Abstract
The correct localization of myosin II to the equatorial cortex is crucial for proper cell division. Here, we examine a collection of genes that cause defects in cytokinesis and reveal with live cell imaging two distinct phases of myosin II localization. Three genes in the rho1 signaling pathway, pebble (a Rho guanidine nucleotide exchange factor), rho1, and rho kinase, are required for the initial recruitment of myosin II to the equatorial cortex. This initial localization mechanism does not require F-actin or the two components of the centralspindlin complex, the mitotic kinesin pavarotti/MKLP1 and racGAP50c/CYK-4. However, F-actin, the centralspindlin complex, formin (diaphanous), and profilin (chickadee) are required to stably maintain myosin II at the furrow. In the absence of these latter genes, myosin II delocalizes from the equatorial cortex and undergoes highly dynamic appearances and disappearances around the entire cell cortex, sometimes associated with abnormal contractions or blebbing. Our findings support a model in which a rho kinase-dependent event, possibly myosin II regulatory light chain phosphorylation, is required for the initial recruitment to the furrow, whereas the assembly of parallel, unbranched actin filaments, generated by formin-mediated actin nucleation, is required for maintaining myosin II exclusively at the equatorial cortex.
Keywords: contractile ring, rho1
During cytokinesis in eukaryotic cells, nonmuscle myosin II (hereafter called myosin II) accumulates in the cleavage furrow, where it assembles with F-actin into a contractile ring and provides the force for constricting the midzone of the cell. The mitotic spindle is crucial in determining the placement of the cleavage furrow during cytokinesis. In particular, the central spindle, a bundled microtubule structure that forms during anaphase, seems to direct the formation and/or maintenance of the furrow in most animal cells (for review, see refs. 1 and 2). The formation of the central spindle depends on the microtubule bundling protein complex centralspindlin, which contains two proteins: the mitotic kinesin pavarotti/MKLP1 (3, 4) and racGAP50c/CYK-4 (5). Recent studies have illuminated how the spindle may direct F-actin remodeling at the furrow. F-actin remodeling at the cleavage furrow is stimulated by the activation of a small GTPase rho1/rhoA by the pebble/ect2 guanidine nucleotide exchange factor (6-8). When bound to GTP, rho1 binds and activates diaphanous/mDia1, which is also localized at the cleavage furrow (9, 10). Diaphanous is a formin protein that contains an FH1 and FH2 domain. These domains have been shown to directly nucleate F-actin filament formation and, in conjunction with profilin, another protein essential for cytokinesis in many organisms (11, 12), result in the formation of unbranched F-actin filaments (13-15). How the spindle directs pebble localization and stimulation is unclear, but recent data showing that pebble forms a complex with racGAP50c points to a possible mechanism (16). Indeed, it has been hypothesized that the centralspindlin complex provides the major furrow positioning signal in Drosophila (1).
Few studies have characterized the recruitment of myosin II to the furrow, although growing evidence suggests that myosin II localizes to the cleavage furrow independently of F-actin. Myosin II accumulation precedes accumulation of F-actin in the furrow in many cells (17-19). Furthermore, myosin II remains in the furrow after depletion of F-actin in echinoderm eggs (20), fission yeast (21), and budding yeast cells (22). Perhaps most striking, C-terminal tail domains of myosin II that completely lack the actin binding N-terminal head domain still localize to the cell midzone in both Dictyostelium cells (23-25) and fission yeast (26).
Functional myosin II molecules are hexameric proteins containing two heavy chains, two essential light chains, and two regulatory light chains (RLCs). Rho1 activates rho kinase and citron kinase, both of which phosphorylate and activate myosin II RLC (27-29). However, nonphosphorylatable mutants of the Drosophila myosin II RLC are still correctly localized in egg chambers (30). Thus, it is unclear whether rho1 signaling affects myosin II localization directly or through its effect on the F-actin network. Here, we show that rho1 signaling is indeed required for the recruitment of myosin II to the cleavage furrow of Drosophila S2 cells through an F-actin-independent process. Using real-time imaging of GFP-tagged myosin, we also show that F-actin and F-actin nucleating and regulatory proteins, including the formin diaphanous, are needed to stably maintain myosin II at the midzone.
Materials and Methods
Plasmid Construction and Cell Culture. Plasmid pUbp-mRFP-α tubulin 84b was created as follows. Monomeric red fluorescent protein (mRFP)-α tubulin 84B was amplified from pMT-mRFP-α tubulin, digested with NheI and SacII, and ligated into pUbp-EGFP, a modified version of pEGFP-C1 (Clontech) in which the CMV promoter has been replaced with the Drosophila ubiquitin promoter.
