Abstract
Sputum and serum from patients with active pulmonary tuberculosis (TB), healthy purified protein derivative-positive adults, and patients with bacterial pneumonia were collected to simultaneously assess local immunity in the lungs and peripheral blood. To determine whether cytokine profiles in sputum from TB patients and control subjects were a reflection of its cellular composition, cytospin slides were prepared in parallel and assessed for the presence of relative proportions of epithelial cells, neutrophils, macrophages, and T cells. Gamma interferon (IFN-γ) in sputum from TB patients was markedly elevated over levels for both control groups. With anti-TB therapy, IFN-γ levels in sputum from TB patients decreased rapidly and by week 4 of treatment were comparable to those in sputum from controls. Further, IFN-γ levels in sputum closely followed mycobacterial clearance. Although detected at fourfold-lower levels, IFN-γ immunoreactivities in serum followed kinetics in sputum. TNF-α, interleukin 8 (IL-8) and IL-6 also were readily detected in sputum from TB patients at baseline and responded to anti-TB therapy. In contrast to IFN-γ, however, TNF-α and IL-8 levels also were elevated in sputum from pneumonia controls. These data indicate that sputum cytokines correlate with disease activity during active TB of the lung and may serve as potential early markers for sputum conversion and response to anti-TB therapy.
Despite efforts to improve diagnosis and treatment, tuberculosis (TB) remains a major health problem worldwide, especially in developing countries. Obstacles to TB control include the long duration of therapy and the lack of concrete markers indicating success or failure of treatment early during the course of active disease.
Sputum culture conversion following 8 weeks of treatment has been used as a surrogate of response to antituberculous chemotherapy (16). However, cultures require up to 6 weeks to perform and, therefore, are not ideally suited for real-time assessment of response to treatment. By contrast, assessment of immunological parameters in biological fluids can be accomplished within days of sample collection and, if validated as a surrogate marker, may be particularly useful in settings where the activity of short-term administration of new drugs (early bactericidal activity studies) or of immunoadjuvants to standard anti-TB therapy is tested. Further, identifying immunological parameters that correlate with culture sterilization may provide important information about host factors most relevant to anti-Mycobacterium tuberculosis (MTB) immunity.
Since TB predominantly affects the lung, assessment of specimens recovered from this site may best reflect the interaction between the host and MTB during active disease. Fiber optic bronchoscopy and bronchoalveolar lavage (BAL) has been used to assess anti-MTB immunity in situ (18, 19). However, this invasive technique cannot be applied serially during treatment. As a result we investigated alternative approaches for evaluating anti-MTB immune responses at sites of active MTB-infection.
Recent evidence from other pulmonary conditions, such as asthma and chronic obstructive pulmonary disease, indicates that sputum may provide a ready alternative to BAL for serially evaluating factors involved in lung immunity (8, 14). Even though induced sputum is favored over spontaneous sputum by some investigators for study of lower airway inflammation, since it may better resemble findings in BAL (3, 7), others have shown that total cell differential and cytokine content are comparable in induced and spontaneous sputum and BAL (7, 11). To investigate whether sputum specimens also could be used to study immune parameters at sites of active TB, we collected spontaneous and induced sputum and serum from patients with newly diagnosed pulmonary TB in Vitória, Brazil, serially throughout the first 12 weeks of antituberculous chemotherapy. To study whether sputum cytokine responses at the time of diagnosis of active TB are specific for this disease, biological fluids from healthy purified protein derivative (PPD)-positive controls and from patients with purulent bacterial pneumonia also were collected once and assessed. To establish whether cytokine profiles in sputum reflect the cellular composition of sputum, cytospin slides were prepared in parallel and assessed for the relative proportions of epithelial cells, neutrophils, macrophages, and T-cells.
MATERIALS AND METHODS
Study subjects.
