Abstract
Diagnosis of tuberculosis is time-consuming and requires infrastructures which are often not available in countries with high incidences of the disease. In the present study, an 82-kDa protein antigen was isolated by affinity chromatography and was identified by peptide mass fingerprinting as isocitrate dehydrogenase II, which is encoded by the icd2 gene of Mycobacterium bovis BCG. The icd2 gene of BCG was cloned by PCR, and the product of recombinant gene expression was purified and analyzed by two-dimensional polyacrylamide gel electrophoresis. The recombinant protein, named rICD2, was tested for its recognition by immunoglobulin G (IgG) antibodies from the sera of 16 patients with tuberculosis (TB) and 23 healthy individuals by Western blotting. The results showed that rICD2 is recognized by IgG antibodies from the sera of all TB patients tested at serum dilutions of ≥1:640. At a serum dilution of 1:1,280, the sensitivity was 50% and the specificity was 86.9%. These results indicate that rICD2 might represent a candidate for use in a new assay for the serodiagnosis of TB.
Tuberculosis (TB) remains a major cause of death and disabilities in developing countries, where over 90% of global cases occur, and is now also a cause for growing concern in industrialized countries, where the incidence of the disease has also increased (6). Diagnosis of TB in developing countries mainly relies on examination of chest X rays and/or examination of smears under a microscope for detection of acid-fast bacilli. However, only about 50% of the patients with pulmonary TB are smear positive, and chest X rays can detect advanced pulmonary TB only after extensive damage of lung tissues has already occurred (22). At present, the most reliable method for diagnosis of TB is still isolation of organisms by culture and biochemical identification of the tubercle bacilli, but because of the slow growth rate of Mycobacterium tuberculosis, results are not seen until several weeks after specimen collection. The time to detection might be shortened to 1 week by the use of radiometric systems and nucleic acid probes, but these techniques are still too expensive for use by laboratories in developing countries. A simple and inexpensive diagnostic assay might be of great benefit for global control of the disease (9). Considerable efforts have been made to evaluate assays based on specific recognition of selected mycobacterial antigens (Ags) in vitro by serum antibodies (Abs) (16, 17, 25, 34) or the release of gamma interferon by peripheral blood mononuclear cells stimulated with M. tuberculosis-specific Ags (33, 34). In the latter case, the use of cocktails of immunodominant Ags of M. tuberculosis has been indicated to increase the sensitivity of the assay significantly without affecting the specificity of the assay (33), and the same strategy has been suggested for use in the diagnosis of TB based on detection of specific Ab responses (13, 14). As the pattern of Ag recognition by patient Abs may be influenced by the stage of the disease (15, 28) and by the immunocompetence of the patients (5, 14), an ideal combination might comprehend Ags recognized at different stages of M. tuberculosis infection and should be able to detect M. tuberculosis-specific Abs in sera from human immunodeficiency virus (HIV)-infected patients with TB (14).
Compelling evidence shows that the 38-kDa PhoS homologue of M. tuberculosis is recognized by Abs in the sera of TB patients with moderate to high degrees of sensitivity and high specificity, and several investigators have proposed its use as a serodiagnostic reagent (1, 2, 8, 15, 25, 35). A few other mycobacterial proteins identified more recently have also been proposed as promising candidates for a multicomponent serodiagnostic assay for TB (5, 14, 20, 29). The completion of the determination of the sequence of the M. tuberculosis genome (4) and the rapid progress in protein identification and molecular cloning that followed (26, 27, 30) are providing new candidates for such a multicomponent serodiagnostic assay.
In the present study, identification and molecular cloning of isocitrate dehydrogenase II (ICD-II), encoded by the icd2 gene of M. bovis BCG, were carried out. A potential application of the recombinant ICD-II protein (rICD2) for the serodiagnosis of TB was also evaluated. The recombinant protein Ag exhibited good sensitivity and specificity, suggesting its possible use as a component of a serodiagnostic test for TB.
MATERIALS AND METHODS
Bacterial strains.
M. bovis BCG, strain Pasteur, was originally supplied by Pasteur Merieux (Lyon, France). Escherichia coli TOP10 competent cells were from Invitrogen (Groningen, The Netherlands).
Human sera.
Sera were obtained from 16 patients with TB and 23 healthy donors. Diagnosis of TB was confirmed by a positive culture for M. tuberculosis. The patients were 22 to 78 years old; 15 were male and 1 was female. Eleven blood samples were obtained at the beginning of drug therapy, three samples were collected 2 weeks after initiation of therapy, and two samples were collected after 4 weeks of therapy. The healthy individuals whose sera were evaluated were 22 to 34 years old; 9 were male and 14 were female. Eight of them had an anamnesis of BCG vaccination, and 15 did not; 1 had been vaccinated with BCG twice. Delayed-type hypersensitivity to purified protein derivative (PPD) was unknown for most of the healthy individuals. Informed consent was obtained from all the donors, and the protocol was approved by the local ethical committee.
Mouse MAbs and polyclonal Abs.