Drosophila S2 cells were maintained on plates in Schneider's Drosophila Media (GIBCO) supplemented with 10% FBS at 27°C. The RLC-GFP stable cell line was created as described in ref. 31. Stable S2 cell lines expressing both RLC-GFP and mRFP-α tubulin were created by transfection of the RLC-GFP stable line with pUbp-mRFP-α tubulin by using Cellfectin Reagent (Invitrogen) and selection with 1 mg/ml G418 (Life Technologies).
RNA Interference (RNAi). Approximately 5 × 105 cells were plated in each well of a 24-well tissue culture plate. Each well was treated with 5-10 μg of the appropriate double-stranded RNA as described in ref. 32 for 5-6 days before fixation and staining or live cell imaging.
Immunofluorescence and Microscopy. For fixed cell imaging, cells were transferred to 22-mm clean glass coverslips, fixed in 3% formaldehyde, and permeablized with 1% Triton X-100. For drug treatment experiments, cells were incubated on coverslips with 20 μM Latrunculin A (Molecular Probes) for 30 min before fixation. Fixed cells were incubated with 1:300 dilution of DM1α monoclonal α-tubulin antibody (Sigma), washed, and incubated with 1:1,000 Rhodamine Red X-conjugated goat anti-mouse antibody (Jackson ImmunoResearch)/1 μg/ml DAPI (Molecular Probes)/165 nM AlexaFluor 633-phalloidin (Molecular Probes). For live cell imaging, cells were transferred to imaging chambers (Applied Scientific) in Schneider's Drosophila media. Cells were imaged on an Axiovert 200 inverted epifluorescence microscope equipped with a ×100 objective lens.
Immunoblotting. Cells were lysed and protein levels were quantitated by using Bradford Reagent (Bio-Rad). A dilution series of control cell lysates as well as RNAi'd cell lysates were loaded on each gel, separated by PAGE, and transferred to nitrocellulose membranes. The membranes were blocked and then incubated with the primary antibodies diluted as follows: 1:100 α-rho1 (33) (Developmental Studies Hybridoma Bank, Iowa City, IA), 1:1,000 α-pebble (6), 1:60,000 α-diaphanous (9) 1:20 α-chickadee (34) (Developmental Studies Hybridoma Bank), 1:2,000 α-racGAP50c (16), 1:1,000 α-zipper (30), and 1:1,000 α-pavarotti (3). Membranes were washed and incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (Pierce). Signals were detected by ECL (Amersham Pharmacia Biosciences).
Results
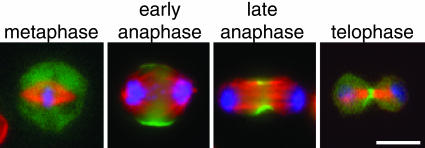
Myosin II RLC-GFP Reveals Myosin II Localization Through Mitosis and Cytokinesis. To follow myosin II localization throughout cell division, we used a stable S2 cell line expressing the regulatory light chain of nonmuscle myosin II fused to GFP (RLC-GFP) (31). To correlate myosin II localization with the stages of cell division, we fixed the RLC-GFP-expressing cells and stained for DNA and microtubules. In fixed cells, myosin II is diffusely localized in the cytoplasm through metaphase (Fig. 1). At early anaphase, myosin II is recruited to the cortex as a broad equatorial band and is excluded from the poles. At later stages of cell division when the furrow is further contracted, myosin II is concentrated at a narrower equatorial band. Finally, at the late stages of cytokinesis, myosin II is found at the center of the midbody.
Fig. 1.
Myosin II regulatory light chain-GFP (RLC-GFP) reveals the localization of myosin II during cell division. S2 cells expressing RLC-GFP (green) were fixed and stained for DNA (blue) and α-tubulin (red). RLC-GFP was found in the cytoplasm in metaphase cells but was localized to the midzone cortex at anaphase and through telophase/cytokinesis. (Scale bar: 5 μm.).
