteria were present. In this case, the IFN-γ-inductive capacity was maximal. It was recently shown that expression of pattern recognition receptors, including the mannose receptor and Toll-like receptors on monocytes (and dendritic cells) is downregulated by Th1 cytokines such as TNF and IFN-γ (16, 44). However, it appears that both L. johnsonii La1 and S. aureus could still efficiently trigger phagocytosis in cytokine-pretreated monocytes, most likely involving complement receptor CR3 and/or CD11b/c. Moreover, the combination of monocyte-derived signals and direct recognition of L. johnsonii La1 by NK cells via complement receptor type III (CR3 or CD18/CD11b) (12) or carbohydrate receptors, such as asialo-GM1, could not be rigorously excluded (45, 51).
It was further demonstrated that activation of NK cells by whole gram-positive cells was CD14 independent. These data are in general agreement with those of Cauwels et al. (8) and Cuzzola et al. (12), who described CD14-independent responses to gram-positive bacteria, including pneumococci, streptococci, and staphylococci. It is well documented that Toll-like receptor 2 is implicated in the recognition of gram-positive-bacterium-derived LTA and PGs (38, 63).
Interestingly, purified CW components and LTA did not induce NK cell activation, suggesting that integral bacterial CW structure rather than isolated molecules is relevant for activation. This is confirmed by the recent finding that LTA and PG act synergistically to modulate monocyte phenotype in human whole blood (28).
Fehniger et al. (15) demonstrated that combinations of IL-12 and IL-18 are potent inducers of IFN-γ in human CD56bright NK cells, whereas IL-12 in combination with IL-15 skewed NK cells to secrete IL-10. Individually, IL-12, IL-15, and IL-18 failed to induce effector responses in NK cells, indicating that cytokines as well as other factors, including microbial stimuli, contribute to the establishment of a particular microenvironment for the regulation of NK cell activation (2, 6, 7).
There is increasing evidence that NK cell activation is regulated by the balance between inhibitory and activating signals. In addition, contact-based costimulatory signals derived from activated monocytes, dendritic cells (19), or NKT cells (5) seem to be necessary for early IFN-γ secretion and thus an appropriate protective innate response in the absence of antigen (46). This in turn may profoundly influence the nature of the adaptive immune response. Thus, further to their role in innate defenses against infections, NK cells might be relevant for the microenvironmental regulation of T-cell polarization (3, 32). Experimental evidence that selected lactobacilli may participate in the establishment of a particular microenvironment leading to a protective immune response was provided.
REFERENCES
- 1.Bernet, M. F., D. Brassart, J. R. Neeser, and A. L. Servin. 1994. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut 35:483-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluman, E. M., K. J. Bartynski, B. R. Avalos, and M. A. Caligiuri. 1996. Human natural killer cells produce abundant monocyte inflammatory protein-1 alpha in response to monocyte-derived cytokines. J. Clin. Investig. 97:2722-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrnes, A. A., X. Ma, and P. Cuomo. 2001. Type I interferons and IL-12: convergence and cross-regulation among mediators of cellular immunity. Eur. J. Immunol. 31:2026-2034. [DOI] [PubMed] [Google Scholar]
- 4.Cantoni, C., C. Bottino, M. Vitale, A. Pessino, R. Augugliaro, A. Malaspina, S. Parolini, L. Moretta, A. Moretta, and R. Biassoni. 1999. NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J. Exp. Med. 189:787-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carnaud, C., D. Lee, and O. Donnars. 1999. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol 163:4647-4650. [PubMed] [Google Scholar]