Monoclonal Ab (MAb) WB8A11 was generated from BALB/c mice immunized intraperitoneally (i.p.) with a culture filtrate (CF) Ag of BCG in incomplete Freund's adjuvant (IFA), as described previously (11). A hybridoma cell culture supernatant was used as the MAb in Western blotting assays, and ascitic fluid was used as the MAb in immunoaffinity chromatography assays.
A mouse polyclonal serum, anti-ICD-II, was obtained from C57BL/6 mice (male and female) that had been immunized i.p. with 8 μg of immunoaffinity chromatography-purified ICD-II of BCG in IFA. The mice were boosted twice before the collection of blood samples.
Purification of native Ag of M. bovis BCG.
CFs were prepared from 12-day-old cultures of BCG as described previously (10). MAb WB8A11-reacting Ag was purified by immunoaffinity chromatography from CFs of BCG. To this end, ascitic fluid containing MAb WB8A11 was added to Sepharose-protein A at 2 mg/ml of gel slurry and was covalently bound to Sepharose-protein A by use of dimethyl pimelidate as described previously (10). CFs of BCG were added to the gel slurry at 0.5 mg/ml in phosphate-buffered saline (PBS), and the mixture was incubated for 6 h at 4°C with gentle agitation. After washing of the gel with PBS, the protein Ag was eluted with 100 mM Na3PO4 (pH 12.5). One-fifth volume of 1 M sodium phosphate (pH 6.8) was added to the eluate to lower the pH, and the mixture was frozen at −20°C.
Protein identification.
Affinity-purified protein that reacted with MAb WB8A11 was loaded onto a 12.5% polyacrylamide gel, and electrophoresis was carried out as described below. The gel was stained with Coomassie brilliant blue R-250 by standard procedures, and the relevant band was excised from the gel. Protein identification was performed by peptide mass fingerprinting with a Voyager Spec mass spectrometer and searches of the TrEMBL (SmartIdent) database at the Swiss-2D-service, Central Laboratory for Clinical Chemistry, University Hospital of Geneva.
Cloning and expression of icd2 gene of M. bovis BCG.
The icd2 gene was obtained by PCR amplification from genomic DNA of BCG with a high-fidelity DNA polymerase with proofreading activity (Expand High Fidelity PCR system; Boehringer Mannheim, Mannheim, Germany). Genomic DNA of BCG was obtained as described previously (10). The upstream primer was UPPER24 (5′-GCCTTGGACAGCCTCCAGCGCTGC-3′), and the downstream primer was LOWER25 (5′-ATGAGCGCCGAACAGCCGACCATCA-3′). Amplification reaction mixtures consisted of 20 ng of DNA, 5 μl of 10× Expand buffer (Boehringer Mannheim), 1.5 mM MgCl2, 200 μM deoxynucleoside triphosphates, each primer (primers UPPER24 and LOWER25) at a concentration of 0.1 μM, and 3.5 U of polymerase (Expand High Fidelity PCR System; Boehringer Mannheim) in a final volume of 50 μl. The reaction mixtures were subjected to 30 cycles of 1 min at 95°C, 2 min at 67.5°C, and 3 min at 72°C, followed by 1 cycle of 10 min at 72°C. Ten reaction mixtures were pooled, and the PCR product was purified with GENECLEAN SPIN (Bio 101, Inc., La Jolla, Calif.) according to the supplier's protocol B and inserted into the pBAD TOPO vector (Invitrogen) according to the instructions of the manufacturer. The product from the cloning reaction was used for transformation of One Shot competent E. coli TOP10 cells (Invitrogen). Screening and determination of the insert orientation were performed by restriction analysis of the transformants with restriction endonucleases BamHI and EcoRI (data not shown). The recombinant plasmid containing the icd2 gene of BCG in the correct orientation for regulated expression driven by the araBAD promoter was named pBicd2. The identity of the PCR-amplified icd2 gene was verified by digestion with restriction endonucleases KpnI, EcoRI, BclI, BglI, BglI-KpnI, BclI-KpnI, BclI-BglI, and BclI-EcoRI, followed by electrophoresis on 1.6 to 2% agarose gels (data not shown).
To check for expression of icd2, overnight cultures of E. coli TOP10/pBicd2 in Luria-Bertani broth containing 50 μg of ampicillin per ml were inoculated 1:25 into 5 ml of fresh medium. The cultures were incubated at 37°C with vigorous shaking until the optical density at 600 nm reached 0.6, and then l-arabinose was added to a final concentration of 0.2, 0.02, 0.002, 0.0002, 0.00002, or 0% (negative control). The cultures were incubated for an additional 2 h and 30 min, and then the bacterial cells were pelleted by centrifugation (12,000 × g, 5 min), resuspended in 200 μl of Laemmli sample buffer containing 40 mM dithiothreitol (DTT), boiled for 5 min, and eventually analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Western blotting with anti-His tag MAb (Sigma). Optimal expression of rICD2 was obtained with concentrations of l-arabinose ≥0.002% (data not shown).
Purification of rICD2.