Patients with newly diagnosed initial episodes of pulmonary TB were identified at the outpatient TB clinic of the Hospital Universitário Cassiano Antonio de Morais (HUCAM), a large public teaching hospital in Victoria, Brazil, between June and December 2000. Following informed consent for study participation and human immunodeficiency virus (HIV) testing, approved by the institutional review boards at the Federal University of Espírito Santo, Case Western Reserve University/University Hospitals of Cleveland, and the University of Medicine and Dentistry of New Jersey, potential study participants were evaluated by medical history, physical examination, chest X-ray, sputum acid-fast bacilli (AFB) smear, mycobacterial culture and drug susceptibility testing, complete blood count, and chemistry panel. Fifteen HIV-uninfected AFB smear-positive patients met eligibility criteria (12) for participation in this study and are included in this analysis. A diagnosis of TB was confirmed in all TB patients by mycobacterial culture. The mean age of TB patients was 34 ± 10 years (range, 18 to 52 years). Twelve patients were male, and three were female. One patient showed minimal disease, eight showed moderately advanced TB, and six showed far-advanced TB radiographically (2). Cavities were not part of the radiographic presentation of TB for two patients (one with minimal disease and another with moderately advanced TB). None of the patients studied had extrapulmonary TB. After pretreatment specimens were collected, patients were started on standard short-course chemotherapy consisting of 2 months of daily isoniazid (INH), rifampin, pyrazinamide, and ethambutol followed by 4 months of daily INH and rifampin. Treatment was ambulatory and unsupervised. Compliance was monitored by patient interview and urine INH metabolite testing (Mycodyn Uritec; Symcon, Solana Beach, Calif.).
Ten HIV-uninfected, PPD-positive healthy adults, age 33 ± 3 years (range, 22 to 51 years), without clinical or laboratory evidence of active TB were recruited among laboratory and hospital staff at HUCAM. Seven healthy controls were male, and three were female.
Six patients with purulent bacterial pneumonia of age 41 ± 7 years (range, 23 to 65 years) presenting to the emergency room at HUCAM for evaluation of respiratory symptoms were enrolled as disease controls. Four disease controls were male, and two were female. All six patients had lobar infiltrates, consistent with bacterial community-acquired pneumonia, and responded rapidly to appropriate antibiotic treatment. Active TB in these subjects was ruled out by AFB smears and MTB culture.
Sample collection.
TB patients were hospitalized overnight for blood and sputum collection and studied before anti-TB therapy and at 2, 4, 8, and 12 weeks thereafter. After an overnight fast, patients were asked to rinse their mouth with water and collect any sputum they could produce (spontaneous sputum). Induced sputum collection was done 2 h later. Patients were asked to brush their teeth and rinse their mouth several times with water. Sputum induction was performed in a negative-pressure room. Patients were instructed to inhale sterile 3% saline for up to 30 min via a DeVilbiss Ultra-Neb Large Volume Ultrasonic Nebulizer (no. 99HD; DeVilbiss Health Care, Somerset, Pa.) with a calibrated mass median aerodynamic diameter particle size of less than 4 μm and an output of 4 ml/min. Disposable, single-use mouthpieces, tubing, and nebulizer cups were used for each sample collection. All sputum produced during induction was collected. Spontaneous and induced sputum samples were collected directly into 50-ml sterile disposable polypropylene centrifuge tubes, kept at 4°C, and promptly transported to the laboratory for processing within 2 h of collection. Sputum specimens from healthy control subjects (induced sputum only) and patients with bacterial pneumonia (spontaneous sputum only) were collected and processed using the same techniques as for sputum from TB patients. Because preliminary experiments indicated that cytokine concentrations in sputum samples from healthy control subjects collected on two separate occasions were comparable, these subjects were studied only once.
Processing of sputum and serum.
Spontaneous and induced sputum specimens were processed according to the method of Bhowmik et al. (3) with minor modifications. Briefly, spontaneous and induced sputum was diluted with an equal amount of 0.1% dithiothreitol in Hanks solution and incubated on a rocking platform for 15 min at room temperature to digest mucus. Following digestion, half of the sputum was removed and submitted to the microbiology laboratory for AFB smear and quantitative culture. Remaining sputum digests were centrifuged at 275 × g to sediment cellular constituents. Cell supernatants were collected and stored frozen at −70°C until assessment of cytokine levels. Cell pellets were resuspended in RPMI containing 10% fetal calf serum (5 × 104/ml) and used to prepare cytospin slides for later assessment of cellular composition by Wright stain as described previously (21). Slides were evaluated by a pathologist blinded to the identity of the samples. Phenotypic parameters evaluated included numbers of epithelial cells, total leucocytes, neutrophils, macrophages, and lymphocytes.