- 6.Carson, W. E., J. G. Giri, M. J. Lindemann, M. L. Linett, M. Ahdieh, R. Paxton, D. Anderson, J. Eisenmann, K. Grabstein, and M. A. Caligiuri. 1994. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 180:1395-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carson, W. E., M. E. Ross, R. A. Baiocchi, M. J. Marien, N. Boiani, K. Grabstein, and M. A. Caligiuri. 1995. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J. Clin. Investig. 96:2578-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cauwels, A., E. Wan, M. Leismann, and E. Tuomanen. 1997. Coexistence of CD14-dependent and independent pathways for stimulation of human monocytes by gram-positive bacteria. Infect. Immun. 65:3255-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers, B. J., M. Salcedo, and H. G. Ljunggren. 1996. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1). Immunity 5:311-317. [DOI] [PubMed] [Google Scholar]
- 10.Chan, S. H., B. Perussia, J. W. Gupta, M. Kobayashi, M. Pospisil, H. A. Young, S. F. Wolf, D. Young, S. C. Clark, and G. Trinchieri. 1991. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J. Exp. Med. 173:869-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colonna, M. 1996. Natural killer cell receptors specific for MHC class I molecules. Curr. Opin. Immunol. 8:101-107. [DOI] [PubMed] [Google Scholar]
- 12.Cuzzola, M., G. Mancuso, C. Beninati, C. Biondo, F. Genovese, F. Tomasello, T. H. Flo, T. Espevik, and G. Teti. 2000. Beta 2 integrins are involved in cytokine responses to whole Gram-positive bacteria. J. Immunol. 164:5871-5876. [DOI] [PubMed] [Google Scholar]
- 13.Czachor, J. S., T. E. Herchline, R. N. Husni, S. M. Gordon, J. A. Washington, and D. L. Longworth. 2001. Bacteremic nonmenstrual staphylococcal toxic shock syndrome associated with enterotoxins A and C. Clin. Infect. Dis. 32:E53-E56. [DOI] [PubMed] [Google Scholar]
- 14.D'Orazio, J. A., G. W. Burke, and J. Stein-Streilein. 1995. Staphylococcal enterotoxin B activates purified NK cells to secrete IFN-gamma but requires T lymphocytes to augment NK cytotoxicity. J. Immunol. 154:1014-1023. [PubMed] [Google Scholar]
- 15.Fehniger, T. A., M. H. Shah, M. J. Turner, J. B. VanDeusen, S. P. Whitman, M. A. Cooper, K. Suzuki, M. Wechser, F. Goodsaid, and M. A. Caligiuri. 1999. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J. Immunol. 162:4511-4520. [PubMed] [Google Scholar]
- 16.Flo, T. H., O. Halaas, and S. Torp. 2001. Differential expression of Toll-like receptor 2 in human cells. J. Leukoc. Biol. 69:474-481. [PubMed] [Google Scholar]
- 17.Galea-Lauri, J., D. Darling, S. U. Gan, L. Krivochtchapov, M. Kuiper, J. Gaken, B. Souberbielle, and F. Farzaneh. 1999. Expression of a variant of CD28 on a subpopulation of human NK cells: implications for B7-mediated stimulation of NK cells. J. Immunol. 163:62-70. [PubMed] [Google Scholar]
- 18.Granato, D., F. Perotti, I. Masserey, M. Rouvet, M. Golliard, A. Servin, and D. Brassart. 1999. Cell surface-associated lipoteichoic acid acts as an adhesion factor for attachment of Lactobacillus johnsonii La1 to human enterocyte-like Caco-2 cells. Appl. Environ. Microbiol. 65:1071-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granucci, F., C. Vizzardelli, E. Virzi, M. Rescigno, and P. Ricciardi-Castagnoli. 2001. Transcriptional reprogramming of dendritic cells by differentiation stimuli. Eur. J. Immunol. 31:2539-2546. [DOI] [PubMed] [Google Scholar]
- 20.Haller, D., C. Bode, W. P. Hammes, A. M. A. Pfeifer, E. J. Schiffrin, and S. Blum. 2000. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 47:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haller, D., C. Bode, and W. P. Hammes. 1999. Cytokine secretion by stimulated monocytes depends on the growth phase and heat treatment of bacteria: a comparative study between lactic acid bacteria and invasive pathogens. Microbiol. Immunol. 43:925-935. [DOI] [PubMed] [Google Scholar]
- 22.Haller, D., S. Blum, C. Bode, W. P. Hammes, and E. J. Schiffrin. 2000. Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro: evidence of NK cells as primary targets. Infect. Immun. 68:752-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harty, D. W., H. J. Oakey, M. Patrikakis, E. B. Hume, and K. W. Knox. 1994. Pathogenic potential of lactobacilli. Int. J. Food Microbiol. 24:179-189. [DOI] [PubMed] [Google Scholar]
- 24.Hazenbos, W. L., J. E. Gessner, F. M. Hofhuis, H. Kuipers, D. Meyer, I. A. Heijnen, R. E. Schmidt, M. Sandor, P. J. Capel, M. Daeron, J. G. van de Winkel, and J. S. Verbeek. 1996. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity 5:181-188. [DOI] [PubMed] [Google Scholar]
