Abstract
A strategy was developed for the control, standardization, and critical evaluation of an enzyme-linked immunosorbent assay (ELISA) for the detection of human papillomavirus-specific immunoglobulin G in human sera. Control human sera, polyclonal animal sera, and monoclonal antibodies were used to establish optimal assay parameters, including antigen coating, serum dilutions, and criteria for daily reproducibility, monitoring, and rejection of assays. Three evaluation techniques were used in parallel to define an optimal cutoff absorbance value that yields greater than 93% sensitivity and 98.5% specificity in the assay's ability to discriminate positive and negative control sera. This strategy provides an optimal method by which to determine cutoff absorbance values for ELISA.
Detection of human papillomavirus (HPV) DNA in cervical samples has been considered a hallmark for the diagnosis of infection. However, since the detection of HPV DNA is often transient (2), measurement of virus-specific immunity may be a better indicator of HPV exposure since a prior or current infection is likely to be detected. Enzyme-linked immunosorbent assays (ELISA) based on the use of viruslike particles (VLPs) have been described (3, 4). However, many studies using serologic VLP ELISAs have provided insufficient details for laboratories to implement, monitor, and control assays. For example, while some studies use a variety of methods to determine a cutoff value (COV), others rely solely on COVs determined in previous studies (8-13). Due to variations in technique related to antigen lot, environmental conditions, and operator, COVs generated by ELISA may vary from study to study. To minimize such discrepancies, control and standardization methods and multiple evaluation techniques can be used to determine an optimal ELISA COV for each study independently. This report describes a method for VLP ELISA optimization, standardization, and quality control.
MATERIALS AND METHODS
Serum samples.
A total of 109 (43 positive, 66 negative) serum samples were used as controls. Human serum samples were kindly provided by Mike Hagensee at Louisiana State University, by Howard Strickler at the National Cancer Institute (currently at the Albert Einstein School of Medicine), and by Egleston Children's Hospital (Atlanta, Ga.). Sera obtained from Louisiana State University and the National Cancer Institute were received along with an indication of their reactivity (positive or negative) to HPV type 16 (HPV-16) L1 VLPs as determined in the laboratory of origin. For negative sera obtained from Egleston Hospital, Western blotting and our ELISA were used to determine the reactivity of children's sera to HPV-16 L1 VLP antigen.
In addition, both preimmune rabbit sera and rabbit anti-HPV-11 L1-specific, anti-HPV-18 L1-specific, anti-HPV-31 L1-specific, and anti-HPV-16 L1-specific sera were kindly provided by Robert Rose, University of Rochester Medical Center (University of Rochester, Rochester N.Y.). Monoclonal antibodies (MAbs; kindly provided by Niel Christensen, University of Pennsylvania, Hershey) H16.V5 (anti-HPV-16) and H11.F1 (anti-HPV-11) and a commercially available MAb against HPV-16 from Biodesign International (Kennebunk, Maine) were also used for standardization of the assay and cross-reactivity studies (1).
VLP production.
HPV-16 VLPs were constructed by using the BacPAK baculovirus expression system (Clontech, Palo Alto, Calif.). Major structural protein L1 was cloned into the BacPAK system to generate a recombinant baculovirus expressing the L1 protein. The cloned L1-encoding gene originated from a viral sequence isolated from a CIN (cervical intraepithelial neoplasia) III lesion and was kindly provided by Dennis McCance (University of Rochester). DNA sequencing of the cloned insert was performed. The sequence is 100% identical to the HPV-16 L1-encoding gene of the reference sequence with GenBank accession no. U34179, AF125673, and K02718 over a 394-bp region spanning bp 6634 to 7028 of the genome map. For HPV-11 L1, a recombinant baculovirus construct was kindly provided by Robert Rose (6).
