Abstract
To define the virus specificity of the immunoglobulin M (IgM) antibody-capture enzyme-linked immunosorbent assay (MAC-ELISA) among the medically important members of the Japanese encephalitis (JE) virus serocomplex of flaviviruses, 103 IgM-positive human serum samples from patients with confirmed West Nile (WN) virus, St. Louis encephalitis (SLE) virus, or JE virus infections were assembled and simultaneously tested against all three viral antigens in a standardized MAC-ELISA. Of the serum samples tested, 96 (93%) showed higher positive-to-negative absorbance ratios (P/Ns) with the infecting virus antigen compared to those obtained with the other two virus antigens. Of the seven specimens with higher P/Ns with heterologous virus antigens, six were from patients with SLE virus infections (the serum samples had higher levels of reactivity with WN virus antigen) and one was from a patient with a JE virus infection (this serum sample also had a higher level of reactivity with WN virus antigen). Not surprisingly, similar virus specificity was observed with WN virus-elicited IgM in cerebrospinal fluid. As shown in previous studies, a subset of these specimens was even less reactive in the MAC-ELISA with dengue virus, a member of a different flavivirus serocomplex. The degree of virus cross-reactivity did not appear to be related to days postonset, at least during the first 40 days of infection. Infections with WN virus could be correctly distinguished from infections with SLE virus on the basis of the observed anti-viral IgM cross-reactivities alone 92% of the time. Infections with SLE virus resulted in antibody that was more cross-reactive, so identification of SLE virus as the infecting agent by use of MAC-ELISA cross-reactivity alone was more problematic.
Flaviviruses, which are significant causes of disease worldwide, are divided into several complexes (7). Medically important members of the Japanese encephalitis (JE) virus serocomplex include the JE, Murray Valley encephalitis, St. Louis encephalitis (SLE), and West Nile (WN) viruses. Each of these viruses causes similar disease syndromes in humans, ranging from an asymptomatic or mild flulike illness to clinical encephalitis. Until 1999, SLE virus was the only mosquito-borne flavivirus causing human morbidity and mortality in the United States. WN virus, traditionally found in Europe, Africa, the Middle East, and Asia, appeared in New York City in the late summer of 1999, creating the first opportunity for WN and SLE viruses to cocirculate (2, 9, 15). By 2001 the distribution of WN virus had expanded into areas of known recent SLE virus activity such as the states of Florida and Louisiana (5).
Cross-reactivity of anti-flaviviral immunoglobulin G (IgG) has been well documented; however, IgM is known to be more specific (3, 13, 16). Traditionally, only the plaque reduction neutralization test (PRNT) (1) or virus isolation provided a means for the unambiguous identification of flaviviral infections. In 1982, Burke and Nisalak (3) reported that IgM reactivity could differentiate between infections with JE and dengue (DEN) viruses. Subsequent investigations showed that IgM elicited by viruses from different flaviviral antigenic complexes were complex specific (13). A recently published study demonstrated the value of the IgM antibody-capture enzyme-linked immunosorbent assay (MAC-ELISA) for the diagnosis of human WN virus infections in Romania (16). That study demonstrated that the MAC-ELISA had acceptable sensitivity and specificity, showing little cross-reactivity of WN virus-elicited IgM with members of the yellow fever, tick-borne encephalitis, and DEN virus serocomplexes of flaviviruses. The extent of IgM cross-reactivity within the JE virus serocomplex (e.g., between WN and SLE viruses or WN and JE viruses) was not determined.
Using specimens from patients with serologically confirmed flaviviral encephalitis, we were able to examine systematically the cross-reactivity of IgM in serum and cerebrospinal fluid (CSF) among the members of the JE virus serocomplex by MAC-ELISA. We analyzed these data to determine the usefulness of MAC-ELISA in the identification of the infecting virus based solely on a single-dilution IgM test. If testing for these flaviviruses is done simultaneously in a standardized MAC-ELISA format, our results suggest that the specificity of the MAC-ELISA is sufficient to differentiate WN virus infections from SLE virus (and JE virus) infections.
MATERIALS AND METHODS
Serum specimens.