Cytospin slides prepared from both spontaneous and induced sputum from TB patients were studied. Most healthy PPD-positive control subjects were unable to expectorate sputum spontaneously. Therefore, only slides prepared from induced sputum samples of these individuals were used for comparison. Spontaneous sputum only was used to prepare cytospin slides from patients with bacterial pneumonia.
Serum was obtained by centrifugation at 500 × g and stored frozen at −70°C until use.
Cytokine assays.
Cytokine levels in biological fluids were assessed using commercially available enzyme-linked immunosorbent assay (ELISA) kits. Based on previous experience by others (19), indicating that cytokine levels can be detected only in concentrated BAL samples, sputum specimens were concentrated (10-fold) prior to assessment of cytokines using Centricon YM10 filters (Millipore, Bedford, Mass.). Serum samples were used unconcentrated. To control for differences in volumes of sputum or serum originally obtained, results are reported as cytokine content/milliliter of original volume of sputum or serum. Kits from Medgenix (Biosource International, Camarillo, Calif.) were used to determine gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) immunoreactivities. These assays have lower limits of detection of <1.5 pg of IFN-γ/ml and <3 pg of TNF-α/ml, respectively. Interleukin 8 (IL-8) and interleukin 6 (IL-6) levels were measured using commercial assays from R&D Systems (Minneapolis, Minn.) with sensitivities of <10 pg/ml and <0.7 pg/ml, respectively.
Statistics.
Results were analyzed by Student's t test, paired t test, analysis of variance (ANOVA) (where indicated), and linear regression and correlation analysis. A P value of ≤0.05 was considered significant.
RESULTS
IFN-γ levels in sputum from TB patients, healthy control subjects, and disease controls.
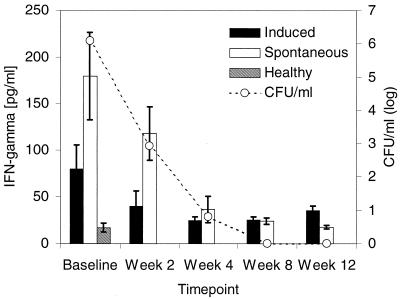
IFN-γ has been used previously as a marker of anti-MTB immunity in human and murine models of MTB infection (5, 9, 13, 19). Therefore, we first assessed IFN-γ levels in biological fluids from TB patients, healthy PPD-positive control subjects, and patients with purulent bacterial pneumonia. IFN-γ was readily detectable in sputum from all TB patients and ranged from 9 to 481 pg/ml in spontaneous sputum and from 7 to 413 pg/ml in induced sputum. Levels in spontaneous and induced sputum from TB patients were highest at the time of diagnosis and before treatment was initiated (Fig. 1). Cytokine immunoreactivities in spontaneous sputum exceeded those in induced sputum specimens by 2.5-fold (P ≤ 0.02; n = 15; ANOVA). IFN-γ was detectable only at low levels in induced sputum from control subjects (12 ± 2 pg/ml [mean ± standard error of the mean {SEM}]; range, 0 to 27 pg/ml) (Fig. 1) and was undetectable in sputum specimens from all six pneumonia controls (data not shown).
FIG. 1.
Correlation between IFN-γ levels and mycobacterial load in sputum from TB patients during the first 12 weeks of anti-MTB treatment. Duplicate aliquots of spontaneous and induced sputum from TB patients (n = 15) were used for assessment of IFN-γ levels by ELISA and quantitative mycobacterial culture. Results are expressed as picograms of IFN-γ/milliliter (left y axis) and log CFU (CFU, right y axis). Sputum IFN-γ levels from healthy PPD-positive controls (n = 10) are included for comparison. Cytokine data are presented as mean ± SEM. ▪, induced sputum from TB patients; □, spontaneous sputum from TB patients; ▧, induced sputum from healthy controls.
To evaluate whether IFN-γ immunoreactivities in sputum from TB patients correlated with intensity of infection, we next stratified cytokine levels by mycobacterial load (expressed as log CFU) in sputum. When sputum bacterial load in spontaneous or induced sputum (log CFU) at the time of the initial evaluation was plotted against IFN-γ levels in corresponding sputum specimens, a positive correlation was observed (for spontaneous sputum, r = 0.52 and P ≤ 0.04; for induced sputum, r = 0.55 and P ≤ 0.03). Further, IFN-γ immunoreactivities were lower in sputum from patients with noncavitary disease (n = 2) than from those with more advanced cavitary disease (n = 13) (spontaneous sputum, 51 ± 42 versus 204 ± 56 pg/ml [mean ± SEM], P ≤ 0.02; induced sputum, 16 ± 8 versus 80 ± 32 pg/ml [mean ± SEM]), P = 0.07).