For HPV-16 VLP production, SF21cells were infected with recombinant baculovirus at a multiplicity of infection of 0.1 for 5 days. Infected insect cell cultures were harvested, and nuclei were lysed, releasing VLPs into culture supernatant. VLPs were purified by cesium chloride (CsCl) gradient centrifugation. Briefly, nuclear lysates were clarified at 3,000 × g and the VLPs were pelleted by centrifugation at 8,000 × g. The VLP pellet was resuspended in 4 ml of phosphate-buffered saline (PBS) and 2.7 g of CsCl (Ultrapure; catalog no. 5507UB; Life Technologies, Rockville, Md.) was added and mixed by vortexing. The volume was adjusted to 10 ml with PBS, and 5 ml was added to each 5-ml thin-walled centrifuge tube (UltraPlus Centrifuge Ware; Nalgene, Rochester, N.Y.). Samples were centrifuged at 243,356 × g for 48 h. The VLP bands were removed by using an 18-gauge needle inserted into the opening of the tube to just below the VLP band approximately 1.2 cm from the bottom of the tube. Extracted bands were dialyzed against PBS for 24 to 48 h with two changes of buffer by using Pierce dialysis slides (Slide-A-Lyzer unit; molecular weight cutoff, 10,000; Pierce, Rockford, Ill.). Analysis of the L1 protein was performed by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and Western blot analysis with MAbs H16.V5 and H11.F1, followed by protein assays (Pierce) to determine concentrations. Electron microscopy and ELISA were used to assess the structural integrity of VLPs. Once purified, VLP preparations were pooled to form a single lot of antigen, the protein concentration was determined, and the preparations were divided into aliquots at a concentration of 250 μg/ml and frozen at −70°C for use throughout the study.
ELISA.
A direct-coat ELISA provided optimal reproducibility and consistency and produced lower background readings and larger positive versus negative optical density (OD) ranges than did a capture ELISA (data not shown); therefore, the direct-coat ELISA was used throughout this study. In addition, PBS served as a better coating buffer for direct VLP coating than did carbonate buffer regarding the level of reactivity observed. A range of average absorbance values from triplicate runs of 16 interlaboratory control sera was 0.051 to 2.49 for PBS coating solution versus 0.049 to 0.729 for carbonate buffer coating solution. This difference in observed intensity may reflect a denaturing effect of the VLP's conformational epitope due to the use of carbonate buffer. Microtiter plates (Immulon II) were coated overnight at 4°C with VLP diluted to 0.1 μg/well (50 μl of a 2-μg/ml stock per well) in PBS. A range of 0.2 to 0.05 μg of VLP antigen per well was shown to be effective for coating, as indicated by discrimination between positive and negative control samples and MAb H16.V5. Absorbance levels at VLP coat concentrations of 0.1 and 0.2 μg/well were similar, resulting in the use of VLP at 0.1 μg/well to conserve the antigen while maintaining optimal levels of sensitivity. Plates were then washed three times with PBST (PBS-0.1% Tween 20) and blocked for 1 h with blocking solution (TBS [10 mM Tris, 0.15 M NaCl, pH 8.0] with 10% goat serum [Life Technologies], 50% SuperBlock [Pierce], and 0.5% Tween 20 [J. T. Baker, Phillipsburg, N.J.]) for immediate use or stored overnight for use within 24 h. After the blocking step, plates were washed three times with PBST using Ultrawash Plus (Dynex Corp., Chantilly, Va.). Serum or MAbs were diluted in TBS with 10% goat serum, 10% SuperBlock, and 10% insect cell lysate and incubated at 37°C for 1 h. Several dilutions of human sera were tested to determine an optimal dilution for the assay. The absorbance of many serum specimens remains at negative or background levels at a 1:100 dilution, while at a 1:20 dilution, values are well above the background. In an effort to increase sensitivity, a 1:20 dilution was chosen for the remainder of the study. Polyclonal rabbit antisera were used at 5 × 10−3 and 10−4 dilutions, and MAbs H16.V5 and H11.F1were used at 10−6, 5 × 10−3, and 10−4 dilutions as indicated. Plates were washed three times with PBST, and alkaline phosphatase conjugate was added in accordance with the manufacturer's (Boehringer Mannheim, Indianapolis, Ind.) recommendation. Following 2 h of incubation with conjugate at 37°C, plates were washed three times with PBST and 100 μl of substrate solution (Phosphatase Substrate; Sigma) was added to each well. Plate reactions were detected at 45 min by absorbance readings at 405 nm with an automated plate reader (Dynex MRX II Revelation; Dynex Corp.).