Unique, well-characterized, blind-coded human serum sets from patients with WN, JE, and SLE virus infections were assembled. These infections were diagnosed by virus isolation or MAC-ELISA, IgG ELISA, and serum dilution PRNT (90% endpoints) in Vero cells with SLE (TBH-28), JE (Nakayama), and WN (Eg 101) viruses (1, 11). All specimens had been submitted to the Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, for diagnostic testing (10). Storage of these specimens occurred at either 4°C or −20°C for no longer than 2 years, with a mean storage time of 9 months. Freeze-thaw cycles were limited to two per specimen.
MAC-ELISA.
The MAC-ELISA-positive and -negative antigens were prepared as sucrose-acetone extracts of virus-infected suckling mouse brains or unpurified C6/36 cell culture supernatants by using the aforementioned viral strains. In addition, the DEN virus antigen was a tetravalent mixture of C6/36 tissue culture supernatant antigen prepared from the following DEN virus serotypes: DEN virus serotype 1 (DEN-1; Hawaii), DEN-2 (New Guinea C), DEN-3 (H-87), and DEN-4 (H-241). The MAC-ELISA was performed as described previously (11). Briefly, coating antibody, conjugate, and antigens were independently titrated against a positive control serum sample and standardized by choosing reagent dilutions that yielded an A450 in the range of 0.8 to 1.2. Plates were coated with goat anti-human IgM, incubated overnight at 4°C, and subsequently blocked with phosphate-buffered saline containing 0.5% Tween 20 and 5% nonfat dry milk. Serum specimens and positive and negative control specimens were tested in triplicate at a 1:400 dilution. This dilution has previously been shown to be satisfactory for testing of most serum specimens and results in virtual elimination of false-negative results and minimizes the number of false-positive results (11). Related CSF specimens were tested undiluted. Bound IgM was detected by stepwise addition of either positive or negative antigen, followed by the addition of a flavivirus group-reactive monoclonal antibody (SLE 6B6C-1) conjugated to horseradish peroxidase. Bound conjugate was detected by adding 3,3′5,5′-tetramethylbenzidine (Neogen Corporation, Lexington, Ky.) substrate, and the A450 was measured with a microplate reader.
MAC-ELISA data calculations.
Positive-to-negative absorbance ratios (P/Ns) were determined by dividing the average A450 for the unknown serum sample tested with positive antigen by the average A450 for the negative serum sample tested with positive antigen. A positive test was obtained when the P/N of the test serum sample was >3.0 and the mean A450 for the test serum sample that reacted with the viral antigen was at least twice the mean A450 for the same serum sample that reacted with the negative antigen.
Statistical analysis.
A virus-specific IgM result was recorded when the MAC-ELISA P/Ns for the infecting viruses were statistically different, as determined by use of the bootstrap confidence intervals for the ratio of the mean P/N for homologous antigen to the mean P/N for heterologous antigen for the heterologous antigens, or when the P/Ns for the heterologous antigens were negative (<3.0). Statistical bootstrap methods were used to determine significance (6, 14). Standard receiver operating characteristic (ROC) curve analysis, a plot of the true-positive rate versus the false-positive rate across a range of positivity cutoffs, was used to quantify the diagnostic ability of the testing of specimens against different heterologous flaviviral antigens at a single dilution in the same test (6, 12). A useful measure of diagnostic ability is the area under the ROC curve (AUC), with larger areas associated with greater diagnostic ability. The AUC can be interpreted as the probability that any randomly selected positive serum specimen has a higher P/N than any randomly selected negative serum specimen. The maximum AUC is 1.
