Abstract
CD40 ligand (CD40L or CD154) is a costimulatory molecule expressed mainly on activated CD4+ T cells. Concentrations of the soluble form of CD40L (sCD40L) in serum were determined for a cohort of 77 human immunodeficiency virus type 1 (HIV-1)-infected patients before and after initiation of highly active antiretroviral treatment (HAART) by a quantitative enzyme-linked immunosorbent assay. Circulating sCD40L levels were higher by twofold in untreated patients than in healthy controls (means ± standard deviations [SD]: 1.41 ± 1.48 versus 0.69 ± 0.59 ng/ml; P < 0.001). HIV-1-infected patients classified as CD4 T-cell category 1 had significantly higher sCD40L levels than patients classified as CD4 categories 2 and 3 (mean ± SD: 2.08 ± 1.46 ng/ml versus 1.57 ± 1.58 [category 2] and 0.94 ± 1.25 ng/ml [category 3]; P = 0.046), while no correlation with clinical categories A, B, and C was found. Individual serum sCD40L levels correlated with CD4+ T-cell counts (P = 0.039) but not with viral load, gamma globulin levels, or acute-inflammatory-response markers. After 8 to 12 months of HAART, a further threefold increase of serum sCD40L levels, which paralleled the increase of CD4+ T-cell counts, was observed. These novel findings suggest that sCD40L measurement in HIV-1-infected patients could serve as a new surrogate marker useful in the assessment of treatment efficacy, especially in settings where well-equipped laboratories and funding required for CD4+ T-cell count and viral load measurements are not available.
The costimulatory molecule CD40 ligand (CD40L or CD154) is transiently expressed on the surfaces of T cells following activation. However, the molecule is barely detectable on resting cells (14). The interaction between CD40L and its counterreceptor, CD40, which is expressed on the surfaces of antigen-presenting cells, is required for the productive B-cell activation, antibody production, and isotype class switching. This interaction may also play a key role in many diseases characterized by immune system activation (5, 6, 10), including human immunodeficiency virus type 1 (HIV-1) infection (9, 17). In fact, CD40L is overexpressed on the surfaces of CD4+ T cells in patients infected with HIV-1, and this overexpression is corrected after antiretrovirus therapy, suggesting a role for this molecule in the recovery of cell traffic disturbances and in the T-cell renewal capacity (17).
A soluble isoform of CD40L (sCD40L), resulting from cleavage of the surface molecule expressed on activated T cells, is able to replace, at least in vitro, the normal T-cell-derived CD40L signal to CD40-bearing cells (11). sCD40L can be detected in the serum of healthy individuals (7, 19, 21), and elevated levels, as well as functional capacity of sCD40L, have been reported in patients with systemic lupus erythematosus (7, 19), rheumatoid arthritis, systemic vasculitis, hepatitis B virus infection (19), and lymphoproliferative disorders (21). To the best of our knowledge, no data regarding the serum sCD40L levels in HIV-1-infected patients have been reported.
Levels of soluble markers of T-cell activation in serum, CD4+ T-cell counts, and HIV viral load represent the three categories of markers which are used for the prognosis of disease progression and for assessment of treatment efficacy in HIV-1 infection (4, 12). Both CD4+ T-cell and viral-load measurements are expensive tests, requiring sophisticated methods, and are not available to the majority of HIV-1-infected patients. The relatively inexpensive and easily performed measurements of T-cell activation-related soluble molecules can be used as additional markers for disease progression and might replace CD4+ T-cell counts in resource-deprived settings.
This study was undertaken to determine serum sCD40L levels in HIV-1-infected patients, to study the impact of highly active antiretrovirus treatment (HAART) on them, and to search for possible correlations between circulating sCD40L levels and established surrogate markers, such as CD4+ T-cell counts and viral load.
MATERIALS AND METHODS
Study population.
Seventy-seven consecutive HIV-1-infected patients were enrolled in this study. The inclusion criteria were confirmed HIV-1 infection and no prior antiretrovirus treatment. All patients with fever, neoplasm, or concurrent opportunistic infection at the time of initial presentation were excluded from the study. After the initial evaluation, patients were placed on triple HAART and were followed up with clinic visits at 4-month intervals. Blood samples were obtained at study entry and after 8 to 12 months of HAART. Flow cytometry measurements of CD4+ T lymphocytes were performed as described previously (2). In the analysis of our data CD4+ T-cell counts always represent the absolute number of cells per microliter. Viral load was measured by a branched-DNA signal amplification assay (Chiron Corp., Emeryville, Calif.). Acute-inflammatory-response markers, such as erythrocyte sedimentation rate (ESR) and levels of C-reactive protein (CRP) in serum, as well as serum gamma globulin levels, were determined in a clinical laboratory by using standard methods. Serum from each blood sample was separated, aliquoted, frozen, and stored at −80°C. Frozen sera from 20 healthy blood donors, age and sex matched, were used as controls.