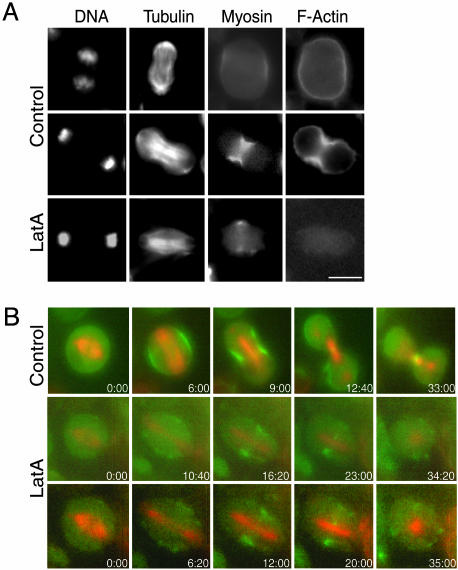
F-Actin Is Not Required for the Recruitment of Myosin II to the Cleavage Furrow. We investigated the role of F-actin in localization of myosin II to the cleavage furrow. First, S2 cells expressing RLC-GFP were fixed and stained for DNA, microtubules, and F-actin. F-actin was found in the cortex of cells in all stages of cell division but was only visibly enriched at the equator of cells that had begun to contract significantly before fixation (Fig. 2A). However, myosin II was found at the equatorial cortex of cells in early anaphase before any contraction was visible (Figs. 1 and 2 A). Thus, myosin II accumulation at the furrow precedes F-actin enrichment. Treatment of S2 cells with 20 μM Latrunculin A (LatA) for 30 min before fixation completely disrupted actin filaments as measured by staining with fluorescent phalloidin. Myosin II, however, was found at the midzone of the anaphase/telophase cells treated with LatA (Fig. 2 A). These fixed cell results suggest that F-actin is not required for the localization of myosin II to the equatorial region of the cell destined to become a cleavage furrow.
Fig. 2.
F-actin is not required for recruitment of myosin II to the cleavage furrow. (A) S2 cells stably expressing RLC-GFP were treated with DMSO only (Top and Middle) or 20 μM LatA to depolymerize F-actin (Bottom) and then fixed and stained for F-actin, DNA, and α-tubulin. Control cells in early anaphase (Top) show that RLC-GFP is enriched at the midzone cortex earlier than F-actin. Cells treated with LatA (Bottom) did not show F-actin staining, but myosin II is still enriched at the equator. (B) Time-lapse epifluorescence microscopy was used to follow S2 cells stably expressing both RLC-GFP (green) and mRFP-α-tubulin (red) as they transitioned into anaphase. In control cells (Top) RLC-GFP was localized normally at the midzone equator through the final stages of cytokinesis. In cells treated with 20 μM LatA (Middle and Bottom) myosin II is still recruited to the midzone, but it disperses into patches in the membrane after many minutes. (Scale bar: 5 μm.).
To examine the effect of F-actin disassembly in more detail, we followed the initial recruitment of myosin II by live cell imaging. For this purpose, a stable S2 cell line expressing both RLC-GFP and mRFP-α-tubulin (35) was prepared. mRFP-α-tubulin in these cells showed a similar distribution to anti-α-tubulin antibodies in fixed cells, and the presence of the mRFP moiety did not appear to perturb mitosis or cytokinesis. In addition, it is clear from the live cells that myosin II remains at the midbody connecting the daughter cells for many hours until they finally complete abscission (Fig. 2B; see also Movie 1, which is published as supporting information on the PNAS web site). RLC-GFP and mRFP-α-tubulin expressing cells were treated with LatA, and metaphase cells were filmed as they transitioned into anaphase. All eight cells filmed after treatment with LatA failed to contract as they transitioned into anaphase or telophase, but myosin II was still recruited initially to the equatorial cortex (Fig. 2B; see also Movie 2, which is published as supporting information on the PNAS web site). This localization, however, was not stable. RLC-GFP was found at the equator for an average of 19 min (± 3 min, n = 6 cells) before it became dispersed in patches around the cell cortex. Thus, F-actin is not required for the initial recruitment of myosin II to the equatorial cortex, but it is required for the maintenance of myosin II in the furrow region thereafter.
Essential Cytokinesis Genes Have Different Effects on Myosin II Localization and Furrow Contraction. Drosophila S2 cells depleted of myosin II cannot undergo cytokinesis (36-38). Therefore, genes necessary for proper myosin II localization to the furrow should also fail at cytokinesis. Thus, we treated RLC-GFP-expressing S2 cells with double-stranded RNAs for eight genes shown in previous studies to be required for cytokinesis and scored them for myosin II localization in fixed cells. RNAi of all eight genes resulted in a penetrant (>10%), reproducible multinucleate phenotype indicative of a cytokinesis defect (Table 1). In addition, depletion of all eight of the genes caused diffuse myosin II localization, a poorly formed central spindle, and little or no furrow contraction in fixed cells (Table 1; see also Fig. 6, which is published as supporting information on the PNAS web site).