Quality control and analysis of data.
Initial assay standardization and reproducibility studies included triplicate wells for each of these controls on each plate. The assay was reduced to duplicate wells per sample after small standard deviations were observed. Inclusion of pooled and individual controls allowed monitoring of day-to-day and plate-to-plate variations. Lack of concordance among controls was used as a way to determine the need for replicate experiments (5, 7, 14). Criteria were set to either accept or fail any given day of ELISA testing. An acceptable range of absorbance values was determined as the mean for any particular control throughout the study plus or minus two times the standard deviation of the mean of that control (14). On any given day, if more than four of the control sera were outside of this acceptable range, the ELISAs for that day were classified as failed and test samples from that day were reanalyzed until the controls were within the acceptable range. In addition, wells without VLP coating were tested for ELISA reactivity to human sera with absorbance values of less than 0.1.
Three methods were used to determine an acceptable ELISA COV based on ELISA data generated from control sera. The first method consisted of histogram analysis of absorbance readings to observe the distribution of the control serum values. Negative control sera define a single distribution with a narrow absorbance range, whereas positive controls define a broader range distribution. The overlap between these two distributions indicates the region in which a COV will fall. After a COV was selected, the corresponding rates of false-positive or false-negative readings were evaluated. The second method (negative control method) used the average absorbance value of the negative control sera plus two or three times the standard deviations of those controls. This method was used when negative control samples were available for repeated analysis over the duration of the testing period.
The third, and perhaps the most stringent, method was based on the use of receiver operating characteristic (ROC) plots (15). The ROC plot is a graph of sensitivity (probability of true positivity) versus 1 − specificity (probability of false positivity). It displays estimated percentages of sensitivity and specificity at a specified COV selected from the entire range of observed results. We estimated sensitivity and specificity by using a logistic regression model in which the control sera were positive or negative according to the referring laboratory and the predictor was the log10 of the raw ODs. This procedure allows optimization of the assay with regard to COV determination to achieve desired levels of sensitivity and specificity.
RESULTS
HPV type specificity.
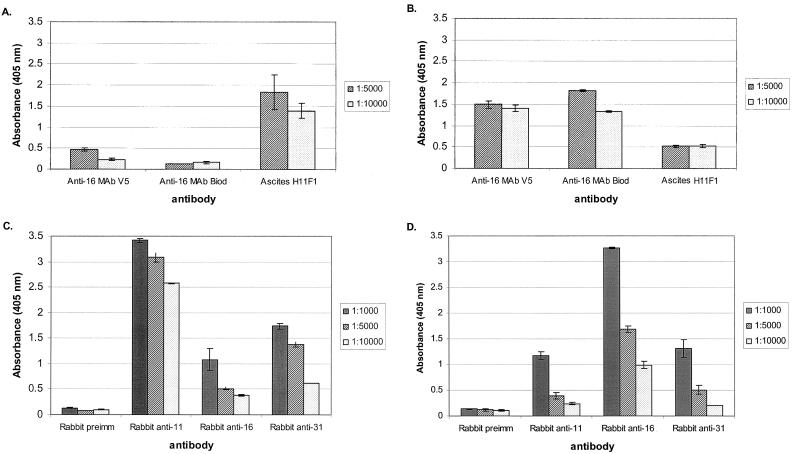
Type specificity of the ELISA was tested by using HPV-16 and HPV-11 VLPs reacted with rabbit preimmune serum and antiserum generated against HPV-11, HPV-16, and HPV-31 VLPs, as well as MAbs against HPV-11 and HPV-16 VLPs (Fig. 1). MAb V5, the anti-HPV-16 MAb from Biodesign International, and H11F1 ascites (anti-HPV-11) were tested by ELISA against HPV-11 (Fig. 1A) and HPV-16 (Fig. 1B) VLPs. Results showed a high type-specific reactivity against both HPV-11 and HPV-16 VLPs. However, some cross-reactivity between HPV-16 VLPs and ascites H11F1 was suggested by an absorbance value near 0.5 for both of the dilutions tested (Fig. 1B, last column set). These cross-reactivity levels are greater than twofold lower than the specific reactivity against HPV-16 VLPs (Fig. 1B).
