Abstract
Background:
Chronic hepatitis B (CHB) infection results in persistent liver inflammation, which ultimately leads to liver fibrosis and increases the risk of cirrhosis. Recruitment of circulating monocytes to the liver is an essential aspect that exacerbates liver fibrosis; however, the mechanism underlying their dysregulation, which contributes to this progression, remains unclear.
Methods:
Single-cell RNA sequencing was performed to characterize the landscape of circulating monocytes from patients with CHB and liver fibrosis (CHB group) and healthy controls (HC group). Conventional techniques were performed to validate the findings.
Results:
Monocytes significantly expanded in the CHB group. The proto-oncogene LIM domain only 2 (LMO2) was highly expressed in monocytes from the CHB group, which may be associated with their expansion. In addition, we noticed that a classical monocyte subcluster surged in the CHB group and highly expressed platelet-related genes such as ITGA2B, which was identified as monocyte-platelet aggregates (MPA). The frequency of MPA was significantly higher in the CHB group, positively associated with platelet and white blood cell, and negatively associated with liver fibroscan and age, which indicates that MPA may play an important role in liver inflammation in the early liver fibrosis stage. Moreover, we found that MPA displays the enrichment of chemokine signaling-associated genes, such as C-C chemokine motif ligand 5 (CCL5), and showed an increased adhesion capacity to endothelial cells. After incubation with MPA cell supernatants, pro-inflammatory factors such as IL-8 and IL-1β were upregulated in LX-2 cells, which were reversed by the addition of anti-CCL5 antibodies.
Conclusions:
Our data suggest that enhanced LMO2 expression in circulating monocytes may be associated with their expansion, and an increased MPA subset may participate in liver fibrosis progression. These results provide valuable insights into the etiology of liver fibrosis in patients with CHB.
Keywords: Chronic hepatitis B, liver fibrosis, single cell sequencing, monocyte, monocyte-platelet aggregates
INTRODUCTION
Chronic HBV infection remains a major health concern in the world. Failure of timely treatment or intervention in chronic HBV infection results in persistent liver inflammation, which subsequently triggers the differentiation of activated HSCs into myofibroblasts. This process further accelerates the progression of liver fibrosis through increased production of extracellular matrix, marking the initiation of both cirrhosis and HCC. 1 Therefore, studies are warranted to investigate the immunopathogenesis of chronic HBV infection and its correlation with liver fibrosis.
Macrophages, which consist of infiltrating monocyte-derived macrophages and tissue-resident KCs, play a pivotal role in liver inflammation and fibrosis. It was reported that the recruitment of monocyte-derived macrophages to the liver is crucial for fostering the inflammatory response observed in NASH and contributes to fibrotic progression through the modulation of growth factors and cytokines.2,3 The severity of NASH and the stage of fibrosis strongly correlated with the presence of CCR2+ macrophage, and the CCR2/CCR5 receptor antagonist impeded monocyte infiltration and ameliorated liver inflammation and fibrosis. 2 The heterogeneity of macrophages and their high adaptability to the microenvironment endow them with diverse characteristics, enabling them to regulate liver fibrosis caused by different triggers through different mechanisms. Thus, an in-depth analysis of the circulating monocytes is helpful in unveiling the etiology of liver fibrosis for patients with CHB.
Single-cell RNA sequencing (scRNA-seq) has been used in the investigation of HBV-related diseases across various conditions. During the immune active phase, FCGR3A+ macrophages modulate the functionality of antiviral immune responses by regulating the interaction with exhausted CD8+, leading to immune dysfunction. 4 In the blood of patients with CHB, the HBsAg levels showed an impact on nature killer cells and CD8+ T cells gene expression. 5 In HBV-related HCC, macrophages suppress tumor T-cell infiltration and modulate the immunosuppressive milieu by means of TIGIT-NECTIN2 interaction. 6 In pregnant women infected with HBV, monocytes exhibit notable immune deficiencies and reduced interferon responsiveness. 7 Administering PegIFN-α treatment for CHB decreases the proportion of over-activated monocytes and shifts the TNF-driven state to an IFN-α-driven state, which enhances innate antiviral immunity. 8 This approach has expanded our understanding of the pathological characteristics of CHB across different clinical stages.
In this study, we performed single-cell transcriptome profiling of peripheral blood mononuclear cells (PBMCs) to discern the immunological distinctions of circulating monocytes between healthy individuals and patients with CHB and liver fibrosis. Additionally, the external CHB-associated scRNA-seq data sets were analyzed and the conventional techniques were performed to validate the findings. Our study elucidates the transcriptional heterogeneity of circulating monocytes and their association with liver fibrosis and thus, will provide a rich resource for understanding the etiology and developing effective prevention strategies for liver fibrosis in patients with CHB.
METHODS
Ethics approval and participant consent
All patients were recruited at Changsha Xiangya Second Hospital, and peripheral blood samples were collected. This study was approved by the hospital’s ethics committee (2021-146, 2024-0370) and conducted in accordance with the Declarations of Helsinki and Istanbul. Informed consent was obtained from all participants.
Study population
Single-cell sequencing data were obtained from 5 patients with CHB and liver fibrosis and 3 healthy adult volunteers. The inclusion criteria for patients with CHB were as follows: (1) positive for HBsAg, HBeAg, anti-HBc, and at least 6 months without antiviral treatment; (2) the absence of liver disease caused by factors other than CHB; (3) nonpregnant patients, age >18 years. Detailed clinical information is provided in Supplemental Table S1, http://links.lww.com/HC9/B932. Flow cytometry analysis comprised 132 blood samples, including 31 healthy individuals, 66 patients with CHB and liver fibrosis, 17 patients with non-HBV–related liver fibrosis, and 18 patients with HBV-related liver cancer. The detailed clinical information is provided in Supplementary Table S2, http://links.lww.com/HC9/B932.
PBMCs preparation
PBMCs were isolated using a PBMC isolation kit (TBDscience, LDS1075-200 mL). Briefly, the mixture of diluent and whole blood was slowly added to Ficoll. After centrifuging at 450 g for 30 min at 25oC, the second layer was aspirated and washed twice by PBS with 2% fetal bovine serum. The obtained PBMCs were used immediately for 3’ 10X RNA-seq procedures or cryopreserved for future use.
10 × scRNA-seq and data processing
ScRNA-seq libraries were constructed using the Chromium Next GEM Single-Cell 3’ Kit v3.1 (10x Genomics, PN-1000268). The purified libraries were sequenced on an Illumina sequencing platform with 150 bp paired-end reads. Raw gene expression matrices were produced per sample using the Cell Ranger (v.5.0.1) Pipeline, aligned with the human reference version GRCh38, and imported into R4.0.3 for gene expression analysis using R packages/Seurat. 9 Gene and UMI counts were quantified for each cell after removing low-quality cells. Subsequently, the ‘Normalization’ function of the Seurat package employing the LogNormalize method was used for normalizing the expression levels of each gene in each cell. Integration of data was performed using the ‘SelectIntegrationFeatures,’ ‘FindIntegrationAnchors’,’ and ‘IntegrateData’ functions. The resulting integrated data was scaled using the ‘ScaleData’ function. Dimensionality reduction analysis was performed using the ‘RunPCA’ and ‘RunUMAP’ functions. Unsupervised clustering analysis was executed using the ‘FindNeighbors’ and ‘FindClusters’ functions with resolution parameter as 0.8.