Measurement of serum sCD40 ligand levels.
The detection and measurement of serum sCD40L levels were carried out by using a commercially available enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle, according to the manufacturer's instructions (sCD40L ELISA test kit; Bender Medsystems, Vienna, Austria). This assay is highly specific for the quantitative determination of sCD40L in human sera and has a minimum sensitivity of 0.095 ng/ml. Intra- and interassay coefficients of variation were 4.0 and 6.8%, respectively, and mean recovery was 91%, according to the manufacturer.
Statistical analysis.
Means and standard deviations (SD) were computed for each variable. The mean values between groups of patients at baseline were compared by using the Student t test for unpaired data and the one-way analysis of variance test. Spearman's rank correlation coefficient for nonparametric variables was used to assess possible correlations between individual serum sCD40L levels and CD4+ T-cell count, viral-load, gamma globulin, CRP, and ESR determinations. Baseline values and after-treatment values were compared by using the t test for paired differences. Data are expressed as the means ± SD. The level of significance was a P value of 0.05. All analyses were conducted with SPSS software (SPSS Inc., Chicago, Ill.) and reported P values are two-tailed.
RESULTS
Patient characteristics at baseline.
We studied 77 patients, of whom 67 (87%) were men and 10 (13%) were women (mean age ± SD, 37 ± 10 years; range, 20 to 69 years). Regarding the healthy controls, there were 17 (85%) men and 3 (15%) women, (mean age ± SD, 35 ± 10 years; range 18 to 64 years). The two populations (patients and controls) did not differ significantly by sex or age. The study group included 56 homosexual or bisexual men, 6 intravenous drug users, and 15 patients infected by heterosexual contact. The mean CD4 count (absolute number) ± SD was 335 ± 248 cells/μl, with a range of 24 to 1,139 cells/μl, and the median HIV-1 RNA level was 33,556 copies/ml, with an interquartile range of 13,194 to 75,978 copies/ml. Thirty-seven (48%) of the patients had AIDS at initial evaluation, according to the 1993 Centers for Disease Control and Prevention revised classification system for HIV infection (1). Thirty-six patients were classified as clinical category A, 22 were classified as clinical category B, and 19 were classified as clinical category C. Regarding the CD4 T-cell category, 13 patients were classified as category 1 (CD4+ T-cell counts, >500 cells/μl), 34 were classified as category 2 (200 to 500 cells/μl), and 30 were classified as category 3 (<200 cells/μl).
Serum sCD40L levels are increased in HIV-1-infected patients.
Serum sCD40L levels in the 77 HIV-1-infected patients ranged from 0.10 to 6.80 ng/ml. Mean values of sCD40L were significantly higher (by twofold) in HIV-1-infected patients than in healthy controls (means ± SD: 1.41 ± 1.48 versus 0.69 ± 0.59 ng/ml; P < 0.001), and they did not differ significantly among patients classified as clinical categories A, B, and C (means ± SD: 1.56 ± 1.51, 1.31 ± 1.55, and 1.25 ± 1.38 ng/ml, respectively; P = 0.72).
Correlation of serum sCD40L levels with cellular and serologic markers at baseline.
Serum sCD40 ligand levels were significantly higher in HIV-1-infected patients classified as CD4+ T-cell category 1 than in patients classified as CD4 categories 2 and 3 (means ± SD: 2.08 ± 1.46 ng/ml versus 1.57 ± 1.58 [category 2] and 0.94 ± 1.25 ng/ml [category 3]; P = 0.046) (Table 1). As shown in Table 2, a significant positive correlation between individual serum sCD40L levels and CD4+ T-cell counts (P = 0.039) was observed, but no correlation with viral load, gamma globulin levels, or acute-phase response markers, such as CRP and ESR, was found.
TABLE 1.