FIG. 1.
ELISA analysis using HPV-11 and HPV-16 VLPs to determine cross-reactivity among designer antibodies. Anti-HPV-16 MAb V5, an anti-HPV-16 MAb from Biodesign International (Biod), and ascites H11F1 were tested against HPV-11 (A) and HPV-16 (B) VLPs. Rabbit preimmune (preimm), anti-HPV-11, anti-HPV-16, and anti-HPV-31 sera were tested against HPV-11 (C) and HPV-16 (D) VLPs.
Absorbance levels observed when using rabbit sera versus HPV-11 VLPs indicated type specificity since anti-HPV-11 sera produced values greater than twofold higher than those observed when anti-HPV-16 or anti-HPV-31 rabbit sera were used. However, the results suggest some cross-reactivity, particularly at lower dilutions, since the values appear to be substantially higher than those for preimmune rabbit control sera (Fig. 1C, first column set versus third and fourth column sets). This cross-reactivity was reduced by dilution to 1:10,000, suggesting that the observed cross-reactivity is dilution dependent, whereas dilutions of anti-HPV-11 sera to 1:10,000 failed to reduce reactivity substantially from that observed at a 1:1,000 dilution (Fig. 1C, second column set). Similarly, some cross-reactivity against HPV-16 VLPs versus rabbit sera was observed at lower dilutions, whereas type-specific reactivity remained greater than twofold higher for all dilutions (Fig. 1D). Levels resulting from anti-HPV-11 and anti-HPV-31 sera declined to levels similar to those of preimmune sera at a dilution of 1:10,000.
Assay reproducibility.
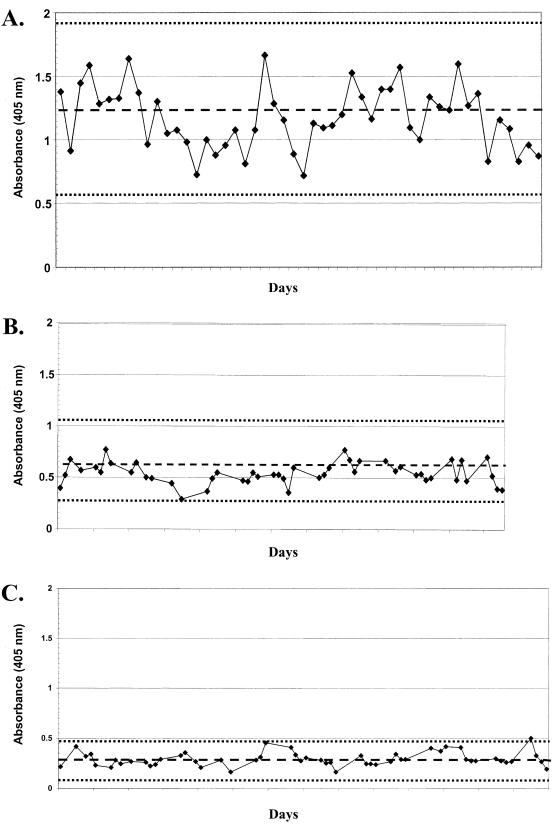
ELISA absorbance values for control sera were plotted daily, and values were observed for compliance with an established acceptable range (e.g., pooled control plots, Fig. 2A, B, and C; plots for individual controls are not shown). Daily assays were rejected when more than four of the control samples fell outside of the acceptable range (see Materials and Methods). On the basis of this strategy, repeat analysis was done on 4 (∼4%) of 90 test days.