Table 1. RNAi reveals genes essential for myosin II localization.
| Gene RNAi'd | FlyBase ID | Protein function | Fraction binucleate cells | Myosin localized: fixed cells | Myosin recruited: live cells | Myosin retained: live cells | Furrow contraction: live cells |
|---|---|---|---|---|---|---|---|
| pebble | FBgn0003041 | Rho1 GTP exchange factor | 0.44 | — | — | — | — |
| rho kinase | FBgn0026181 | Kinase; activation of myosin light chain | 0.33 | — | — | — | — |
| rho 1 | FBgn0014020 | Small GTPase | 0.38 | — | — | — | — |
| zipper | FBgn0005634 | Nonmuscle myosin II heavy chain | 0.33 | — | — | — | — |
| actin | FBgn0000042 | Structural component of cytoskeleton | 0.46 | +* | —* | —* | |
| pavarotti | FBgn0011692 | Mitotic kinesin | 0.49 | — | + | — | — |
| racGAP50c | FBgn0033881 | G-protein inactivation | 0.21 | — | + | — | — |
| chickadee | FBgn0000308 | Profilin (actin polymerization) | 0.41 | — | + | — | + |
| diaphanous | FBgn0011202 | Formin (actin polymerization) | 0.51 | — | + | — | + |
Live cell data is from cells treated with LatA to depolymerize actin filaments, not from cells in which actin is RNAi depleted.
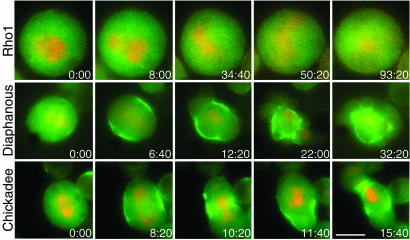
To better understand the failure of myosin II localization, we observed live RLC-GFP and mRFP-α-tubulin-expressing cells after RNAi treatment for 5 days. Antibodies were available for seven of the eight genes assayed, and we verified the efficacy of RNAi in this cell line by Western blot. All seven were depleted to <10% of control levels (data not shown). When rho1, pebble, rho kinase, or the myosin II heavy chain were depleted, RLC-GFP did not localize to the cortex or the midzone, and the cells did not contract in 10 of 10, 4 of 5, 6 of 8, and 7 of 7 cells filmed, respectively (Table 1 and Fig. 3; see also Movies 3 and 4, which are published as supporting information on the PNAS web site).
Fig. 3.
Live cell imaging reveals that some genes are required for recruitment of RLC-GFP to the equatorial cortex and others only for its retention there. An S2 cell line stably expressing both RLC-GFP (green) and mRFP-α-tubulin (red) was treated with double-stranded RNAs for genes that showed an effect on myosin II localization in fixed cells, and then epifluorescence microscopy was used to determine RLC-GFP localization in cells as they transitioned out of metaphase. Cells depleted of rho1 (Top) did not contract and showed no RLC-GFP localization or central spindle formation. RNAi of rho kinase, pebble, and the myosin II heavy chain showed similar phenotypes (data not shown). Cells depleted of diaphanous (Middle) contract after myosin II becomes localized to the equatorial cortex, but localization is transient, and because myosin II disperses into patches about the cortex, those regions of the cortex contract. Chickadee-depleted cells (Bottom) show a similar phenotype. (Scale bar: 5 μm.).
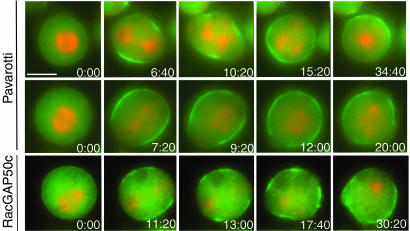
Surprisingly, in contrast to what we inferred from our more limited fixed cell data, live cell imaging revealed that myosin II was recruited normally to the cortical equatorial band at anaphase when pavarotti, racGAP50c, diaphanous, or chickadee were depleted (Table 1 and Figs. 3 and 4). As in untreated cells, myosin II first appeared as a broad band at the midzone equator that was excluded from the poles. However, this localization was transient, and the myosin II subsequently dispersed in patches throughout the cell cortex. Moreover, these patch-like accumulations of myosin II on the cortex were themselves transient and either moved or were continually formed and reformed in new locations in the cell cortex. Thus, live cell imaging clearly shows that myosin II is at least transiently localized to the midzone equator when these proteins are depleted. However, like F-actin itself, these genes are essential for retaining myosin II at the cell midzone.
Fig. 4.
Cells depleted of pavarotti or racGAP50c recruit myosin II to the equatorial cortex but do not contract significantly. Cells depleted of pavarotti (Top and Middle) or racGAP50c (Bottom) did not exhibit significant equatorial contraction. However, they did show initial recruitment of RLC-GFP (green) to a broad equatorial cortical band. This band then dispersed in patches throughout the cortex. (Scale bar: 5 μm.).