Functional enrichment analysis
Differentially expressed genes (DEGs) between selected cell clusters were identified using the ‘FindMarkers’ function in Seurat with Wilcoxon Rank Sum test statistics. P values were corrected for multiple testing using Bonferroni correction, and genes with corrected p values that were ≤ 0.05 were considered to be significant DEGs. Then, those DEGs were loaded into the R package clusterProfiler 10 for Gene Ontology (GO)/Disease Ontology (DO)/Kyoto Encyclopedia of Genes and Genomes enrichment analysis. The pathway with adjusted p value < 0.05 was considered significantly enriched.
Gene expression score for hepatitis B infectious disease
All hepatitis B infectious disease-related genes were extracted from the Disease Ontology database with DOID:2043. All cells in each cell type were calculated by AddModuleScore for these HBV infectious disease-related gene expressions, and the scores of all cells in each cell type were shown in the violin plot. Student t test was calculated by comparing all cells’ scores in each cell type between healthy controls (HC) and CHB groups.
Flow cytometric analysis and cell sorting
PBMCs were incubated with LIVE/DEAD Fixable Aqua Dead Cell Stain (Invitrogen, CAT# L34962) for 15 min at 4 °C. After washing, cells were labeled with anti-human CD14, anti-human CD41, anti-human CD16 antibodies at 4 °C for 30 min. After washing, the cells were analyzed using a BD LSRFortessa flow cytometer. Antibodies are described in Supplemental Table S3, http://links.lww.com/HC9/B932. For cell sorting, CD14++CD16-CD41+ and CD14++CD16-CD41- subpopulations were sorted using the BD FACSAria Fusion flow cytometer and collected for further experiments.
Culture LX-2 cells with MPA supernatant
LX-2 cells were seeded at a density of 1.5×105 cells/well in 24-well plates. Flow cytometry sorted monocyte-platelet aggregates (MPA) or non-MPA cells from patients with CHB were cultured at a density of 0.75×105 cells/well in 96-well plates at 37 oC for 48 hours. Culture supernatants were collected after centrifugation at 1000 g for 10 min at 4 oC, and were stored at −80 oC until use. The anti-C-C chemokine motif ligand 5 (CCL5) antibody (Biolegnd, 620253) with 10 μg/mL was added to the collected medium for 1 hour at 4 oC. Then, the collected medium with or without anti-CCL5 antibody pretreatment was added to LX-2 cells. After incubation for 24 hours, total RNA was extracted from LX-2 cells for gene expression analysis.
RNA extraction and real-time quantitative PCR
Total RNA was extracted from LX-2 cells using the SPARKeasy Cell RNA Rapid Extraction Kit (SparkJade, AC0205-13). First-strand cDNA was synthesized from total RNA using Hifair® Ⅲ 1st Strand cDNA Synthesis SuperMix Kits (Yeasen, 11141ES60). The mRNA levels were determined using real-time qPCR analysis on an Applied Biosystems 7500 real-time PCR system using Hieff UNICON® Universal Blue qPCR SYBR Green Master Mix (Yeasen, 11202ES08). GAPDH was used as the internal reference gene, and the mRNA relative expression level was quantified by 2−△△CT. The primer sequences are shown in Supplemental Table S4, http://links.lww.com/HC9/B932.
ELISA
Human PDGF-A (Mlbio, ml436985-J) and CCL5 ELISA Kit (Mlbio, ml253256-J) were used following the kit’s instructions. Briefly, the culture supernatants from flow cytometry sorted MPA or non-MPA cells were added to the pre-coated plate. After incubation at 37 °C for 1 h, the plate was washed for 5 times. Then, enzymic reagent was added and incubated at 37 °C for 30 min. After washing 5 times, substrate A and substrate B were added to each well. After the reaction in the dark at 37 °C for 15 min, a stop solution was added, and the absorbance was measured at 450 nm. The PDGF-A and CCL5 concentrations were calculated using a standard curve.
Monocyte and HuVec cell adhesion analysis
HuVec cells were inoculated into a 24-well plate and cultured until cell convergence reached 70%. Flow cytometry sorted MPA and non-MPA cells were collected and stained with VybrantTM DiI (ThermoFisher, V-22885) at 37 °C for 15 min. After washing by PBS, 0.4×105 cells/well were seeded on the surface of HuVec cells and co-cultured at 37 °C for 2 h. After washing with PBS, cell adhesion was observed under a fluorescence microscope, and images were acquired for further cell counts.
Immunofluorescence assay
PBMCs were incubated with anti-human CD14 antibody at 4 °C for 0.5 h. Then, they were fixed and permeabilized with FOXP3 working solution (Invitrogen, 00-5523-00) and blocked for 30 min at room temperature with 5% BSA. The cells were incubated with anti-LIM domain only 2 (LMO2) antibodies for 1 h at 4 °C and then with donkey anti-mouse IgG (H+L) and donkey anti-rabbit IgG (H+L) antibodies at room temperature for 30 min. Antibodies are described in Supplemental Table S3, http://links.lww.com/HC9/B932. After washing with PBS, the liquid-containing cells were dropped on the slide, and then the immunofluorescence images were acquired, and the integrated optical density within each cell was analyzed using Image J.
Generation of THP1 cells stably expressing LMO2
The PLVX-IRES-Puro-LMO2 plasmid was obtained from Tsingke Biotechnology. Lentiviruses were produced from 293T cells using PEI (Polysciences, 24765-1). After 48 h, the viral suspension was collected and filtered through a 0.45 μm filter. The viral suspension was diluted at a 1:1 ratio with RPMI medium and used to infect THP1 cells along with 10 ng/mL of polybrene (Beyotime, C0351). After 48 h, the cells were collected, and the overexpression efficiency was verified by western blotting.
Western blotting
Cells were lysed with RIPA buffer (Beyotime, P0013K) containing protease inhibitors (Roche, 11836153001). Then, the protein samples were denatured for 10 min at 10 °C. Whole-cell lysates were separated by electrophoresis on a 12% SDS-PAGE gel, then transferred onto a PVDF membrane and probed with primary antibodies against LMO2 (Santa Cruz, sc-65736) or β-actin (Proteintech, HRP60008). Detection was performed using a goat anti-mouse IgG H&L (HRP) secondary antibody (Abcam, Ab6789). The target bands were analyzed by Image J.