FIG. 2.
Plots of the absorbance values of control sera obtained daily by ELISA against HPV-16 L1 VLP. Panels: A, high responder serum pool; B, intermediate responder serum pool; C, negative responder serum pool. The mean values (broken line) and acceptability range (dotted lines) are indicated.
Determination of a COV.
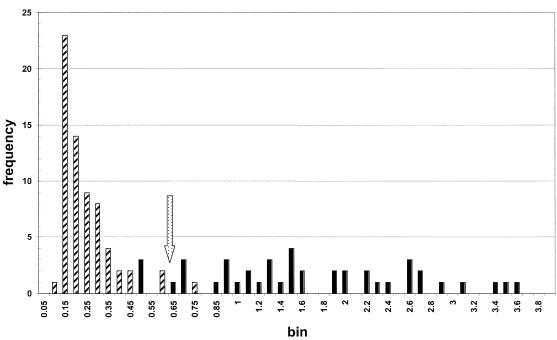
Plotting of control serum data by histogram analysis revealed the distributions associated with either the negative (n = 66) or the positive (n = 43) control sera tested throughout the study (Fig. 3). Negative control values are represented by absorbance values in a range of 0.1 to 0.75, while the distribution of positive control sera covered a range of 0.5 to 3.6. The distribution of the controls allowed estimation of a COV based on the area of overlap (0.5 to 0.75) in the OD readings between positive and negative controls. Estimation of a COV providing the lowest rate of both false-positive and false-negative readings (5 and 7%, respectively) yielded an OD value of 0.6.
FIG. 3.
Histogram plot of control serum test results obtained throughout the study. Hatched bars represent negative controls, and solid bars represent positive controls. bin, 0.05.
The second method of COV estimation used is based on the cumulative average for all of the negative controls used throughout the study and is referred to as a negative control method (see Materials and Methods). Considering the stringency required to avoid false-positive results and the approximated COV determined by histogram analysis (0.6), a COV can be derived by using the average value of the negative controls plus 3 times the standard deviation, resulting in an estimated COV of 0.616.
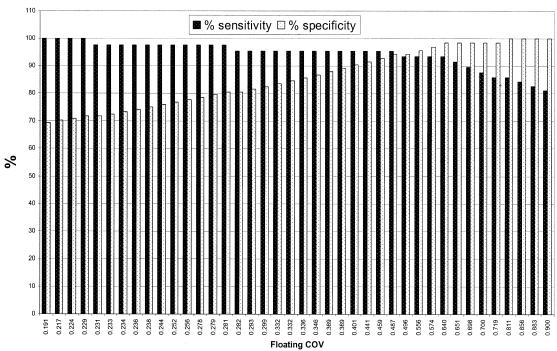
The third and most statistically based approach to COV determination was used to complement the histogram and negative control methods. ROC analysis of control sera was performed to plot the range of sensitivity and specificity obtained when using the ELISA as a discriminating tool (Fig. 4). Thus, with a floating COV scale, greater than 90% specificity and sensitivity can be observed when any absorbance value between 0.4 and 0.65 is chosen as a COV (Fig. 4). In consideration of the values observed when using the histogram method as well as the negative control method, a value can be determined from the ROC plot that correlates with these values while minimizing the rate of false-positive and false-negative readings in the assay. With this strategy, a COV of 0.64 was chosen to coincide with other methods of COV determination and maintain both high sensitivity (93%) and high specificity (98.5%).
FIG. 4.