For purification of rICD2, an overnight culture of E. coli TOP10/pBicd2 was inoculated 1:100 (vol/vol) into Luria-Bertani broth containing 70 μg of ampicillin per ml. The bacteria were incubated at 37°C with shaking until the optical density at 600 nm reached 0.5 to 0.6, and then the inducer, l-arabinose, was added to a final concentration of 0.005% and the cultures were incubated for an additional 3 h before they were harvested. The bacterial cells were harvested by centrifugation (9,700 × g, 10 min, 25°C), washed once with 1/5 volume of 5 mM ɛ-amino-n-caproate (Sigma), and then resuspended in 1/40 volume of 5 mM ɛ-amino-n-caproate and put on ice; 1 N NaOH was added to adjust the pH to 8, and lysozyme (Sigma) was added to a final concentration of 0.1 mg/ml. The bacterial suspension was incubated for 10 min on ice and then divided into 7-ml aliquots. Each aliquot was sonicated with a probe sonicator at intervals of 1 min with the pulser on and 10 s with the pulser off for a total pulse time of 10 min on ice. One-tenth volume of 200 mM sodium phosphate (pH 7.8)-5 M NaCl (10× native binding buffer) was added to the bacterial lysate, and the mixture was then mixed with ProBond resin (Invitrogen) for batch binding of the His-tagged recombinant protein. Batch binding, washing of the resin, and elution of the recombinant protein were performed by the protocols of the supplier. The eluted protein was precipitated by adding saturated ammonium sulfate to a final saturation concentration of 75%, pelleted by centrifugation, and resuspended in sterile PBS or Laemmli sample buffer. As a final purification step, the recombinant protein was separated from contaminants by preparative SDS-PAGE followed by electroelution with an electroeluter (model 422; Bio-Rad Laboratories, Hercules, Calif.).
Two-dimensional PAGE, SDS-PAGE and Western blotting.
For two-dimensional PAGE, the protein was suspended in two-dimensional sample buffer, which consisted of 8 M urea, 0.5% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, 40 mM DTT, and 2% IPG buffer (pH 4 to 7) (Amersham Pharmacia Biotech). Immobiline Dry Strips (length, 7 cm; pH range, 4 to 7; Amersham Pharmacia Biotech) were rehydrated overnight in a total volume of 150 μl of the protein suspension in two-dimensional sample buffer. Isoelectric focusing was carried out with a Multiphor II apparatus (Amersham Pharmacia Biotech). For separation in the second dimension, strips were equilibrated in 50 mM Tris-HCl (pH 6.8)-8 M urea-30% glycerol-1% SDS-5 mg of DTT per ml for 5 min at room temperature and then for 10 min in 50 mM Tris-HCl (pH 6.8)-10% glycerol-2% SDS and were eventually loaded onto 12.5% polyacrylamide gels. Electrophoresis was carried out in a discontinuous buffer system as described by Laemmli (18). Samples for SDS-PAGE were suspended in Laemmli sample buffer, boiled for 5 min, and then loaded onto 12.5% gels. After electrophoretic separation, the proteins were electrotransferred onto an Immun-Blot polyvinylidene difluoride membrane (Bio-Rad Laboratories) with a Trans-Blot cell (Bio-Rad Laboratories). After transfer onto filters, the proteins were visualized by staining with ponceau S, destained by washing with Tris-buffered saline (TBS; 20 mM Tris, 0.5 M NaCl [pH 7.4]), and then incubated overnight in blocking solution: TBS (pH 7.4) containing 4% bovine serum albumin (BSA). For Western blotting with human sera, each filter with immobilized rICD2 was cut into strips of 2 to 2.5 mm in width, and single serum samples at given dilutions were tested for their abilities to recognize the recombinant protein on each strip. Mouse MAbs, mouse polyclonal Abs, and human sera were diluted in TBS containing 3% BSA. MAb WB8A11 was diluted 1:100, and anti-His tag MAbs and mouse polyclonal sera were diluted 1:1,000; human sera were tested at dilutions ranging from 1:20 to 1:10,240. Peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) and goat anti-human IgG were used at dilutions of 1:1,000. The blots were developed with 4-chloro-1-naphthol as the substrate.
Statistical analysis.
Chi-square analysis and Fischer's exact test were used for statistical evaluation of the results. A P value <0.05 was considered significant.
RESULTS
Purification of MAb WB8A11-reacting antigen and identification of ICD-II of M. bovis BCG.
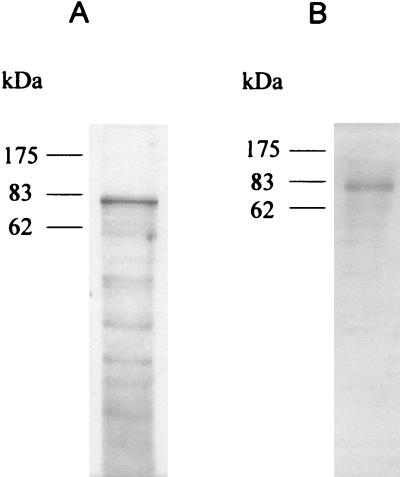
A new MAb, WB8A11, that recognizes a protease-sensitive Ag with an apparent molecular mass of 82 kDa present in CFs as well as in whole-cell lysates of BCG was previously described in our laboratory (11). The Ag that reacted with MAb WB8A11 was not recognized by MAbs directed against previously described Ags of similar molecular masses, suggesting that MAb WB8A11 might identify a novel mycobacterial protein Ag (11).