Although the dynamic movement of myosin II around the cortex was similar for RNAi of pavarotti, racGAP50c, diaphanous, and chickadee, the contractile response of the cells to this mislocalization differed. Cells depleted of diaphanous (seven of eight cells) and chickadee (four of four cells) displayed significant contractions as myosin II was first recruited into the broad cortical equatorial band and then moved throughout the cortex (Table 1 and Fig. 3; see also Movies 5 and 6, which are published as supporting information on the PNAS web site). Although cells depleted of pavarotti or its binding partner racGAP50c showed some membrane contractile activity, they did not contract significantly despite the transient localization of myosin II (Table 1 and Fig. 4; see also Movies 7 and 8, which are published as supporting information on the PNAS web site). Because cells depleted of pavarotti or racGAP50c rarely displayed contractions, only the earliest stage of myosin II localization is apparent: the initial recruitment of a broad band at the equator and exclusion at the poles. This initial transient localization, but failure to contract, was seen in seven of eight cells depleted of pavarotti and five of eight cells depleted of racGAP50c, whereas the remaining cells showed some contraction.
Discussion
The results reported here provide insight into the molecular events involved in myosin II localization and activation during cytokinesis. Using live cell imaging of myosin II, we have discovered three steps in the myosin II localization/activation process that involve distinct groups of genes: (i) an initial recruitment of myosin II to the equatorial cortex that is independent of F-actin and centralspindlin but requires rho1 signaling, (ii) a secondary stabilization of myosin II at the midzone that requires F-actin and a second set of genes that are likely involved in building a specific type of actin network, and (iii) the activation of furrowing once myosin II is localized that depends on centralspindlin.
Initial Recruitment of Myosin II to the Equatorial Cortex. Rho1, its activating guanidine nucleotide exchange factor pebble, and rho kinase are each required for the initial recruitment of myosin II to the equatorial cortex. Rho1 has been implicated in two pathways that are important for cytokinesis (Fig. 5A). In the first pathway, rho1 signals to F-actin through the formin diaphanous. However, we found that proteins on this F-actin pathway, including F-actin itself, are not essential for the initial myosin II recruitment to the equatorial cortex. However, we found that rho kinase, another downstream target of rho1, is essential. Because rho kinase phosphorylates the myosin II RLC (Fig. 5A), it is possible that phosphorylation of the RLC is essential for myosin II recruitment to the furrow. We were unable to directly test this hypothesis, because the myosin II heavy chain formed large aggregates when the RLC was depleted by RNAi (data not shown).
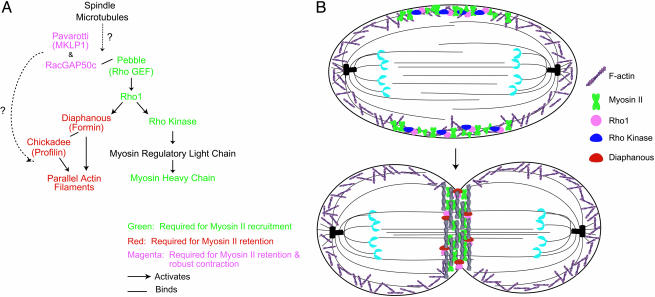
Fig. 5.
Myosin II localization to the equatorial cortex involves two steps, recruitment and retention, that have different molecular determinants. (A) Schematic of known proteins involved in cytokinesis, their relationships to one another, and their effect on myosin II localization. Proteins that are essential for myosin II recruitment are marked in green, those necessary for myosin II retention in the equatorial cortex are marked in red, and those essential for both myosin II retention and significant contraction are indicated in magenta. In black are proteins not assayed for their effect on myosin II localization. The two signaling pathways downstream of rho1 have different effects on myosin II localization. The pathway that leads to F-actin stimulation is necessary only for retention, whereas the pathway that leads to RLC phosphorylation is necessary for the initial recruitment of myosin II. (B) Model for the recruitment and retention of myosin II to the cleavage furrow. Localized activation of rho kinase by rho1 is necessary for the initial recruitment of myosin II to the equatorial cortex at anaphase. At later stages, rho1-activated diaphanous, in conjunction with chickadee, mediates the assembly of parallel F-actin filament structures that are necessary for the retention of myosin II in the equatorial cortex.
Phosphorylation of the RLC both activates the motor domain and, in some myosins, increases bipolar thick filament formation (39). Because F-actin is not required for myosin II recruitment, activation of the motor is unlikely to be the mechanism by which phosphorylation of the RLC would cause recruitment of myosin II to the equatorial cortex. It is quite possible, however, that the rho kinase-mediated myosin II phosphorylation leads to thick filament assembly and that this assembly is important for localization of myosin to the equatorial cortex. Indeed, in Dictyostelium, it is clear that bipolar thick filament formation is sufficient for myosin II localization to the midzone of a mitotic cell (23, 25). The nonactin-based mechanism of recruitment of myosin II filaments remains unknown.
