Abstract
The parasite Toxoplasma gondii can infect most mammals and birds, sometimes causing severe pathology. Primary infection during pregnancy can result in abortion or fetal defects. Host immunity, particularly cellular immunity towards antigenic peptides, can control infection, but an efficient vaccine is not yet available. We have evaluated T-cell responses to a crude soluble toxoplasma antigen (ST-Ag) and to five recombinant peptide antigens of cells in whole-blood cultures from 22 pregnant women with preexisting infections and from 7 pregnant negative controls. Cells from all infected patients but from none of the controls responded specifically to ST-Ag by expressing surface CD25 on culture. Responses to the recombinant antigens showed considerable variation between individuals. rGRA1 elicited a response in 16 of the 22 samples (73%), rSAG1 in 13, rGRA7 in 9, rGRA6-CT in 4, and rGRA6-NT in only 1. Most responding cells were CD4+. Cells from infected subjects cultured with ST-Ag all released high levels of gamma interferon (IFN-γ) into the culture supernatant (4,343 ± 2,536 pg/ml). Cells from 12 patients released IFN-γ after culture with rGRA1 (130 ± 98 pg/ml), those from 10 patients released it after culture with rSAG1 (183 ± 128 pg/ml), and those from 4 patients released it after culture with rGRA7 (324 ± 374 pg/ml). Intensity of IFN-γ production in response to the latter two recombinant antigens correlated with responses to ST-Ag (r = 0.61 and 0.53, respectively; P < 0.01). Interleukin-4 was always absent from supernatants of cells stimulated with toxoplasma antigens. The heterogeneity of human responses to individual recombinant toxoplasma antigens should be considered in the design of potential vaccines.
The protozoan parasite Toxoplasma gondii infests a wide range of mammalian hosts and some birds, usually with few pathological consequences. Human infestation is widespread and originates mainly, but not only, with cats. Incidence varies widely between geographical sites, but disease is rare, except in immunodepressed individuals and, especially, as a result of congenital infection. Cellular immunity provides an essential component of protection against T. gondii, and J. K. Frenkel (reviewed by Darcy and Santoro [2]) first demonstrated the key role played by T lymphocytes. Infection during pregnancy, especially during the third trimester, carries a high risk of mother-to-fetus transmission with severe consequences for the child. Immunocompetent mothers infected before pregnancy do not pass the parasite to their offspring in utero, even if reexposure to the parasite occurs during the critical period. The crucial T-cell responses to T. gondii may be measured (10), and we have recently determined that cytofluorimetric detection of CD25 expression after stimulation of whole-blood cultures with T. gondii antigen for 7 days is a sensitive and specific test, comparable to the 3H-thymidine incorporation assay (5). The assay, which uses a crude soluble T. gondii antigen preparation (ST-Ag), was found to be useful for the diagnosis of congenital toxoplasmosis in infants born to mothers who had seroconverted during pregnancy (6).
It is not known which of the several antigens present in ST-Ag are recognized by a protective T-cell response and whether an effective immunity requires recognition of particular antigens or can be mounted against a number of alternatives. We have therefore tested the in vitro cellular responses of several women with established antitoxoplasma immunity against ST-Ag and a range of recombinant T. gondii antigens. Three of the antigens chosen had been reported (6, 17) to induce protection in immunized mice (SAG1, GRA1, and GRA7), and GRA6 is recognized by nearly all (96%) human immune sera (8). Responses were evaluated by detection of CD25 expression by stimulated whole-blood cultures compared to that with appropriate negative controls.
MATERIALS AND METHODS
Peripheral blood samples.
Blood was drawn into Vacutainer tubes containing lithium heparin (Becton Dickinson, Meylan, France) from 22 women known to have encountered T. gondii before pregnancy and from 7 unexposed controls as part of a routine screening program for toxoplasmosis in pregnancy. T. gondii antibodies in the plasma were measured by enzyme-linked immunosorbent assay (ELISA) (Enzygnost; Dade Behring, Paris La défence, France) at the parasitology service, Croix Rousse Hospital, Lyon, France. Immunoglobulin G (IgG) and IgM antibodies were evaluated separately by indirect immunofluorescence and cytofluorimetry as described previously (1).