CCK-8 cell proliferation
THP1 cells were incubated at 37 °C under 5% CO2 and divided into vector control and LMO2 overexpression groups. Each group was seeded at the density of 30,000 cells/well in a 96-well plate with 5 replicates per group. After incubation for 24, 48, and 72 h, 20 μl of CCK-8 reagent (NCM Biotech, C6008) was added to each well. Two hours later, absorbance was measured at 450 nm.
Statistical analysis
The statistical significance was assessed using 1-way ANOVA or Student t test by GraphPad Prism (version 10.1.2). A p value <0.05 was considered statistically significant.
RESULTS
Single-cell transcriptomic PBMC landscape reveals a pronounced increase in monocyte proportion in patients with CHB
PBMCs collected from 5 patients with CHB and liver fibrosis (CHB group) and 3 healthy individuals (HC group) were subjected to scRNA-seq analysis (Figure 1A). The clinical information of the patients with CHB is shown in Supplemental Table S1. After quality control, we performed data integration analysis using 46,122 cells from 8 samples to eliminate batch effects. After applying an unsupervised clustering algorithm and removing small parts of neutrophils, eosinophils, and platelets, we identified 8 major cell types, including B cells (CD79A), plasma B cells (Jchain), T cells (CD3D+), monocytes (CD14+, CD16+), natural killer T cells (CD3D+, NKG7+), nature killer cells (CD3D-, NCR1+), dendritic cell (DC) cells (CD11C+), and pDC cells (IRF7) (Figure 1B, C).
FIGURE 1.
Single-cell profiling of PBMCs in patients with CHB and liver fibrosis. (A) Schematic depiction of the overall experiment design of this study. (B) The UMAP plots of the 46,122 cells derived from patients with CHB and liver fibrosis (CHB, n=5) and healthy individuals (HC, n=3). Coloration based on clustering into 8 major cell types. (C) UMAP feature plots illustrating marker genes of distinct cell types. (D, F) The proportions of each cell type. Student t test. ** p < 0.01. (E) UMAP visualization of 8 samples. (G) Venn diagram showing the shared upregulated DEGs across 5 cell types in the CHB group. Circle plot showing the top 5 enriched GO pathways for upregulated DEGs in the CHB group. Abbreviations: CHB, chronic hepatitis B; DEG, differentially expressed genes; GO, Gene Ontology; HC, healthy controls; NKT, natural killer T cells; UMAP, uniform manifold approximation and projection.
As shown in Figure 1D, E, monocyte proportion was obviously increased in the CHB group compared with that in the HC group. Upon further comparison of cellular composition, the proportion of monocytes was 34.3% in the CHB group, which was significantly increased compared with 6.5% in the HC group (p < 0.01) (Figure 1F). Simultaneously, we analyzed the external data set GSE247322, which encompasses PBMC single-cell transcriptomic data with HBV infection. Consistency between our data and the external results was noted, and the monocyte proportion increased significantly in the HBsAg high group, 24.8%, compared to the HC group, 5.5% (p < 0.05) (Supplemental Figure S4B, http://links.lww.com/HC9/B932).
HBV genes detected mainly in monocytes and B cells
By analyzing the DEGs from different cell types, a total of 192 shared upregulated genes in the CHB group were found. GO analysis showed that “viral process” and “viral genome replication” were significantly enriched from these shared upregulated genes (Figure 1G). To further detect the HBV gene expression in PBMCs, the single-cell transcriptomic sequenced raw reads were aligned to the HBV reference (MF674506.1, Hepatitis B virus isolate VN05T0217) and quantified per cell barcode using Cellranger v7.2.0. According to the filtered clean data, HBV genes were mostly found in monocytes and B cells (Supplemental Figure S1A, B, http://links.lww.com/HC9/B932), which were consistent with previous reports.11,12 HBx genes were the most detected, followed by HBc and Polymerase genes (Supplemental Figure S1C, D, http://links.lww.com/HC9/B932). According to the unfiltered raw data, HBV genes are mainly found in monocytes, and HBx was also the most detected genes (Supplemental Figure S1E, http://links.lww.com/HC9/B932).
To further investigate the association between cell subtypes and the disease, we analyzed the expression profiles of hepatitis B infectious disease-related genes obtained from the Disease Ontology database across a range of cell subtypes. The gene expression scores for HBV infectious disease were significantly higher in monocytes followed by T cells and DCs in the CHB group than those in the HC group (Supplemental Figure S1F, http://links.lww.com/HC9/B932), which implies a potential association between monocytes and HBV infection.
Heterogeneity analysis reveals MPA subcluster increase in monocytes
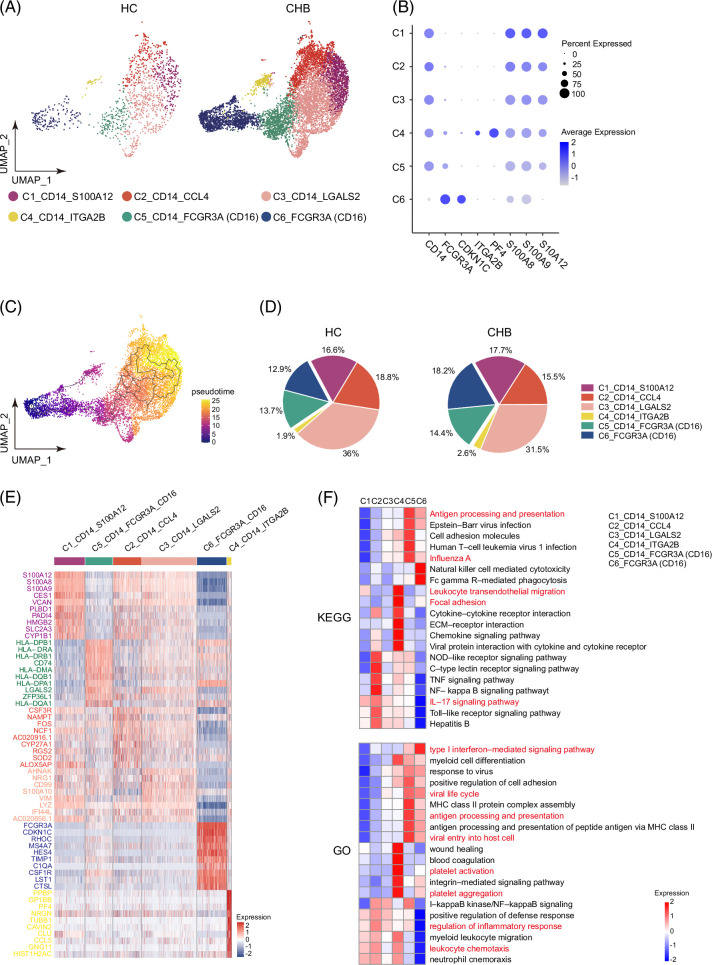
Due to the fact that monocytes are closely related to the disease, we next separated monocytes from the entire pool and re-clustered them into 6 subclusters (Figure 2A). FCGR3A is an alias for CD16. According to CD14 and FCGR3A (CD16) expression profiling, 6 subclusters are further divided into 3 categories (Figure 2B). Subcluster C1-C4 show characteristics as classical monocytes (CD14H, CD16L), subcluster C5 shows characteristics as intermediate monocytes (CD14H, CD16M), and subcluster C6 shows characteristics as non-classical monocytes (CD14L, CD16H). Choosing C6 as the root, development trajectory analysis revealed that the fate is mainly from non-classical monocytes, intermediate monocytes, and with classical monocytes as the end (Figure 2C). Interestingly, subcluster C4_CD14_TGA2B displayed upregulated expression of platelet-related genes such as ITGA2B and PF4, suggesting their classification as MPA, which were obviously scarce in the HC group (Figure 2A, B).
