Abstract
YKL-40, a member of the family 18 glycosyl hydrolases, is secreted by activated neutrophils and macrophages. It is a growth factor for connective tissue cells and a potent migration factor for endothelial cells and may function in inflammation and tissue remodeling. YKL-40 was determined in 134 cerebrospinal fluid (CSF) samples taken on admission from patients suspected of having meningitis (48 with purulent meningitis, 49 with lymphocytic meningitis, 5 with encephalitis, and 32 without evidence of meningitis). YKL-40 levels in CSF were significantly higher in patients with purulent meningitis (median, 663 μg/liter [range, 20 to 8,960]) and encephalitis (5,430 μg/liter [620 to 11,600]) than in patients with lymphocytic meningitis (137 μg/liter [41 to 1,865]) or patients without meningitis (167 μg/liter [24 to 630]) (Kruskal-Wallis and Dunn multiple comparison tests, P < 0.001). CSF YKL-40 levels were also determined for 26 patients with purulent meningitis having a repuncture, and patients who died (n = 5) had significantly higher YKL-40 levels than patients who survived (n = 21) (2,100 μg/liter [1,160 to 7,050] versus 885 μg/liter [192 to 15,400], respectively; Mann-Whitney test, P = 0.018). YKL-40 was most likely locally produced, since patients with infections of the central nervous system had CSF YKL-40 levels that were at least 10-fold higher than the corresponding levels in serum (2,033 μg/liter [470 to 11,600] versus 80 μg/liter [19 to 195]). The CSF neopterin level was the biochemical parameter in CSF and blood that correlated best with CSF YKL-40 levels, indicating that YKL-40 may be produced by activated macrophages within the central nervous system. In conclusion, high levels of YKL-40 in CSF are found in patients with purulent meningitis.
Central nervous system (CNS) infections are characterized by an inflammatory response within the subarachnoidal space and/or the brain parenchyma. As in other inflammatory reactions, a complex network occurs, involving local production of soluble mediators and recruitment of white blood cells (WBC) from the bloodstream (31). The inflammatory response to the invading pathogens rather than the pathogen itself appears to be largely responsible for the brain damage following CNS infections (4). Therefore, studies of the release within the cerebrospinal fluid (CSF) of potential mediators during CNS infections are still required for a comprehensive understanding of the mechanism behind this process.
YKL-40 is a mammalian member of family 18 of glycosyl hydrolases (9, 11, 27, 29), a protein family that also includes bacterial chitinases and chitinase-related proteins. The protein has been named YKL-40 from its molecular mass (40 kDa) and the one-letter code for its three N-terminal amino acids (19). The protein is also called human cartilage glycoprotein-39 (8) or 38-kDa heparin-binding glycoprotein (30). The sequence of the gene for YKL-40 is known, but promoter analysis and regulatory factors have not been described (28). YKL-40 is a matrix protein of the specific granules of human neutrophils and is released after activation of the neutrophils (33). Activated macrophages also secrete YKL-40 (20, 21, 28), and it has been shown that in patients with rheumatoid arthritis YKL-40 is expressed by CD14+/CD16+ monocytes/macrophages in peripheral blood and in synovial tissue (1). The physiological function of YKL-40 is not completely known. YKL-40 is a heparin- and chitin-binding lectin (29, 30), but it has no chitinase activity (9, 11, 29). Recently, it has been shown that YKL-40 is a growth factor for connective tissue cells (7) and a potent migration factor for endothelial cells (23). Furthermore, the patterns of its secretion in normal and disease states suggest that YKL-40 may have a function in inflammation and tissue remodeling (16, 17, 28-30, 32).
High concentrations of YKL-40 in serum are correlated to morbidity of such different diseases as community-acquired pneumonia (24), active rheumatoid arthritis (10, 18), and ongoing hepatic fibrosis (13, 17), as well as to mortality of recurrent breast cancer (14) and colorectal cancer (6). Moreover, high levels of YKL-40 in serum were recently found to be an independent predictor of death in patients with pneumococcal bacteremia (22), suggesting an important role of YKL-40 in infectious diseases also. However, levels of YKL-40 within the compartment of a local infection, such as meningitis, have not yet been determined.
The aim of the present study was to evaluate levels of YKL-40 in CSF in patients clinically suspected of having meningitis. YKL-40 levels in CSF obtained on admission and during the course of hospitalization were determined.
MATERIALS AND METHODS
CSF YKL-40 levels in patients suspected of having meningitis on admission.
CSF samples were obtained by lumbar puncture from 134 patients clinically suspected of having meningitis on admission to the Department of Infectious Diseases at Hvidovre University Hospital in the period from 1988 to 2000. Fifty-two of these patients admitted to the department in the period from 1988 to 1994 have previously been described elsewhere (26). The lumbar puncture was performed as a routine diagnostic procedure for meningitis and was done in accordance with the ethical standards of the hospital. All patients with available CSF samples were included except patients infected with human immunodeficiency virus.
Based on clinical, microbiological, and biochemical characterization, the 134 patients could be divided in the following four groups.
(i) Forty-eight patients had purulent meningitis. Thirty of these patients had known bacterial etiology; 26 patients had a positive CSF culture (median number of CSF WBC, 3,040 cells/μl [range, 16 to 15,858]), and 4 patients had neutrophil pleocytosis (mean number of CSF WBC, 4,421 cells/μl [27 to 18,485]) and positive blood culture or a significant increase in antibody titers against Neisseria meningitidis. Thirteen cases were due to Neisseria meningitidis, 11 were due to Streptococcus pneumoniae, 3 were due to Haemophilus influenzae, 1 was due to Klebsiella pneumoniae, 1 was due to Staphylococcus aureus, and 1 was due to Listeria monocytogenes. Eighteen patients had negative CSF and blood cultures, neutrophil pleocytosis (mean number of CSF WBC, 460 cells/μl [range, 63 to 6,920] with >80% neutrophils, except for one patient with 1,210 cells/μl and 45% neutrophils), a quick response to antibiotic therapy, and exclusion of other etiologies. The initial or empirical antibiotic therapy was intravenous ceftriaxone and ampicillin. If bacteria were demonstrated in the CSF and/or blood, antibiotic therapy was changed according to the susceptibility of the pathogen. The duration of antibiotic therapy was usually 7 to 10 days.