ST-Ag.
Cultures of murine WEHI 164 cells (ATCC CRL1751) were infected (three tachyzoites/cell) with T. gondii strain RH propagated in the peritoneal cavity of OF1 mice (Iffa Credo, St. Germain sur l'Arbresle, France), and harvested 24 h postinfection. Tachyzoites were recovered from the cell culture after 2 days, washed in phosphate-buffered saline (PBS) (Biomérieux, Marcy l'Etoile, France) and disrupted by five freeze-thaw cycles after adjustment to 108 tachyzoites/ml of PBS. The crude extract was clarified by centrifugation at 2,500 × g for 15 min and then filtered through 0.2-μm-pore-size membranes (Millipore, Guyancourt, France). Protein content of the soluble extract was estimated using a commercial kit (Bio-Rad DC Protein Assay; Bio-Rad, Ivry-sur-Seine, France).
Recombinant antigens.
GRA1 and GRA6 (kindly provided by M.-F. Cesbron-Delauw, Institut Pasteur, Lille, France) and GRA7 (4) (a gift from D. Jacobs and C. Dello, Immunogenetics, Ghent, Belgium) were expressed as fusion proteins with glutathione S-transferase (GST) in Escherichia coli. SAG1 was kindly provided by D. Rolland (Biomérieux) and produced in Schizosaccharomyces pombe yeast (15). Native GST or PBS served as negative controls.
Blood cultures and stimulation.
Whole-blood samples (50 μl) were incubated in sterile 45-by-88-mm propylene tubes with an equal volume of ST-Ag (final concentration, 3.5 μg/ml), recombinant antigen (final concentration, 10 μg/ml), PBS, or GST as a negative control (final concentration, 10 μg/ml). On day one, 500 μl of RPMI medium (Bio-Whittaker, Verviers, Belgium) was added to each tube, and the samples were then incubated for 7 days at 37°C in 5% CO2 in air. On the seventh day, culture supernatants were collected and frozen at −20°C for later determination of cytokines.
Immunophenotypic studies.
Erythrocytes in the cell button were lysed with NH4Cl (155 mM), KHCO3 (10 mM), and EDTA (0.1 mM), and the leukocytes were recovered by centrifugation. The leukocytes were triply stained with fluorescein isothiocyanate-conjugated anti-CD25 (Dako; Trappes, France), phycoerythrin-conjugated anti-CD4 (Dako), and cyanin 5-phycoerythrin-conjugated anti-CD3 (Sigma; St. Quentin Fallavier, France) for 15 min at 4°C in the dark. The stained cells were washed and resuspended in PBS supplemented with 0.1% bovine serum albumin and EDTA (5 mM) and then analyzed in a FacScan cytofluorimeter (Becton Dickinson) to detect CD25-positive T cells. The percentage of T cells expressing CD25 was considered to be elevated if it was greater than the average of values from the uninfected control subjects after the same antigenic stimulation, by at least 3 standard deviations.
Detection of cytokines in the culture supernatants.
The cytokines gamma interferon (IFN-γ) and interleukin-4 (IL-4) present in the 7-day culture supernatants were measured by ELISA using a commercial kit (Biosource, Nivelles, Belgium) following the manufacturer's procedures. Threshold detection for IFN-γ was 42 pg/ml, and that for IL-4 was 14 pg/ml.
Statistical analysis.
Differences between groups were evaluated by Student's t test using Statview software (Abacus, Berkley, Calif.); P values of <0.05 were considered significant.
RESULTS
Serological status of the subjects.
All 22 women with documented exposure to T. gondii predating pregnancy had raised titers of specific IgG but not IgM antibodies. None of the seven unexposed controls had significant titers of IgG or IgM antibodies to T. gondii.
