Abstract
A lateral-flow immunoassay (LFT) was developed to detect bovine rotavirus in fecal samples. Using samples (n = 74) from diarrheic calves, a comparison of the LFT with a commercial latex agglutination test (LAT) and transmission electron microscopy (EM) was conducted. When EM was used as the reference method, initial studies of 29 samples indicated 70 and 80% sensitivities of the LFT and LAT, respectively, with both being 100% specific. When the LAT was the reference test, the LFT was 75% sensitive and 91% specific. Additional specimens (n = 45) were tested by the LFT and LAT alone, and results were identical for both methods.
Rotaviruses are the major cause of severe gastroenteritis in human infants and animals worldwide (2, 5-9). Bovine rotavirus (BRV) is responsible for approximately 30% of calf enteritis (3); thus, accurate and rapid diagnostic tests are important for proper treatment and prevention. The objective of this study was to develop and evaluate a lateral-flow immunoassay (LFT) for rapid detection of BRV in fecal samples. Evaluation of the LFT was conducted by comparing its performance to that of transmission electron microscopy (EM) and the latex agglutination test (LAT).
Seventy-four fecal samples from calves with acute diarrhea were tested. Fecal samples and supernatants of BRV-infected cell cultures previously tested by enzyme-linked immunosorbent assay (ELISA) with the Rotazyme II kit (Abbott Laboratories, Abbott Park, Ill.) were used as positive and negative controls for this study (1).
For examination by EM, 10% (wt/vol) suspensions of calf feces were homogenized in 0.01 M phosphate-buffered saline (PBS) (pH 7) and were centrifuged at 1,500 × g for 5 min. The supernatant was removed and centrifuged at 28,000 × g for 1 h. A 35-μl aliquot of pelleted material was treated with 0.7 ml of double-distilled water, 140 μl of phosphotungstic acid (pH 7), and 35 μl of 1% bovine serum albumin for 5 min and then was sprayed onto carbon-coated grids for viewing at ×30,000 magnification. The manufacturer's instructions for testing of human fecal samples were followed to test the bovine feces using the Virogen Rotatest (Wampole Laboratories, Cranbury, N.J.), a rapid LAT which utilizes latex particles coated with antibodies specific for group A rotavirus antigens.
All components of the LFT system, including BRV antiserum, anti-mouse immunoglobulin G (IgG), antirotavirus antibody-gold conjugated, membrane-blocking reagent, and known positive and negative controls, were evaluated in a series of titration experiments to determine optimum concentrations for the assay. Criteria used in these experiments were the attainment of maximum signal strength for specific line formation (true-positive/true-negative result) with an absence of nonspecific line formation (false-positive/false-negative result) and low background staining. The colloidal gold particles (2 nm; BBI International, Cardiff, United Kingdom) and monoclonal antibody used to prepare the LFT indicator conjugate were handled following the manufacturer's instructions. Monoclonal antibody clone 3C10 (Research Diagnostics, Inc., Flanders, N.J.) specific to the BRV inner capsid protein p42 was titrated to determine the appropriate concentration needed to stabilize the colloidal gold prior to conjugation. The sensitivity of the resulting antibody-gold conjugate was assayed by immunoblotting onto nitrocellulose. Fecal samples were initially prepared as either 10% (wt/vol) suspensions from solid or semisolid feces or as 20% (vol/vol) suspensions from liquid feces in 0.01 M PBS (pH 7) that were centrifuged at 1,500 × g for 10 min, and the supernatants were stored in sterile vials at −80°C until used. After the LFT was optimized, the centrifugation step was eliminated on fresh samples.