(ii) Forty-nine patients had lymphocytic meningitis (pleocytosis with a predominance of mononuclear cells). Forty-seven cases were due to acute aseptic meningitis, and one case was due to recurrent chronic aseptic meningitis. A known viral etiology (in most cases, enterovirus) was established in nine cases. All patients fully recovered without antibiotic treatment, except for 13 patients who received one dose of antibiotics immediately after the lumbar puncture was performed and before the results of the CSF analysis were known (usually <30 min after lumbar puncture). One case was due to Borrelia burgdorferi, which was treated with ceftriaxone for 10 days.
(iii) Five patients had encephalitis (decreased sensorium, abnormal electroencephalogram, and/or abnormal computed-tomography-magnetic resonance imaging scan). The patients were treated with acyclovir. A viral etiology (herpes simplex virus) was found in two cases.
(iv) Thirty-two patients were suspected to have meningitis but did not show evidence of meningitis (i.e., no CSF pleocytosis); these included 5 patients with septic shock (3 cases were due to N. meningitidis, 1 was due to Streptococcus pyogenes, and 1 was due to Escherichia coli), 2 with acute tonsillitis, 8 with fever of unknown origin, 2 with influenza, 3 with pneumonia, 2 with urinary tract infection, 1 with cytomegalovirus hepatitis, 1 with polyneuropathy, and 8 with torticollis/cephalgia.
CSF YKL-40 levels in noninfectious control patients.
As controls, we also measured YKL-40 levels in CSF from 20 patients with various noninfectious diseases (e.g., headache, lower back pain, or neuropathy). The CSF samples were obtained when the patients had myelography for diagnostic purposes.
CSF YKL-40 levels during antibiotic treatment of purulent meningitis.
CSF samples were obtained by repuncture from 26 patients with purulent meningitis during the course of hospitalization and at the time when therapy was initiated. None of the patients having a repuncture had available CSF samples on admission, and in most cases they were transferred from other departments or hospitals, where the diagnosis was established based on the evaluation of an initial CSF sample. The repeated CSF examination was performed for diagnostic purposes to reconfirm the diagnosis and/or treatment efficacy. Four cases were due to N. meningitidis, 5 were due to Streptococcus pneumoniae, 1 was due to H. influenzae, 2 were due to S. pyogenes, 1 was due to Pseudomonas aeruginosa, 1 was due to S. aureus, and 1 was due to L. monocytogenes. Eleven patients had negative CSF and blood cultures and neutrophil pleocytosis (with >80% neutrophils) in the CSF sample at the time of diagnosis.
Blood YKL-40 levels.
Due to the study design, blood samples were not routinely collected from the patients in the present study. However, eight patients (one with encephalitis, two with purulent meningitis, one with lymphocytic meningitis, and four without evidence of meningitis) had an available blood sample that was taken at admission and at the same time as the CSF sample.
CSF analysis.
CSF samples were analyzed by routine laboratory methods to determine glucose and total protein levels, total leukocyte count, and differential count. In the period from 1988 to 1993, the differential count was recorded only as segment-nuclear cells (neutrophils) and mononuclear cells (monocytes and lymphocytes). Levels of lactoferrin and neopterin in CSF were determined by enzyme-linked immunosorbent assay (by routine laboratory methods at National University Hospital, Rigshospitalet, and at Statens Serum Institut, Copenhagen, Denmark, respectively).
Measurement of YKL-40.
Levels of YKL-40 in CSF were determined by radioimmunoassay as previously described (16). The lower limit of detection was 20 μg/l. The intra- and interassay coefficients of variation were <6.6 and 12%, respectively.
Stability of YKL-40 in CSF.
The stability of YKL-40 levels in purulent CSF was evaluated during a 2-h period. CSF was obtained from pooled patient samples and was passed through a 0.2-μm-pore-size sterile filter (Millipore, Bedford, Mass.). The concentration of YKL-40 in the pooled CSF was ∼700 μg/liter. Neutrophils, isolated from healthy volunteers by dextran (Statens Serum Institut)-induced sedimentation followed by layering of the leukocyte-rich plasma over Lymphoprep (1.077 g/ml; Sigma Chemical Co., St. Louis, Mo.), with subsequent removal, washing, and resuspension of the neutrophil pellet (25), were added to the CSF to final concentrations of 1,000, 100, 10, 1, and 0 cells/μl. The CSF was kept at room temperature for 2 h, and samples were taken at 0, 15, 30, 60, and 120 min and processed as for routine CSF samples (centrifugation and storage of the supernatants at −20°C for subsequent YKL-40 analysis).
Statistical analysis.
All results are provided as medians, with minimum and maximum values also given. Comparisons between two groups were performed by the nonparametric Mann-Whitney test, whereas comparisons between more than two groups were performed by the nonparametric Kruskal-Wallis test. If the comparison between groups showed a significant difference, Dunn's multiple-comparison test was performed to compensate for multiple comparisons. For correlation analysis, the nonparametric Spearman test was used. The Pearson chi-square test was used for comparison between categorical data. Logistic regression analysis was applied for multivariate analysis. A P value of <0.05 was considered significant.
RESULTS
Clinical outcomes for 134 patients with CSF samples taken on admission.