Cellular response to ST-Ag.
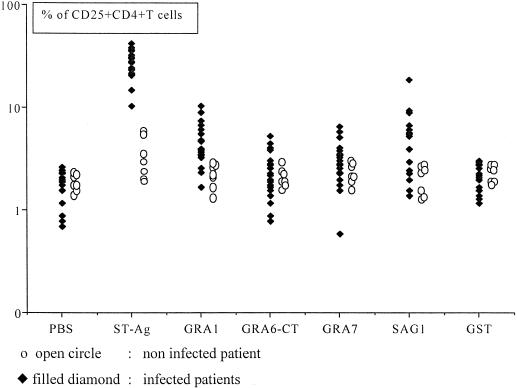
As shown in Fig. 1, cells from infected patients were induced to express CD25 on a large proportion (28% ± 8%) of their CD4+ T cells by incubation with ST-Ag. Only a few CD25+ CD4+ T cells were seen in cultures exposed to PBS (1.8% ± 0.6%; P < 0.001) or to GST (2% ± 0.6%; P < 0.001). Cells from the unexposed controls did not respond significantly to ST-Ag stimulation.
FIG. 1.
Expression of CD25 on CD4+ T-cells from 22 chronically T. gondii-infected (filled diamonds) and 7 uninfected (open circles) pregnant women after a 7-day culture of whole blood with ST-Ag, recombinant antigens (GRA1, GRA6-CT, GRA7, and SAG1) and negative controls (PBS and GST).
Cellular response to recombinant antigens.
Stimulation of whole-blood cultures from exposed women by the five recombinant antigens tested induced CD25 expression on a raised percentage of CD4-positive T cells in a proportion of subjects (Fig. 1). Overall, 21 of the 22 infected subjects, but none of the unexposed controls, responded to at least one of the tested antigens. GRA1 induced a response in 16 of 22 (73%) samples, SAG1 in 13 of 22 (59%), GRA7 in 9 of 22 (41%) and GRA6 in 4 of 22 (18%). The positive responses to single antigens always induced a lower percentage of CD25+ CD4+ T cells than did ST-Ag (Fig. 1), and summing the responses to individual antigens might account for from 6 to 66% of the total response to ST-Ag in different subjects. The intensities of the responses to ST-Ag and to the individual antigens were poorly correlated; only the response to SAG1 showed a weakly significant relationship (r = 0.42; P < 0.04). ST-Ag induced CD25 expression on 28% of CD4+ T cells from the one subject who showed no significant responses to any of the individual antigens, suggesting the presence of a normal overall cellular immune response to T. gondii in this patient.
Cytokine production by stimulated blood cultures.
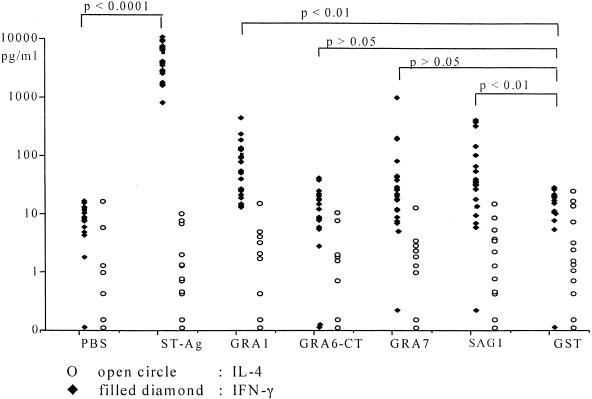
Cell supernatants collected after 7 days of culture never contained detectable IL-4 (<14 pg/ml), whether they came from exposed patients or unexposed controls and whether they had been stimulated by ST-Ag, individual antigens, or control preparations (Fig. 2). IFN-γ, however, was produced in large quantities by cultures of cells from infected patients in the presence of ST-Ag (4,373 ± 2,536 pg/ml) but not in control cultures (Fig. 2). Lesser, but significant, quantities of IFN-γ were produced by certain exposed subjects' cells after incubation with individual recombinant antigens. GRA1 induced IFN-γ production in 12 of 22 (55%) cultures, SAG1 in 10 of 22 (45%) cultures, and GRA7 in 4 of 22 (18%) cultures. IFN-γ production after stimulation by SAG1 or GRA7 correlated with response to ST-Ag by the same patients (r = 0.61 and 0.53, respectively; P < 0.01 in both cases). A correlation between IFN-γ production and the percentage of T4 cells stimulated to express CD25 by the same antigen was seen only for SAG1 (r = 0.61; P < 0.01).