Maintenance of Myosin II at the Contractile Ring. In contrast to the lack of F-actin involvement in the early recruitment of myosin II to the equatorial cortex at anaphase, F-actin disruption by LatA results in a failure to maintain myosin II in the equatorial region. Interestingly, the downstream rho1 effectors diaphanous/formin and chickadee/profilin are also necessary for myosin II maintenance at the equatorial midzone. Although the loss of these genes could deplete F-actin, phalloidin staining showed that F-actin is still present in all of our RNAi-treated cells (data not shown). In addition, these RNAi-treated cells still contract, unlike when F-actin is completely disrupted with LatA. Thus, myosin II appears to be interacting with F-actin in the cortex as it disperses in dynamic patches throughout the cortex of these diaphanous- or chickadee-depleted cells.
We suggest that the role of diaphanous/formin and chickadee/profilin in maintaining the myosin II contractile ring is through the creation of specific F-actin structures. In particular, formin- and profilin-mediated nucleation results in unbranched actin filaments (40) because profilin promotes the barbed-end growth of formin-capped actin filaments (15, 41). Indeed, electron microscopy has shown that F-actin in the cleavage furrow mainly consists of unbranched, bundled filaments (42-44). These parallel filaments contrast with Arp2/3-mediated nucleation, which creates a highly branched actin filament network. Indeed, Arp2/3, although essential for lamellipodia formation, is not required for cytokinesis in Drosophila cells (37, 38, 45). Our hypothesis, diagrammed in Fig. 5B, is that once myosin II is recruited to the equatorial cortex of the cell by a rho kinase-dependent mechanism, possibly localized activation of RLC phosphorylation, it is retained there because of its higher affinity for parallel, unbranched actin filaments than to branched actin networks. Consistent with our hypothesis, myosin II is depleted from the lamellipodia in migrating cells where Arp2/3 is localized and branched F-actin networks are formed (46) but is enriched in the lamella where F-actin filaments are more likely to be aligned in parallel bundles (47). Thus, we propose that high rho1 signaling to diaphanous at the cleavage furrow maintains a higher concentration of parallel actin filaments in this region compared with the rest of the cortex, and these parallel filaments serve to selectively retain myosin II at the equator to form a stable contractile ring. In the absence of these parallel actin filaments, myosin II can bind branched F-actin throughout the cortex, perhaps occasionally organizing them into parallel bundles that cause increased myosin recruitment corresponding to the flashes of cortical myosin accumulation, but these interactions are unstable.
Contraction of Actomyosin Structures During Cell Division and the Role of Centralspindlin. Live-cell imaging shows that when pavarotti or racGAP50c are depleted, the cells do not display significant contractions despite recruiting myosin II to the equatorial cortex. Although there is some modest membrane contractile activity in these cells, it is clear that significant contraction or furrowing requires both components of the centralspindlin complex. It is surprising that only these proteins were found to be necessary for cortical contraction at sites of myosin II localization. Our data from fixed cells, as well as earlier studies, indicated that Drosophila cells do not undergo equatorial contractions during mitosis when diaphanous or chickadee is depleted (12). However, our live-cell imaging shows that when either of these two genes is depleted in S2 cells, not only is myosin II transiently localized to the equatorial cortex before dispersing, but cells do indeed display transient equatorial contraction. It is difficult to recognize these events in fixed cells because of their transient nature and the somewhat irregular shapes of cells depleted of these proteins. This work highlights the importance of live-cell imaging in the study of dynamic processes such as cytokinesis.
In addition to the suppression of furrowing, depletion of centralspindlin also led to an inability to retain F-actin exclusively at the equatorial cortex during cytokinesis. This similar phenotype of the centralspindlin complex and the F-actin affecting proteins suggests that centralspindlin may be an upstream regulator of F-actin filament formation. Indeed kinase-dead mutants of pavarotti have been shown to accumulate at the spindle poles and are associated with an abnormal accumulation of F-actin near the centrosomes (48). Centralspindlin may be acting indirectly by helping to localize an important actin-affecting protein at the central spindle, or it may act more directly on the cortex. Because racGAP50c has been shown to bind pebble in vitro (16) (Fig. 5A), it has been hypothesized that centralspindlin affects the F-actin cortex through rho1 signaling by the localization and/or activation of pebble (1, 16). However, racGAP50c depletion does not lead to a lack of myosin II recruitment as does pebble or rho1 depletion, and, thus, centralspindlin must act in a rho1-independent manner (Fig. 5A). For instance, the racGAP activity of centralspindlin may itself be important for signaling to the F-actin cortex. Finally, centralspindlin cannot be the major actomyosin ring positioning signal because myosin II is properly recruited in its absence.