In the present study, we purified the WB8A11-reacting Ag by immunoaffinity chromatography from CFs of BCG (Fig. 1). Initial attempts at N-terminal sequencing of the Ag failed, as the native protein was probably being blocked at the amino terminus. Therefore, the purified protein was separated from contaminants by SDS-PAGE, and the relevant band was excised and processed for peptide mass fingerprinting. Searches of the TrEMBL database allowed identification of the protein as ICD-II, encoded by the icd2 gene of M. tuberculosis (TrEMBL accession number O53611). The sequence obtained was used for searches of homologous sequences in the Sanger database, which confirmed the presence of the gene in the M. tuberculosis chromosome (Sanger database accession number Rv 0066c) and the homologous gene in M. bovis. The open reading frame of 2,238 bp, starting with ATG and ending with TGA, codes for a polypeptide of 745 amino acids with a theoretical molecular weight of 82,550 in M. bovis, a theoretical molecular weight of 82,551 in M. tuberculosis H37Rv, a theoretical pI of 5.24 in M. bovis, and a theoretical pI of 5.16 in H37Rv. Comparison of the nucleotide sequence of icd2 of M. tuberculosis with the homologous sequence in M. bovis revealed a very high degree of homology, with the two genes differing from each other at only 2 nucleotides: nucleotide 351, which is C in M. bovis and G in M. tuberculosis, and nucleotide 420, which is A in M. bovis and G in M. tuberculosis. The different nucleotide at position 351 results in a difference in amino acid 117, which is asparagine in M. bovis and lysine in M. tuberculosis, whereas the different nucleotide at position 420 does not result in any difference at the amino acid level, as it is the third nucleotide of redundant codons for leucine (Leu140). Therefore, the predicted proteins differ from each other at only 1 amino acid, which is basic in both organisms. The sequences of the predicted proteins show high degrees of homology with those of ICD-II proteins from other bacteria, as assessed by searches of the National Center for Biotechnology Information database with BLAST software, but not with the sequences of isocitrate dehydrogenase I (ICD-I) proteins from several different bacterial organisms.
FIG. 1.
Immunoaffinity chromatography-purified MAb WB8A11-reacting Ag. (A) Silver staining of a 12.5% polyacrylamide gel; (B) Western blotting with MAb WB8A11. A total of 2 μg of protein of purified Ag was loaded for both SDS-PAGE and Western blotting. The positions of the protein molecular mass markers are indicated on the left of each panel.
Cloning and expression of the icd2 gene in E. coli and purification of rICD2.
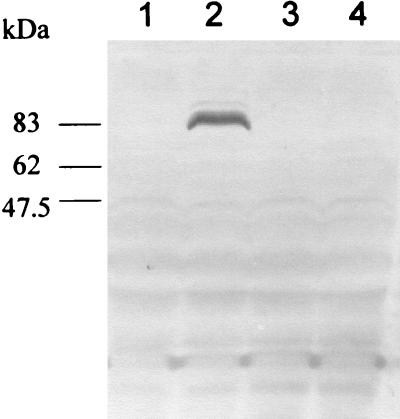
The icd2 gene of BCG was cloned by PCR. To minimize the possibility of errors caused by amplification, we used a high-fidelity DNA polymerase with proofreading activity. The PCR-amplified coding region of the icd2 gene was inserted into the pBAD TOPO vector under the transcriptional control of the araBAD promoter (PBAD), regulated by the AraC gene product encoded by the vector. Expression from PBAD is turned on in the presence of l-arabinose. The resulting construct consisted of a short N-terminal translation leader, followed by the PCR product (i.e., the whole open reading frame of icd2 except for the stop codon) and a short C-terminal stretch of amino acids ending with a polyhistidine (six-His) tag. Expression of the recombinant protein in E. coli was assessed in the presence or absence of the inducer, l-arabinose, by Western blotting of whole-cell lysates (Fig. 2). As expected, a protein with an apparent molecular mass of approximately 85 kDa that was recognized by the anti-His tag MAb was detectable in lysates of TOP10/pBicd2 after induction with l-arabinose but not in lysates of TOP10/pBicd2 incubated in the absence of the inducer or in lysates of TOP10/pBAD TOPO (negative control) induced with l-arabinose or uninduced (Fig. 2).
FIG. 2.
Expression of rICD2 in E. coli was assessed by Western blotting with anti-His tag MAb of lysates of TOP10/pBicd2 and TOP10/pBAD TOPO (negative control) induced with l-arabinose or uninduced. Lanes 1 and 2, whole-cell lysate of TOP10/pBicd2; lanes 3 and 4, TOP10/pBAD TOPO; lanes 1 and 3, uninduced; lanes 2 and 4, induced with l-arabinose. The positions of the protein molecular weight markers are indicated on the left.