FIG. 2.
In vitro production of IFN-γ (filled diamonds) and IL-4 (open circles) after a 7-day culture of whole blood from 22 chronically T. gondii-infected pregnant women in the presence of soluble ST-Ag, recombinant antigen (GRA1, GRA6-CT, GRA7, and SAG1) or controls (PBS and GST). Cytokines were detected by using an ELISA (see Materials and Methods).
DISCUSSION
Preexisting infection of a pregnant woman by T. gondii prevents transmission of the parasite to her fetus, and this protection appears to be essentially mediated by cellular acquired immunity to toxoplasma antigens. The antigenic preparation used to evaluate the overall cellular response is a complex mixture derived from solubilized tachyzoites, and the protective response might involve many of the component elements or only a few dominant antigens. We have therefore compared the responses of cells from a series of chronically infected pregnant women to ST-Ag and to four cloned recombinant antigens from T. gondii.
The cellular immune response was detected by measuring the percentage of T cells induced to express CD25 after 7 days of culture with antigen, using flow cytometry (5). Although a small number of circulating T cells may express CD25 at low levels (16), induction of strong expression on a large percentage of T cells after exposure to a specific antigen (Fig. 1) is an indicator of activation. In the present series, expression of CD25 was generally accompanied by production of large amounts of IFN-γ (Fig. 2), strongly suggesting activation. Cells from seronegative women never expressed raised levels of CD25 or of IFN-γ after exposure to toxoplasma antigens. The series included no women who were infected for the first time during their pregnancy.
Studies of candidate toxoplasma antigens for vaccine development using a mouse model have identified SAG1, GRA1, GRA7, and ROP2 as conferring protection (9, 17). T-cell clones derived from three chronically infected human subjects have been shown to recognize GRA2 (24% of clones) and SAG1 (16% of clones) (13). We here show that not all patients respond significantly to SAG1, GRA1, GRA6, or GRA7 and that different patients showed quite individual patterns of response to the antigens tested. The lack of apparent response to individual antigens in some patients might be due to insensitivity of the method to very small numbers of responding cells. It is unlikely to be due to insufficient amounts of the recombinant antigens, which were present at 10 pg/ml, compared to ST-Ag, which was used at only 3.5 pg/ml and always induced a strong response. The antigen GRA6 was tested as two separate constructs containing the N-terminal or the C-terminal portion of the molecule fused to GST. The N-terminal construct is recognized by antibodies from 96% of seropositive subjects (8) but induced CD25 expression only in 1 of 22 tested patients. In contrast, the C-terminal construct, which is poorly recognized by antibodies, induced CD25 expression on cells from four cases. The major B-cell epitopes on GRA6 appear to be concentrated on the N-terminal peptide, whereas the C-terminal portion may carry T-cell epitopes.