Serum sCD40 ligand levels in 77 HIV-1-infected patients classified according to their CD4 cell category
| CD4 cell categorya | No. of patients | Mean sCD40 ligand level (ng/ml) ± SDb |
|---|---|---|
| 1 (>500 cells/μl) | 13 | 2.08 ± 1.46 |
| 2 (200-500 cells/μl) | 34 | 1.57 ± 1.58 |
| 3 (<200 cells/μl) | 30 | 0.94 ± 1.25 |
Classification according to the 1993 revised Centers for Disease Control and Prevention classification system.
For all values P = 0.046 by one-way analysis of variance.
TABLE 2.
Correlation of serum sCD40L levels with other cellular and serologic markers at baseline in 77 HIV-1-infected patients before HAART initiation
| Variable (units) | Spearman rank correlation coefficient | P |
|---|---|---|
| CD4+ T cells (cells/μl) | 0.235 | 0.039 |
| HIV-1 RNA (copies/ml) | −0.100 | 0.386 |
| CRP (mg/dl) | −0.050 | 0.670 |
| ESR (mm/1st h) | −0.106 | 0.364 |
| Gamma globulins (g/dl) | −0.126 | 0.283 |
Effect of HAART on sCD40L levels and CD4+ T-cell counts.
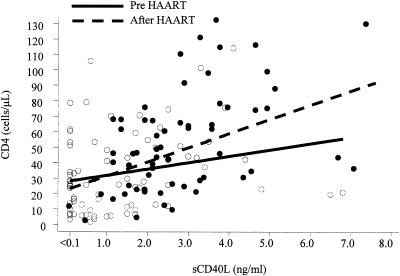
In 62 of the 77 patients sCD40L levels were measured again at 8 to 12 months after the initiation of a HAART regimen. Serum sCD40L levels ranged from 0.10 to 7.60 ng/ml and were significantly higher than baseline levels (means ± SD: 2.91 ± 1.54 versus 1.16 ± 1.4 ng/ml; P < 0.001 by paired t test). As expected, CD4+ T-cell counts were also significantly higher than baseline (means ± SD: 488 ± 296 versus 295 ± 248 cells/μl; P < 0.001). Figure 1 shows the scatter plot of CD4+ T-cell counts versus sCD40L levels before and after HAART initiation. A clear shift in the distribution of both markers toward higher levels after HAART initiation was observed. Finally, while these 62 patients were under treatment, there was again a significant correlation of sCD40L levels with CD4+ T-cell counts (P = 0.01) but no correlation with viral load, gamma globulin levels, or CRP and ESR was noted.
FIG. 1.
Scatter plot of CD4+ T-cell counts versus serum sCD40L levels before (open circles) and after (solid circles) HAART initiation in 62 HIV-1-infected patients. Superimposed are the corresponding regression lines. Both parameters are significantly higher after treatment (P < 0.001 by paired t test).
DISCUSSION
The multiple roles that the CD40L/CD40 system may play in HIV-1 infection have been reviewed recently (9). The emerging significance of CD40L function in these patients is emphasized by in vitro findings showing that direct cell-cell contact between normal donor-derived macrophages and cells engineered to express CD40L resulted in the production of large amounts of HIV-1-suppressive beta-chemokines (8). Moreover, treating macrophages with sCD40L also induced them to secrete high levels of beta-chemokines, which were able to protect CEMx174-CCR5 cells from infection by HIV-1 (3).
We have shown here that concentrations of circulating sCD40L are elevated in patients infected with HIV-1, compared to those in healthy controls. This finding of elevated levels of sCD40L in spite of low numbers of CD4+ T cells is not surprising, taking into consideration that the CD40L molecule is expressed on activated T cells and not on resting T cells (5, 14) and that HIV-1 infection is characterized by extreme T-cell activation (13). Sousa et al. have indeed found significantly enhanced proportions and counts of CD40L-expressing cells within the CD4+ T-cell subset in asymptomatic, untreated, HIV-1-infected patients (17). Elevated serum sCD40L levels in our patients, most probably resulting from cleavage of the surface molecule, should be attributed solely to HIV-1 infection because patients with other conditions causing immune system activation, such as fever, neoplasm, and concurrent opportunistic infection, were excluded from the study.
