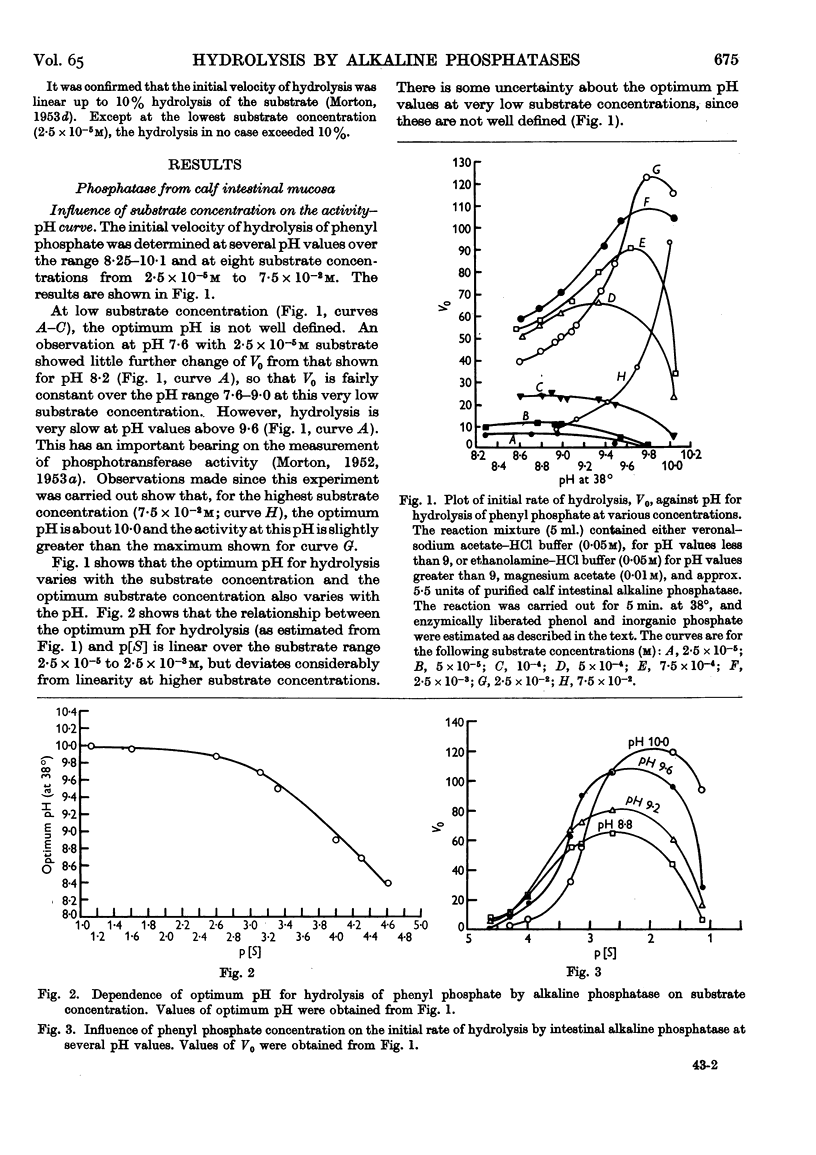
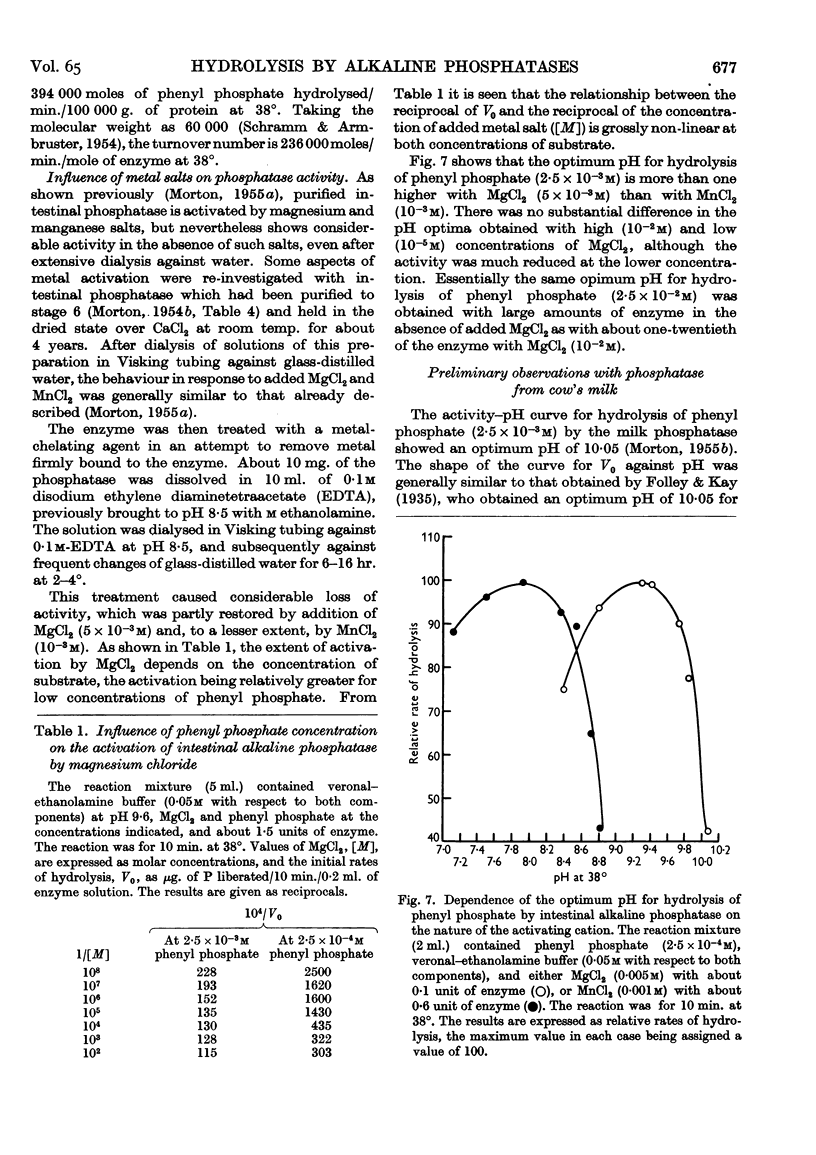
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DIXON M. The effect of pH on the affinities of enzymes for substrates and inhibitors. Biochem J. 1953 Aug;55(1):161–170. doi: 10.1042/bj0550161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delory G. E., King E. J. The rate of enzymic hydrolysis of phosphoric esters: 2. Relation of structure to dissociation constant, Michaelis constant, and rate of hydrolysis. Biochem J. 1943;37(5):547–550. doi: 10.1042/bj0370547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folley S. J., Kay H. D. The alkaline phosphomonoesterase of the mammary gland. Biochem J. 1935 Aug;29(8):1837–1850. doi: 10.1042/bj0291837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- HORWITT B. N. Determination of inorganic serum phosphate by means of stannous chloride. J Biol Chem. 1952 Dec;199(2):537–541. [PubMed] [Google Scholar]
- MORTON R. K. Alkaline phosphatase of milk. 2. Purification of the enzyme. Biochem J. 1953 Dec;55(5):795–800. doi: 10.1042/bj0550795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. Microsomal particles of normal cow's milk. Nature. 1953 Apr 25;171(4356):734–735. doi: 10.1038/171734a0. [DOI] [PubMed] [Google Scholar]