FIGURE 2.
Assessment of changes in monocyte clusters. (A) UMAP plots showing monocyte subclusters. Different colors indicate different cell types. (B) Dot plots showing the expression of marker genes. (C) Cellular trajectory of monocytes by Monocle 3. (D) Pie chart showing the percentage of monocyte subcluster. Student t test was performed. (E) Heatmap showing the top 10 DEGs of each monocyte subcluster. (F) The selected GO and KEGG pathways were shown by Heatmap. The vital pathways were highlighted in red. Abbreviations: DEG, differentially expressed gene; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; UMAP, uniform manifold approximation and projection.
Further analysis revealed a discrepancy in monocyte composition between the CHB and HC groups. Total classical monocyte ratios, including C1-C4, decreased in the CHB group (67.4%) compared to the HC group (73.3%) (p = 0.028), while non-classical monocytes C6 increased in the CHB group (18.2%) compared to the HC group (12.9%) (p = 0.081). MPA C4 comprised a small fraction of the monocyte population but showed a higher proportion in the CHB group (2.6%) than in the HC group (1.9%); however, it is not statistically significant (Figure 2D).
Hierarchical cluster analysis of DEGs revealed that each monocyte subcluster expressed distinct transcriptional modules containing cluster-specific genes (Figure 2E). Then, comprehensive enrichment analyses using GO and Kyoto Encyclopedia of Genes and Genomes databases revealed distinct biological functionalities and signaling pathways for each cell subcluster (Figure 2F). Notably, MPA subcluster C4 exhibited pronounced associations with “platelet activation,” “focal adhesion,” and “leukocyte transendothelial migration” pathways. “Antigen processing and presentation,” “viral life cycle,” and “type I interferon-mediated signaling pathways” were obviously enriched in intermediate monocytes C5 and non-classical monocytes C6. Classical monocytes C1, C2, and C3 exhibited pronounced associations with inflammatory response, leukocyte chemotaxis, and IL-17 signaling pathway.
To further analyze alterations of monocyte subsets, flow cytometry was performed using the gating strategy illustrated in Figure 3A, including classical monocytes (CD14++CD16-), intermediate monocytes (CD14++CD16+), and non-classical monocytes (CD14+CD16++) in 31 healthy individuals, 18 patients with HBV-related liver cancer, 17 patients with non-HBV–related liver fibrosis and 66 patients with CHB and liver fibrosis. The findings revealed that all 3 monocyte subtypes exhibited significantly higher counts in the CHB group than those in the HC group. Similarly, the counts of some monocyte subclusters showed a significant increase in the non-HBV–related liver fibrosis group or HBV-related liver cancer group compared to the HC group (Figure 3B). The percentage of classical monocytes decreased significantly in the CHB group compared to the HC group (p < 0.05), while the percentage of non-classical monocytes increased significantly in the CHB group compared to the HC group (p < 0.05) or HBV-related liver cancer group (p < 0.001) (Figure 3C). These results were consistent with the scRNA-seq data, which indicates that the frequency of virus-related non-classical monocytes increases and the frequency of inflammatory response-related classical monocytes decreases in patients with CHB.
FIGURE 3.
Flow cytometry analysis of 3 monocyte subclusters. PBMCs were from HC (n = 31), patients with HBV-related liver cancer (n = 18), patients with non-HBV–related liver fibrosis (n = 17), and patients with CHB and liver fibrosis (n = 66). (A) Representative flow cytometry gating of 3 monocyte subsets in PBMCs from patients with the CHB and HC group. (B) Absolute counts of 3 monocytes subcluster (classical, CD14++CD16-; non-classical, CD14+CD16+; and intermediate, CD14++CD16+). (C) Percentages of 3 monocytes subcluster in total monocytes. Data are presented as the median with an interquartile range. Data were analyzed by one-way ANOVA with Tukey correction according to the data distribution. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Abbreviations: CHB, chronic hepatitis B; HC, healthy controls; PBMC, peripheral blood mononuclear cells.
MPA frequency detection and correlation analysis with clinical parameters
Since MPA shows characteristics as classical monocytes through scRNA analysis, the percentage of MPA (CD14++CD16-CD41+) in classical monocytes was analyzed by flow cytometry. The average MPA proportion in classical monocytes was significantly higher in the CHB group (21.24 ± 1.08%) than in the HC group 8.83 ± 0.64% (p < 0.0001) (Figure 4A), which was consistent with the findings from scRNA-seq analysis. Meanwhile, the CD41 expression in total, intermediate, and non-classical monocytes was significantly higher in the CHB group than in the HC group. These results demonstrate that MPA is not only formed in classical monocytes but also in intermediate or non-classical monocytes from patients with CHB.
FIGURE 4.
CD41 expression in 3 monocyte subclusters. (A) The percentages of CD41+ cells in total monocytes, CD14++CD16- monocytes, CD14++CD16+ monocytes, or CD14+CD16+ monocytes. Data are presented as the median with an interquartile range. Data were analyzed by one-way ANOVA with Tukey correction according to data distribution. (B) Pearson correlation analysis of the percentages of CD41+ in monocyte subsets and clinical parameters in patients with CHB. Only p < 0.05 were denoted in corresponding circles with asterisk. Circle color and size are represented as r values. * p < 0.05, ** p < 0.01, *** p < 0.001.
To further explore the variations of MPA levels in different liver diseases, we analyzed MPA proportion in 17 non-HBV–induced liver fibrosis cases and 18 HBV-induced liver cancer cases. The representative CD41 gating strategy on monocytes and the subpopulations is shown in Fig. S5, http://links.lww.com/HC9/B932. The results revealed a significant increase in MPA proportion in total monocytes and 3 subclusters following liver injury caused by various factors compared with the HC group. However, liver injury due to HBV infection resulted in a significant increase in MPA levels than in non-HBV–induced liver fibrosis and HBV-induced liver cancer (Figure 4A), which demonstrates that MPA significantly increased in patients with CHB and the concurrent liver fibrosis may serve as an indicator of inflammation and liver function impairment.