Next, we compared serum and sputum IFN-γ levels. Serum from TB patients contained measurable amounts of IFN-γ (48 ± 15 pg/ml [mean ± SEM]; range, 0 to 244 pg/ml). However, the mean concentration of IFN-γ in serum was 1.6-fold lower than that in induced sputum (not significant) and 3.9-fold lower (P ≤ 0.02, n = 15; ANOVA) than that in spontaneous sputum. In contrast to findings with sputum, however, there was no detectable correlation between serum IFN-γ levels and mycobacterial load (as assessed by log CFU) in spontaneous or induced sputum from TB patients. IFN-γ was undetectable in sera from both PPD-positive healthy subjects and pneumonia controls (data not shown).
The effect of anti-TB treatment on sputum and serum IFN-γ levels.
IFN-γ levels in sputum and serum from TB patients during the first 12 weeks of anti-TB treatment were assessed next. IFN-γ levels in spontaneous and induced sputum declined rapidly following initiation of anti-MTB therapy (Fig. 1). At 2 weeks, mean IFN-γ levels in spontaneous and induced sputum had decreased by 38% (not significant) and by 52% (P < 0.01; n = 15; paired t test), respectively (Fig. 1). By week 4, levels had declined by an additional 45 and 18%, respectively (P < 0.01 [spontaneous sputum] and P < 0.06 [induced sputum]) and were no longer significantly different from those in sputum of control subjects (Fig. 1). IFN-γ levels in serum decreased with kinetics similar to those observed with sputum (data not shown). Since there was a wide range of IFN-γ levels in biological fluids at baseline, we also evaluated dynamic changes of IFN-γ immunoreactivities in sputum and serum of individual patients. We found that the degree of decline of IFN-γ in specimens from individuals with high and low baseline values was proportional.
To assess whether there was a direct relationship between changes in mycobacterial load and IFN-γ content in sputum, we plotted mean log CFU in spontaneous and induced sputum over time against mean IFN-γ immunoreactivities at each time point. Overall, a positive correlation was observed between mean IFN-γ levels and mean log CFU in both spontaneous and induced sputum (for spontaneous sputum, r = 0.98 and P ≤ 0.004; for induced sputum, r = 0.96 and P ≤ 0.01) (Fig. 1). Interestingly, culture conversion (the microbiologic surrogate for response to treatment) in spontaneous and induced sputum did not occur until week 8 of therapy in 5 of 15 subjects studied, whereas mean IFN-γ levels in sputum from both culture-positive and -negative TB patients were no longer statistically significantly different from background levels following 4 weeks of treatment (for spontaneous sputum, 56 ± 20 versus 15 ± 15 pg/ml; for induced sputum, 26 ± 6 versus 20 ± 5 pg/ml). Taken together, these data suggest that not only does IFN-γ in sputum reflect the intensity of MTB infection and lung inflammation, but because of its rapid response to effective anti-TB therapy it may be a more sensitive marker for response to treatment than MTB sputum culture conversion.
Cellular composition of sputum.
Results of studies using bronchoscopy and BAL indicate that levels of IFN-γ and frequencies of IFN-γ-producing cells are increased in sputum from TB patients compared to that from healthy control subjects (19, 20). To establish whether increased sputum IFN-γ levels were associated with expansion of numbers of T-lymphocytes in sputum, we examined morphology and phenotype of cells in sputum from TB patients and control subjects next.
Epithelial cells accounted for 25 to 50% of cells on cytospin slides prepared from spontaneous and induced sputum from TB patients at baseline and from control subjects. Nonepithelial cells in spontaneous and induced sputum from TB patients at baseline contained 50 to 75% neutrophils, 25 to 50% macrophages, and less than 1% lymphocytes. By contrast, >75% of nonepithelial cells in sputum from PPD-positive control subjects were macrophages, < 25% of cells evaluated exhibited characteristics of neutrophils, and less than 1% of cells resembled lymphocytes. Neutrophils represented 75 to 90% of nonepithelial cells on cytospin slides from patients with bacterial pneumonia, whereas the remaining 10 to 25% of cells had the morphological characteristics of macrophages.