Clinical and demographic data for the134 patients with available CSF samples on admission are shown in Table 1. Six of 48 patients with purulent meningitis died (mortality rates were as follows: total, 13%; meningococcal meningitis, 8%; pneumococcal meningitis, 18%; H. influenzae meningitis, 0%; staphylococcal meningitis, 100%; K. pneumoniae meningitis, 100%; and unknown etiology, 6%). Two of 32 patients without meningitis died (they had septic shock due to N. meningitidis or S. pyogenes), whereas all patients with encephalitis or lymphocytic meningitis survived. A clinical parameter of prognostic importance in terms of fatal outcome for patients with purulent meningitis was impaired consciousness (Pearson chi-square test, P = 0.01), and patients who died had significantly higher age than patients who survived (69 years [10 to 84] versus 24 years [1 to 87], respectively; P = 0.02). Underlying disease, sex, back rigidity, need for assisted ventilation, or any biochemical parameter in the CSF tested (including YKL-40, [see below]) was not of significant importance for fatal outcome of purulent meningitis (P > 0.05). A logistic multivariate regression analysis including the significant risk factors for fatal outcome (i.e., advanced age and impaired consciousness) showed that advanced age was the only independent predictor of fatal outcome in patients with purulent meningitis (P = 0.04).
TABLE 1.
Demographic and clinical characteristics of 134 patients suspected of having meningitis on admission
| Characteristic | Value for groupa
|
|||
|---|---|---|---|---|
| Purulent meningitis | Lymphocytic meningitis | Encephalitis | Nonmeningitis | |
| No. of patients | 48 | 49 | 5 | 32 |
| Female/male | 24/24 | 22/27 | 2/3 | 19/13 |
| Age (yr) | 26 (1-87) | 24 (3-42) | 55 (13-62) | 17 (1-76) |
| Underlying illnessf (%) | 23b | 4e | 0 | 16 |
| CSF WBC (106 cells/liter) | 1,350 (16-18,485)b, c, d | 130 (7-903)d, e | 93 (0-267)e | 1 (0-10)b, e |
| CSF neutrophils (106 cells/liter) | 1,080 (15-17,561)b, c, d | 21.6 (0-695.3)d, e | 8.5 (0-154.9)e | 0 (0)b, e |
| CSF lymphocytes (106 cells/liter) | 128.2 (0-1,657.1)d | 58.7 (2.4-452.1)d | 84.3 (0-104.1) | 0 (0)b, e |
| CSF monocytes (106 cells/liter) | 35.7 (0-326.4)d | 12.6 (1.2-1,889.4)d | 4.7 (0-8) | 0 (0)b, e |
| CSF glucose (mmol/liter) | 2.8 (0.1-6)d | 3.3 (2.5-4.6)d, e | 3.0 (2.1-3.1)e | 3.7 (2.9-7.2)d, c, e |
| CSF/blood glucose ratio | 0.42 (0.01-1.48)b, d | 0.61 (0.26-0.83)e | 0.54 (0.53-0.55) | 0.61 (0.45-1)e |
| CSF protein (g/liter) | 1.75 (0.1-18)b, d | 0.7 (0.2-2.2)d | 1.5 (0.8-10.7)d | 0.4 (0.1-0.9)b, c, e |
| Blood WBC (109 cells/liter) | 17.1 (5.5-220)b, c, d | 8.8 (2.8-16.9)e | 6.9 (5.6-8.1)e | 9.0 (3.3-24)e |
| Blood neutrophils (109 cells/liter) | 14.8 (3.5-31.7)b, d | 6.7 (2-14.2)e | 4.5 (4.2-4.8) | 5.9 (2.5-18.4)e |
| Blood lymphocytes (109 cells/liter) | 1.3 (1-193.5) | 1.4 (0.4-2.9) | 1.0 (0.6-1.4) | 1.6 (0.2-3.2) |
| Blood monocytes (109 cells/liter) | 0.7 (0-5.2)b | 0.4 (0.1-1.3)e | 0.4 (0.3-0.4) | 0.4 (0.1-3.1) |
| Back rigidity (%) | 85c, d | 91c, d | 25b, e | 41b, e |
| Decreased sensorium (%) | 53b, d | 0c, e | 100b, d | 11c, e |
| Days in hospital | 12 (7-53)b, d | 4 (1-10)b, d | 11 (11-65)c, e | 5 (1-29)c, e |
| Assisted ventilation (%) | 31b | 0c, d, e | 20b | 16b |
| Fatal outcome (%) | 13b | 0e | 0 | 6 |
Unless otherwise indicated, values are medians with minimums and maximums given in parentheses. Statistically significant differences between groups (Kruskal-Wallis and Dunn multiple-comparison tests for continual data, P <0.05; Pearson chi-square square test corrected for multiple comparisons with the Bonferroni coefficient for categorical data, P <0.05) are indicated.
Significant difference versus lymphocytic meningitis.
Significant difference versus encephalitis.
Significant difference versus nonmeningitis.
Significant difference versus purulent meningitis.
For example, malignancy, diabetes, drug abuse, or alcoholism.
CSF YKL-40 levels at admission.