- 25.Hessle, C., B. Andersson, and A. E. Wold. 2000. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infect. Immun. 68:3581-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hessle, C., L. A. Hanson, and A. E. Wold. 1999. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin. Exp. Immunol. 116:276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husni, R. N., S. M. Gordon, J. A. Washington, and D. L. Longworth. 1997. Lactobacillus bacteremia and endocarditis: review of 45 cases. Clin. Infect. Dis. 25:1048-1055. [DOI] [PubMed] [Google Scholar]
- 28.Jorgensen, P. F., J. E. Wang, and M. Almlof. 2001. Peptidoglycan and lipoteichoic acid modify monocyte phenotype in human whole blood. Clin. Diagn. Lab. Immunol. 8:515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang, S., N. L. Vujanovic, B. Wollenberg, and T. L. Whiteside. 1998. Absence of B7.1-CD28/CTLA-4-mediated co-stimulation in human NK cells. Eur. J. Immunol. 28:780-786. [DOI] [PubMed] [Google Scholar]
- 30.Lanier, L. L. 2001. On guard--activating NK cell receptors. Nat. Immunol. 2:23-27. [DOI] [PubMed] [Google Scholar]
- 31.Lanier, L. L. 1998. NK cell receptors. Annu. Rev. Immunol. 16:359-393. [DOI] [PubMed] [Google Scholar]
- 32.Lertmemongkolchai, G., G. Cai, C. A. Hunter, and G. J. Bancroft. 2001. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J. Immunol. 166:1097-1105. [DOI] [PubMed] [Google Scholar]
- 33.Lidbeck, A., and C. E. Nord. 1993. Lactobacilli and the normal human anaerobic microflora. Clin. Infect. Dis. 16(Suppl. 4):S181-S187. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Botet, M., M. Carretero, T. Bellon, J. J. Perez-Villar, M. Llano, and F. Navarro. 1998. The CD94/NKG2 C-type lectin receptor complex. Curr. Top. Microbiol. Immunol. 230:41-52. [DOI] [PubMed] [Google Scholar]
- 35.Martin-Fontecha, A., E. Assarsson, E. Carbone, K. Karre, and H. G. Ljunggren. 1999. Triggering of murine NK cells by CD40 and CD86 (B7-2). J. Immunol. 162:5910-5916. [PubMed] [Google Scholar]
- 36.Medzhitov, R., and C. A. Janeway. 1997. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9:4-9. [DOI] [PubMed] [Google Scholar]
- 37.Mehrotra, P. T., R. P. Donnelly, S. Wong, H. Kanegane, A. Geremew, H. S. Mostowski, K. Furuke, J. P. Siegel, and E. T. Bloom. 1998. Production of IL-10 by human natural killer cells stimulated with IL-2 and/or IL-12. J. Immunol. 160:2637-2644. [PubMed] [Google Scholar]
- 38.Michelsen, K. S., A. Aicher, and M. Mohaupt. 2001. The role of Toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCS). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J. Biol. Chem. 276:25680-25686. [DOI] [PubMed] [Google Scholar]
- 39.Miettinen, M., A. Lehtonen, I. Julkunen, and S. Matikainen. 2000. Lactobacilli and streptococci activate NF-κB and STAT signaling pathways in human monocytes. J. Immunol. 164:3733-3740. [DOI] [PubMed] [Google Scholar]
- 40.Miettinen, M., J. Vuopio-Varkila, and K. Varkila. 1996. Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect. Immun. 64:5403-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miettinen, M., S. Matikainen, J. Vuopio-Varkila, J. Pirhonen, K. Varkila, M. Kurimoto, and I. Julkunen. 1998. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect. Immun. 66:6058-6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moretta, A., C. Bottino, and M. Vitale. 2001. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19:197-223. [DOI] [PubMed] [Google Scholar]
- 43.Moretta, A., and L. Moretta. 1997. HLA class I specific inhibitory receptors. Curr. Opin. Immunol. 9:694-701. [DOI] [PubMed] [Google Scholar]
- 44.Muzio, M., D. Bosisio, and N. Polentarutti. 2000. Differential expression and regulation of Toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J. Immunol. 164:5998-6004. [DOI] [PubMed] [Google Scholar]
- 45.Neeser, J. R., D. Granato, M. Rouvet, A. Servin, S. Teneberg, and K. A. Karlsson. 2000. Lactobacillus johnsonii La1 shares carbohydrate-binding specificities with several enteropathogenic bacteria. Glycobiology 10:1193-1199. [DOI] [PubMed] [Google Scholar]
