Abstract
The predisposition to develop periodontitis is partly genetically determined in humans as well as in animals. Here we demonstrate, however, that early manipulations in the maternal environment of an animal (rat) model of periodontitis can fully reverse the genetic predisposition to develop periodontitis at adult age.
Periodontitis is a disease of the tooth-supporting tissues that, in the most severe cases, leads to the loss of teeth. The starting point is an abundant growth of naturally occurring gram-negative anaerobic bacteria. Most of the tissue destruction, however, seems to be caused by components released by immune cells in response to the overgrowth of these microorganisms (13). Hence, the bacteria trigger the disease, but immune reactions seem to be responsible for the tissue damage and the outcome and progression of the disease. Studies with humans (14) and animals (1, 2) have demonstrated that individuals differ considerably in their predisposition to periodontitis and that this variation is partly genetic.
However, as in many inflammatory diseases, environmental factors also play an important role. Smoking and diabetes are clear examples of this, as are old age and negative life events, all of which increase the individual's sensitivity. Yet we know of no studies that have examined the effects of early life experiences on susceptibility to periodontitis. To investigate this, we manipulated the maternal environment of newborn rats and looked at the development of periodontitis of the same rats at adult age. We used two genetically distinct rat lines that were originally selected for high and low susceptibility to apomorphine (abbreviated APO-SUS and APO-UNSUS, respectively; for more details, see reference 6). These lines also differ in many other (neuro)physiological aspects (7), one of them being their immunological profile and their predisposition to develop periodontitis. Thus, APO-SUS males generate a stronger, Th2-dependent, immunoglobulin E response than do APO-UNSUS rats after injection with the nematode Trichinella spiralis. By contrast, APO-UNSUS males are more susceptible to Th1-mediated experimental autoimmune encephalomyelitis (11). In line with these results is the finding that the ratio of mRNA expression for gamma interferon (Th1) and interleukin 4 (Th2) is significantly higher in APO-UNSUS than in APO-SUS rats (11). APO-SUS rats are also more susceptible to periodontitis than are APO-UNSUS rats (1), which is not surprising, as there are strong indications that Th1-dominated responses tend to protect against periodontal breakdown, whereas Th2-dominated responses tend to increase periodontitis (15, 18).
In the first experiment we fostered APO-SUS litters to APO-UNSUS mothers (crossfostering). Besides a normal control group that was left undisturbed at birth, an extra group was added in which APO-SUS litters were moved to other but also APO-SUS mothers (infostering). So these animals were reared by mothers other than their biological mothers but of the same genotype. Hence, while the comparison between control and infostered groups gives information about the fostering effect per se, the comparison between infostered and crossfostered animals is informative about the effect of the maternal environment (APO-SUS versus APO-UNSUS). For a detailed description of the crossfostering procedure, see reference 5. Adoption took place approximately 24 h after parturition. In the second experiment APO-UNSUS litters were taken away from their mother for 24 h at day 9 of age (maternal deprivation). Previous experiments have shown that maternal deprivation on this day produces the strongest effects in the Nijmegen Wistar population, from which APO-SUS and APO-UNSUS rats were selected (9). Twenty-four hours later they were put back. Litters were kept in their home cage at room temperature (22 ± 2°C). This two-sided procedure (crossfostering in one line, maternal deprivation in the other) has been shown to influence the original selection criterion in these lines, i.e., the susceptibility to apomorphine. APO-SUS rats are sensitive to crossfostering but not to maternal deprivation. Conversely, APO-UNSUS animals are sensitive to maternal deprivation but not to crossfostering (10). This sensitivity to maternal deprivation is also reflected in a marked increase in the severity of experimental autoimmune encephalomyelitis and an alteration of macrophage reactivity (17).
Periodontitis was experimentally induced by tying a silk ligature around the neck of the maxillary right second molar teeth and measured as the extent of bone loss. This method has been used extensively in different species and accelerates but does not affect the natural development of periodontitis (12, 16), After 7 weeks animals were sacrificed, and the severity of the disease was measured as the extent of bone loss. The exact procedure has been extensively described in a previous study on periodontitis in these rats (1). Data were analyzed using a two-way analysis of variance with gender and treatment as the main factors.