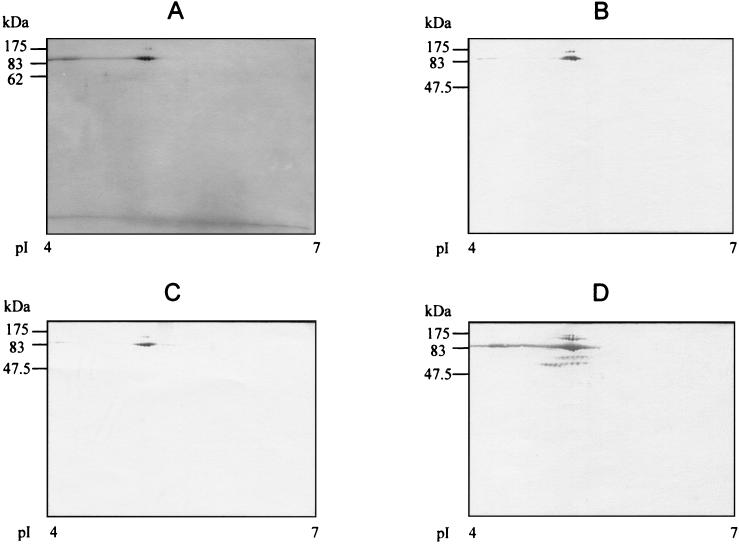
The recombinant protein was purified by Ni-affinity chromatography from lysates of E. coli TOP10/pBicd2, followed by preparative SDS-PAGE and electroelution of the relevant band. This resulted in a recombinant protein with a high degree of purity, as assessed by two-dimensional PAGE and silver staining of the purification product (Fig. 3A). The purified recombinant protein was recognized by the anti-His tag MAb (Fig. 3B), MAb WB8A11 (Fig. 3C), and a polyclonal mouse serum raised against native, immunoaffinity chromatography-purified ICD-II of BCG (Fig. 3D), whereas it was not recognized by nonimmune mouse serum at the same dilution (data not shown). Additional spots, in addition to the main spot, were detected by the immune mouse serum. These spots were assumed to represent degradation products or aggregate forms of rICD2.
FIG. 3.
Silver-stained two-dimensional gel (A) and two-dimensional Western blot of purified rICD2 with MAb WB8A11 (B), anti-His tag MAb (C), or anti-ICD-II polyclonal serum from a C57BL/6 mouse (D). The positions of the protein molecular mass markers are indicated on the left of each panel; the pI ranges are indicated at the bottom of each panel.
Reactivity of human serum IgG to rICD2 by Western blotting.
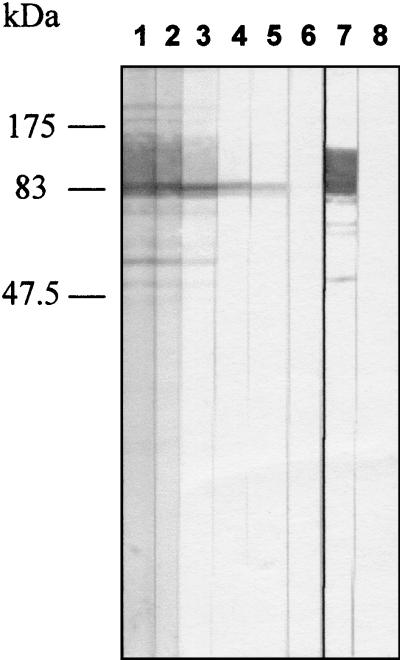
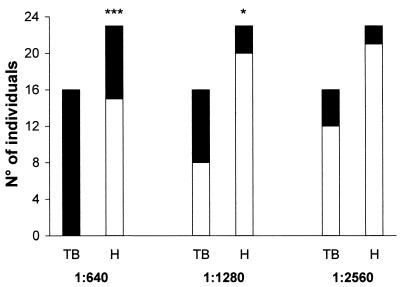
In order to investigate the potential use of rICD2 in an assay for the serodiagnosis of TB, we tested the reactivities of IgG antibodies from the sera of 16 TB patients and 23 healthy donors to rICD2 by Western blotting. The recombinant protein was subjected to preparative SDS-PAGE and was electrotransferred onto a polyvinylidene difluoride membrane. The filter with immobilized rICD2 was cut into strips, and each strip was tested with a single serum sample at a given dilution. The sera were tested for their abilities to recognize rICD2 at dilutions ranging from 1:20 to 1:10,240. The serum anti-rICD2 IgG titer was defined as the highest dilution of serum that gave a clearly visible band in a reproducible manner. An example of the results of an experiment with serum diluted by titration as described above is shown in Fig. 4; in that experiment, strips with electrotransferred immobilized rICD2 were tested with serum from a TB patient at dilutions ranging from 1:80 to 1:5,240, and the anti-rICD2 Ab titer was estimated to be 1:2,560 (Fig. 4). At a serum dilution of 1:640, 16 of 16 (100%) serum specimens from patients with TB and 8 of 23 (34.7%) serum specimens from healthy donors had positive reactions (P < 0.001) (Fig. 5), with a sensitivity of 100% and a specificity of 65.3%. At a dilution of 1:1,280, the sensitivity and the specificity were 50 and 86.9%, respectively (P < 0.05) (Fig. 5). Finally, at a serum dilution of 1:2,560, the sensitivity decreased to 25% and the specificity reached 91.3%, but these results were not significant. Only one serum sample, from a patient with TB, was positive at a dilution of 1:5,120 (data not shown). There were no significant differences in anti-rICD2 Ab titers between sera collected at the beginning of drug therapy and sera collected 2 or 4 weeks after the initiation of therapy (data not shown).