At baseline, serum sCD40L levels were significantly higher among patients classified at CD4+ T-cell category 1 than among patients classified as CD4 categories 2 and 3. Conversely, there was no significant difference in sCD40L levels among clinical categories. When the association of sCD40L levels with other laboratory parameters was examined, it was found that circulating sCD40L levels correlated significantly with CD4+ T-cell counts. Taken together, these data suggest that the progressive decline of CD4+ T-cell counts caused by HIV-1 infection is followed by a decline in serum sCD40L levels, since CD40L is expressed mainly on CD4+ T cells. A defective in vitro induction of CD40L expression on the surfaces of anti-CD3-stimulated T cells in more-advanced stages of HIV-1 infection has been observed (18, 20), while in patients who have developed AIDS a selective depletion of CD4+ CD40L+ T cells has been reported (9). On the other hand, the lack of association of sCD40L levels with acute-inflammatory-response markers (CRP and ESR) or B-cell activation (serum gamma globulin levels) suggests that serum sCD40L levels reflect specifically the presence of activated CD4+ T cells and not immune system activation in general.
In 62 patients serum sCD40L levels were determined before and after treatment with a HAART regimen. Serum sCD40L concentrations in individual patients were significantly higher after treatment, and this increase paralleled the expected increase in CD4+ T-cell counts. Moreover, after treatment serum sCD40L levels correlated again to CD4+ T-cell counts but not to other markers of immune system activation. The finding that HAART results in even higher sCD40L levels may seem paradoxical, especially in light of the observation that surface CD40L overexpression on CD4+ T cells is corrected during HAART (17). However, this is not the case because increased levels of circulating CD40L might simply result from the HAART-induced increase in the absolute numbers of CD4+ T cells. A portion of these cells are still activated, expressing the CD40L surface molecule (albeit downregulated), since the virus is not eradicated. Although we have not addressed T-cell surface expression of CD40L in our patients, an alternative hypothesis is that downregulation of the surface CD40L molecule might be associated with increased cleavage, thus resulting in elevated serum sCD40L levels. In any case, the HAART-induced increase in CD4+ T cells is associated with a parallel increase in serum sCD40L levels. Therefore, we propose that sCD40L levels could be used as an additional marker to assess the efficacy of treatment. A significant difference between pre- and post-HAART measurements, which reflects the presence of rising CD4+ T-cell counts after HAART, could be useful even in patients with very low CD4 counts.
Although the immunological correlates of the elevated serum sCD40L levels associated with HIV-1 infection will require further study, we conclude that this molecule, unlike other non-T-cell-specific soluble molecules, which reflect pan-immune system activation (15, 16), could serve as an additional surrogate marker for the assessment of treatment efficacy. Such measurements could be useful in settings where well-equipped laboratories and funding required for CD4+ T-cell and viral-load measurements are not available. Their usefulness for patients with other concurrent infections may be limited, since studies on the levels of sCD40L in patients coinfected with other microorganisms common in developing countries (e.g., the malaria parasite) are lacking. Also, prospective studies of large numbers of patients are required to evaluate whether sCD40L levels at the initial presentation can predict disease progression. Finally, studies to elucidate a possible protective biological role, as suggested by in vitro experiments (3, 8), for the increased sCD40L concentrations in the circulation of patients infected with HIV-1 are warranted.
Acknowledgments
We thank Giota Touloumi for help in statistical analysis
REFERENCES
- 1.Centers for Disease Control. 1992. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbid. Mortal. Wkly. Rep. 41:1-18. [PubMed] [Google Scholar]
- 2.Choremi, H., V. Viglis, P. Gargalianos, T. Kordossis, A. Iniotaki, and J. Kosmidis. 1994. Downregulation of CD28 surface antigen on CD4+ and CD8+ T lymphocytes during HIV-1 infection. J. Acquir. Immune Defic. Syndr. 7:245-253. [PubMed] [Google Scholar]