Most of the cells responding to stimulation by toxoplasma antigens by raised CD25 expression were CD4+ T cells. This agrees with recent observations that antigen-presenting cells infected by T. gondii induce more proliferation in CD4+ T cells than in CD8+ T cells (12, 14). Production of IFN-γ did not always accompany CD25 induction in response to the recombinant antigens. IFN-γ was never produced after exposure to GRA6, presented as either the N-terminal or the C-terminal portion, and it accompanied CD25 expression in only 9 of 16 responders to GRA1, in 10 of 13 responders to SAG1, and in 4 of 9 responders to GRA7. Some cases of IFN-γ expression in the absence of significant CD25 stimulation were observed: three after culture with GRA1 and one after culture with SAG1. In these cases, a different cell population, for example, natural killer cells or CD8+ T cells, might produce the IFN-γ (3, 11). None of the toxoplasma antigens used ever induced IL-4 production. Similar IFN-γ and IL-4 responses have been observed in both acquired (19) and congenital (7) chronic infections. TH2 responses to T. gondii appear to be limited to the acute phase in the presence of specific IgE antibodies (18) and to congenitally infected children during serological rebound (7). We saw a certain correlation between the percentages of CD25-expressing activated T cells from individual patients stimulated with ST-Ag or with SAG1 (r = 0.42; P < 0.04). This might suggest that SAG1 is a major determinant of the response, but nine of our patients showed no response at all to this antigen presented alone. The production of IFN-γ after culture with SAG1 or GRA7 also correlated with that induced by ST-Ag and, in the case of SAG1, with the percentage of CD4+ T cells induced to express CD25. The activated cells are thus a likely source of IFN-γ.
In conclusion, we show that the antigens GRA1, GRA7, and SAG1 are recognized by, and produce a stimulatory response with production of a TH1 cytokine in, cells from a number of patients with a chronic toxoplasma infection. These antigens are candidates for the induction of a protective response that can prevent the tragedy of congenital toxoplasmosis, but none of them was recognized by all of our subjects. Indeed, cells from one of our patients responded to none of the individual recombinant antigens, although they showed a strong response to the crude toxoplasma extract. Other antigens may need to be studied to ensure an adequate protective response, and the reactions to individual antigens during acute infection should be investigated. The considerable variation in individual cellular responses to toxoplasma antigens that we describe here needs to be considered in the development of a future toxoplasma vaccine.
Acknowledgments
We are grateful to M.-F. Cesbron-Delauw from Institut Pasteur, D. Jacobs and C. Dello from Innogenetics, and D. Rolland from BioMérieux for their gift of recombinant antigens.
REFERENCES
- 1.Cozon, G. J. N., C. Roure, G. Lizard, T. Greenland, D. Larget-Piet, F. Gandillon, and F. Peyron. 1993. An improved assay for the detection of Toxoplasma gondii antibodies in human serum by flow cytometry. Cytometry 14:569-575. [DOI] [PubMed] [Google Scholar]
- 2.Darcy, F., and F. Santoro. 1994. Toxoplasmosis, p. 163-201. In F. Kierszenbaum (ed.), Parasitic infections and the immune system. Academic Press, Inc., San Diego, Calif.
- 3.Hunter, C. A., C. S. Subauste, V. H. Van Cleave, and J. S. Remington. 1994. Production of γ interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect. Immun. 62:2818-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs, D., J.-F. Dubremetz, A. Loyens, F. Bosman, and E. Saman. 1998. Identification and heterologous expression of a new dense granule protein (GRA7) from Toxoplasma gondii. Mol. Biochem. Parasitol. 91:237-249. [DOI] [PubMed] [Google Scholar]