FIG. 4.
Recognition of rICD2 by IgG from human serum by Western blotting. A serum sample from a patient with TB was tested for recognition of purified rICD2 at dilutions ranging from 1:80 to 1:5,260 (lanes 1 to 6); lane 7, positive control (MAb WB8A11); lane 8, negative control (incubation in TBS-3% BSA followed by incubation with peroxidase-conjugated goat anti-human IgG). The positions of the protein molecular mass markers are indicated on the left.
FIG. 5.
Number of TB patients (TB) and healthy subjects (H) giving positive reactions for anti-rICD2 by Western blotting with different serum dilutions (indicated below). The purified rICD2 for which the results are shown in Fig. 3 was used in these experiments. ▪, positive result; □, negative result; ∗∗∗, P ≤ 0.001; ∗, P ≤ 0.05.
DISCUSSION
In the present study, a previously described MAb (MAb WB8A11) was used for the isolation and identification of a new antigen, ICD-II of M. bovis BCG. ICD-II is a monomeric-type, NADP+-dependent isocitrate dehydrogenase which catalyzes the oxidation of d-isocitrate to form 2-oxoglutarate, CO2, and NADPH. Although the dimeric isozyme ICD-I is ubiquitous, ICD-II has been identified in a limited number of unrelated bacterial species, including Azotobacter vinelandii (3), Rhodomicrobium vannielii (19), Vibrio parahaemolyticus (12), Vibrio sp. strain ABE-1 (31), and Corynebacterium glutamicum (7). The genes encoding both isozymes are present in the chromosomes of M. tuberculosis and M. bovis. Purification of mycobacterial ICD by combined dye-affinity and ion-exchange chromatographies has previously been reported to yield an antigenic fraction that was recognized by Abs in the sera of TB patients with a sensitivity of 60% and a specificity of 92% in an enzyme-linked immunosorbent assay (24). Analysis of that fraction by SDS-PAGE demonstrated the presence of multiple bands. Additional bands besides the main ICD-II band were also observed in the present study in the immunoaffinity chromatography-purified Ag preparation that reacted with MAb WB8A11 (Fig. 1A). These bands might represent degradation products of ICD-II, but they might also include possible contaminants that bind to MAb WB8A11 and coelute with ICD-II during purification by immunoaffinity chromatography. Therefore, we decided to use the highly purified recombinant protein (Fig. 3A) for the testing of the reactivity of IgG in human serum to this Ag by Western blotting. The results showed that rICD2 is recognized by IgG in the sera of TB patients at serum dilutions up to 1:5,120, with a sensitivity of 50% and a specificity of 86.9% at a serum dilution of 1:1,280. These results are consistent with those previously reported by Öhman and Ridell (24), showing that ICD-II is preferentially recognized by sera from TB patients instead of sera from healthy individuals.
In a previous study, analysis of fractions enriched in components of different cellular compartments of BCG with MAb WB8A11 indicated the presence of an Ag in the cell wall as well as other cellular compartments that reacted with MAb WB8A11 (11). Identification of the ICD-II enzyme suggested that the presence of an Ag in the cell wall fraction that reacted with MAb WB8A11 might be due to protein precipitation during preparation of the fractions. This hypothesis is reinforced by our observation that rICD2 of BCG tends to precipitate after freezing-thawing (data not shown). MAb WB8A11 recognized the recombinant protein, indicating that the epitope that reacts with MAb WB8A11 is present in the recombinant protein, and antibodies in the sera of genetically different mice immunized with the purified native protein also recognized the recombinant protein (Fig. 3).
Considering the limited distribution of ICD-II among prokaryotes and its ability to elicit Ab responses, in order to evaluate its potential use for serodiagnosis of TB we cloned the icd2 gene of BCG, purified the recombinant protein, and tested the recombinant protein for its ability to recognize IgG Abs in the sera of TB patients as well as healthy subjects. Several Ags have been tested for their abilities to recognize Abs in the sera of TB patients and healthy individuals in different laboratories, but no test with a single Ag has proved to be able to achieve sensitivities and specificities comparable to those of isolation by culture and identification of the bacillus in a study population suitably large and heterogeneous (21, 29, 34). Because of the diversity of humoral responses to infection with tubercle bacilli (21, 23, 29), serological tests based on a single Ag have been reported to be unsuited for the diagnosis of TB, as they are unlikely to be confirmatory (25, 32).
Few known Ags of tubercle bacilli in CFs are recognized by Abs in the sera of TB patients and PPD-positive individuals by Western blotting with a serum dilution of 1:200 (28, 29), and recognition of a very limited number of Ags has been observed in PPD-negative healthy individuals even at a serum dilution of 1:50 (29). On the other hand, the pattern of recognition of Ags of tubercle bacilli might be variable depending on the genetic background and other variables of the study population (15, 34), the stage of the disease and the location of the infection (32), and the specific characteristics of the assay (25). A multicomponent serological test might overcome some of the variables mentioned above (13). Ideally, the single components should have complementary properties (5) that allow differentiation of HIV-positive and HIV-negative TB patients (14, 29) and patients with different genetic backgrounds, patients at different stages of the disease, and patients with different locations of infection (29).