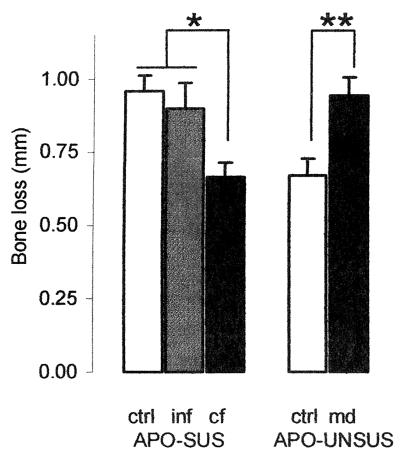
Figure 1 displays the amount of bone loss for control and manipulated APO-SUS and APO-UNSUS rats. Males and females are shown pooled, as no gender differences were observed, either as a main effect or in interaction. Both manipulations appear to affect the susceptibility to periodontitis. While infostered animals did not differ from control animals, susceptible animals raised by unsusceptible mothers showed less bone loss than those raised by susceptible mothers (crossfostered versus infostered: F1,21 = 5.6, P = 0.027). Also, maternal deprivation increased the extent of bone loss in unsusceptible animals (F1,21 = 9.8, P = 0.005). In fact, both manipulations completely overruled the genetic predisposition to minor or major periodontitis. Post hoc analysis using the Duncan multiple-range test supported a distinction between two different subsets of animals (see also Fig. 1; P < 0.05). First, there are the APO-SUS control and infostered groups and the maternally deprived APO-UNSUS animals, which all readily develop periodontitis. Second, there are the (control) APO-UNSUS and crossfostered APO-SUS animals, which develop periodontitis to a lesser extent.
FIG. 1.
Bone loss in breakdown in millimeters (mean ± standard error of the mean) of control and manipulated susceptible (APO-SUS, left panel) and unsusceptible rat lines (APO-UNSUS, right panel). ctrl, control; inf, infostered; cf, crossfostered; and md, maternal deprivation. Males and females were pooled; each group contained at least five litters; 10 to 15 animals per group.
Given the underlying brain-immune system interactions (3, 4), we suspect that these findings are not limited to periodontitis alone. The early maternal environment may affect other inflammatory disorders, as well as more complex diseases (8). More important, perhaps, is that these results indicate that the Damoclean sword of genetically predisposed diseases can be fully shielded by environmental manipulations.
Acknowledgments
F.S. was supported by a PULS Grant (48.001) from the Life Sciences Foundation (SLW), which is subsidized by The Netherlands Organization for Scientific Research (NWO).
REFERENCES
- 1.Breivik, T., F. Sluyter, M. Hof, and A. R. Cools. 2000. Differential susceptibility to periodontitis in genetically selected Wistar rat lines that differ in their behavioral and endocrinological response to stressors. Behav. Genet. 30:123-130. [DOI] [PubMed] [Google Scholar]
- 2.Breivik, T., P. S. Thrane, P. Gjermo, P. Opstad, R. Pabst, and S. von Hoersten. 2001. Hypothalamic-pituitary-adrenal axis activation by experimental periodontal disease in rats. J. Periodontal Res. 36:297-300. [DOI] [PubMed] [Google Scholar]
- 3.Breivik, T., and P. S. Thrane. 2000. Psychoneuroimmune interactions in periodontal disease, p. 627-644. In R. Ader, D. L. Felten, and N. Cohen (ed.), Psychoneuroimmunology, 3rd ed. Academic Press, San Diego, Calif.
- 4.Breivik, T., P. S. Thrane, R. Murison, and P. Gjermo. 1996. Emotional stress effects on immunity, gingivitis and periodontitis. Eur. J. Oral Sci. 104:327-334. [DOI] [PubMed] [Google Scholar]
- 5.Carlier, M., M. Nosten-Bertrand, and C. Michard-Vanhee. 1992. Separating genetic effects from maternal environmental effects, p. 111-125. In D. Goldowitz, D. Wahlsten, and R. Wimer (ed.), Techniques for the genetic analysis of brain and behavior: focus on the mouse, vol. 8. Techniques in the behavioral and neural sciences. Elsevier, Amsterdam, The Netherlands.