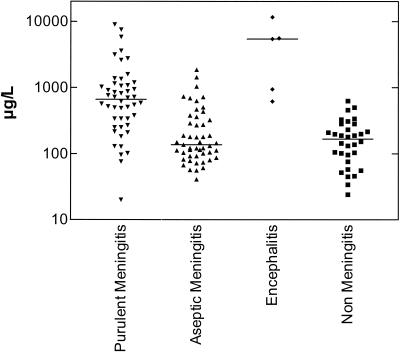
There was a significant difference in YKL-40 levels among patient groups (purulent meningitis, lymphocytic meningitis, encephalitis, and nonmeningitis) at time of admission (Kruskal-Wallis test, P < 0.0001), with significantly higher CSF YKL-40 levels in patients with purulent meningitis (n = 48; 663 μg/liter [20 to 8,960]) and encephalitis (n = 5; 5430 μg/liter [620 to 11,600]) than in patients with lymphocytic meningitis (n = 49; 137 μg/liter [41 to 1,865]) and patients without meningitis (n = 32; 167 μg/liter [24 to 630]) (Dunn's multiple-comparison test. P < 0.001) (Fig. 1). There was no significant difference in CSF YKL-40 levels between patients with purulent meningitis and patients with encephalitis or between patients with lymphocytic meningitis and patients without meningitis (Dunn's multiple-comparison test, P > 0.05) (Fig. 1). To distinguish purulent meningitis from viral meningitis with a cutoff point of 340 μg/liter or more, the sensitivity, specificity, and positive predictive value were 75, 76, and 75%, respectively. Among patients with purulent meningitis, a significant difference in CSF YKL-40 levels was found between cases of different etiology (Kruskal-Wallis test, P = 0.0028), with significantly higher CSF YKL-40 levels in patients with pneumococcal meningitis (n = 11) than in patients with meningococcal meningitis (n = 13) (1,150 μg/liter [380 to 8.960] versus 340 μg/liter [20 to 1,060], respectively; Dunn's multiple-comparison test, P < 0.01). CSF YKL-40 levels in patients with meningococcal meningitis were higher than those in patients with meningococcemia but without evidence of meningitis (n = 3) (144 μg/liter [34 to 210]; due to the small number of patients in this group, no statistical analysis was performed).
FIG. 1.
YKL-40 levels in CSF from patients with suspected or confirmed meningitis at admission. Patients with purulent meningitis (n = 48) and patients with encephalitis (n = 5) had significantly higher levels of YKL-40 in CSF than patients with lymphocytic meningitis (n = 49) (Dunn's multiple-comparison test, P < 0.001) and than patients suspected of having but without evidence of having meningitis (n = 32) (P < 0.001). Horizontal lines indicate medians.
CSF YKL-40 levels in the noninfectious control group.
Patients having myelography (n = 20) had CSF YKL-40 levels of 305 μg/liter (78 to 540), which were significant lower than the CSF YKL-40 levels in patients with purulent meningitis or encephalitis (Mann-Whitney test, P < 0.001 and P = 0.01, respectively) but significantly higher than those in patients suspected of having but without evidence of having meningitis (P < 0.05).
Blood YKL-40 levels.
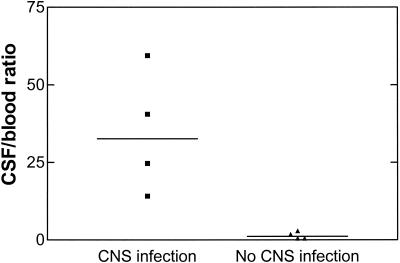
Patients with CNS infections (n = 4) had serum YKL-40 levels that were more than 10-fold lower than those in the corresponding CSF samples (80 μg/liter [19 to 195] versus 2,033 μg/liter [470 to 11,600], respectively), whereas such difference was not observed in patients without CNS infection (n = 4) (148 μg/liter [92 to 526] versus 234 μg/liter [102 to 284], respectively). Figure 2 shows the ratio of YKL-40 in CSF to that in serum in the two groups of patients.
FIG. 2.
Ratio of YKL-40 in CSF to that in blood in patients with and without CNS infection. Ratios were significantly higher in patients with CNS infections (n = 4) than in patients without CNS infection (n = 4) (Mann-Whitney test, P = 0.029).
Association of CSF YKL-40 levels at admission in patients with meningitis with outcome, clinical parameters, and laboratory findings.
To further explore a role of YKL-40 in the pathophysiology of meningitis, we studied the association between CSF YKL-40 levels and various clinical parameters as well as various parameters for the meningeal and systemic inflammatory responses. Because several other clinical and biochemical parameters besides the observed difference in CSF YKL-40 levels were significantly different among the four diagnostic groups (Table 1), only results from analyses performed within each diagnostic group are shown here.
(i) Patients with purulent meningitis (n = 48).
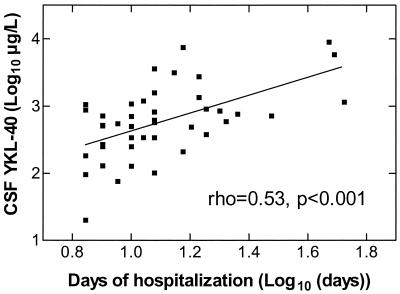
There was an association between CSF YKL-40 levels and the severity of the disease. A significant correlation was found between CSF YKL-40 levels and the number of days of hospitalization among survivors (n = 42; ρ = 0.53; P < 0.001) (Fig. 3) as well as the duration of antibiotic therapy (n = 42; ρ = 0.33; P < 0.05). Six patients died, and they had higher CSF YKL-40 levels than patients who survived (n = 42) (1,090 μg/liter [216 to 2,610] versus 580 μg/liter [20 to 8,960]; P = 0.13). Patients with impaired consciousness had higher CSF YKL-40 levels than conscious patients (820 μg/liter [128 to 3,600] versus 425 μg/liter [20 to 8,960]; P = 0.057). CSF YKL-40 levels were associated with the meningeal inflammatory response (e.g., number of CSF neutrophils and lymphocytes, levels of neopterin and lactoferrin in CSF, and CSF/blood glucose ratio) and with the systemic inflammatory response (number of blood neutrophils) (P < 0.05) (Table 2). CSF YKL-40 levels correlated significantly with the age of the patients (ρ = 0.56, P < 0.001).
FIG. 3.
Association between YKL-40 levels in CSF from patients with purulent meningitis at admission and number of days of hospitalization. Only results for patients who survived (n = 42) are shown and were included in the analysis.