Supplementary Material
Acknowledgments
We thank Patrick Heun and Gary Karpen (Lawrence Berkeley National Laboratory, Berkeley, CA) for the pMT-mRFP-α-tubulin 84B plasmid; Aaron Straight (Stanford University) for the pUbp-EGFP plasmid; and Robert Saint (Australian National University, Canberra, Australia), David Glover (Cambridge University, Cambridge, U.K.), Roger Karess (Centre de Genetique Moleculaire, Gif-sur-Yvette, France), Hugo Bellen (Baylor University, Waco, TX), Steve Wasserman (University of California at San Diego, La Jolla), Dan Kiehart (Duke University, Durham, NC), and Tsien Hsu (Medical University of South Carolina, Charleston, SC) for kindly providing antibodies. This work was supported by the National Institutes of Health Cell and Molecular Biology Training Grant and Genome Training Grant HG00044 (to S.O.D.), National Institutes of Health Grant GM46551 (to J.A.S.), and the Howard Hughes Medical Institute, Sandler Program in Basic Sciences, and National Institutes of Health Grant 38499 (to R.D.V.).
Abbreviations: LatA, latrunculin A; mRFP, monomeric red fluorescent protein; RLC, myosin II regulatory light chain; RNAi, RNA interference.
References
- 1.D'Avino, P. P., Savoian, M. S. & Glover, D. M. (2005) J. Cell Sci. 118, 1549-1558. [DOI] [PubMed] [Google Scholar]
- 2.Glotzer, M. (2004) J. Cell Biol. 164, 347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams, R. R., Tavares, A. A., Salzberg, A., Bellen, H. J. & Glover, D. M. (1998) Genes Dev. 12, 1483-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishima, M., Kaitna, S. & Glotzer, M. (2002) Dev. Cell. 2, 41-54. [DOI] [PubMed] [Google Scholar]
- 5.Jantsch-Plunger, V., Gonczy, P., Romano, A., Schnabel, H., Hamill, D., Schnabel, R., Hyman, A. A. & Glotzer, M. (2000) J. Cell Biol. 149, 1391-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prokopenko, S. N., Brumby, A., O'Keefe, L., Prior, L., He, Y., Saint, R. & Bellen, H. J. (1999) Genes Dev. 13, 2301-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madaule, P., Eda, M., Watanabe, N., Fujisawa, K., Matsuoka, T., Bito, H., Ishizaki, T. & Narumiya, S. (1998) Nature 394, 491-494. [DOI] [PubMed] [Google Scholar]
- 8.Bement, W. M., Benink, H. A. & von Dassow, G. (2005) J. Cell Biol. 170, 91-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afshar, K., Stuart, B. & Wasserman, S. A. (2000) Development (Cambridge, U.K.) 127, 1887-1897. [DOI] [PubMed] [Google Scholar]
- 10.Kato, T., Watanabe, N., Morishima, Y., Fujita, A., Ishizaki, T. & Narumiya, S. (2001) J. Cell Sci. 114, 775-784. [DOI] [PubMed] [Google Scholar]
- 11.Severson, A. F., Baillie, D. L. & Bowerman, B. (2002) Curr. Biol. 12, 2066-2075. [DOI] [PubMed] [Google Scholar]
- 12.Giansanti, M. G., Bonaccorsi, S., Williams, B., Williams, E. V., Santolamazza, C., Goldberg, M. L. & Gatti, M. (1998) Genes Dev. 12, 396-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pruyne, D., Evangelista, M., Yang, C., Bi, E., Zigmond, S., Bretscher, A. & Boone, C. (2002) Science 297, 612-615. [DOI] [PubMed] [Google Scholar]
- 14.Sagot, I., Rodal, A. A., Moseley, J., Goode, B. L. & Pellman, D. (2002) Nat. Cell Biol. 4, 626-631. [DOI] [PubMed] [Google Scholar]
- 15.Kovar, D. R., Kuhn, J. R., Tichy, A. L. & Pollard, T. D. (2003) J. Cell Biol. 161, 875-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somers, W. G. & Saint, R. (2003) Dev. Cell 4, 29-39. [DOI] [PubMed] [Google Scholar]
- 17.Lippincott, J. & Li, R. (1998) J. Cell Biol. 140, 355-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motegi, F., Nakano, K. & Mabuchi, I. (2000) J. Cell Sci. 113, 1813-1825. [DOI] [PubMed] [Google Scholar]
- 19.Noguchi, T. & Mabuchi, I. (2001) J. Cell Sci. 114, 401-412. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder, T. E. & Otto, J. J. (1988) Zool. Sci. 5, 713-725. [Google Scholar]