The ICD-II protein of tubercle bacilli would need further investigation to confirm its potential for use as a component of a test for the serodiagnosis of TB. The limited reactivity of MAb WB8A11 with CF preparations from a few species of pathogenic mycobacteria (11), together with the results of this study, suggest that ICD-II might contain pathogen-specific epitopes. These hypothetical epitopes might be identified by testing the abilities of Abs in the sera of TB patients as well as healthy donors to recognize partially overlapping synthetic peptides covering the polypeptide sequence of ICD-II.
Acknowledgments
The present study received support from the Istituto Superiore di Sanità, Progetto Tubercolosi, Rome, Italy; EU Biomed II Programme (contract BMH4-CT97-2671); and Progetti M.U.R.S.T. (protocols MM06248818-001 and 2001063758-002), Rome, Italy.
REFERENCES
- 1.Andersen, A. B., and E. B. Hansen. 1989. Structure and mapping of antigenic domains of protein antigen b, a 38,000-molecular-weight protein of Mycobacterium tuberculosis. Infect. Immun. 57:2481-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang, Z., A. Choudhary, R. Lathigra, and F. A. Quiocho. 1994. The immunodominant 38-kDa lipoprotein antigen of Mycobacterium tuberculosis is a phosphate-binding protein. J. Biol. Chem. 269:1956-1958. [PubMed] [Google Scholar]
- 3.Chung, A. E., and J. S. Franzen. 1969. Oxidized triphosphopiridine nucleotide specific isocitrate dehydrogenase from Azotobacter vinelandii: isolation and characterization. Biochemistry 8:3175-3184. [DOI] [PubMed] [Google Scholar]
- 4.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Conner, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M.-A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 5.Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. W. Skeiky, T. Vedvick, R. Badaro, S. G. Reed, and R. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Global burden of tuberculosis. Estimated incidence, prevalence, and mortality by country. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 7.Eikmanns, B. J., D. Rittmann, and H. Sahm. 1995. Cloning, sequence analysis, expression, and inactivation of the Corynebacterium glutamicum icd gene encoding isocitrate dehydrogenase and biochemical characterization of the enzyme. J. Bacteriol. 117:774-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espitia, C., I. Cervera, R. Gonzales, and R. Mancila. 1989. A 38 kDa Mycobacterium tuberculosis antigen associated with infection. Its isolation and serological evaluation. Clin. Exp. Immunol. 77:373-377. [PMC free article] [PubMed] [Google Scholar]
- 9.Foulds, J., and R. O'Brien. 1998. New tools for the diagnosis of tuberculosis: the perspective of developing countries. Int. J. Tuberc. Lung Dis. 2:778-783. [PubMed] [Google Scholar]
- 10.Freer, G., W. Florio, B. Dalla Casa, D. Bottai, G. Batoni, G. Maisetta, S. Senesi, and M. Campa. 1998. Identification and molecular cloning of a novel secretion antigen from Mycobacterium tuberculosis and Mycobacterium bovis BCG. Res. Microbiol. 149:265-275. [DOI] [PubMed] [Google Scholar]
- 11.Freer, G., W. Florio, B. Dalla Casa, B. Castagna, G. Maisetta, G. Batoni, V. Corsini, S. Senesi, and M. Campa. 1998. Characterization of antigens recognized by new monoclonal antibodies raised against culture filtrate proteins of Mycobacterium bovis bacillus Calmette-Guérin. FEMS Immunol. Med. Microbiol. 20:129-138. [DOI] [PubMed] [Google Scholar]
- 12.Fukunaga, N., S. Imagawa, T. Sahara, A. Ishii, and M. Suzuki. 1992. Purification and characterization of monomeric isocitrate dehyderogenase with NADP+-specificity from Vibrio parahaemolyticus. J. Biochem. 112:849-855. [DOI] [PubMed] [Google Scholar]
- 13.Gennaro, M. L. 2000. Immunologic diagnosis of tuberculosis. Clin. Infect. Dis. 30(Suppl. 3):S243-S246. [DOI] [PubMed] [Google Scholar]
- 14.Hendrickson, R. C., J. F. Douglass, L. D. Reynolds, P. D. McNeill, D. Carter, S. G. Reed, and R. L. Houghton. 2000. Mass spectrometric identification of Mtb81, a novel serological marker for tuberculosis. J. Clin. Microbiol. 38:2354-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackett, P. S., G. H. Bothamley, H. V. Batra, A. Mistry, D. B. Young, and J. Ivanyi. 1988. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J. Clin. Microbiol. 26:2313-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laal, S., K. M. Samanich, M. G. Sonnenberg, J. T. Belisle, J. O'Leary, M. S. Simberkoff, and S. Zolla-Pazner. 1997. Surrogate marker of preclinical tuberculosis in human immunodeficiency virus infection: antibodies to an 88-kDa secreted antigen of Mycobacterium tuberculosis. J. Infect. Dis. 176:133-143. [DOI] [PubMed] [Google Scholar]