TABLE 2.
Association between CSF YKL-40 levels at admission and biochemical parameters in CSF and blooda
| Parameter | ρ value for CSF YKL-40-level
|
||
|---|---|---|---|
| Purulent meningitis (n =48) | Lymphocytic meningitis (n =49) | Nonmeningitis (n =32) | |
| CSF WBC | 0.31b | 0.28 | 0.08 |
| CSF neutrophils | 0.31b | −0.27 | NAe |
| CSF lymphocytes | 0.46b | 0.48c | NA |
| CSF monocytes | 0.11 | 0.16 | NA |
| CSF glucose | −0.29 | −0.08 | 0.41b |
| CSF/blood glucose ratio | −0.47c | −0.37b | −0.10 |
| CSF protein | 0.25 | 0.15 | 0.62b |
| CSF lactoferrin | 0.35b | −0.13 | 0.07 |
| CSF neopterin | 0.52d | 0.55d | 0.52b |
| CSF interleukin-8 | −0.12 | 0.02 | 0.27 |
| Blood WBC | 0.35b | 0.09 | −0.22 |
| Blood neutrophils | 0.38c | 0.005 | −0.13 |
| Blood lymphocytes | −0.12 | 0.07 | −0.29 |
| Blood monocytes | 0.18 | −0.17 | −0.10 |
Patients with encephalitis were not studied due to the small number of patients (n =5).
Spearman test, P <0.05.
Spearman test, P <0.01.
Spearman test, P <0.001.
NA, not analyzed
(ii) Patients with lymphocytic meningitis (n = 49).
CSF YKL-40 levels were associated with the meningeal inflammatory response (e.g., number of CSF lymphocytes, CSF neopterin levels, CSF/blood glucose ratio) (P < 0.05) but not with the systemic inflammatory response (number of WBC in blood) (P > 0.05) (Table 2). CSF YKL-40 levels correlated significantly with the age of the patients (ρ = 0.42; P = 0.003) and with the number of days of hospitalization (ρ = 0.293; P = 0.041).
(iii) Patients suspected of having but without evidence of having meningitis (n = 32).
CSF YKL-40 levels correlated significantly with CSF neopterin levels, CSF protein levels, and CSF glucose levels (P < 0.05) (Table 2). There was a significant correlation between CSF YKL-40 levels and number of days of hospitalization (ρ = 0.53; P = 0.002) and between CSF YKL-40 levels and the age of the patients (ρ = 0.78; P < 0.001). In contrast, no significant correlation was found between CSF YKL-40 levels and the age of the patients in the noninfectious control group (n = 20) (ρ = 0.39; P > 0.05).
(iv) Patients with encephalitis (n = 5).
No analysis was performed due to small number of patients in this group.
Clinical outcome for patients with purulent meningitis who had a repuncture during hospitalization.
Among those with a repuncture, 5 of 26 patients with purulent meningitis died (mortality rates were as follows: total, 19%; meningococcal meningitis, 0%; pneumococcal meningitis, 0%; H. influenzae meningitis,0%; L. monocytogenes meningitis, 100%; P. aeruginosa meningitis. 100%; and unknown etiology, 27%) (Table 3). Underlying disease, sex, advanced age, decreased sensorium, back rigidity, need for assisted ventilation, or any biochemical CSF parameter tested (except YKL-40 [see below]) was not of significant importance for fatal outcome (P > 0.05) (Table 3).
TABLE 3.
Demographic and clinical characteristics of 26 patients with purulent meningitis having a repuncture
| Parameter | Valuea |
|---|---|
| No. of patients | 26 |
| Female/male | 11/15 |
| Age (yr) | 57 (4-75) |
| Underlying illness (%)b | 35 |
| Day of CSF sample (from admission) | 8 (2-19) |
| YKL-40 level (μg/liter) | 965 (192-15,400) |
| CSF WBC (106 cells/liter) | 59.5 (0-3,210) |
| CSF neutrophils (106 cells/liter) | 37.8 (0-2,921.1) |
| CSF lymphocytes (106 cells/liter) | 32.4 (0-99.4) |
| CSF monocytes (106 cells/liter) | 2.8 (0-16.4) |
| CSF glucose (mmol/liter) | 3.1 (0.4-6.2) |
| CSF/blood glucose ratio | 0.47 (0.05-0.82) |
| CSF protein (g/liter) | 1.0 (0.3-10.9) |
| Blood WBC (109 cells/liter) | 12.6 (6.2-28.9) |
| Blood neutrophils (109 cells/liter) | 9.1 (5.3-25.9) |
| Blood lymphocytes (109 cells/liter) | 1.7 (0.6-3.4) |
| Blood monocytes (109 cells/liter) | 0.7 (0.4-2.2) |
| Days in hospital | 22 (7-86) |
| Fatal outcome (%) | 19 |
Unless otherwise indicated, values are medians with minimums and maximums given in parentheses.
For example, malignancy, diabetes, drug abuse, or alcoholism.
CSF YKL-40 levels during hospitalization in patients with purulent meningitis having a repuncture (n = 26) and association of YKL-40 with outcome, clinical parameters, and laboratory findings.
CSF YKL-40 levels in patients with purulent meningitis having a repuncture were highly elevated (965 μg/liter [192 to 15,400]), despite the fact that number of leukocytes in the CSF was low (59.5 cells/μl [0 to 3,210]). There was no significant correlation between YKL-40 levels and the day of CSF tapping (from admission) (ρ = −0.29; P > 0.05). Five patients died, and they had significantly higher CSF YKL-40 levels than patients who survived (n = 21) (2,100 μg/liter [1,160 to 7,050] versus 885 μg/liter [192 to 15,400]; P = 0.018). CSF YKL-40 levels correlated significantly with CSF neopterin levels and CSF glucose levels (ρ = 0.42 and ρ = −0.44, respectively; P < 0.05) but not with any other clinical or biochemical parameter (P > 0.05). Indeed, no correlation between YKL-40 and the age of the patients was observed (ρ = 0.32; P > 0.05).
