Abstract
The aim of this work was to assess the usefulness of hydatid cyst fluid (HCF) of Echinococcus granulosus, obtained from mice experimentally infected with hydatid cyst tissue homogenates, for the serodiagnosis of cystic echinococcosis (CE) in humans. The sensitivity and specificity of HCF obtained from mice for the detection of immunoglobulin G (IgG) antibodies in the sera of CE patients were compared with those of HCF from sheep and/or from a human CE patient by using immunoblotting (IB) and an enzyme-linked immunosorbent assay (ELISA). HCFs obtained from three different host species all were highly useful for immunoblotting, and sera from 19 (95%) of 20 CE patients equally recognized the antigen B subunit (approximately 8 kDa). HCF from mice showed a cross-reaction with 9 of 20 alveolar echinococcosis (AE) sera (45%), whereas HCFs from two other host species cross-reacted with 14 of the AE sera (70%). Although 2 (10%) of 20 sera from neurocysticercosis (NCC) patients were false positive with HCF from both sheep and humans, none of these sera showed a positive reaction with HCF from mouse origin. ELISAs with HCFs from both mouse and sheep origins detected all 20 CE and AE sera; however, these ELISAs showed 45% (9 of 20) and 60% (12 of 20) false-positive reactions with 20 NCC sera, respectively. The presence of nonspecific human IgG in HCF obtained from a CE patient prevented us from applying it to the ELISA. HCF of E. granulosus, obtained from laboratory mice with a secondary infection with hydatid cyst tissue homogenates, appears to be highly useful for the serodiagnosis of CE in humans and may be useful in domestic animals.
Cystic echinococcosis (CE), caused by infection with larval Echinococcus granulosus, has public health importance not only in areas of endemicity but also in countries or regions without endemicity due to the migration of infected people (1, 7, 13) and livestock exchanges. Movement of infected livestock also raises the potential for transmission, creating new areas of endemicity. Immunodiagnosis of CE is commonly performed as a primary screening test with crude antigens and a confirmatory test with specific antigens (8). For preparation of such crude or purified native antigens, most researchers collect hydatid cyst fluid (HCF) from naturally infected intermediate hosts, such as sheep, cattle, horses, camels, pigs, or moose, or from CE patients (4, 5, 6, 9, 10, 15). However, the nature and quality of the antigens are variable among the host species (11). This may be one of the reasons why different laboratories obtain different results for the detection of anti CE antibodies with antigens prepared from different host species. In order to standardize the origin of HCF of E. granulosus, we have investigated the antigenic quality of HCF prepared from mice experimentally infected with hydatid cyst tissue homogenates and have evaluated its usefulness for the serodiagnosis of CE in humans.
MATERIALS AND METHODS
HCF was collected from cysts (2 to 3 cm in diameter) developed in ICR female mice after 12 months of secondary infection with hydatid cyst tissue homogenates originally obtained from a sheep naturally infected with E. granulosus in China. For comparison of reactivity, HCF from naturally infected sheep was obtained at a slaughter house in Urumqi, China, and HCF from a patient at Osaka Red Cross Hospital, Osaka, Japan, was obtained from a 16-year-old Chinese boy living in Osaka. Twenty serum samples from surgically confirmed CE patients were obtained from Australia; 20 serum samples from alveolar echinococcosis (AE) cases were obtained from Asahikawa Medical College, Asahikawa, Japan (n = 10) and from the Centers for Disease Control and Prevention (n = 10); 20 serum samples from neurocysticercosis (NCC) cases were obtained from Korea (n = 10), China (n = 7), and the Centers for Disease Control and Prevention (n = 3); and 7 serum samples were obtained from healthy Japanese donors. An additional 30 individual normal serum samples from Japanese students were used as negative controls for determination of the cutoff values of the enzyme-linked immunosorbent assay (ELISA).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting were carried out as described by Ito et al. (6) with commercially available precast 4 to 20% polyacrylamide gradient gels (01026, for two dimensions with a 6-cm width; Tefco Co. Ltd., Tokyo, Japan) under reducing conditions. A prestained low-range marker (Bio-Rad) was used for monitoring electrophoresis and transblotting. Approximately 150 μl of HCFs prepared from mice, sheep, and humans was loaded into large sample wells with a width of 6 cm. Electrophoresis was carried out at a constant 20 mA for approximately 90 min. Transfer to Immobilon transfer membranes (polyvinylidene difluoride; Millipore) was carried out at a constant 40 mA for 15 h. Immunoblotting was carried out with human sera at a 1:50 dilution. Horseradish peroxidase (HRP)-labeled polyclonal antibodies against human immunoglobulin G (IgG) at a 1:1,000 dilution were used as a conjugate as reported previously (6).