RESULTS
The results of testing for cross-reactivity by MAC-ELISA by virus infection and days postonset are shown in Table 1. By use of a P/N cutoff of 3.0, of the 36 WN virus IgM-positive serum samples tested (average P/N, 10.27; standard deviation [SD], 3.17; range, 4.04 to 14.48), 6 (17%) reacted with SLE virus antigen (average P/N, 3.88; SD, 0.66; range, 3.0 to 4.95) and 19 (53%) reacted with JE virus antigen (average P/N, 4.35; SD, 1.25; range, 3.16 to 7.73). Of the 32 SLE virus IgM-positive serum samples (average P/N, 5.47; SD, 1.3; range, 3.06 to 7.73), 20 (63%) reacted with WN virus antigen (average P/N, 5.17; SD, 2.14; range, 3.04 to 11.36) and 7 (22%) reacted with JE virus antigen (average P/N, 4.06; SD, 1.65; range, 3.04 to 7.63). Of the 35 JE virus IgM-positive serum samples (average P/N, 5.28; SD, 1.65; range, 3.05 to 9.16), 1 (3%) reacted with SLE virus antigen and 11 (31%) reacted with WN virus antigen (average P/N, 4.4; SD, 0.85; range, 3.19 to 5.6). Table 2 provides P/Ns for a set of WN virus SD IgM-positive CSF specimens tested against WN and SLE virus antigens. For the 28 WN-positive CSF specimens (average P/N, 17.44; SD, 9.22; range, 6.14 to 34.48), 14 (50%) reacted with SLE virus antigen (average P/N, 4.79; SD, 1.47; range, 3.19 to 7.84). All WN virus IgM-positive serum and CSF specimens had P/Ns significantly higher with the WN virus antigen than with the two heterologous flaviviral antigens. The P/Ns produced with SLE virus IgM-positive serum samples with SLE virus antigen were more comparable to the P/Ns observed with the other two flaviviral antigens. Six of these specimens had greater reactivities with WN virus antigen. Thirty-four of the 35 JE virus IgM-positive serum samples yielded higher P/Ns with JE virus antigen than with the heterologous virus antigens. One of these specimens had a greater reactivity with WN virus. The timing of serum collection postonset did not correlate directly to either the IgM cross-reactivity or the magnitude of the P/N in the first 40 days of infection.
TABLE 1.
P/N for confirmed flavivirus-positive human serum specimens tested against three related flaviviruses
| Viral infection and sample no. | Day postonset | P/N for the following antigena:
|
Viral infection and sample no. | Day postonset | P/N for the following antigena
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| WN virus | SLE virus | JE virus | WN virus | SLE virus | JE virus | |||||
| WN virus infections | ||||||||||
| 1 | 4 | 7.27 | 1.93 | 2.58 | ||||||
| 2 | 5 | 14.48 | 3.69 | 5.27 | ||||||
| 3 | 6 | 13.96 | 2.03 | 2.66 | ||||||
| 4 | 6 | 12.64 | 2.6 | 3.54 | ||||||
| 5 | 6 | 7.76 | 2.31 | 3.51 | ||||||
| 6 | 6 | 11.77 | 2.39 | 4.49 | ||||||
| 7 | 7 | 10.59 | 2.59 | 2.39 | ||||||
| 8 | 7 | 9.86 | 2.08 | 3.47 | ||||||