Next, the correlation analysis was performed between MPA proportion and clinical parameters among the 66 patients with CHB (Figure 4B, Supplemental Figure S2, http://links.lww.com/HC9/B932). The analysis of the correlation between platelet and white blood cell counts in blood and the MPA proportion in total monocytes showed a significant positive correlation, indicating that higher MPA proportions in total monocytes were associated with inflammatory responses. Analysis of the correlation between age, liver fibroscan, and AST showed a significant positive correlation with each other, which indicates that liver function gradually deteriorates with age. However, MPA proportions in total monocytes significantly negatively correlated with liver fibroscan, AST, and age. The MPA proportion in classical and non-classical monocytes showed a similar association with clinical parameters. These data indicate that MPA may play an important role in liver inflammation in the early liver fibrosis stage or at younger ages.
MPA exhibits pro-inflammatory characteristics
To further delineate the function of the MPA subcluster, we bifurcated the C4_CD14_TGA2B subcluster from total monocytes, compared their DEGs with other subclusters, and performed functional enrichment analysis with upregulated DEGs in MPA. The results demonstrate that the upregulated gene functions in MPA cells include the positive regulation of platelet activation, cell adhesion, leukocyte transendothelial migration, and chemotaxis signaling (Figure 5A, B, C). Among the upregulated genes in MPA cells, we found the upregulated expression of the C-C chemokine motif ligand (CCL5), which has been identified as a pivotal regulator for liver fibrosis. 13 Additionally, we observed the upregulation of the gene encoding PDGF-A (Figure 5D), which is associated with hepatic fibrosis through the TGF-beta 1 signaling pathway.14,15 Similarly, CCL5 and PDGF-A levels in the supernatant of the isolated MPA cells were significantly higher compared with non-MPA cells from patients with CHB, which are in accordance with scRNA-seq analysis (Figure 5F).
FIGURE 5.
MPA exhibits pro-inflammatory characteristics. (A) Volcano plot showing DEGs between MPA and non-MPA cells. (B) GO functional and (C) KEGG pathway enrichment of up-regulated genes in MPAs. The vital pathways are highlighted in different colors. (D) UMAP feature plots illustrating CCL5, PDGF-A, PF4, and ITGA2B (CD41) expression in total monocytes. (E) Adhesion capability of MPA and non-MPA cells to HuVec cells. Representative images are shown on the left. The average cell counts per field of view from 4 patients with HC or CHB were shown on the right. Six images from each individual were randomly selected. (F) The concentration of CCL5 and PDGF-A in the supernatant of isolated MPA and non-MPA cells from patients with CHB detected by ELISA. Each dot represents the average data from each patient. (G) Relative expression levels of pro-inflammatory genes in LX-2 cells. Supernatants were collected from MPA and non-MPA monocytes from patients with CHB. With or without anti-CCL5 antibody pretreatment, the collected supernatants were added to LX-2 cells. Twenty-four hours later, gene expression levels were measured by RT-PCR. Each dot represents data from each individual. Data are presented as mean ± SEM. Student t test. * p < 0.05, ** p < 0.01. Abbreviations: CHB, chronic hepatitis B; DEG, differentially expressed gene; GO, Gene Ontology; HC, healthy controls; KEGG, Kyoto Encyclopedia of Genes and Genomes; MPA, monocyte-platelet aggregates; UMAP, uniform manifold approximation and projection.
To assess the potential function of the MPA subset, we explored the adhesion capacity of MPA cells (CD14++CD16-CD41+) and non-MPA cells (CD14++CD16-CD41-) with HuVec cells. Our results demonstrated that MPA cells isolated from the patients with CHB displayed significantly heightened adhesion capacity to HuVec cells (p < 0.01, vs. HC) (Figure 5E). To further elucidate the potential role of MPA cells in the development of liver fibrosis, LX-2 cells were cultured with MPA or non-MPA cell supernatants from patients with CHB. After 24 h of incubation, the LX-2 cells were collected for gene expression by RT-PCR. The results showed that LX-2 cells cultured with MPA cell supernatants showed the upregulation of pro-inflammatory factors such as IL-8, IL-1β, CXCL1, IL-6, and CCL2 compared to non-MPA cell supernatants from patients with CHB, however, they were not statistically significant. The addition of anti-CCL5 antibodies significantly reduced the expressions of these cytokines (p < 0.05 for IL-8, IL-6, IL-1β, p < 0.01 for CCL2) (Figure 5G). These results demonstrate that MPA may induce pro-inflammatory cytokine production in LX-2 cells by CCL5 secretion, thus exacerbating liver inflammation and contributing to the progression of liver fibrosis.
LMO2 upregulated in monocytes from patients with CHB and was associated with its proliferation
Owing to the close correlation between monocytes and the disease, characteristic genetic changes in total monocytes were analyzed by comparing DGEs between the HC and CHB groups. The results revealed that inflammation-related genes such as IL-1β, CCL3, and NFκBIZ were significantly downregulated in the CHB group. Meanwhile, interferon-stimulated genes, such as ISG15, IFI6, and IFI27, were significantly upregulated in the CHB group (Figure 6A). Then, GO enrichment analysis showed that “leukocyte chemotaxis” and “antigen processing and presentation” were enriched from downregulated genes in the CHB group (Figure 6B), while “response to virus”, “histone modification”, “type I interferon production,” and “myeloid leukocyte differentiation” pathways were enriched from the upregulated genes in the CHB group (Figure 6C).
FIGURE 6.
LMO2 is highly expressed in monocytes from patients with CHB. (A) Heatmap showing DEGs of monocytes between the HC and CHB groups. The top 25 downregulated and upregulated DEGs in the CHB groups were shown. The GO functional enrichment of downregulated (B) and upregulated DEGs (C) of monocytes from the CHB group compared to the HC group. The vital pathways are highlighted in red. (D) Violin plot showing the expression levels of LMO2 in different cell clusters in the HC and CHB groups. (E) UMAP feature plots illustrating LMO2 expression in total PBMCs from each healthy individual and patient with CHB. (F) Representative IF staining images showing the localization of CD14 (red) and LMO2 (green) in PBMCs; cell nuclei counterstained with DAPI (blue). (G) Integrated optical density (IOD) within each cell was analyzed in all images using the Image J software. Five images from each individual were randomly selected, and each dot represents the average IDO for each individual. (H) The violin plot illustrating LMO2, HHEX, and LYL1 expression in total monocytes. (I) Western blotting analysis of LMO2 expression in THP1-LMO2+ cells or THP1 cells transduced by control lentivirus. (J) The proliferation levels of THP1 cells by CCK-8 assay. Data is represented as the mean ± SEM from triplicate experiments. Student t test. * p < 0.05. Abbreviations: CHB, chronic hepatitis B; DEG, differentially expressed gene; GO, Gene Ontology; HC, healthy controls; IF, immunofluorescence; IOD, integrated optical density; LMO2, LIM domain only 2; PBMCs, peripheral blood mononuclear cells; UMAP, uniform manifold approximation and projection.