The ELISA was performed as described by Verastegui et al. (15) with slight modifications. Briefly, 96-well microtitration plates (Nunc; Nalge Nunc International, Roskilde, Denmark) were incubated with 100 μl of HCF/well (diluted in 10 mM carbonate buffer [pH 9.6] in order to give protein concentrations of 1.2 μg/ml for the detection of E. granulosus-specific total IgG and 10 μg/ml for the detection of E. granulosus-specific IgG subclasses) overnight. Excess antigen was removed by washing the wells five times with phosphate-buffered saline-Tween 20 (pH 7.4; containing 10 mM Na2HPO4, 1 mM KH2PO4, 140 mM NaCl, 3 mM KCl, and 0.05% Tween 20), and then blocking was done with 250 μl of 1% casein in 140 mM NaCl-200 mM Tris-HCl (pH 7.6) at 37°C for 1 h. The wells were dried, and 100 μl of serum samples at a 1:200 dilution in 1% casein buffer was loaded into duplicate wells and incubated for 1 h at 37°C. Positive and pooled negative control sera were used in each plate. After 1 h of incubation, the plates were washed with phosphate-buffered saline-Tween 20 as described above, and 100 μl of HRP-labeled polyclonal antibodies against human IgG (Zymed Laboratories) at a 1:1,000 dilution in 1% casein buffer was loaded into all the wells and incubated for 1 h at 37°C for the detection of E. granulosus-specific total IgG. For the detection of E. granulosus-specific IgG subclasses, 100 μl of HRP-labeled monoclonal antibodies against human IgG1, IgG2, IgG3, or IgG4 (Zymed Laboratories) at a 1:250 dilution was loaded into respective wells and incubated for 1 h at 37°C. The plates were washed as described above to remove the excess conjugate. For color development, 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid; Sigma) was added to each well as a substrate (0.3 mg/ml, 100 μl/well), and the reaction was terminated after 15 min or after 25 to 30 min by the addition of 100 μl of 1% sodium dodecyl sulfate solution to each well. The absorbance at 405 nm was monitored with a Bio-Rad microplate reader (model 450). The cutoff points were established as two times and three times the optical density of a pool of serum samples obtained from 30 healthy donors and reacted with HCFs from mice and sheep, respectively (17).
RESULTS AND DISCUSSION
Immunoblotting with HCF antigens from mice, sheep, and humans (Fig. 1A to C, respectively) showed very similar patterns of recognition by almost all of the CE sera. As described in previous reports (6, 10, 11), the smallest subunit of antigen B (8 kDa) is highly sensitive and is expected to be Echinococcus genus specific (6). Our immunoblotting results obtained with HCFs from three different host origins (summarized in Table 1) indicated that 19 (95%) of the 20 confirmed CE sera reacted with the antigen B subunit (approximately 8 kDa) in the HCF. The HCF from a human CE patient showed a relatively stronger positive reaction (Fig. 1C). This result appeared to be due mainly to its higher protein concentration. Antigen B in HCF from mice was recognized by 9 (45%) of 20 AE sera, whereas antigen B in HCFs from both sheep and humans was recognized by 14 (70%) of the AE sera. Although antigen B of HCFs from sheep and humans showed a cross-reaction with two (10%) of 20 NCC sera, antigen B of HCF from mice was not recognized by any of the NCC sera. According to our observations, the immunoblots used to detect antibodies against the antigen B subunit (8 kDa) in HCFs from three different host origins exhibited almost the same sensitivity for the diagnosis of CE. The HCF from mice appeared to be more specific for CE or for echinococcosis at least, with the fewest nonspecific background banding patterns (Fig. 1A and Table 1). When we applied HCFs from three different host origins in the ELISA, HCFs from both mice and sheep showed very similar results with all 20 CE sera and all 20 AE sera (Fig. 2 and Table 1). In agreement with other reports (11, 15), HCFs from both mice and sheep exhibited relatively higher levels of cross-reactivity (45 and 60%) with NCC sera, respectively. This result may have been due to the presence in HCF of some components other than antigen B that are homologous to antigens of the closely related parasite Taenia solium.