- 46.Nilsson, N., T. Bremell, A. Tarkowski, and H. Carlsten. 1999. Protective role of NK1.1+ cells in experimental Staphylococcus aureus arthritis. Clin. Exp. Immunol. 117:63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pende, D., C. Cantoni, and P. Rivera. 2001. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur. J. Immunol. 31:1076-1086. [DOI] [PubMed] [Google Scholar]
- 48.Pessino, A., S. Sivori, C. Bottino, A. Malaspina, L. Morelli, L. Moretta, R. Biassoni, and A. Moretta. 1998. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J. Exp. Med. 188:953-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reynolds, C. W., and R. B. Herberman. 1981. In vitro augmentation of rat natural killer (NK) cell activity. J. Immunol. 126:1581-1585. [PubMed] [Google Scholar]
- 50.Rosenthal, R. S., and R. Dziarski. 1994. Isolation of peptidoglycan and soluble peptidoglycan fragments. Methods Enzymol. 235:253-285. [DOI] [PubMed] [Google Scholar]
- 51.Saubermann, L. J., P. Beck, and Y. P. De Jong. 2000. Activation of natural killer T cells by alpha-galactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology 119:119-128. [DOI] [PubMed] [Google Scholar]
- 52.Schiffrin, E. J., F. Rochat, H. Link-Amster, J. M. Aeschlimann, and A. Donnet-Hughes. 1995. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J. Dairy Sci. 78:491-497. [DOI] [PubMed] [Google Scholar]
- 53.Scott, P., and G. Trinchieri. 1995. The role of natural killer cells in host-parasite interactions. Curr. Opin. Immunol. 7:34-40. [DOI] [PubMed] [Google Scholar]
- 54.Shepherd, V. L., K. B. Lane, and R. Abdolrasulnia. 1997. Ingestion of Candida albicans down-regulates mannose receptor expression on rat monocytes. Arch. Biochem. Biophys. 344:350-356. [DOI] [PubMed] [Google Scholar]
- 55.Sivori, S., M. Vitale, L. Morelli, L. Sanseverino, R. Augugliaro, C. Bottino, L. Moretta, and A. Moretta. 1997. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J. Exp. Med. 186:1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spits, H., L. L. Lanier, and J. H. Phillips. 1995. Development of human T and natural killer cells. Blood 85:2654-2670. [PubMed] [Google Scholar]
- 57.Stevens, D. L., A. E. Bryant, S. P. Hackett, A. Chang, G. Peer, S. Kosanke, T. Emerson, and L. Hinshaw. 1996. Group A streptococcal bacteremia: the role of tumor necrosis factor in shock and organ failure. J. Infect. Dis. 173:619-626. [DOI] [PubMed] [Google Scholar]
- 58.Takai, T., M. Li, D. Sylvestre, R. Clynes, and J. V. Ravetch. 1994. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell 76:519-529. [DOI] [PubMed] [Google Scholar]
- 59.Takeuchi, O., K. Hoshino, and T. Kawai. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 60.Tannock, G. W. 1997. Probiotic properties of lactic-acid bacteria: plenty of scope for fundamental R & D. Trends Biotechnol. 15:270-274. [DOI] [PubMed] [Google Scholar]
- 61.Tarkkanen, J., H. Saxen, M. Nurminen, P. H. Makela, and E. Saksela. 1986. Bacterial induction of human activated lymphocyte killing and its inhibition by lipopolysaccharide (LPS). J. Immunol. 136:2662-2669. [PubMed] [Google Scholar]
- 62.Trinchieri, G. 1994. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 84:4008-4027. [PubMed] [Google Scholar]
- 63.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in monocytes. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Voss, S. D., J. Daley, J. Ritz, and M. J. Robertson. 1998. Participation of the CD94 receptor complex in costimulation of human natural killer cells. J. Immunol. 160:1618-1626. [PubMed] [Google Scholar]
- 65.Wilson, J. L., J. Charo, A. Martin-Fontecha, P. Dellabona, G. Casorati, B. J. Chambers, R. Kiessling, M. T. Bejarano, and H. G. Ljunggren. 1999. NK cell triggering by the human costimulatory molecules CD80 and CD86. J. Immunol. 163:4207-4212. [PubMed] [Google Scholar]
- 66.Wu, J., H. Cherwinski, T. Spies, J. H. Phillips, and L. L. Lanier. 2000. DAP10 and DAP12 form distinct, but functionally cooperative, receptor complexes in natural killer cells. J. Exp. Med. 192:1059-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yokoyama, W. M. 1998. Natural killer cell receptors. Curr. Opin. Immunol. 10:298-305. [DOI] [PubMed] [Google Scholar]