Among the top 25 upregulated genes in monocytes, proto-oncogene LMO2 exhibited significant upregulation in the CHB group. Simultaneous analysis of LMO2 expression across various cell types showed that LMO2 was highly expressed in monocytes and DCs in the CHB group compared with those in the HC group (Figure 6D, E). LYL1, which is the concordant expression gene of LMO2, 16 and HHEX, which is a direct transcriptional target of LMO2, 17 were simultaneously upregulated in total monocytes from the CHB group (Figure 6H).
To ascertain the LMO2 expression in the monocytes, we conducted immunofluorescence staining of CD14 and LMO2 in PBMCs. The results confirmed that the fluorescence intensity of LMO2 was obviously higher in monocytes from the CHB group than those in the HC group (Figure 6F). Moreover, pixel density analysis of LMO2 expression showed that the mean integrated optical density value for LMO2 was significantly elevated in the CHB group than in the HC group (p < 0.05) (Figure 6G). Additionally, the analysis of 2 exogenous data sets (GSE:182159, GSE:247322) showed that the LMO2 expression levels obviously increased in monocytes from patients infected with HBV compared with healthy individuals (Supplemental S3, S4, http://links.lww.com/HC9/B932). Collectively, these findings suggest a significant upregulation of LMO2 expression in monocytes from patients with CHB.
To further investigate the effect of LMO2 in monocytes, lentivirus was used to introduce LMO2 genes into THP1 cells. Western blotting revealed a distinct band at approximately 28 kDa, indicating the presence of the LMO2 protein. Notably, a higher band intensity was observed in THP1 cells transfected with lentivirus overexpressing the LMO2 gene (THP1-LMO2+) than in those transfected with lentivirus carrying control vectors (THP1-vector), suggesting the successful overexpression of LMO2 in the former (Figure 6I). CCK-8 assays were performed at various time points post-seeding. The proliferation rate of the LMO2-overexpressing cells was consistently higher than the THP1-vector cells across different time points (Figure 6J). These observations suggest that LMO2 overexpression may be associated with monocyte proliferation.
DISCUSSION
Macrophages and monocytes are pivotal modulators of hepatic inflammation through the orchestration of interactions with hepatocytes and HSCs, which facilitates collagen deposition and plays a dominant role in liver fibrosis progression.18,19 In this study, we revealed a pronounced increase in circulating monocytes from patients with CHB and liver fibrosis. This result was verified by flow cytometric analysis of PBMCs from 66 patients with CHB. Consistent results were obtained using exogenous HBV-infected peripheral immune single-cell data. 5 After hepatic inflammation, expansion of the hepatic macrophage pool predominantly originates from circulating monocytes. 20 Not only did the proportion of circulating monocytes increase, but a significant alteration of their gene regulation was also observed. Additionally, we found that the MPA subset surged in classical monocytes in the CHB group, as confirmed by both scRNA-seq and flow cytometry analysis. However, the frequency of MPA cells in the CHB group was relatively lower in the scRNA-seq analysis (approximately 2.6% of total monocytes) compared to the flow cytometry analysis (approximately 15% of total monocytes). In scRNA-seq, the Chromium 10 × system was used to create emulsions that encapsulate single cells along with hydrogel beads and reagents in droplets. 21 Given the larger volume of monocyte-platelet aggregates, their likelihood of being encapsulated may be lower than that of single monocytes. The MPA frequency in total monocytes is negatively associated with liver fibroscan and age, which indicates that MPA may play an important role in liver inflammation at early liver fibrosis stage or at younger ages. These data further support the notion that monocyte reprogramming may contribute to the progression of liver fibrosis.
In this study, we found that LMO2 expression was significantly upregulated in monocytes from patients with CHB. The hematopoietic transcription factor LMO2, which is a member of the TAL1 transcriptional complex, plays a crucial role in early hematopoiesis and acts as an oncogenic driver of T cell acute lymphoblastic leukemia. 22 The LMO2 protein functions as a bridging molecule in multiprotein complexes.23–25 There are 2 E-box binding sites in the bipartite DNA binding elements, which are restricted to CD4 and CD8 double-negative immature thymocytes. The recognition of dual E-box oligo motif by the LMO2-containing oligomeric complex may lead to abnormal regulation of downstream target genes and result in partially T cell development blocked.26,27 Only T cell tumors arise in transgenic mice with enforced LMO2 expression, even if a general transcription promoter controls the LMO2 expression.28–30 Clonal T cell leukemias arose in a high proportion of mice with long latency, and cooperation with the LMO2 binding partner Tal1 was manifested by the more rapid development of T cell tumors in transgenic mice. 30 Accordingly, we found monocyte expansion with LMO2 upregulation in patients with CHB; however, no monocytic leukemia was found in these patients. Transgenic mice with enforced expression of LMO2 in T cells developed highly penetrant T-cell lymphoblastic leukemia, and HHEX was found as a crucial mediator of LMO2’s oncogenic function. 17 In this study, we found that monocytes were significantly expanded with higher LMO2 overexpression and HHEX and LYL1 were simultaneously upregulated in monocytes from patients with CHB. Therefore, we hypothesize that aberrant monocyte expansion in patients with CHB may be associated with LMO2 overexpression. However, the mechanism for LMO2 regulating monocyte expansion needs to be further explored. In this study, the THP-1 cell line, derived from the peripheral blood of acute monocytic leukemia patients, was used to demonstrate that LMO2 overexpression could enhance monocyte proliferation. Several reports have shown that just a small subset of human monocytes, ranging from 1%–10%, can enter the cell cycle in vitro in response to factors such as M-CSF.31–33 Thus, modulating LMO2 overexpression in human primary monocytes to detect the changes in proliferation is a challenge due to their low proliferation rate. However, further evidence from primary monocytes is still needed, and the reasons for LMO2 upregulation in monocytes from patients with CHB need to be further explored in the future.
Human monocytes are categorized into 3 subsets based on CD14 and CD16 expression. CD14++CD16- classical monocytes represent the predominant type of monocytes. 34 The classical monocytes represent a heterogeneous cell population with antiviral, interferon-responsive, immunomodulatory, inflammatory, and tissue repair functions. 35 The intermediate monocytes (CD14++CD16+) usually accumulate in inflamed livers through direct recruitment from blood or from local differentiation from CD14++CD16- classical monocytes. 36 And the intrahepatic intermediate monocytes showed high levels of phagocytic activity, antigen presentation, and secreted pro-inflammatory and profibrogenic cytokines and chemokines such as IL-8, IL-13, or CCL5. 36 By analyzing the scRNA-seq data, MPA subpopulation C4 showed characteristics as classical monocytes. In coronary artery disease, MPA cells preferentially migrate to plaques and subsequently differentiate into macrophages. 37 In this study, MPA cells isolated from classical monocytes from patients with CHB and liver fibrosis exhibited strong adhesion capacity to HUVEC cells. Additionally, MPA cells could produce more abundant CCL5 and PDGF-A than non-MPAs. CCL5 could exacerbate liver inflammation and serve as a crucial mediator of HSC activation.38,39 PDGF plays a crucial role in wound healing and tissue repair and acts as a significant fibrotic factor.40,41 Thus, MPAs may play a vital role during the progression of liver fibrosis.