During TB treatment relative proportions of mononuclear cells in both spontaneous and induced sputum from TB patients decreased and those of epithelial cells increased. Further, numbers of neutrophils, both in spontaneous and induced sputum, decreased progressively, whereas those of macrophages increased. A strong negative correlation existed between frequencies of neutrophils and macrophages throughout the first 12 weeks of therapy (r = −0.97; P < 0.005). By week 12 of treatment, macrophages represented more than 75% of nonepithelial cells. Interestingly, the relative proportions of lymphocytes remained unchanged during follow-up.
To further evaluate the phenotype of sputum cells, we also stained aliquots of spontaneous and induced sputum cells from a subset of TB patients with fluorochrome-conjugated antibodies to the major T-cell subsets, B-cells, monocytes/macrophages, and neutrophils and submitted them to fluorescence-activated cell sorter analysis. In contrast to findings in a recent report by Alexis et al., who assessed activation status of macrophages and neutrophils in sputum from healthy subjects by flow cytometry (1), we were unable to use sputum from TB patients for phenotypic analysis of leukocyte populations.
Levels of TNF-α, IL-8, and Il-6 in sputum.
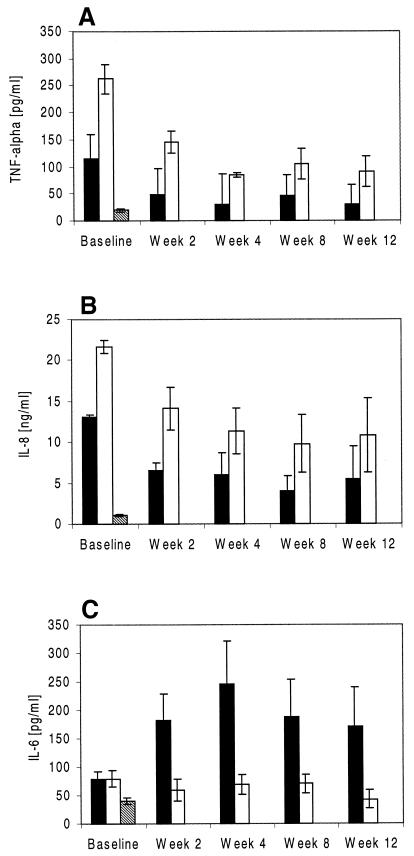
Since macrophages and neutrophils, which are potent producers of cytokines, such as TNF-α, IL-8, and IL-6, represented the majority of cells found in sputum from TB patients and control subjects, we next examined their levels in sputum. The level of TNF-α in spontaneous (274 ± 42 pg/ml; range, 20 to 549 pg/ml) and induced (125 ± 35 pg/ml; range, 20 to 483 pg/ml) sputum from TB patients was highest at the time of diagnosis. Further, the level of TNF-α in induced sputum from TB patients exceeded that in induced sputum from PPD-positive control subjects by 11-fold (P ≤ 0.01 [ANOVA]) (Fig. 2A). In contrast to findings for IFN-γ, TNF-α immunoreactivity in spontaneous sputum from pneumonia controls was comparable (315 ± 60 pg/ml) to that in spontaneous sputum from TB patients. In TB patients, a positive correlation existed between baseline TNF-α levels and mycobacterial load in spontaneous and induced sputum (spontaneous sputum, r = 0.54 and P ≤ 0.03; induced sputum, R = 0.64 and P ≤ 0.01). In contrast to findings for IFN-γ, however, levels of TNF-α did not differ significantly in sputum from patients with noncavitary (n = 2) disease compared to those with more advanced cavitary disease (n = 13) (for spontaneous sputum, 271 ± 51 versus 275 ± 40 pg/ml [mean ± SEM]; for induced sputum, 117 ± 93 versus 151 ± 46 pg/ml [mean ± SEM]).
FIG. 2.
Levels of TNF-α (A), Il-8 (B), and IL-6 (C) in sputum from TB patients and healthy PPD-positive control subjects. Spontaneous and induced sputum from TB patients (n = 15) was collected at the time of diagnosis of TB and at 2, 4, 8, and 12 weeks thereafter, and cytokine levels were assessed by ELISA. Data are shown in comparison to TNF-α, IL-8, and IL-6 levels in sputum from healthy control subjects (n = 10). Cytokine data are presented as mean ± SEM. ▪, induced sputum from TB patients; □, spontaneous sputum from TB patients; ▧, induced sputum from healthy controls.