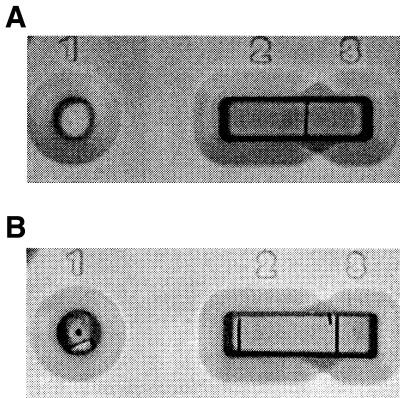
Hi-flow Plus Mylar-backed nitrocellulose membranes (Millipore Corp., Bedford, Mass.) were cut into 6-cm by 6-mm strips and were etched at 2.1 and 3.3 cm from the end designated zone 1, the sample application site. Ten microliters of anti-BRV polyclonal antiserum (1:1,000) was spotted at the 2.1-cm mark (zone 2), and 10 μl of goat anti-mouse IgG (1:5,000) was spotted from 2.2 to 3.3 cm (zone 3). The membranes were allowed to air dry for 45 min at room temperature (RT). They were blocked by incubating with 1% casein enzymatic hydrolysate (Sigma, St. Louis, Mo.) for 1/2 h and were then washed twice for 5 min with 0.01 M Tris-buffered saline. After drying for 45 min at RT, the membranes were secured in plastic cassettes. The LFT was performed by adding a mixture of 30 μl of test sample, 30 μl of anti-BRV-gold colloid conjugate, and 140 μl of transport-facilitating agent (1% casein and polyethylene glycol 20,000 in PBS, pH 7.0) to the loading window (zone 1) of the plastic cassette. The sample is wicked by the Hi-flow Plus membrane from zone 1 into zones 2 and 3, which, respectively, are saturated with anti-BRV and anti-mouse IgG antibodies. Following incubation for 30 min at RT, the membrane was evaluated visually. A single colored line appearing in zone 3 (Fig. 1A) indicates the absence of BRV. The concurrent presence of colored lines in zones 2 and 3 (Fig. 1B) indicates the presence of BRV. The absence of line formation indicates an invalid test that must be repeated. Once the test result has developed, the reaction lines, which form within 2 min of sample application, are permanent.
FIG. 1.
LFT true-negative (A) and -positive (B) results.
Rapid, accurate diagnosis of BRV as a cause of diarrhea is required to manage disease and to prevent loss of animals. Virus isolation (VI), fluorescent antibody (FA) testing, EM, ELISA, and the LAT are presently used by diagnostic laboratories for detection of BRV in feces (1, 3, 4), but EM, VI, and FA testing require expensive equipment and skilled personnel. Transmission EM is often used as the “gold standard” for virus detection, but it achieves definitive results only when greater than 106 to 108 virus particles are present per ml of feces (3). VI and follow-up FA testing are more sensitive than EM because virus is rapidly amplified to detectable numbers in culture, but it takes 3 to 8 days before the BRV cytopathic effect develops in the cells (1). FA testing of fresh intestinal tissue is rapid, but the tissues must be retrieved 4 to 6 h after the onset of diarrhea because the infected villous epithelial cells are rapidly destroyed. ELISA is commonly performed because even small amounts of BRV can be detected in feces 4 to 9 days after the onset of diarrhea and because the test can be completed in less than 4 h (4). The LAT is rapid and sensitive for detection of BRV in feces, but due to rapid dissociation of the latticed latex-virus precipitate, LAT slides must be read without delay. This is the first report on the development of an LFT for the detection of BRV in fecal samples and evaluation of the LFT in comparison to the LAT and EM.
Twenty-nine specimens were evaluated by comparing EM (reference method), the LAT, and the LFT (Table 1). When EM was the reference method, initial studies of 29 samples indicated 70 and 80% sensitivities of the LFT and LAT, respectively, with both being 100% specific. When the LAT was the reference test, the LFT was 75% sensitive and 91% specific. Forty-five additional specimens were evaluated by the LFT (Table 2) with the Virogen Rotatest LAT as the reference test, and there was complete agreement between the test results.
TABLE 1.
Comparison of EM (reference standard) to LAT and LFT for BRV (n = 29)
| Result by: | Frequency | πia | ||
|---|---|---|---|---|
| EM | LAT | LFT | ||
| + | + | + | 6 | 0.206 |
| + | + | − | 2 | 0.070 |
| + | − | − | 1 | 0.030 |
| + | − | + | 1 | 0.034 |
| − | − | − | 19 | 0.655 |
πi = frequency/n, where n is the portion of samples having a particular outcome.
TABLE 2.