- 3.Di Marzio, P., R. Mariani, R. Lui, E. K. Thomas, and N. R. Landau. 2000. Soluble CD40 ligand induces beta-chemokine production by macrophages and resistance to HIV-1 entry. Cytokine 12:1489-1495. [DOI] [PubMed] [Google Scholar]
- 4.Fahey, J. L., J. M. G. Taylor, B. Manna, P. Nishanian, N. Aziz, J. V. Giorgi, and R. Detels. 1998. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS 12:1581-1590. [DOI] [PubMed] [Google Scholar]
- 5.Foy, T. M., A. Aruffo, J. Bajorath, J. E. Buhlmann, and R. Noelle. 1996. Immune regulation by CD40 and its ligand gp39. Annu. Rev. Immunol. 14:591-617. [DOI] [PubMed] [Google Scholar]
- 6.Grewal, I. S., and R. A. Flavell. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16:111-135. [DOI] [PubMed] [Google Scholar]
- 7.Kato, K., E. Santana-Sahagún, L. Z. Rassenti, M. H. Weisman, N. Tamura, S. Kobayashi, H. Hashimoto, and T. J. Kipps. 1999. The soluble CD40 ligand in systemic lupus erythematosus. J. Clin. Investig. 104:947-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornbluth, R. S., K. Kee, and D. D. Richmant. 1998. CD40 ligand (CD154) stimulation of macrophages to produce HIV-1-suppressive b-chemokines. Proc. Natl. Acad. Sci. USA 95:5205-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornbluth, R. S. 2000. The emerging role of CD40 ligand in HIV infection. J. Leukoc. Biol. 68:373-382. [PubMed] [Google Scholar]
- 10.Koshy, M., D. Berger, and M. K. Crow. 1996. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J. Clin. Investig. 98:826-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane, P., T. Brocker, S. Hubele, E. Padovan, A. Lanzavecchia, and F. McConnell. 1993. Soluble CD40 ligand can replace the normal T cell derived CD40 ligand signal to B cells in T cell-dependent activation. J. Exp. Med. 177:1209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellors, J. W., A. Munoz, J. V. Giorgi, J. B. Margolick, C. J. Tassoni, P. Gupta, L. A. Kingsley, J. A. Todd, A. J. Saah, R. Detels, J. P. Phair, and C. R. Rinaldo, Jr. 1997. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann. Intern. Med. 126:946-954. [DOI] [PubMed] [Google Scholar]
- 13.Pantaleo, G., C. Graziosi, and A. Fauci. 1993. The immunopathogenesis of human immunodeficiency virus infection. N. Engl. J. Med. 328:327-339. [DOI] [PubMed] [Google Scholar]
- 14.Roy, M., T. Waldschmidt, A. Aruffo, A. J. Ledbetter, and J. R. Noelle. 1993. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J. Immunol. 151:2497-2510. [PubMed] [Google Scholar]
- 15.Sfikakis, P. P., V. Tzavara, N. Sipsas, O. Kosmopoulou, and T. Kordossis. 1995. Levels of the circulating cell adhesion molecule E-selectin and disease progression in HIV infection. Infection 23:207-211. [DOI] [PubMed] [Google Scholar]
- 16.Sipsas, N., P. P. Sfikakis, P. Sfikakis, H. Choremi, and T. Kordossis. 1994. Serum concentrations of soluble intercellular adhesion molecule-1 and progress towards disease in patients infected with HIV. J. Infect. 29:271-282. [DOI] [PubMed] [Google Scholar]
- 17.Sousa, A. E., A. F. Chaves, M. Doroana, F. Antunes, and R. M. Victorino. 1999. Early reduction of the over-expression of CD40L, OX40, and Fas on T cells in HIV-1 infection during triple anti-retroviral therapy: possible implications for lymphocyte traffic and functional recovery. Clin. Exp. Immunol. 116:307-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subauste, C. S., M. Wessendarp, A. G. Smulian, and P. T. Frame. 2001. Role of CD40 ligand signalling in defective type-1 cytokine response in human immunodeficiency virus infection. J. Infect. Dis. 183:1722-1731. [DOI] [PubMed] [Google Scholar]
- 19.Vakkalanka, R. K., C. Woo, K. A. Kirou, M. Koshy, D. Berger, and M. K. Crow. 1999. Elevated levels and functional capacity of soluble CD40 ligand in systemic lupus erythematosus sera. Arthritis Rheum. 42:871-881. [DOI] [PubMed] [Google Scholar]
- 20.Vanham, G., L. Penne, J. Devalck, L. Kestens, R. Colebunders, E. Bosmans, K. Thielemans, and J. L. Ceuppens. 1999. Decreased CD40 ligand induction in CD4 T cells and dysregulated IL-12 production during HIV infection. Clin. Exp. Immunol. 117:335-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Younes, A., V. Snell, U. Consoli, K. Clodi, S. Zhao, J. L. Palmer, E. K. Thomas, R. J. Armitage, and M. Andreeff. 1998. Elevated levels of biologically active soluble CD40 ligand in the serum of patients with chronic lymphocytic leukaemia. Br. J. Haematol. 100:135-141. [DOI] [PubMed] [Google Scholar]