- 21.Naqvi, N. I., Eng, K., Gould, K. L. & Balasubramanian, M. K. (1999) EMBO J. 18, 854-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bi, E., Maddox, P., Lew, D. J., Salmon, E. D., McMillan, J. N., Yeh, E. & Pringle, J. R. (1998) J. Cell Biol. 142, 1301-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zang, J.-H. & Spudich, J. A. (1998) Proc. Natl. Acad. Sci. USA 95, 13652-13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shu, S., Liu, X. & Korn, E. D. (2003) Proc. Natl. Acad. Sci. USA 100, 6499-6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hostetter, D., Rice, S., Dean, S., Altman, D., McMahon, P. M., Sutton, S., Tripathy, A. & Spudich, J. A. (2004) PLoS Biol. 2, e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motegi, F., Mishra, M., Balasubramanian, M. K. & Mabuchi, I. (2004) J. Cell Biol. 165, 685-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishizaki, T., Maekawa, M., Fujisawa, K., Okawa, K., Iwamatsu, A., Fujita, A., Watanabe, N., Saito, Y., Kakizuka, A., Morii, N. & Narumiya, S. (1996) EMBO J. 15, 1885-1893. [PMC free article] [PubMed] [Google Scholar]
- 28.Mizuno, T., Amano, M., Kaibuchi, K. & Nishida, Y. (1999) Gene 238, 437-444. [DOI] [PubMed] [Google Scholar]
- 29.Yamashiro, S., Totsukawa, G., Yamakita, Y., Sasaki, Y., Madaule, P., Ishizaki, T., Narumiya, S. & Matsumura, F. (2003) Mol. Biol. Cell 14, 1745-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jordan, P. & Karess, R. (1997) J. Cell Biol. 139, 1805-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers, S. L., Wiedemann, U., Hacker, U., Turck, C. & Vale, R. D. (2004) Curr. Biol. 14, 1827-1833. [DOI] [PubMed] [Google Scholar]
- 32.Clemens, J. C., Worby, C. A., Simonson-Leff, N., Muda, M., Maehama, T., Hemmings, B. A. & Dixon, J. E. (2000) Proc. Natl. Acad. Sci. USA 97, 6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magie, C. R., Pinto-Santini, D. & Parkhurst, S. M. (2002) Development (Cambridge, U.K.) 129, 3771-3782. [DOI] [PubMed] [Google Scholar]
- 34.Verheyen, E. M. & Cooley, L. (1994) Development (Cambridge, U.K.) 120, 717-728. [DOI] [PubMed] [Google Scholar]
- 35.Campbell, R. E., Tour, O., Palmer, A. E., Steinbach, P. A., Baird, G. S., Zacharias, D. A. & Tsien, R. Y. (2002) Proc. Natl. Acad. Sci. USA 99, 7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Somma, M. P., Fasulo, B., Cenci, G., Cundari, E. & Gatti, M. (2002) Mol. Biol. Cell 13, 2448-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Echard, A., Hickson, G. R., Foley, E. & O'Farrell, P. H. (2004) Curr. Biol. 14, 1685-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eggert, U. S., Kiger, A. A., Richter, C., Perlman, Z. E., Perrimon, N., Mitchison, T. J. & Field, C. M. (2004) PLoS Biol. 2, e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scholey, J. M., Taylor, K. A. & Kendrick-Jones, J. (1980) Nature 287, 233-235. [DOI] [PubMed] [Google Scholar]
- 40.Wallar, B. J. & Alberts, A. S. (2003) Trends Cell Biol. 13, 435-446. [DOI] [PubMed] [Google Scholar]
- 41.Kovar, D. R., Wu, J. Q. & Pollard, T. D. (2005) Mol. Biol. Cell 16, 2313-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schroeder, T. E. (1973) Proc. Natl. Acad. Sci. USA 70, 1688-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanger, J. M. & Sanger, J. W. (1980) J. Cell Biol. 86, 568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maupin, P. & Pollard, T. D. (1986) J. Ultrastruct. Mol. Struct. Res. 94, 92-103. [DOI] [PubMed] [Google Scholar]
- 45.Rogers, S. L., Wiedemann, U., Stuurman, N. & Vale, R. D. (2003) J. Cell Biol. 162, 1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponti, A., Machacek, M., Gupton, S. L., Waterman-Storer, C. M. & Danuser, G. (2004) Science 305, 1782-1786. [DOI] [PubMed] [Google Scholar]
- 47.Verkhovsky, A. B., Svitkina, T. M. & Borisy, G. G. (1995) J. Cell Biol. 131, 989-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minestrini, G., Harley, A. S. & Glover, D. M. (2003) Mol. Biol. Cell 14, 4028-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.