We also assessed pretreatment TNF-α levels in serum from patients with TB and compared them with those in serum from PPD-positive and pneumonia controls. TNF-α was detectable in serum from PPD-positive control subjects (9 ± 1 pg/ml; range, 0 to 20), but was 14-fold lower than levels in serum from TB patients (129 ± 24 pg/ml; range, 13 to 361 pg/ml; P ≤ 0.001 [ANOVA]). In contrast, TNF-α levels in serum from pneumonia patients (78 ± 45 pg/ml; range, 8 to 162 pg/ml) were not statistically significantly different from those of TB patients.
TNF-α levels in sputum specimens decreased rapidly following initiation of treatment. By week 12, mean TNF-α immunoreactivities in spontaneous and induced sputum had declined by 80 to 90% compared to starting levels (P ≤ 0.01 and P ≤ 0.05, respectively) (Fig. 2A). As was the case for IFN-γ, the degree of decline of TNF-α in sputum specimens from individual patients with high and low baseline values was proportional. Further, a positive correlation existed between mycobacterial load and TNF-α immunoreactivities in spontaneous and induced sputum throughout the first 12 weeks of treatment (for spontaneous sputum, r = 0.98 and P ≤ 0.005; for induced sputum, r = 0.97 and P ≤ 0.007). TNF-α levels in serum did not change significantly between baseline and week 12 and did not correlate with decreases in sputum mycobacterial load.
Baseline levels of IL-8 were comparable in spontaneous sputum specimens from TB patients (Fig. 2B) and pneumonia controls (16 ± 6.0 ng/ml; range, 6 to 29 ng/ml). Kinetics of IL-8 in sputum from TB patients followed a pattern similar to that seen for TNF-α and IFN-γ and correlated with mycobacterial clearance as assessed by determining the number of CFU (Fig. 2B). IL-8 was undetectable in sputum from healthy PPD-positive controls (data not shown).
In contrast to findings for TNF-α and IL-8 described above, IL-6 in sputum did not follow the same pattern as IFN-γ. In fact, IL-6 levels in spontaneous and induced sputum from TB patients were comparable at baseline and exceeded those in induced sputum from controls by only twofold (Fig. 2C). Further, IL-6 immunoreactivities in spontaneous sputum remained essentially unchanged, whereas levels in induced sputum increased (fivefold) between baseline and week 4 and then remained elevated (at 170 pg/ml by week 12).
DISCUSSION
Whether and how host responses to MTB correlate with clearance of the organism from the lung during therapy has not been established. To date, efforts at gaining a better understanding of the relationship between immunologic and microbiologic parameters during therapy for active TB have been hampered by the fact that invasive procedures, such as bronchoscopy and BAL, have been required to collect specimens adequate for evaluating anti-MTB immunity in the lung. As a result of its invasive nature, bronchoscopy can't be repeated serially, thus precluding its use in the longitudinal evaluation of immunologic surrogates. In contrast, even complex microbiologic markers indicative of response to TB treatment (6) can be studied in sputum, which is readily accessible.
Results of several recent publications support the notion that collection of spontaneously expectorated and induced sputum may provide a viable alternative to bronchoscopy and BAL for assessing immune function in the lung, both in healthy individuals and in patients with lung disease (3, 7, 8, 14). Induced sputum, in particular, may provide information qualitatively similar to that obtained from BAL (7, 8, 11, 17). Data presented here indicate that both spontaneous and induced sputum from TB patients can be used to simultaneously evaluate levels of cytokines previously implicated in anti-MTB immunity (IFN-γ and TNF-α) and to assess microbiologic parameters during the course of anti-MTB therapy.
Interestingly, levels of inflammatory cytokines (TNF-α and IL-8), which are produced both by macrophages and neutrophils (4), were comparable in sputum from TB patients and from patients with purulent bacterial pneumonia, indicating that their production may be a nonspecific response associated with any pulmonary infection. In contrast, IFN-γ, which is produced by lymphocytes and NK cells, was easily detectable in sputum from TB patients but not the control groups, implicating cellular immunity involving activated lymphocytes. Therefore, it is possible that during active TB, IFN-γ levels in sputum represent a specific marker of recruitment of MTB-reactive T-cells to the site of active MTB infection and their activation.