Stability of YKL-40 in CSF.
YKL-40 levels in pooled CSF with various numbers of neutrophils did not change significantly during a 2-h study period (data not shown).
DISCUSSION
In the present study, we found that YKL-40 levels in CSF samples taken on admission were significantly higher in patients with purulent meningitis and in patients with encephalitis than in patients with lymphocytic meningitis or patients without meningitis. However, the use of CSF YKL-40 for diagnostic purposes to distinguish purulent from lymphocytic meningitis cannot be recommended, because of a significant overlap between the two groups. Moreover, we found that YKL-40 levels were at least 10-fold higher in CSF than in blood obtained from patients with CNS infection, which was not the case for patients without CNS infection. Systemic levels of YKL-40 have previously been shown to be elevated in patients with community-acquired pneumonia (24). To our knowledge, this is the first study to show that local levels of YKL-40 are elevated at the site of an infection and that YKL-40 most likely is produced or released within the compartment of a local infection.
To further study the cellular source of YKL-40 production, we investigated the association between YKL-40 and the various cell lines of the CSF infiltrate. A weak association of YKL-40 with the number of neutrophils and more specifically with levels of lactoferrin, also a matrix protein of the specific granules like YKL-40 (33), was detected in CSF samples taken on admission from patients with purulent meningitis but not from those with lymphocytic meningitis. No such correlation was detected in patients with purulent meningitis having a repuncture.
A more consistent finding, in purulent meningitis as well as in lymphocytic meningitis, was a positive correlation between YKL-40 and the number of lymphocytes in CSF samples taken on admission. However, no such correlation was detected in patients with purulent meningitis having a repuncture, and YKL-40 has so far never been detected in lymphocytes.
Another possible candidate could be the monocyte, since YKL-40 is expressed by CD14+/CD16+ monocytes/macrophages in peripheral blood and in synovial tissue from patients with rheumatoid arthritis (1). However, no association between YKL-40 and monocytes was observed in the present study. On the other hand, activated macrophages synthesize YKL-40 in vitro during the late stage of activation (21, 28, 29). Furthermore, in vivo YKL-40 is produced by giant cells and a subpopulation of macrophages located in the media of arteritic vessels of patients with giant cell arteritis (12), by macrophages in inflamed synovial membranes from patients with rheumatoid arthritis (20), and by a subpopulation of macrophages in atherosclerotic plaques (3). To address this issue in CNS infections, we correlated levels of YKL-40 to levels of neopterin, a marker of CNS macrophage activation (8), in CSF. Indeed, we found that levels of neopterin and YKL-40 in CSF were highly interrelated in all patient groups tested (patients with purulent meningitis [with CSF samples taken on admission or during hospitalization] as well as patients with lymphocytic meningitis) and that the correlation coefficient was the highest among those for the different biochemical parameters tested in CSF and blood. Interestingly, a similar association was found in patients without evidence of meningitis, who had no cells detected in the CSF, indicating that patients with systemic infections also may have an activation of the immune system within the CNS. Also, patients with encephalitis, who have a high degree of parenchymal involvement but little CSF infiltrate, all had very high levels of YKL-40 in the CSF, supporting the idea that YKL-40 most likely is produced by resident macrophages rather than by the cells of the CSF infiltrate. However, to confirm this hypothesis, immunohistochemical analyses of brain tissue and CSF from patients with CNS infections are warranted.
In patients with purulent meningitis having a repuncture, we found that the CSF YKL-40 level was the only clinical and biochemical parameter that was associated with mortality. However, future larger prospective studies are required to determine whether YKL-40 should be recommended as a prognostic indicator in repeated CSF examination of severely ill patients with purulent meningitis. Our results are in accordance with previous observations of a predictive value of YKL-40 in serum for fatal outcome in disseminated diseases like cancer (6, 14) and pneumococcal bacteremia (22). Unfortunately, the present study, as well as a previous study on community-acquired pneumonia (24), was not designed to resolve whether levels of YKL-40 in serum also would be a valuable biochemical marker for prognosis of local infections.
CSF YKL-40 levels taken at admission from patients with purulent meningitis were not significantly associated with fatal outcome in the present study. Interestingly, advanced age, which was the only independent predictor for fatal outcome, was the clinical or biochemical parameter that correlated best with YKL-40. The importance of this finding remains to be defined. However, it is well known that elderly individuals with an infection, including patients with meningitis, have a poorer prognosis than younger patients (2). In addition, we found that there was an interrelationship between age, YKL-40 level, and severity of disease (number of days hospitalized) in the other patient groups with an infection (lymphocytic meningitis or nonmeningitis) who were having a lumbar puncture at admission, which was not the case for patients without an infection (noninfectious control group having a myelography). This is in accordance with previous studies of serum YKL-40 levels in healthy subjects (15).
Patients with pneumococcal meningitis had significantly higher CSF YKL-40 levels than patients with meningococcal meningitis. Indeed, patients with pneumococcal meningitis had significantly higher age and were more severely ill (number of days of hospitalization) than patients with meningococal meningitis (P < 0.05) (data not shown). In contrast, patients in the noninfectious control group did not have higher age than patients suspected of having but without evidence of having meningitis, despite the fact that CSF YKL-40 levels were significantly different between the two groups.