FIG. 1.
Immunoblotting with HCFs of E. granulosus from three different hosts. The reactivities of selected serum samples from patients with CE (12 sera), AE (12 sera), and NCC (12 sera) and serum samples from healthy controls (N; 3 sera) are shown. (A) HCF from mice. (B) HCF from sheep. (C) HCF from humans. Ag, antigen. Lanes M, markers.
TABLE 1.
Comparison of antigenicity of HCFs of E. granulosus obtained from three different hosts
| Serum sample source (no. of sera examined) | No. (%) of samples found positive with HCFs from the indicated source by:
|
||||
|---|---|---|---|---|---|
| Immunoblotting
|
ELISA
|
||||
| Mice | Sheep | Humans | Mice | Sheep | |
| CE (20) | 19 (95) | 19 (95) | 19 (95) | 20 (100) | 20 (100) |
| AE (20) | 9 (45) | 14 (70) | 14 (70) | 21 (100) | 20 (100) |
| NCC (20) | 0 (0) | 2 (10) | 2 (10) | 9 (45) | 12 (60) |
| Normal controls (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
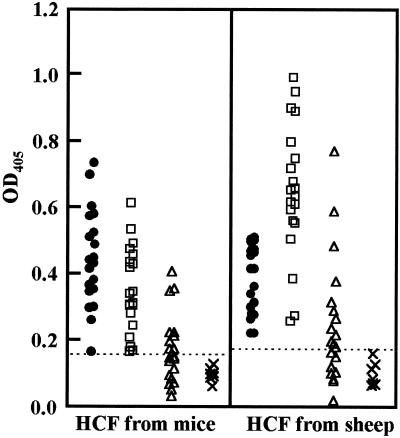
FIG. 2.
Comparison of reactivities of HCFs from mice and sheep in an ELISA with serum samples from patients with CE (•), AE (□), and NCC (▵) and serum samples from normal controls (×). The cutoff values are displayed as broken horizontal lines. The mean and standard deviation values for optical density at 405 nm (OD405) for CE, AE, and NCC patients and for normal controls with HCFs from mice and sheep were 0.46 ± 0.14 and 0.39 ± 0.11, 0.35 ± 0.13 and 0.65 ± 0.20, 0.17 ± 0.10 and 0.25 ± 0.19, and 0.098 ± 0.02 and 0.1 ± 0.04, respectively.
When we used HCF from humans in the ELISA, we were unable to differentiate the negative control from the positive control due to the presence of nonspecific human IgG components (16). Since the nonspecific human IgG in HCF obtained from a CE patient may also be used to coat ELISA microplate wells together with the HCF, it then can be recognized by an anti-human IgG conjugate without incubation with human serum. Many nonspecific bands were detected in immunoblots probed with normal sera, as illustrated in Fig. 1C. Hence, HCF from CE patients cannot be used in an ELISA to detect anti-Echinococcus antibodies in humans.