- 5.Kahi, S., G. J. N. Cozon, T. Greenland, M. Wallon, F. Gay-Andrieu, and F. Peyron. 1998. Rapid flow cytometric method to explore cellular immunity against Toxoplasma gondii in humans. Clin. Diagn. Lab. Immunol. 5:745-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahi, S., G. J. N. Cozon, and F. Peyron. 1999. Early detection of cellular immunity in congenital Toxoplasma gondii-infected children. Pediatr. Infect. Dis. J. 18:846-847. [DOI] [PubMed] [Google Scholar]
- 7.Kahi, S., G. J. N. Cozon, J. M. Pinon, T. Greenland, M. Wallon, M. Al-Kurdi, J. Ferrandiz, and F. Peyron. 1999. A switch towards Th2 during serological rebound in children with congenital toxoplasmosis. Clin. Exp. Immunol. 117:524-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lecordier, L., M.-P. Fourmaux, C. Mercier, E. Dehecq, E. Masy, and M.-F. Cesbron-Delauw. 2000. Enzyme-linked immunosorbent assay using the recombinant dense granule antigens GRA6 and GRA1 of Toxoplasma gondii for detection of immunoglobulin G antibodies. Clin. Diagn. Lab. Immunol. 7:607-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Letscher-Bru, V., O. Villard, B. Risse, M. Zauke, J. P. Klein, and T. T. Kien. 1998. Protective effect of vaccination with a combination of recombinant surface antigen and interleukin-12 against toxoplasmosis in mice. Infect. Immun. 66:4503-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLeod, R., M. O. Beem, and R. G. Estes. 1985. Lymphocyte anergy specific to Toxoplasma gondii antigens in a baby with congenital toxoplasmosis. J. Clin. Lab. Immunol. 17:149-153. [PubMed] [Google Scholar]
- 11.Montoya, J. G., K. E. Lowe, C. Clayberger, D. Moody, D. Do, J. S. Remington, S. Talib, and C. S. Subauste. 1996. Human CD4+ and CD8+ T lymphocytes are both cytotoxic to Toxoplasma gondii-infected cells. Infect. Immun. 64:176-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pistoia, I., P. Facchetti, M.-F. Cesbron-Delauw, and I. Prigione. 1996. Characterization of human T-cell clones specific for Toxoplasma gondii. Curr. Top. Microbiol. 219:165-173. [DOI] [PubMed] [Google Scholar]
- 13.Prigione, I., P. Facchetti, L. Lecordier, D. Deslle, S. Chiesa, M.-F. Cesbron-Delauw, and V. Pistoia. 2000. T-cell clones raised from chronically infected healthy humans by stimulation with Toxoplasma gondii excretory-secretory antigen cross-react with live tachyzoite: characterization of the fine antigenic specificity of the clones and multiplication for vaccine development. J. Immunol. 164:3741-3748. [DOI] [PubMed] [Google Scholar]
- 14.Purner, M. B., R. L. Berens, P. B. Nash, A. Van-Linden, E. Ross, C. Kruse, E. C. Krug, and T. J. Curiel. 1996. CD4-mediated and CD8-mediated cytotoxic and proliferative immune responses to Toxoplasma gondii in seropositive humans. Infect. Immun. 64:4330-4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raymond, F., D. Rolland, M. Gauthier, and M. Jolivet. 1998. Purification of a recombinant protein expressed in yeast: optimization of analytical and preparative chromatography. J. Chromatogr. B Biomed. Sci. Appl. 706:113-121. [DOI] [PubMed] [Google Scholar]
- 16.Shevach, E. M. 2000. Regulatory T cells in autoimmunity. Annu. Rev. Immunol. 18:423-449. [DOI] [PubMed] [Google Scholar]
- 17.Vercammen, M., A. Scorza, K. Huygen, J. De-Braekeleer, D. Diet Jacobs, E. Saman, and H. Verschueren. 2000. DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and Rop2 induces partially protective immunity against lethal challenge in mice. Infect. Immun. 68:38-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villena, I., D. Aubert, V. Brodard, C. Quereux, B. Leroux, D. Dupouy, G. Remy, F. Foudrinier, C. Chemla, and J. E. Gomez-Marin. 1999. Detection of specific immunoglobulin E during maternal, fetal, and congenital toxoplasmosis. J. Clin. Microbiol. 37:3487-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto, J. H., A. L. Vallochi, C. Silveira, J. K. Filho, R. B. Nussenblatt, E. Cunha-Neto, R. T. Gazinelli, R. Belfort, and L. V. Rizzo. 2000. Discrimination between patients with acquired toxoplasmosis and congenital toxoplasmosis on the basis of the immune response to parasite antigens. J. Infect. Dis. 181:2018-2022. [DOI] [PubMed] [Google Scholar]