Increased levels of IFN-γ also have been found in BAL fluid from patients with active TB (20), whose bronchoalveolar cells contain increased frequencies of MTB-reactive IFN-γ-producing T cells (19). In this study less than 1% of cells identified on sputum cytospin slides represented lymphocytes, and overall numbers of lymphocytes did not change with therapy. However, a predisposition of lymphocytes at sites of active MTB infection to undergo programmed cell death has been described (13, 15), which may explain the above finding. Alternatively, IFN-γ in sputum may be produced by cells other than lymphocytes. Thus, further studies are necessary to determine the cellular source of IFN-γ in sputum.
Interestingly, the pattern of IL-6 in spontaneous and induced sputum from TB patients was markedly different from that of all other cytokines studied. In particular, IL-6 levels did not decrease following initiation of anti-MTB therapy, and they actually increased in induced sputum. IL-6 has been implicated in healing responses (10). Therefore, increasing IL-6 levels in induced sputum, which more accurately reflects local immunity in the alveolar space, may be a first indicator of success of anti-MTB therapy. Further, since the relative proportions of mononuclear phagocytes and epithelial cells in sputum increased throughout follow-up, these cells may be the predominant source of IL-6 during active TB. Additional TB patients are currently being recruited to further evaluate and substantiate this hypothesis.
In summary, data presented here indicate that sputum may be an alternative to BAL for studying host immune responses in the lung during treatment for TB. This approach is particularly attractive, since it allows for the simultaneous assessment of immunologic and microbiologic parameters. Since decreases in sputum IFN-γ, TNF-α, and IL-8 in sputum closely parallel and even precede mycobacterial clearance, these cytokines may be markers of disease activity and inflammation and may be useful early surrogates of response to anti-TB therapy in future studies evaluating new treatment modalities for TB.
Acknowledgments
This work was supported by a contract from the National Institutes of Health: AI-45244/AI-95383 (Tuberculosis Research Unit) and K08 award AI-01514.
We thank the TB patients and control subjects in Vitória, Brazil, who graciously provided sputum and blood specimens, as well as the clinical and laboratory staff of the Núcleo Doenças Infecciosas not included as authors. Without their contribution this study would not have been possible.
REFERENCES
- 1.Alexis, N., J. Soukup, and S. Becker. 2000. Sputum phagocytes from healthy individuals are functional and activated: a flow cytometric comparison of cells in bronchoalveolar lavage and peripheral blood. Clin. Immunol. 97:21-32. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1969. Classification of pulmonary tuberculosis, p. 68-76. In Diagnostic standards and classification of tuberculosis. National Tuberculosis and Respiratory Disease Association, New York, N.Y.
- 3.Bhowmik, A., T. A. Seemungal, R. J. Sapsford, J. L. Devalia, and J. A. Wedzicha. 1998. Comparison of spontaneous and induced sputum for investigation of airway inflammation in chronic obstructive pulmonary disease. Thorax 53:953-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassatella, M. A. 1999. Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 73:369-509. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, A. M., D. K. Dalton, A. T. Steward, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in IFN-γ gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desjardin, L. E., M. D. Perkins, K. Wolski, S. Haun, L. Teixeira, Y. Chen, J. L. Johnson, J. J. Ellner, J. Bates, M. D. Cave, and K. D. Eisenach. 1999. Measurement of sputum Mycobacterium tuberculosis messenger RNA as a surrogate for response to chemotherapy. Am. J. Respir. Crit. Care Med. 160:203-210. [DOI] [PubMed] [Google Scholar]