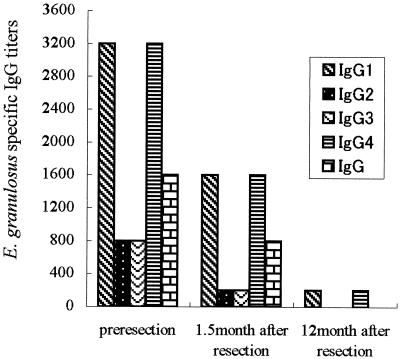
Although hydatid cysts which develop in laboratory mice are typically sterile, they can produce antigen B (12). In addition, there was no critical difference among HCFs obtained from mice, sheep, and humans in their ability to detect specific IgG antibodies in CE patients. Furthermore, HCF from mouse origin showed less or no cross-reaction with serum samples other than those from echinococcosis cases in either an ELISA or an immunoblot system, respectively. In order to confirm the applicability of HCF of mouse origin for serodiagnosis in humans, the dynamics of E. granulosus-specific IgG and IgG subclasses in serum samples obtained from a CE patient prior to surgical resection and at 45 days and 1 year after surgical resection were monitored by an ELISA. Gradual decreasing specific IgG titers were observed (Fig. 3). The titers of IgG1 and IgG4 decreased from 1:3,200 to 1:200 while those of total IgG or both IgG2 and IgG3 decreased from 1:1,600 or 1:800 to undetectable 1 year after resection. As E. granulosus-specific IgG1 and IgG4 titers are usually higher than other IgG subclasses in CE patient sera (14), dynamic changes in the titers of these IgG subclasses are expected to be useful for monitoring CE prognosis. This strategy seems to be effective when mouse HCF is used as the antigen.
FIG. 3.
Dynamics of E. granulosus-specific total IgG and IgG subclasses in serum samples from a CE patient before and after surgical resection, as determined by an ELISA with HCF from mice.
In conclusion, the advantage of using HCF of E. granulosus from mice is that mice can be maintained in the laboratory under controlled conditions for a standard inoculation period, allowing for standardization of the quality of HCF. A more uniform standard of mouse HCF may offer advantages for the primary screening of both CE and AE until other sources of antigen, such as recombinant antigen B (2) or its synthetic peptides (3), become available for routine use.
Acknowledgments
We sincerely thank P. M. Schantz, S. Y. Cho, Y. H. Liu, and Y. Hatakeyama for kindly supplying serum samples and M. Izeki for kindly supplying HCF from a patient.
This project was supported in part by grants-in-aid for international scientific research (11694259) and scientific research (12557024) from the Japan Society for the Promotion of Science and for the control of emerging and reemerging diseases in Japan from the Ministry of Health and Welfare, Japan, to A.I.
REFERENCES
- 1.Craig, P. S., M. T. Rogan, and J. C. Allan. 1996. Detection, screening and community epidemiology of taeniid cestode zoonosis: cystic echinococcosis, alveolar echinococcosis and neurocysticercosis. Adv. Parasitol. 38:169-250. [DOI] [PubMed] [Google Scholar]
- 2.Frosch, P., M. Hartmann, F. Mühlschlegel, and M. Frosch. 1994. Sequence heterogeneity of the echinococcal antigen B. Mol. Biochem. Parasitol. 66:171-175. [DOI] [PubMed] [Google Scholar]
- 3.González-Sapienza, G., C. Lorenzo, and A. Nieto. 2000. Improved immunodiagnosis of cystic hydatid disease by using a synthetic peptide with higher diagnostic value than that of its parent protein, Echinococcus granulosus antigen B. J. Clin. Microbiol. 38:3975-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ioppolo, S., S. Notargiacomo, E. Profumo, C. Frenchi, E. Ortona, R. Rigano, and A. Siracusano. 1996. Immunological responses to antigen B from Echinococcus granulosus cyst fluid in hydatid patients. Parasite Immunol. 18:571-578. [DOI] [PubMed] [Google Scholar]
- 5.Irabuena, O., A. Nieto, A. M. Ferreira, J. Battistoni, and G. Ferragut. 2000. Characterization and optimization of bovine Echinococcus granulosus cyst fluid to be used in immunodiagnosis of hydatid disease by ELISA. Rev. Inst. Med. Trop. Sao Paulo 42:255-262. [DOI] [PubMed] [Google Scholar]
- 6.Ito, A., L. Ma, P. M. Schantz, B. Gottstein, Y. H. Liu, J. J. Chai, S. K. Abdelhafez, N. Altintas, D. D. Joshi, M. W. Lightowlers, and Z. S. Pawloski. 1999. Differential serodiagnosis for cystic and alveolar echinococcosis using fractions of Echinococcus granulosus cyst fluid (antigen B) and Echinococcus multilocularis protoscolex (EM18). Am. J. Trop. Med. Hyg. 60:188-192. [DOI] [PubMed] [Google Scholar]
- 7.Ito, A., M. Okamoto, T. Ishiguro, L. Ma, H. Suzuki, A. Yasui, H. Shigeta, T. Matsuura, T. Hosokawa, and J. J. Chai. 1998. An imported case of cystic echinococcosis in Japan diagnosed by imaging and serology with confirmation of Echinococcus granulosus-specific DNA sequences. Am. J. Trop. Med. Hyg. 58:790-792. [DOI] [PubMed] [Google Scholar]
- 8.Lightowlers, M. W., and B. Gottstein. 1995. Echinococcosis/hydatidosis: antigens, immunological and molecular diagnosis, p. 355-410. In R. C. A. Thompson and A. J. Lymbery (ed.), Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom.