| 9 | 8 | 12.76 | 1.65 | 2.11 | ||||||
| 10 | 8 | 8.68 | 2.93 | 4.17 | ||||||
| 11 | 9 | 6.53 | 1.42 | 1.59 | ||||||
| 12 | 9 | 12.07 | 1.95 | 2.34 | ||||||
| 13 | 10 | 8.7 | 2.41 | 2.27 | ||||||
| 14 | 11 | 14.25 | 2.31 | 3.71 | ||||||
| 15 | 13 | 14.17 | 4.95 | 7.73 | ||||||
| 16 | 14 | 11.71 | 2.44 | 3.41 | ||||||
| 17 | 14 | 6.77 | 1.53 | 1.47 | ||||||
| 18 | 15 | 6.8 | 2.27 | 3.21 | ||||||
| 19 | 16 | 8.76 | 1.42 | 1.72 | ||||||
| 20 | 17 | 14.27 | 4.05 | 5.91 | ||||||
| 21 | 21 | 11.42 | 1.59 | 1.98 | ||||||
| 22 | 21 | 14.39 | 4.12 | 5.69 | ||||||
| 23 | 24 | 12.59 | 1.75 | 2.04 | ||||||
| 24 | 25 | 14.46 | 2.1 | 4.26 | ||||||
| 25 | 26 | 5.12 | 1.1 | 1.42 | ||||||
| 26 | 27 | 11.87 | 2.18 | 4.17 | ||||||
| 27 | 29 | 9.15 | 3.47 | 6.16 | ||||||
| 28 | 30 | 13.25 | 3.0 | 3.4 | ||||||
| 29 | 31 | 10.99 | 1.7 | 2.38 | ||||||
| 30 | 32 | 4.04 | 0.99 | 1.37 | ||||||
| 31 | 33 | 7.9 | 1.9 | 3.88 | ||||||
| 32 | 34 | 4.97 | 2.2 | 1.28 | ||||||
| 33 | 39 | 6.42 | 1.11 | 1.16 | ||||||
| 34 | 39 | 13.64 | 1.33 | 3.16 | ||||||
| 35 | 50 | 8.84 | 1.54 | 3.45 | ||||||
| 36 | ? | 7.0 | 2.28 | 2.17 | ||||||
| SLE virus infections | ||||||||||
| 1 | 0 | 2.77 | 5.75 | 2.04 | ||||||
| 2 | 1 | 3.06 | 5.46 | 1.7 | ||||||
| 3 | 3 | 1.7 | 3.81 | 1.05 | ||||||
| 4 | 4 | 3.5 | 5.57 | 1.56 | ||||||
| 5 | 4 | 2.45 | 4.76 | 1.26 | ||||||
| 6 | 5 | 1.91 | 3.62 | 1.2 | ||||||
| 7 | 5 | 2.77 | 7.73 | 1.83 | ||||||
| 8 | 8 | 2.57 | 4.38 | 2.41 | ||||||
| 9 | 10 | 3.79 | 5.37 | 1.26 | ||||||
| 10 | 11 | 4.74 | 5.49 | 2.85 | ||||||
| 11 | 12 | 3.85 | 3.67 | 1.58 | ||||||
| 12 | 12 | 4.79 | 7.05 | 3.04 | ||||||
| 13 | 15 | 3.82 | 3.73 | 1.56 | ||||||
| 14 | 17 | 3.93 | 6.49 | 2.75 | ||||||
| 15 | 34 | 1.56 | 5.09 | 1.74 | ||||||
Boldface data indicate homologous reactions.
?, onset date unknown.
TABLE 2.
P/N for confirmed WN virus-positive human CSF specimens tested against a related flavivirus
| Sample no. | Day postonset | P/N for the following antigena:
|
|
|---|---|---|---|
| WN virus | SLE virus | ||
| 1 | 1 | 25.08 | 3.19 |
| 2 | 2 | 16.89 | 6.95 |
| 3 | 2 | 9.46 | 1.95 |
| 4 | 3 | 7.06 | 3.64 |
| 5 | 3 | 7.83 | 3.52 |
| 6 | 4 | 34.48 | 5.43 |
| 7 | 4 | 32.13 | 5.64 |
| 8 | 6 | 10.11 | 3.91 |
| 9 | 6 | 12.09 | 2.01 |
| 10 | 6 | 15.42 | 3.46 |
| 11 | 7 | 14.21 | 1.98 |
| 12 | 7 | 14.09 | 2.48 |
| 13 | 7 | 24.98 | 2.72 |
| 14 | 8 | 26.91 | 2.48 |
| 15 | 9 | 10.13 | 1.59 |
| 16 | 10 | 34.48 | 4.57 |
| 17 | 12 | 34.48 | 7.84 |
| 18 | 12 | 33.86 | 5.93 |
| 19 | 17 | 12.35 | 1.71 |
| 20 | 17 | 15.63 | 5.59 |
| 21 | 17 | 12.09 | 2.18 |
| 22 | 17 | 15.36 | 4.09 |
| 23 | 17 | 12.98 | 3.26 |
| 24 | 23 | 13.71 | 2.04 |
| 25 | 24 | 12.15 | 1.53 |
| 26 | 29 | 12.66 | 2.61 |
| 27 | 34 | 6.14 | 2.25 |
| 28 | ?b | 11.54 | 1.86 |
Boldface data indicate homologous reactions.
?, onset date unknown.