- 17.Laal, S., K. M. Samanich, M. G. Sonnenberg, S. Zolla-Pazner, J. M. Phadtare, and J. T. Belisle. 1997. Humoral responses to antigens of Mycobacterium tuberculosis: immunodominance of high-molecular-weight antigens. Clin. Diagn. Lab. Immunol. 4:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Leyland, M. L., and D. J. Kelly. 1991. Purification and characterization of a monomeric isocitrate dehydrogenase with dual coenzyme specificity from the photosynthetic bacterium Rhodomicrobium vannielii. Eur. J. Biochem. 202:85-93. [DOI] [PubMed] [Google Scholar]
- 20.Lim, R. L., L. K. Tan, W. F. Lau, M. C. Ming, R. Dunn, H. P. Too, and L. Chan. 2000. Cloning and expression of immunoreactive antigens from Mycobacterium tuberculosis. Clin. Diagn. Lab. Immunol. 7:600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyashchenko, K., R. Colangeli, M. Houde, H. A. Jahdall, D. Menzies, and M. L. Gennaro. 1998. Heterogeneous antibody responses in tuberculosis. Infect. Immun. 66:3936-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Medical Research Council Cardiothoracic Epidemiology Group. 1992. National survey of notifications of tuberculosis in England and Wales in 1988. Thorax 47:770-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris, S. L., L. Bermudez, and S. D. Chaparas. 1991. Mycobacterium avium complex disease in patients with AIDS: seroreactivity to native and recombinant mycobacterial antigens. J. Clin. Microbiol. 29:2715-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Öhman, R., and M. Ridell. 1996. Purification and characterisation of isocitrate dehydrogenase and malate dehydrogenase from Mycobacterium tuberculosis and evaluation of their potential as suitable antigens for the serodiagnosis of tuberculosis. Tuber. Lung Dis. 77:454-461. [DOI] [PubMed] [Google Scholar]
- 25.Pottumarty, S., V. C. Wells, and A. J. Morris. 2000. A comparison of seven tests for serological diagnosis of tuberculosis. J. Clin. Microbiol. 38:2227-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenkrands, I., A. King, K. Weldingh, M. Moniatte, E. Moertz, and P. Andersen. 2000. Towards the proteome of Mycobacterium tuberculosis. Electrophoresis 21:3740-3756. [DOI] [PubMed] [Google Scholar]
- 27.Rosenkrands, I., K. Weldingh, S. Jacobsen, C. V. Hansen, W. Florio, I. Gianetri, and P. Andersen. 2000. Mapping and identification of Mycobacterium tuberculosis proteins by two-dimensional gel electrophoresis, microsequencing and immunodetection. Electrophoresis 21:935-948. [DOI] [PubMed] [Google Scholar]
- 28.Samanich, K. M., J. T. Belisle, M. G. Sonnenberg, M. A. Keen, S. Zolla-Pazner, and S. Laal. 1998. Delineation of human antibody responses to culture filtrate antigens of Mycobacterium tuberculosis. J. Infect. Dis. 178:1534-1538. [DOI] [PubMed] [Google Scholar]
- 29.Samanich, K. M., M. A. Keen, V. D. Vissa, J. D. Harder, J. S. Spencer, J. T. Belisle, S. Zolla-Pazner, and S. Laal. 2000. Serodiagnostic potential of culture filtrate antigens of Mycobacterium tuberculosis. Clin. Diagn. Lab. Immunol. 7:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonnenberg, M. G., and J. T. Belisle. 1997. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect. Immun. 65:4515-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki, M., T. Sahara, J.-I. Tsuruha, Y. Takada, and N. Fukunaga. 1995. Differential expression in Escherichia coli of the Vibrio sp. strain ABE-1 icdI and icdII genes encoding structurally different isocitrate dehydrogenase isozymes. J. Bacteriol. 177:2138-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swaminathan, S., P. Umadevi, S. Shanta, A. Radhakrishnan, and M. Datta. 1999. Sero diagnosis of tuberculosis in children using two ELISA kits. Indian J. Pediatr. 66:837-842. [DOI] [PubMed] [Google Scholar]
- 33.van Pinxteren, L. A. H., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkinson, R. J., K. Hasl/ov, R. Rappuoli, F. Giovannoni, P. R. Narayanan, C. R. Desai, H. M. Vordermeier, J. Paulsen, G. Pasvol, J. Ivanyi, and M. Singh. 1997. Evaluation of the recombinant 38-kilodalton antigen of Mycobacterium tuberculosis as a potential immunodiagnostic reagent. J. Clin. Microbiol. 35:553-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou, A. T., W. L. Ma, P. Y. Zhang, and R. A. Cole. 1996. Detection of pulmonary and extrapulmonary tuberculosis patients with the 38-kilodalton antigen from Mycobacterium tuberculosis in a rapid membrane-based assay. Clin. Diagn. Lab. Immunol. 3:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]