Comparison of LFT and LAT for BRV (n = 45)
| Result by: | Frequency | πia | |
|---|---|---|---|
| LFT | LAT | ||
| + | + | 12 | 0.267 |
| − | + | 0 | − |
| + | − | 0 | − |
| − | − | 33 | 0.73 |
πi = frequency/n, where n is the portion of samples having a particular outcome.
Major advantages of the LFT over other testing formats include rapidity, simplicity, and the need for minimal training of personnel. Unlike the LAT, the formation of permanent lines allows the results to be read at times convenient for the technician. The LFT can be performed virtually anywhere, including laboratories, the office, or the field. Because the LFT is simple to perform, it can be adopted in underdeveloped countries where diagnostic facilities are limited.
Acknowledgments
We thank Jerome Nietfeld, Teresa Yeary, Marge Muenzenberger, and Dave Adams for their assistance in preparing the manuscript.
The project was supported by funds from the KAES and the Dean's Fund Research, by NC-62 Regional USDA funds for enteric diseases of swine and cattle, and by revenue generated by the Kansas State University Veterinary Diagnostic Laboratory, Manhattan, Kans.
Footnotes
Contribution 01-436-J from the Kansas Agricultural Experiment Station, Manhattan.
REFERENCES
- 1.Al-Yousif, Y., J. Anderson, C. Chard-Bergstrom, A. Bustamante, M. Muenzenberger, K. Austin, and S. Kapil. 2001. Evaluation of a latex agglutination kit (Virogen Rotatest) for detection of bovine rotavirus in fecal samples. Clin. Diagn. Lab. Immunol. 8:496-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Athanassious, R., G. Marsolias, R. Assaf, S. Dea, J. Descoteaux, R. Dulude, and C. Montpetit. 1994. Detection of bovine coronavirus and type A rotavirus in neonatal calf diarrhea and winter dysentery of cattle in Quebec: evaluation of three diagnostic methods. Can. Vet. J. 35:163-169. [PMC free article] [PubMed] [Google Scholar]
- 3.Benfield, D. A., I. J. Stotz, E. A. Nelson, and K. S. Groon. 1984. Comparison of a commercial enzyme-linked immunosorbent assay with electron microscopy, fluorescent antibody, and virus isolation for the detection of bovine and porcine rotavirus. Am. J. Vet. Res. 45:1998-2002. [PubMed] [Google Scholar]
- 4.De Beer, M., I. Peenze, V. M. De Costa, and A. D. Steele. 1997. Comparison of electron microscopy, enzyme-linked immunosorbent assay and latex agglutination for the detection of bovine rotavirus in faeces. J. S. Afr. Vet. Assoc. 68:93-96. [DOI] [PubMed] [Google Scholar]
- 5.Kapikian, A. Z., J. Flores, Y. Hoshino, R. I. Glass, K. Midthun, M. Gorziglia, and R. M. Chanock. 1986. Rotavirus: the major etiologic agent in severe infantile diarrhea may be controllable by a ‘Jennerian' approach to vaccination. J. Infect. Dis. 153:815-822. [DOI] [PubMed] [Google Scholar]
- 6.Kapikian, A. Z., and R. M. Chanock. 1990. Rotaviruses, p. 1353-1404. In B. N. Fields (ed.), Virology, 2nd ed., vol. 2. Raven Press, New York, N.Y. [Google Scholar]
- 7.McNulty, M. S., D. Todd, G. M. Allan, J. B. McFerran, and J. A. Green. 1984. Epidemiology of rotavirus infection in broiler chicken: recognition of four serogroups. Arch. Virol. 81:113-121. [DOI] [PubMed] [Google Scholar]
- 8.Saif, L. J. 1990. Nongroup A rotaviruses, p. 73-96. In J. Saif and K. W. Theil (ed.), Viral diarrheas of man and animals. CRC Press, Inc., Boca Raton, Fla.
- 9.Snodgrass, D. R., A. J. Herring, I. Campbell, J. M. Inglis, and F. D. Hargreaves. 1984. Comparison of atypical rotaviruses from calves, piglets, lambs and man. J. Gen. Virol. 65:909-914. [DOI] [PubMed] [Google Scholar]