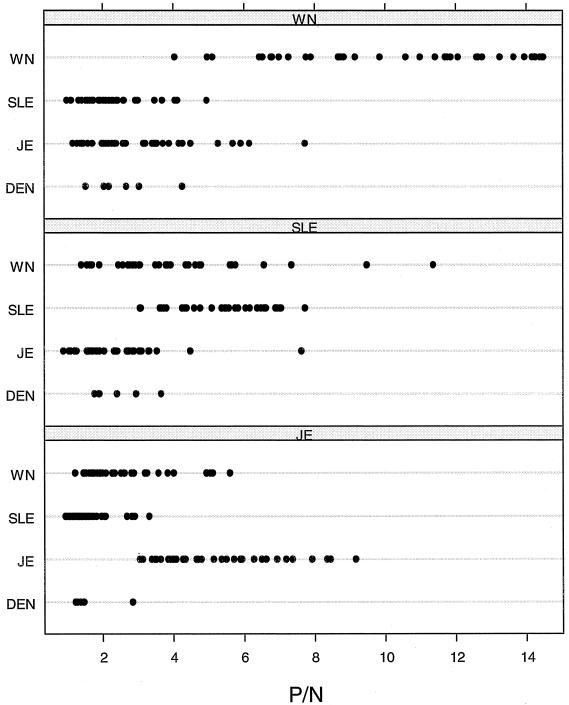
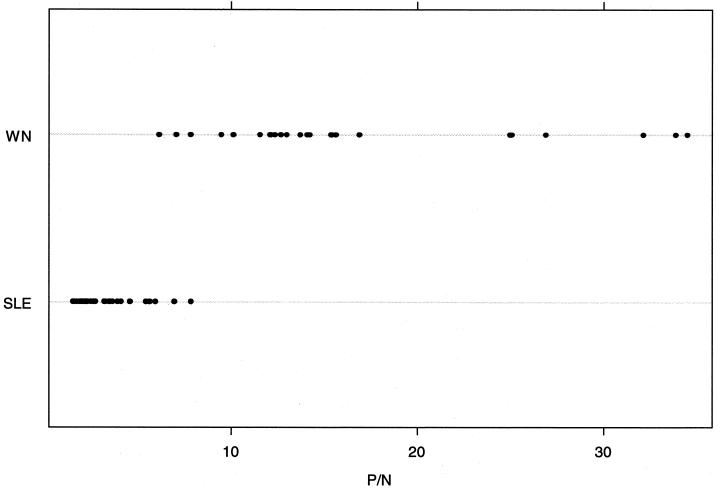
The virus cross-reactivity data, including the reactivities of a subset of each of these serum specimens with antigen from members of another flavivirus serogroup, the DEN serogroup (which includes important human pathogens but not members of the JE virus serocomplex) are depicted in Fig. 1. The WN virus specificity of a set of CSF specimens from WN virus-infected humans was observed to be similar to the WN virus-infected human serum specimens depicted in Fig. 1 (Fig. 2). As observed in previous studies, the MAC-ELISA cross-reactivities of these immune serum specimens from patients with WN, SLE, and JE virus infections with a polyvalent DEN virus antigen were low (Fig. 1).
FIG. 1.
Line scatter graphs illustrating the extent of cross-reaction between four flaviviral antigens and WN, SLE, and JE virus IgM-positive human serum specimens.
FIG. 2.
Line scatter graphs illustrating the extent of cross-reaction between WN virus and SLE virus antigens in WN virus IgM-positive CSF.
Computed bootstrap estimates and associated 95% confidence intervals (CIs) for the ratio of the mean P/N for homologous antigen to the mean P/N for heterologous antigen (11) for WN virus IgM-positive sera were 4.55 (95% CIs = 3.83, 5.32) for SLE virus antigen and 3.22 (95% CIs = 2.66, 3.84) for JE virus antigen. The same ratio for WN virus-infected CSF specimens for SLE virus antigen was even more significant at 5.13 (95% CI = 3.89, 6.67). The ratio of the mean P/N for homologous antigen to the mean P/N for heterologous antigen for SLE virus IgM-positive serum samples were lower: 1.35 (95% CI = 1.04, 1.59) for WN virus antigen and 2.34 (95% CI = 1.84, 2.77) for JE virus antigen. Lastly, JE virus IgM-positive serum samples gave ratios of 1.92 (95% CI = 1.6, 2.3) for WN virus antigen and 3.34 (95% CI = 2.83, 3.88) for SLE virus antigen.
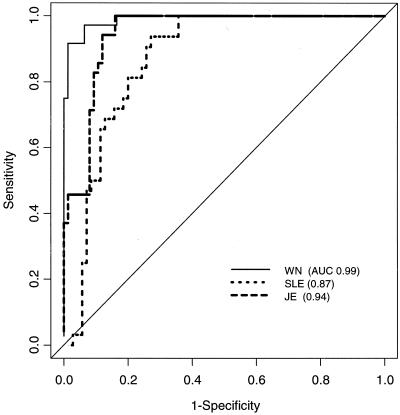
The empirical ROC curves for the three heterologous antigens are plotted in Fig. 3. WN virus had the highest AUC (AUC = 0.99; 95% CI = 0.98, 1.0), followed by JE virus (AUC = 0.94; 95% CI = 0.90, 0.98) and SLE virus (AUC = 0.87; 95% CI = 0.80, 0.94). Positivity was determined by fixing the sensitivity at 95 and 99% to maximize specificity. The resulting estimates of specificity and associated 95% bootstrap confidence intervals were computed by using the methods of Platt et al. (14). The resulting specificities were lowest with SLE virus and highest with WN virus. Use of this assay to detect WN virus infections appears to be highly specific, particularly compared to use of this assay to detect SLE virus (Table 3).