HSC activation, which leads to collagen production, is one of the primary factors that contribute to liver fibrosis. Our data substantiate that MPA may stimulate LX-2 cells to express pro-inflammatory genes. The features of activated HSCs include mitogen-driven proliferation, heightened fibrogenesis driven by connective tissue growth factor and TGFβ1, amplified inflammation and immune modulation, and the alteration of matrix degradation. 42 Thus, in our study, MPA could stimulate HSC activation by expressing vigorous inflammatory cytokine genes. Also, this activation could be reversed by the addition of anti-CCL5 antibodies.
In summary, we observed pronounced alterations in circulating monocytes in patients with CHB and concurrent liver fibrosis. These findings emphasize the MPA subset and their significance in the development of liver fibrosis, which provide valuable insights into therapeutic strategies targeting CHB accompanied by liver fibrosis.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the support from the Seeksoul platform (https://seeksoul.online/index.html#/login) and the High-performance computing platform at Tianjin Medical University for scRNAseq data analysis. They also thank Dr. Xin Liu, Yingxue Sun, and Guangxin Zhang for their kind help and support.
DATA AVAILABILITY STATEMENT
The scRNA-seq data in this study have been deposited into the CNGB Sequence Archive (CNSA) of China National GeneBank DataBase (CNGBdb) with accession number CNP0006627. All of the other data that support the findings of this study are available from the corresponding author upon reasonable request.
AUTHOR CONTRIBUTIONS
Dongming Zhou and Min Wang generated the initial idea. Dongming Zhou, Min Wang, and Jiling Ren designed the experiments and supervised the overall project. Yue Zhuo, Hongzheng Wu, Wenying Zhao, Sheng Yin, and Jiling Ren performed the experiments. Jiling Ren, Yue Zhuo, Lifeng Feng, and Shulei Jia performed the bioinformatics analysis of scRNA-seq. Sheng Yin, Hongzheng Wu, and Fang Lei collected the samples. Fang Lei, Xueyang Pang, Wei Sun, Wanzhen Li, and Yang Li provided technical or material support. Yue Zhuo and Jiling Ren analyzed the data and wrote the manuscript. Dongming Zhou and Min Wang revised the manuscript.
FUNDING INFORMATION
This work was chiefly supported by the funding from the National Natural Science Foundation of China (82241065) to Dongming Zhou and the National Natural Science Foundation of China (82270840) to Min Wang.
CONFLICTS OF INTEREST
The authors have no conflicts to report.
Footnotes
Yue Zhuo, Hongzheng Wu, Wenying Zhao, and Sheng Yin contributed equally to this work.
Abbreviations: CCL5, C-C chemokine motif ligand 5; CHB, chronic hepatitis B; DC, dendritic cell; DEG, differentially expressed gene; GO, Gene Ontology; HC, healthy controls; ISGs, interferon-stimulated genes; LMO2, LIM domain only 2; MPA, monocyte-platelet aggregates; PBMCs, peripheral blood mononuclear cells; scRNA-seq, single-cell RNA sequencing; UMAP, uniform manifold approximation and projection.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.hepcommjournal.com.
Contributor Information
Yue Zhuo, Email: sylm_zhuoyue@163.com.
Hongzheng Wu, Email: 218211076@csu.edu.cn.
Wenying Zhao, Email: zwy1216@yeah.net.
Sheng Yin, Email: shengyinxy@csu.edu.cn.
Fang Lei, Email: leif100@163.com.
Xueyang Pang, Email: pxydem@163.com.
Wei Sun, Email: sunweibio@nankai.edu.cn.
Lifeng Feng, Email: 9820220108@nankai.edu.cn.
Shulei Jia, Email: jiashu320lei@126.com.
Wanzhen Li, Email: 238211110@csu.edu.cn.
Yang Li, Email: 2692380228@qq.com.
Jiling Ren, Email: jlren2020@163.com.
Min Wang, Email: wangmin0000@csu.edu.cn.
Dongming Zhou, Email: zhoudongming@tmu.edu.cn.
REFERENCES
- 1.Li TY, Yang Y, Zhou G, Tu ZK. Immune suppression in chronic hepatitis B infection associated liver disease: A review. World J gastroenterol. 2019;25:3527–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krenkel O, Puengel T, Govaere O, Abdallah AT, Mossanen JC, Kohlhepp M, et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology (Baltimore, Md). 2018;67:1270–1283. [DOI] [PubMed] [Google Scholar]
- 3.Guo Q, Furuta K, Lucien F, Gutierrez Sanchez LH, Hirsova P, Krishnan A, et al. Integrin β(1)-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol. 2019;71:1193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C, Li J, Cheng Y, Meng F, Song JW, Fan X, et al. Single-cell RNA sequencing reveals intrahepatic and peripheral immune characteristics related to disease phases in HBV-infected patients. Gut. 2023;72:153–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beudeker BJB, Osmani Z, van Oord GW, Groothuismink ZMA, de Knegt RJ, Hoogenboezem RM, et al. Association of HBsAg levels with differential gene expression in NK, CD8 T, and memory B cells in treated patients with chronic HBV. JHEP Rep. 2024;6:100980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho DWH, Tsui YM, Chan LK, Sze KMF, Zhang X, Cheu JWS, et al. Single-cell RNA sequencing shows the immunosuppressive landscape and tumor heterogeneity of HBV-associated hepatocellular carcinoma. Nat Commun. 2021;12:3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Li X, Yang K, Guo F, Wang X, Zhao Z, et al. Single-cell RNA sequencing reveals transcriptional profiles of monocytes in HBV-infected pregnant women during mid-pregnancy. J Cell Mol Med. 2023;27:1465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang P, Jia H, Qian X, Tang T, Han Y, Zhang Z, et al. Single-cell RNA sequencing reveals the immunoregulatory roles of PegIFN-α in patients with chronic hepatitis B. Hepatology (Baltimore, Md). 2024;79:167–182. [DOI] [PubMed] [Google Scholar]
- 9.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36:411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu G, Wang LG, Han Y, He QY. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoffe B, Noonan CA, Melnick JL, Hollinger FB. Hepatitis B virus DNA in mononuclear cells and analysis of cell subsets for the presence of replicative intermediates of viral DNA. J Infect Dis. 1986;153:471–477. [DOI] [PubMed] [Google Scholar]
- 12.Chemin I, Vermot-Desroches C, Baginski I, Saurin JC, Laurent F, Zoulim F, et al. Selective detection of human hepatitis B virus surface and core antigens in peripheral blood mononuclear cell subsets by flow cytometry. J Viral Hepat. 1994;1:39–44. [DOI] [PubMed] [Google Scholar]