Sensitivity (solid bars) and specificity (open bars) at various COVs based on a ROC plot of control sera.
DISCUSSION
We developed a protocol that uses a controlled ELISA for detection of HPV-16 antibodies in population-based human studies. While many reports have described the use of ELISAs for the detection of HPV-specific antibodies, few have detailed the validation and standardization of these assays. To define parameters for ELISA analysis of serum samples, we devised a protocol describing conditions found to be optimal for assay reproducibility and standardization. On the basis of an assay format previously described (3, 4), ELISA variables were tested and compared to determine optimal antigen-coating conditions, type specificity, serum dilutions, assay standardization, and, perhaps most significantly, COVs.
Wide acceptance of ELISA for the detection of HPV-specific antibodies has been slow to emerge for several reasons. Induction of specific immunity may be absent since no known viremic stage has been associated with HPV infection. Therefore, confirmation of positive or negative samples for use as controls is difficult. Further complicating the analysis is the inconsistency in determining COVs. In our study, COVs were calculated by using three methods in parallel: histogram plots, a negative control value, and ROC analysis. Similar methods have been used in several studies (8-13). These methods are based on the analysis of control serum raw absorbance levels observed by using the particular protocols at hand. The absorbance levels observed when control serum sets are used might vary depending on the specific protocol, reagents, and operator. This possibility suggests that each study requires the inclusion of positive and negative controls for standardization and validation of the assay and determination of a COV independent of those determined in prior studies. In addition, the use of children's sera to determine a COV based on the negative control method resulted in a value substantially lower than that observed when incorporating interlaboratory negative adult sera (0.450 for children's sera versus 0.694 for adult sera in this study). This result indicates that the use of children's sera alone may result in a COV that is not representative of an adult population. In fact, negative control serum absorbance values may be lower than the lowest values observed in the population being tested. To avoid selection of artificially low COVs, the use of multiple sources of negative controls (adults and children) and multiple methods of COV determination (to include positive controls) is recommended.
This study provides a detailed analytical strategy by which to ensure assay reproducibility over time and to determine an optimal COV for ELISA screening of serologic responses to HPV-16. On the basis of the inclusion of a panel of inter- and intralaboratory control sera, a strategy of assay control and COV determination was used to standardize the analysis. Daily variation was monitored closely by the inclusion of three pooled control sera and at least four individual control sera on each plate. This approach allows the compliance of all ELISAs with designated rejection criteria to ensure reproducibility over time (Fig. 1). In addition, the COV estimated by the negative control method (0.616) and an attempt to minimize the overlap between positive and negative controls observed in the histogram analysis (Fig. 2) suggests that a COV between 0.6 and 0.65 may be optimal, allowing overlap between positive and negative controls of less than 4%. ROC analysis of control sera further supports a COV range of 0.4 to 0.65 (Fig. 3). The highest achievable levels of both sensitivity (93%) and specificity (98.5%) suggested an optimal COV of 0.64.
The data presented here indicate several important parameters for performance of ELISA analysis of HPV exposure. First, the use of proper serum dilutions is required to capture the reactivity of a maximum number of the test sera. In our study, a 1:20 dilution was optimal for the capture of serum reactivity while maintaining low background absorbance. Second, multiple methods should be used in parallel to ensure a concurrence of COV estimates for each study individually to reduce the rates of false-positive or false-negative test results. In addition, the use of ROC curves provides a stringent analysis of the optimal COV based on the controls used in any given study. The use of the strategy described in this work may provide an approach to the standardization and optimization of ELISAs for HPV serologic studies.
Acknowledgments
We thank Suzanne Vernon for critical review of the manuscript, Charles Humphrey for expert electron microscopy analysis of purified VLPs, and John O'Connor for editorial review of the manuscript.