FIG. 3.
Empirical ROC curves by use of P/Ns for WN, SLE, and JE viruses.
TABLE 3.
Observed specificities at fixed sensitivities using P/N for WN virus, SLE virus, and JE virus determined by MAC-ELISA
| Viruses compared | Fixed sensitivity | Estimated specificity | 95% CI |
|---|---|---|---|
| WN virus antibody vs WN virus antigen | 0.95 | 0.94 | 0.75, 0.97 |
| 0.99 | 0.91 | 0.77, 0.97 | |
| WN virus antibody vs SLE virus antigen | 0.95 | 1.00 | 0.90, 1a |
| 0.99 | 0.97 | 0.77, 1 | |
| SLE virus antibody vs SLE virus antigen | 0.95 | 0.68 | 0.54, 0.77 |
| 0.99 | 0.64 | 0.53, 0.74 | |
| SLE virus antibody vs WN virus antigen | 0.95 | 0.43 | 0.24, 0.53 |
| 0.99 | 0.44 | 0.28, 0.56 | |
| JE virus antibody vs JE virus antigen | 0.95 | 0.87 | 0.76, 0.92 |
| 0.99 | 0.84 | 0.73, 0.89 |
Exact binomial confidence interval.
DISCUSSION
The introduction of WN virus into the United States in 1999 significantly altered the landscape of domestic diagnostic arbovirology. Until that time, diagnosis of flaviviral encephalitis in the United States had been relatively straightforward, with most domestically acquired cases of flaviviral encephalitis being caused by SLE virus. A small number of infections are caused by Powassan virus, but the geographic distribution of Powassan virus is limited, and the fact that it is tick borne limits its ability to cause widespread disease. In addition to its limited distribution and low transmission rates, Powassan virus is a member of the tick-borne encephalitis serocomplex of flaviviruses, which makes it serologically distinct from members of the JE virus serocomplex, e.g., SLE virus (16). Occasionally, imported cases of flaviviral encephalitis (usually JE virus infections) might be identified in the United States, but a well-documented travel history would readily implicate JE virus as a possible etiologic agent in these instances.
The most accurate serologic method for the differentiation of infections caused by closely related flaviviruses, such as members of the JE virus serocomplex, is PRNT, performed by testing paired acute- and convalescent-phase serum specimens against a variety of related flaviviruses. The serological cross-reactivity between WN and SLE viruses in the MAC-ELISA, as demonstrated here, was one of the reasons why the 1999 outbreak of WN virus in New York City was initially thought to be caused by SLE virus. The introduction of WN virus into New York City was not substantiated until comparative studies with SLE, WN, and JE viruses by MAC-ELISA, IgG ELISA, and PRNT procedures and identification of virus from human brain and bird tissue were completed. Since PRNT uses live virus, which requires biosafety level 3 containment for WN virus, specimens requiring confirmation are shipped primarily to state and federal reference laboratories with proper containment. Additionally, PRNT is time-consuming, requiring 10 days for test completion for SLE virus and 6 days for test completion for WN virus.
As surveillance systems identify new or recurring areas of WN virus activity in the Western Hemisphere, the decision to implement efforts to control adult mosquitoes is often based on observed rates of virus transmission to equines and humans. Because of this, use of PRNT for real-time decision making for epidemic and epizootic control is often not practical, especially if it is necessary to perform tests in a remote laboratory. The results presented here indicate that MAC-ELISA alone with WN and SLE viruses gives a rapid and reasonably accurate determination of the identity of the infecting virus.