- 13.Berres ML, Koenen RR, Rueland A, Zaldivar MM, Heinrichs D, Sahin H, et al. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J Clin Invest. 2010;120:4129–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thieringer F, Maass T, Czochra P, Klopcic B, Conrad I, Friebe D, et al. Spontaneous hepatic fibrosis in transgenic mice overexpressing PDGF-A. Gene. 2008;423:23–28. [DOI] [PubMed] [Google Scholar]
- 15.Tanikawa AA, Grotto RMT, Silva GF, Ferrasi AC, Sarnighausen VCR, Pardini MIMC. Platelet-derived growth factor A mRNA in platelets is associated with the degree of hepatic fibrosis in chronic hepatitis C. Rev Soc Bras Med Trop. 2017;50:113–116. [DOI] [PubMed] [Google Scholar]
- 16.Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC, et al. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 2002;1:75–87. [DOI] [PubMed] [Google Scholar]
- 17.Smith S, Tripathi R, Goodings C, Cleveland S, Mathias E, Hardaway JA, et al. LIM domain only-2 (LMO2) induces T-cell leukemia by two distinct pathways. PLoS One. 2014;9:e85883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng D, Chai J, Wang H, Fu L, Peng S, Ni X. Hepatic macrophages: Key players in the development and progression of liver fibrosis. Liver Int. 2021;41:2279–94. [DOI] [PubMed] [Google Scholar]
- 19.Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–12. [DOI] [PubMed] [Google Scholar]
- 20.Guilliams M, Scott CL. Liver macrophages in health and disease. Immunity. 2022;55:1515–29. [DOI] [PubMed] [Google Scholar]
- 21.De Simone M, Rossetti G, Pagani M. Chromium 10× Single-Cell 3’ mRNA sequencing of tumor-infiltrating lymphocytes. Methods Mol Biol (Clifton, NJ). 2019;1979:87–110. [DOI] [PubMed] [Google Scholar]
- 22.Morishima T, Krahl AC, Nasri M, Xu Y, Aghaallaei N, Findik B, et al. LMO2 activation by deacetylation is indispensable for hematopoiesis and T-ALL leukemogenesis. Blood. 2019;134:1159–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valge-Archer VE, Osada H, Warren AJ, Forster A, Li J, Baer R, et al. The LIM protein RBTN2 and the basic helix-loop-helix protein TAL1 are present in a complex in erythroid cells. Proc Natl Acad Sci USA. 1994;91:8617–8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadman I, Li J, Bash RO, Forster A, Osada H, Rabbitts TH, et al. Specific in vivo association between the bHLH and LIM proteins implicated in human T cell leukemia. EMBO J. 1994;13:4831–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadman IA. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. EMBO J. 1997;16:3145–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grütz GG, Bucher K, Lavenir I, Larson T, Larson R, Rabbitts TH. The oncogenic T cell LIM-protein Lmo2 forms part of a DNA-binding complex specifically in immature T cells. EMBO J. 1998;17:4594–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nam CH, Rabbitts TH. The role of LMO2 in development and in T cell leukemia after chromosomal translocation or retroviral insertion. Mol Ther. 2006;13:15–25. [DOI] [PubMed] [Google Scholar]
- 28.Neale G, Rehg J, Goorha R. Ectopic expression of rhombotin-2 causes selective expansion of CD4-CD8- lymphocytes in the thymus and T-cell tumors in transgenic mice. Blood. 1995;86:3060–3071. [PubMed] [Google Scholar]
- 29.Larson RC, Osada H, Larson TA, Lavenir I, Rabbitts TH. The oncogenic LIM protein Rbtn2 causes thymic developmental aberrations that precede malignancy in transgenic mice. Oncogene. 1995;11:853–862. [PubMed] [Google Scholar]
- 30.Larson RC, Lavenir I, Larson TA, Baer R, Warren AJ, Wadman I, et al. Protein dimerization between Lmo2 (Rbtn2) and Tal1 alters thymocyte development and potentiates T cell tumorigenesis in transgenic mice. EMBO J. 1996;15:1021–1027. [PMC free article] [PubMed] [Google Scholar]
- 31.Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elliott M, Vadas M, Eglinton J, Park L, To L, Cleland L, et al. Recombinant human interleukin-3 and granulocyte-macrophage colony-stimulating factor show common biological effects and binding characteristics on human monocytes. Blood. 1989;74:2349–2359. [PubMed] [Google Scholar]
- 33.Cheung D, Hamilton J. Regulation of human monocyte DNA synthesis by colony-stimulating factors, cytokines, and cyclic adenosine monophosphate. Blood. 1992;79:1972–1981. [PubMed] [Google Scholar]
- 34.Guilliams M, Mildner A, Yona S. Developmental and functional heterogeneity of monocytes. Immunity. 2018;49:595–613. [DOI] [PubMed] [Google Scholar]
- 35.Mulder K, Patel AA, Kong WT, Piot C, Halitzki E, Dunsmore G, et al. Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity. 2021;54:1883–900.e5. [DOI] [PubMed] [Google Scholar]
- 36.Liaskou E, Zimmermann HW, Li KK, Oo YH, Suresh S, Stamataki Z, et al. Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology (Baltimore, Md). 2013;57:385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oggero S, de Gaetano M, Marcone S, Fitzsimons S, Pinto AL, Ikramova D, et al. Extracellular vesicles from monocyte/platelet aggregates modulate human atherosclerotic plaque reactivity. J Extracell Vesicles. 2021;10:12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie X, Lv H, Liu C, Su X, Yu Z, Song S, et al. HBeAg mediates inflammatory functions of macrophages by TLR2 contributing to hepatic fibrosis. BMC Med. 2021;19:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M, Sun X, Zhao J, Xia L, Li J, Xu M, et al. CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization. Cell Mol Immunol. 2020;17:753–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Wang P, Yu Y, Huang E, Yao Y, Guo D, et al. Hepatocyte Ninjurin2 promotes hepatic stellate cell activation and liver fibrosis through the IGF1R/EGR1/PDGF-BB signaling pathway. Metabolism. 2023;140:155380. [DOI] [PubMed] [Google Scholar]
- 41.Martin IV, Borkham-Kamphorst E, Zok S, van Roeyen CRC, Eriksson U, Boor P, et al. Platelet-derived growth factor (PDGF)-C neutralization reveals differential roles of PDGF receptors in liver and kidney fibrosis. Am J Pathol. 2013;182:107–117. [DOI] [PubMed] [Google Scholar]
- 42.Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473–1492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The scRNA-seq data in this study have been deposited into the CNGB Sequence Archive (CNSA) of China National GeneBank DataBase (CNGBdb) with accession number CNP0006627. All of the other data that support the findings of this study are available from the corresponding author upon reasonable request.