- 9.Margutti, P., E. Ortona, S. Vaccari, S. Barca, R. Rigano, A. Teggi, F. Muhschlegel, M. Frosch, and A. Siracusano. 1999. Cloning and expression of a cDNA encoding an elongation factor 1β/δ protein from Echinococcus granulosus with immunogenic activity. Parasite Immunol. 21:485-492. [DOI] [PubMed] [Google Scholar]
- 10.Meddison, S. E., S. B. Selemanda, P. M. Schantz, J. A. Fried, M. Wilson, and V. C. W. Tsang. 1989. A specific diagnostic antigen of Echinococcus granulosus with an apparent molecular weight of 8 kDa. Am. J. Trop. Med. Hyg. 40:377-383. [DOI] [PubMed] [Google Scholar]
- 11.Poretti, D., E. Felleisen, F. Grimm, M. Pfister, F. Teuscher, C. Zuercher, J. Reichen, and B. Gottstein. 1999. Differential immunodiagnosis between cystic hydatid disease and other cross-reactive pathologies. Am. J. Trop. Med. Hyg. 60:193-198. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez, F., J. Garcia, F. March, N. Cardeñosa, P. Coll, C. Muñoz, C. Auladell, and G. Prats. 1993. Ultrastructural localization of major hydatid fluid antigens in brood capsules and protoscoleces of Echinococcus granulosus of human origin. Parasite Immunol. 15:441-447. [DOI] [PubMed] [Google Scholar]
- 13.Schantz, P. M., J. Chai, P. S. Craig, J. Eckert, D. J. Jenkins, C. N. L. Macpherson, and A. Thakur. 1995. Epidemiology and control of hydatid disease, p. 233-331. In R. C. A. Thompson and A. J. Lymbery (ed.), Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom.
- 14.Shambesh, M. K., P. S. Craig, H. Wen, M. T. Rogan, and E. Paolillo. 1997. IgG1 and IgG4 serum antibody responses in asymptomatic and clinically expressed cystic echinococcosis patients. Acta Trop. 64:53-63. [DOI] [PubMed] [Google Scholar]
- 15.Verastegui, M., P. Moro, A. Guevara, T. Rodriguez, E. Miranda, and R. H. Gilman. 1992. Enzyme-linked immunoelectrotransfer blot test for diagnosis of human hydatid disease. J. Clin. Microbiol. 30:1557-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vidor, E., M. A. Piens, and J. P. Garin. 1987. Host serum protein levels in cysts of human hydatidosis. Trans. R. Soc. Trop. Med. Hyg. 81:669-671. [DOI] [PubMed] [Google Scholar]
- 17.Yamasaki, H., K. Araki, P. K. C. Lim, N. Zasmy, J. W. Mak, R. Taib, and T. Aoki. 2000. Development of a highly specific recombinant Toxocara canis second-stage larva excretory-secretory antigen for immunodiagnosis of human toxocariasis. J. Clin. Microbiol. 38:1409-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]