Our data indicate that the P/Ns obtained with WN virus antigen are roughly three to five times the P/Ns obtained with SLE virus antigen. Moreover, a comparison of the P/Ns generated with WN and SLE virus antigens by use of WN virus-infected human CSF specimens revealed that the P/Ns were much greater (about fivefold) with WN virus antigen than with SLE virus antigen. In a comparison of the ratio of the P/Ns obtained with WN virus antigen versus those obtained with SLE virus antigen, a mean ratio for all WN virus-positive CSF specimens that cross-reacted with the two viruses was 4.76, with all WN virus-positive serum specimens tested having a mean ratio of 3.47. If the ratios of the P/Ns obtained with paired serum and CSF specimens from the same patient are compared, the ratio for the sera was 4.42 and that for the CSF specimens was 6.16. Differential diagnosis of a current infection by cross testing of CSF specimens is even more straightforward because intrathecal synthesis of IgM is indicative of a recent central nervous system infection (4). Significantly higher MAC-ELISA P/Ns for WN virus IgM-positive sera for WN virus antigen than for JE or SLE virus antigens were also illustrated by the large AUCs for WN virus. Conversely, however, testing of sera with antibodies against SLE virus with SLE virus antigen yielded P/Ns only twice the P/Ns obtained with WN virus antigen. While this result could be a useful trend for use in the diagnosis of human SLE virus infections, a comparison of P/Ns generated by MAC-ELISA by reacting serum specimens with closely related flaviviral antigens in a single test could be best used to differentiate WN virus infections from SLE virus infections. As a result, WN virus IgM-positive sera tested against homologous antigen were correctly identified as WN virus infected by the use of the P/N alone for 33 of 36 (92%) serum specimens. Correct diagnoses were also made for JE virus-infected specimens (26 of 35 specimens [74%]) and SLE virus-infected specimens (19 of 32 specimens [59%]). Similarly, WN virus IgM-positive CSF specimens simultaneously tested against homologous and SLE virus antigens correctly predicted WN virus infection in all 28 (100%) specimens.
The MAC-ELISA P/Ns derived from a single screening dilution are usually considered qualitative, not quantitative (8). Nevertheless, P/Ns generated in the same test can be compared if the antigen concentrations have been standardized to similar levels and the antibody is not present in excess. Because the sera used in this study were highly characterized and were confirmed to be positive for antibodies to each virus, the availability of this unique serum set permitted comparisons of cross-reactivities between WN, SLE, and JE virus antigens. The comparison of the P/Ns generated with these three flaviviral antigens demonstrates the value of this approach in establishing the identity of the infecting virus. Although final identification of WN or SLE virus infections may still need to be confirmed by PRNT, the MAC-ELISA for antigenic cross-reactivity testing of WN and SLE viruses described here provides a more rapid and cheaper means of identifying the infecting virus.
It should be stressed that the algorithm described here has proved reliable only when the procedure and standardization described by Martin et al. (11) are used and the antigens are used in the same test. Deviations from these procedures may cause variations in the algorithm. These procedures have been widely distributed to most of the United States since the introduction of WN virus in 1999. Training and the reagents used in the tests are available from the Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention.
JE virus infectionspostonsetWN virusSLE virusJE virus 16355.665.842.71 17417.357.033.53 18486.576.893.29 19?b3.66.051.92 20?5.614.592.89 21?2.866.171.65 22?3.044.321.85 23?2.934.251.61 24?4.436.583.1 25?4.366.953.32 26?1.653.061.09 27?2.726.622.32 28?5.766.392.69 29?4.635.772.37 30?11.367.047.63 31?9.486.384.48 32?1.43.080.89 JE virus infections 101.680.993.51 201.450.993.38 303.571.694.65 401.71.176.5 501.481.974.65 605.62.828.45 705.052.695.94 801.621.173.91 904.931.35.71 1041.741.333.99 1151.531.096.93 1252.271.44.26 1362.31.364.07 1461.921.153.12 1565.112.057.37 1672.881.453.85 1773.251.834.33 1892.521.533.63 19102.081.075.14 20102.341.344.67 21113.192.97.92 22151.851.425.89 23154.951.756.28 24154.943.314.69 25161.981.253.49 26164.012.077.19 27162.611.515.36 28171.761.154.0 29171.221.053.05 30191.931.25.5 31202.621.258.35 32211.470.954.79 33?2.811.596.62 34?2.271.639.16 35?3.841.964.3
Acknowledgments
We acknowledge the contributions of Beth Atkinson and Doug Mahoney of the Mayo Clinic with the S-Plus software to perform the ROC analyses (available at http://www.mayo.edu/hsr/Sfunc.html) and Rebecca Deavours for help with manuscript preparation.