REFERENCES
- 1.Christensen, N. D., J. Dillner, C. Eklund, J. J. Carter, G. C. Wipf, C. A. Reed, N. M. Cladel, and D. A. Galloway. 1996. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 223:174-184. [DOI] [PubMed] [Google Scholar]
- 2.Ho, G. Y., R. Bierman, L. Beardsley, C. J. Chang, and R. D. Burk. 1998. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 338:423-428. [DOI] [PubMed] [Google Scholar]
- 3.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 89:12180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirnbauer, R., N. L. Hubbert, C. M. Wheeler, T. M. Becker, D. R. Lowy, and J. T. Schiller. 1994. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J. Natl. Cancer Inst. 86:494-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey, S., and E. R. Jennings. 1950. The use of control charts in the clinical laboratories. Am. J. Clin. Pathol. 20:1059-1066. [DOI] [PubMed] [Google Scholar]
- 6.Rose, R. C., W. Bonnez, R. C. Reichman, and R. L. Garcea. 1993. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of virus like particles. J. Virol. 67:1936-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shewhart, W. A. 1931. Economic control of quality of the manufactured product. Van Nostrand, New York, N.Y.
- 8.Strickler, H. D., J. Dillner, M. H. Schiffman, C. Eklund, A. G. Glass, C. Greer, D. R. Scott, M. E. Sherman, R. J. Kurman, and M. Manos. 1994. A seroepidemiologic study of HPV infection and incident cervical squamous intraepithelial lesions. Viral Immunol. 7:169-177. [DOI] [PubMed] [Google Scholar]
- 9.Strickler, H. D., A. Hildesheim, R. P. Viscidi, K. V. Shah, B. Goebel, J. Drummond, D. Waters, Y. Sun, N. L. Hubbert, S. Wacholder, L. A. Brinton, C. L. Han, P. C. Nasca, R. McClimens, K. Turk, V. Devairakkam, S. Leitman, C. Martin, and J. T. Schiller. 1997. Interlaboratory agreement among results of human papillomavirus type 16 enzyme-linked immunosorbent assays. J. Clin. Microbiol. 35:1751-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strickler, H. D., M. H. Schiffman, C. Eklund, A. G. Glass, D. R. Scott, M. E. Sherman, S. Wacholder, R. J. Kurman, M. M. Manos, J. T. Schiller, and J. Dillner. 1997. Evidence for at least two distinct groups of humoral immune reactions to papillomavirus antigens in women with squamous intraepithelial lesions. Cancer Epidemiol. Biomarkers Prev. 6:183-188. [PubMed] [Google Scholar]
- 11.Strickler, H. D., M. H. Schiffman, K. V. Shah, C. S. Rabkin, J. T. Schiller, S. Wacholder, B. Clayman, and R. P. Viscidi. 1998. A survey of human papillomavirus 16 antibodies in patients with epithelial cancers. Eur. J. Cancer Prev. 7:305-313. [DOI] [PubMed] [Google Scholar]
- 12.Strickler, H. D., G. D. Kirk, J. P. Figueroa, E. Ward, A. R. Braithwaite, C. Escoffery, J. Drummond, B. Goebel, D. Waters, R. McClimens, and A. Manns. 1999. HPV 16 antibody prevalence in Jamaica and the United States reflects differences in cervical cancer rates. Int. J. Cancer 80:339-344. [DOI] [PubMed] [Google Scholar]
- 13.Viscidi, R. P., K. L. Kotloff, B. Clayman, K. Russ, S. Shapiro, and K. V. Shah. 1997. Prevalence of antibodies to human papillomavirus (HPV) type 16 virus-like particles in relation to cervical HPV infection among college women. Clin. Diagn. Lab. Immunol. 4:122-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westgard, J. O., P. L. Barry, M. R. Hunt, and T. Groth. 1981. A multi-rule Shewhart chart for QC in clinical chemistry. Clin. Chem. 27:493-501. [PubMed] [Google Scholar]
- 15.Zweig, M. H., and G. Campbell. 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39:561-577. [PubMed] [Google Scholar]