- 7.D'Ippolito, R., A. Foresi, A. Chetta, A. Casalini, A. Castagnaro, C. Leone, and D. Oliveri. 1999. Induced sputum in patients with newly diagnosed sarcoidosis. Comparison with bronchial wash and BAL. Chest 115:1611-1615. [DOI] [PubMed] [Google Scholar]
- 8.Fahy, J., H. Wong, and H. Boushey. 1995. Comparison of samples collected by sputum induction and bronchoscopy from asthmatic and healthy subjects. Am. J. Respir. Crit. Care Med. 152:53-58. [DOI] [PubMed] [Google Scholar]
- 9.Flynn, J. L., J. Chan, D. K. Triebold, A. T. Steward, and B. R. Bloom. 1993. An essential role for interferon-γ in resistance to M. tuberculosis. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallucci, R. M., P. P. Simenova, J. M. Matheson, C. Kommineni, J. L. Guriel, T. Sugawara, and M. L. Luster. 2000. Impaired cutaneous wound healing in interleukin-deficient and immunosuppressed mice. FASEB J. 14:2525-2531. [DOI] [PubMed] [Google Scholar]
- 11.Henig, N. R., M. R. Tonelli, M. V. Pier, J. L. Burns, and M. L. Aitgen. 2001. Sputum induction as a research tool for sampling the airways of subjects with cystic fibrosis. Thorax 56:306-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch, C. S., Z. Toossi, G. Vanham, J. L. Johnson, P. Peters, A. Okwera, R. Mugerwa, P. Mugeyenyi, and J. J. Ellner. 1999. Apoptosis and T-cell hyporesponsiveness in pulmonary tuberculosis. J. Infect. Dis. 179:945-953. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch, C. S., Z. Toossi, J. L. Johnson, H. Luzze, E. Ntambi, P. Peters, M. McHugh, A. Okwera, M. Joloba, P. Mugeyenyi, R. D. Mugerwa, P. Terebuh, and J. J. Ellner. 2001. Augmentation of apoptosis and IFN-γ production at sites of active MTB infection in human tuberculosis. J. Infect. Dis. 183:779-788. [DOI] [PubMed] [Google Scholar]
- 14.Keatings, V. M., P. D. Collins, D. M. Scott, and P. J. Barnes. 1996. Differences in IL-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am. J. Respir. Crit. Care Med. 153:530-534. [DOI] [PubMed] [Google Scholar]
- 15.Li, B., H. Bassiri, M. D. Rossman, P. Kramer, A. F. Eyuboglu, M. Torres, E. Sada, T. Imir, and S. R. Carding. 1998. Involvement of the Fas/Fas ligand pathway in activation-induced cell death of mycobacteria-reactive human γδ-T cells: a mechanism for the loss of γδ-T cells in patients with pulmonary tuberculosis. J. Immunol. 161:1558-1567. [PubMed] [Google Scholar]
- 16.Mitchison, D. A. 1993. Assessment of new sterilizing drugs for treating pulmonary tuberculosis by cultures at 2 months. Am. Rev. Respir. Dis. 147:1062-1063. [DOI] [PubMed] [Google Scholar]
- 17.Moodley, J. P., T. Dorasamy, S. Venketsamy, V. Naicker, and V. G. Lalloo. 2000. Correlation of CD4:CD8 ratio and tumour necrosis factor (TNF) alpha levels in induced sputum with bronchoalveolar lavage fluid in pulmonary sarcoidosis. Thorax 55:696-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwander, S. K., E. Sada, M. Torres, D. Escobedo, J. G. Sierra, S. Alt, and E. A. Rich. 1996. T lymphocytic and immature macrophage alveolitis in active pulmonary tuberculosis. J. Infect. Dis. 173:1267-1272. [DOI] [PubMed] [Google Scholar]
- 19.Schwander, S. K., M. Torres, E. Sada, C. Carranza, E. Ramos, M. Tary-Lehmann, R. S. Wallis, J. G. Sierra, and E. A. Rich. 1998. Enhanced responses to M. tuberculosis antigens by human alveolar lymphocytes during active pulmonary tuberculosis. J. Infect. Dis. 178:1434-1445. [DOI] [PubMed] [Google Scholar]
- 20.Somoskovi, A., G. Zissel, P. F. Zipfel, M. W. Ziegenhagen, J. Klaucke, H. Haas, M. Schlaak, and J. Muller-Quernheim. 1999. Different cytokine patterns correlate with the extension of disease in pulmonary tuberculosis. Eur. Cytokine Netw. 10:135-142. [PubMed] [Google Scholar]
- 21.Toossi, Z., P. Gogate, H. Shiratsuchi, T. Young, and J. J. Ellner. 1995. Enhanced production of TGF-beta by blood monocytes from patients with active tuberculosis and presence of TGF-beta in tuberculous granulomatous lung lesions. J. Immunol. 154:465-473. [PubMed] [Google Scholar]