REFERENCES
- 1.Beaty, B. J., C. H. Calisher, and R. E. Shope. 1995. Arboviruses, p. 465-478. In E. H. Lennette, D. A. Lennette, and E. T. Lennette (ed.), Diagnostic procedures for viral, rickettsial and chlamydial infections. American Public Health Association, Washington, D.C.
- 2.Briese, T., W. G. Glass, and W. I. Lipkin. 2000. Detection of West Nile virus sequences in cerebrospinal fluid. Lancet 355:1614-1615. [DOI] [PubMed] [Google Scholar]
- 3.Burke, D. S., and A. Nisalak. 1982. Detection of Japanese encephalitis virus immunoglobulin M antibodies in serum by antibody capture radioimmunoassay. J. Clin. Microbiol. 15:353-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1997. Case definitions for infectious conditions under public health surveillance. Morb. Mortal. Wkly. Rep. 46(RR-10):12-13. [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. 2001. Weekly update: West Nile Virus Activity—United States, September 12-18, 2001. Morb. Mortal. Wkly. Rep. 50:805.. [PubMed] [Google Scholar]
- 6.Davison, A. C., and D. Y. Hinkley. 1997. Bootstrap methods and their application. Cambridge University Press, Cambridge, United Kingdom.
- 7.Heinz, F. X., M. S. Collett, R. H. Purcell, E. A. Gould, C. R. Howard, M. Houghton, J. Moorman, C. M. Rice, and H.-J. Thiel. 1999. Family: Flaviviridae, p. 859-878. In M. H. V. Van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wicker (ed.), Virus taxonomy: classification and nomenclature of viruses. 7th report of the International Committee on Taxonomy of Viruses. Academic Press, Inc., San Diego, Calif.
- 8.Kuby, J. 1997. Immunology, 3rd ed. W. H. Freeman & Co., New York, N.Y.
- 9.Lanciotti, R. S., J. T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K. E. Volpe, M. B. Crabtree, J. H. Scherret, R. A. Hall, J. S. MacKenzie, C. B. Cropp, B. Panigrahy, E. Ostlund, B. Schmitt, M. Malkinson, C. Banet, J. Weissman, N. Komar, H. M. Savage, W. Stone, T. McNamara, and D. J. Gubler. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333-2337. [DOI] [PubMed] [Google Scholar]
- 10.Lowry, P. W., D. H. Truong, L. D. Hinh, J. L. Ladinsky, N. Karabatsos, C. B. Cropp, D. Martin, and D. J. Gubler. 1998. Japanese encephalitis among hospitalized pediatric and adult patients with acute encephalitis syndrome in Hanoi, Vietnam 1995. Am. J. Trop. Med. Hyg. 58:324-329. [DOI] [PubMed] [Google Scholar]
- 11.Martin, D. A., D. J. Muth, T. Brown, A. J. Johnson, N. Karabatsos, and J. T. Roehrig. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J. Clin. Microbiol. 38:1823-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeil, B. J., E. Keeler, and L. J. Adelstein. 1975. Primer on certain elements of medical decision making. N. Engl. J. Med. 293:211-215. [DOI] [PubMed] [Google Scholar]
- 13.Monath, T. P., R. R. Nystrom, R. E. Bailey, C. H. Calisher, and D. J. Muth. 1984. Immunoglobulin M antibody capture enzyme-linked immunosorbent assay for diagnosis of St. Louis encephalitis. J. Clin. Microbiol. 20:784-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platt, R. W., J. A. Hanley, and H. Yang. 2000. Bootstrap confidence intervals for the sensitivity of a quantitative diagnostic test. Stat. Med. 9:313-322. [DOI] [PubMed] [Google Scholar]
- 15.Roehrig, J. T. 1999. Arboviruses, p. 356-373. In S. Specter, R. L. Hodinka, and S. A. Young (ed.), Clinical virology manual, 3rd ed. American Society for Microbiology, Washington, D.C.
- 16.Tardei, G., S. Ruta, V. Chitu, C. Rossi, T. F. Tsai, and C. Cernescu. 2000. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile virus infections. J. Clin. Microbiol. 38:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]