- 6.Cools, A. R., R. Brachten, D. Heeren, A. Willemen, and B. Ellenbroek. 1990. Search after neurobiological profile of individual-specific features of Wistar rats. Brain Res. Bull. 24:49-69. [DOI] [PubMed] [Google Scholar]
- 7.Cools, A. R., and M. A. Gingras. 1998. Nijmegen high and low responders to novelty: a new tool in the search after the neurobiology of drug abuse liability. Pharmacol. Biochem. Behav. 60:151-159. [DOI] [PubMed] [Google Scholar]
- 8.Ellenbroek, B. A., and A. R. Cools. 2000. The long-term effects of maternal deprivation depend on the genetic background. Neuropsychopharmacology 23:99-106. [DOI] [PubMed] [Google Scholar]
- 9.Ellenbroek, B. A., P. T. J. M. van den Kroonenberg, and A. R. Cools. 1998. The effects of an early stressful life event on sensorimotor gating in adult rats. Schizophr. Res. 30:251-260. [DOI] [PubMed] [Google Scholar]
- 10.Ellenbroek, B. A., F. Sluyter, and A. R. Cools. 2000. The role of genetic and early environmental factors in determining apomorphine susceptibility. Psychopharmacology 148:124-131. [DOI] [PubMed] [Google Scholar]
- 11.Kavelaars, A., C. J. Heijnen, B. Ellenbroek, H. van Loveren, and A. Cools. 1997. Apomorphine-susceptible and apomorphine-unsusceptible Wistar rats differ in their susceptibility to inflammatory and infectious diseases: a study on rats with group-specific differences in structure and reactivity of hypothalamic-pituitary-adrenal axis. J. Neurosci. 17:2580-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornman, K. S., S. C. Holt, and P. B. Robertson. 1981. The microbiology of ligature-induced periodontitis in the cynomologus monkey. J. Periodontal Res. 16:363-371. [DOI] [PubMed] [Google Scholar]
- 13.Lamster, I. B., and M. J. Novak. 1992. Host mediators in gingival crevicular fluid: implications for the pathogenesis of periodontal disease. Crit. Rev. Oral Biol. Med. 3:31-60. [DOI] [PubMed] [Google Scholar]
- 14.Michalowicz, B. S., D. Aeppli, J. G. Virag, D. G. Klump, J. E. Hinrichs, N. L. Segal, T. J. Bouchard, Jr., and B. L. Pihlstrom. 1991. Periodontal findings in adult twins. J. Periodontol. 62:293-299. [DOI] [PubMed] [Google Scholar]
- 15.Seymour, G. J., E. Gemmell, M. Kjeldsen, K. Yamazaki, T. Nakajima, and K. Hara. 1996. Cellular immunity and hypersensitivity as components of periodontal destruction. Oral Dis. 2:96-101. [DOI] [PubMed] [Google Scholar]
- 16.Svanberg, G., J. Lindhe, A. Hugosson, and H. G. Gröndahl. 1973. Effect of nutritional hyperparathyroidism on experimental periodontitis in the dog. Scand. J. Dent. Res. 81:155. [DOI] [PubMed] [Google Scholar]
- 17.Teunis, M. A. T., C. J. Heijnen, F. Sluyter, J. M. Bakker, M. Hof, A. R. Cools, and A. Kavelaars. Maternal deprivation of rat pups increases clinical symptoms of experimental autoimmune encephalomyelitis at adult age. J. Neuroimmunol., in press. [DOI] [PubMed]
- 18.Tokoro, Y., Y. Matsuki, T. Yamamoto, T. Suzuki, and K. Hara. 1997. Relevance of local Th2-type cytokine mRNA expression in immunocompetent infiltrates in inflamed gingival tissue to periodontal diseases. Clin. Exp. Immunol. 107:166-174. [DOI] [PMC free article] [PubMed] [Google Scholar]