- MORTON R. K. Separation and purification of enzymes associated with insoluble particles. Nature. 1950 Dec 30;166(4235):1092–1095. doi: 10.1038/1661092a0. [DOI] [PubMed] [Google Scholar]
- MORTON R. K. Some properties of alkaline phosphatase of cow's milk and calf intestinal mucosa. Biochem J. 1955 Aug;60(4):573–582. doi: 10.1042/bj0600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. The lipoprotein particles in cow's milk. Biochem J. 1954 Jun;57(2):231–237. doi: 10.1042/bj0570231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. The purification of aklaline phosphatases of animal tissues. Biochem J. 1954 Aug;57(4):595–603. doi: 10.1042/bj0570595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. The substrate specificity and inhibition of alkaline phosphatases of cow's milk and calf intestinal mucosa. Biochem J. 1955 Oct;61(2):232–240. doi: 10.1042/bj0610232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. Transferase activity of hydrolytic enzymes. Nature. 1953 Jul 11;172(4367):65–68. doi: 10.1038/172065a0. [DOI] [PubMed] [Google Scholar]
- ROCHE J., SARLES H. Spécificité d'organe des phosphatases alcalines et hyperphosphatasémies. II. Bull Soc Chim Biol (Paris) 1954;36(4-5):491–499. [PubMed] [Google Scholar]
- ROSS M. H., ELY J. O., ARCHER J. G. Alkaline phosphatase activity and pH optima. J Biol Chem. 1951 Oct;192(2):561–568. [PubMed] [Google Scholar]