The exact physiological role of YKL-40 is at present unknown. Since YKL-40 is released from the specific granules of the neutrophil granulocyte, it may influence oxygen-dependent and -independent bacterial killing (33). However, in vitro studies have shown that stimulation with YKL-40 does not influence neutrophil chemiluminescence or bactericidal activity (unpublished data). Also, chemotaxis may be influenced by YKL-40, since ECF-L, another member of the mammalian family of 18-glycosyl hydrolases without chitinase activity, showed chemotactic activity for neutrophils, eosinophils, and T lymphocytes (27). However, YKL-40 showed no chemotactic activity for neutrophils as determined using the Boyden chamber method (unpublished data). Recently, it has been shown that YKL-40 is a growth factor for connective tissue cells (7) and a potent migration factor for endothelial cells (23). Fibrinoid necrosis and vascular proliferation are late pathological findings of the brain damage observed in fatal cases of bacterial meningitis (5). In the present study, CSF YKL-40 levels remained elevated late during the course of purulent meningitis, when the cellular infiltrate had ceased. This suggests, in accordance with other studies, that YKL-40 could have a function in inflammation and tissue remodeling. Further studies should be performed to determine whether CSF levels of YKL-40 could be a valuable biochemical marker of brain damage in other CNS diseases.
In conclusion, we found that patients with purulent meningitis or encephalitis have significantly higher levels of YKL-40 in CSF than patients with lymphocytic meningitis or patients without meningitis and that YKL-40 levels were significantly higher in the CSF than in the blood during CNS infection. Further studies are required to determine the biological function of YKL-40 in infectious diseases.
Acknowledgments
This work was supported by grants from Læge Sofus Carl Emil Friis og Hustru Olga Doris Friis' Legat, Ebbe Celinders Legat, Ydes Fond, Købmand Svend Hansen og Hustru Ina Hansens Fond, Michaelsen Fonden, and Overlæge Johan Boserup og Lise Boserups Legat.
We thank Finn Sellebjerg for providing CSF samples from noninfectious control patients and Michael Christiansen and Leif Percival Andersen for the measurement of neopterin and lactoferrin, respectively. Inger Aakard and Susanne Munch are gratefully acknowledged for the measurement of YKL-40.
REFERENCES
- 1.Baeten, D., A. M. Boots, P. G. Steenbakkers, D. Elewaut, E. Bos, G. F. Verheijden, G. Berheijden, A. M. Miltenburg, A. W. Rijnders, E. M. Veys, and F. De Keyser. 2000. Human cartilage gp-39+, CD16+ monocytes in peripheral blood and synovium: correlation with joint destruction in rheumatoid arthritis. Arthritis Rheum. 43:1233-1243. [DOI] [PubMed] [Google Scholar]
- 2.Bohr, V. A., and N. Rasmussen. 1988. Neurological sequelae and fatality as prognostic measures in 875 cases of bacterial meningitis. Dan. Med. Bull. 35:92-95. [PubMed] [Google Scholar]
- 3.Boot, R. G., T. A. van Achterberg, B. E. van Aken, G. H. Renkema, M. J. Jacobs, J. M. Aerts, and C. J. de Vries. 1999. Strong induction of members of the chitinase family of proteins in atherosclerosis: chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler. Thromb. Vasc. Biol. 19:687-694. [DOI] [PubMed] [Google Scholar]
- 4.Braun, J. S., R. Novak, K. H. Herzog, S. M. Bodner, J. L. Cleveland, and E. I. Tuomanen. 1999. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat. Med. 5:298-302. [DOI] [PubMed] [Google Scholar]
- 5.Cairns, H., and D. S. Russell. 1946. Cerebral arteritis and phlebitis in pneumococcal meningitis. J. Pathol. Bacteriol. 58:649-665. [DOI] [PubMed] [Google Scholar]
- 6.Cintin, C., J. S. Johansen, I. J. Christensen, P. A. Price, S. Sørensen, and H. J. Nielsen. 1999. Serum YKL-40 and colorectal cancer. Br. J. Cancer 79:1494-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Ceuninck, F., S. Gaufillier, A. Bonnaud, M. Sabatini, C. Lesur, and P. Pastoureau. 2001. YKL-40 (cartilage gp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem. Biophys. Res. Commun. 285:926-931. [DOI] [PubMed] [Google Scholar]
- 8.Hagberg, L. 1996. The clinical use of cerebrospinal fluid neopterin in central nervous system infections. Pteridines 6:147-152. [Google Scholar]
- 9.Hakala, B. E., C. White, and A. D. Recklies. 1993. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J. Biol. Chem. 268:25803-25810. [PubMed] [Google Scholar]
- 10.Harvey, S., M. Weisman, J. O'Dell, T. Scott, M. Krusemeier, J. Visor, and C. Swindlehurst. 1998. Chondrex: new marker of joint disease. Clin. Chem. 44:509-516. [PubMed] [Google Scholar]
- 11.Hu, B., K. Trinh, W. F. Figueira, and P. A. Price. 1996. Isolation and sequence of a novel human chondrocyte protein related to mammalian members of the chitinase protein family. J. Biol. Chem. 271:19415-19420. [DOI] [PubMed] [Google Scholar]
- 12.Johansen, J. S., B. Baslund, C. Garbarsch, M. Hansen, M. Stoltenberg, I. Lorenzen, and P. A. Price. 1999. YKL-40 in giant cells and macrophages from patients with giant cell arteritis. Arthritis Rheum. 42:2624-2630. [DOI] [PubMed] [Google Scholar]
- 13.Johansen, J. S., P. Christoffersen, S. Møller, P. A. Price, J. H. Henriksen, C. Garbarsch, and F. Bendtsen. 2000. Serum YKL-40 is increased in patients with hepatic fibrosis. J. Hepatol. 32:911-920. [DOI] [PubMed] [Google Scholar]
- 14.Johansen, J. S., C. Cintin, M. Jørgensen, C. Kamby, and P. A. Price. 1995. Serum YKL-40: a new potential marker of prognosis and location of metastases of patients with recurrent breast cancer. Eur. J. Cancer 31A:1437-1442. [DOI] [PubMed] [Google Scholar]
- 15.Johansen, J. S., J. Hvolris, M. Hansen, V. Backer, I. Lorenzen, and P. A. Price. 1996. Serum YKL-40 levels in healthy children and adults. Comparison with serum and synovial fluid levels of YKL-40 in patients with osteoarthritis or trauma of the knee joint. Br. J. Rheumatol. 35:553-559. [DOI] [PubMed] [Google Scholar]
- 16.Johansen, J. S., H. S. Jensen, and P. A. Price. 1993. A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br. J. Rheumatol. 32:949-955. [DOI] [PubMed] [Google Scholar]
- 17.Johansen, J. S., S. Møller, P. A. Price, F. Bendtsen, J. Junge, C. Garbarsch, and J. H. Henriksen. 1997. Plasma YKL-40: a new potential marker of fibrosis in patients with alcoholic cirrhosis? Scand. J. Gastroenterol. 32:582-590. [DOI] [PubMed] [Google Scholar]
- 18.Johansen, J. S., M. Stoltenberg, M. Hansen, A. Florescu, K. Hørslev Petersen, I. Lorenzen, and P. A. Price. 1999. Serum YKL-40 concentrations in patients with rheumatoid arthritis: relation to disease activity. Rheumatology 38:618-626. [DOI] [PubMed] [Google Scholar]
- 19.Johansen, J. S., M. K. Williamson, J. S. Rice, and P. A. Price. 1992. Identification of proteins secreted by human osteoblastic cells in culture. J. Bone Miner. Res. 7:501-512. [DOI] [PubMed] [Google Scholar]
- 20.Kirkpatrick, R. B., J. G. Emery, J. R. Connor, R. Dodds, P. G. Lysko, and M. Rosenberg. 1997. Induction and expression of human cartilage glycoprotein 39 in rheumatoid inflammatory and peripheral blood monocyte-derived macrophages. Exp. Cell Res. 237:46-54. [DOI] [PubMed] [Google Scholar]
- 21.Krause, S. W., M. Rehli, M. Kreutz, L. Schwarzfischer, J. D. Paulauskis, and R. Andreesen. 1996. Differential screening identifies genetic markers of monocyte to macrophage maturation. J. Leukoc. Biol. 60:540-545. [DOI] [PubMed] [Google Scholar]
- 22.Kronborg, G., C. Østergaard, N. Weis, N. Obel, S. S. Pedersen, P. A. Price, and J. S. Johansen. The serum level of YKL-40 is elevated in patients with Streptococcus pneumoniae bacteremia and is associated to the outcome of the disease. Scand. J. Infect. Dis., in press. [DOI] [PubMed]
- 23.Malinda, K. M., L. Ponce, H. K. Kleinman, L. M. Shackelton, and A. J. Millis. 1999. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp. Cell Res. 250:168-173. [DOI] [PubMed] [Google Scholar]
- 24.Nordenbaek, C., J. S. Johansen, P. Junker, N. Borregaard, O. Sorensen, and P. A. Price. 1999. YKL-40, a matrix protein of specific granules in neutrophils, is elevated in serum of patients with community-acquired pneumonia requiring hospitalization. J. Infect. Dis. 180:1722-1726. [DOI] [PubMed] [Google Scholar]
- 25.Østergaard, C., T. Benfield, B. Gesser, A. Kharazmi, N. Frimodt-Møller, F. Espersen, and J. D. Lundgren. 1999. Pretreatment with granulocyte colony-stimulating factor attenuates the inflammatory response but not the bacterial load in cerebrospinal fluid during experimental pneumococcal meningitis in rabbits. Infect. Immun. 67:3430-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Østergaard, C., T. L. Benfield, F. Sellebjerg, G. Kronborg, N. Lohse, and J. D. Lundgren. 1996. Interleukin-8 in cerebrospinal fluid from patients with septic and aseptic meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 15:166-169. [DOI] [PubMed] [Google Scholar]
- 27.Owhashi, M., H. Arita, and N. Hayai. 2000. Identification of a novel eosinophil chemotactic cytokine (ECF-L) as a chitinase family protein. J. Biol. Chem. 275:1279-1286. [DOI] [PubMed] [Google Scholar]
- 28.Rehli, M., S. W. Krause, and R. Andreesen. 1997. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics 43:221-225. [DOI] [PubMed] [Google Scholar]
- 29.Renkema, G. H., R. G. Boot, F. L. Au, W. E. Donker Koopman, A. Strijland, A. O. Muijsers, M. Hrebicek, and J. M. Aerts. 1998. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur. J. Biochem. 251:504-509. [DOI] [PubMed] [Google Scholar]
- 30.Shackelton, L. M., D. M. Mann, and A. J. Millis. 1995. Identification of a 38-kDa heparin-binding glycoprotein (gp38k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J. Biol. Chem. 270:13076-13083. [DOI] [PubMed] [Google Scholar]
- 31.Tauber, M. G., and B. Moser. 1999. Cytokines and chemokines in meningeal inflammation: biology and clinical implications. Clin. Infect. Dis. 28:1-12. [DOI] [PubMed] [Google Scholar]
- 32.Volck, B., K. Østergaard, J. S. Johansen, C. Garbarsch, and P. A. Price. 1999. The distribution of YKL-40 in osteoarthritic and normal human articular cartilage. Scand. J. Rheumatol. 28:171-179. [DOI] [PubMed] [Google Scholar]
- 33.Volck, B., P. A. Price, J. S. Johansen, O. Sørensen, T. L. Benfield, H. J. Nielsen, J. Calafat, and N. Borregaard. 1998. YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc. Assoc. Am. Physicians 110:351-360. [PubMed] [Google Scholar]