Abstract
Screening of genomic expression libraries from Mycobacterium tuberculosis with sera from tuberculosis (TB) patients or rabbit antiserum to M. tuberculosis led to the identification of novel antigens capable of detecting specific antibodies to M. tuberculosis. Three antigens, Mtb11 (also known as CFP-10), Mtb8, and Mtb48, were tested together with the previously reported 38-kDa protein, in an enzyme-linked immunosorbent assay (ELISA) to detect antibodies in TB patients. These four proteins were also produced as a genetically fused polyprotein, which was tested with two additional antigens, DPEP (also known as MPT32) and Mtb81. Sera from individuals with pulmonary and extrapulmonary TB, human immunodeficiency virus (HIV)-TB coinfections, and purified protein derivative (PPD)-positive and PPD-negative status with no evidence of disease were tested. In samples from HIV-negative individuals, the ELISA detected antibodies in >80% of smear-positive individuals and >60% smear-negative individuals, with a specificity of ∼98%. For this group, smears detected 81.6% but a combination of smear and ELISA had a sensitivity of ∼93%. The antigen combination detected a significant number of HIV-TB coinfections as well as antibodies in patients with extrapulmonary infections. Improved reactivity in the HIV-TB group was observed by including the antigen Mtb81 that was identified by proteomics. The data indicate that the use of multiple antigens, some of which are in a single polyprotein, can be used to facilitate the development of a highly sensitive test for M. tuberculosis antibody detection.
Tuberculosis (TB) is a major disease in developing countries and an increasing problem in developed countries, with an estimated 8 million new cases each year (7, 21, 35). The rise in the number of infections is due to drug-resistant strains of Mycobacterium tuberculosis, the causative agent of TB, and to more incidences of coinfections with human immunodeficiency virus (HIV), particularly in sub-Saharan Africa (21). This has prompted the need to develop rapid, inexpensive, but clinically sensitive and specific tests that can improve upon current diagnostic tests used in TB diagnosis, such as culture and acid-fast smear testing (20). PCR methods are available but are expensive, require culture, and are not applicable to field use (14, 33-34, 42). One such approach to providing simple tests has been to develop adjunctive serology assays. However, previous attempts to diagnose TB by serology have met with limited success (2-3, 6,8, 10-12, 15-16, 23-24, 37-38, 44-47). Many of the antigens to date do not have the appropriate clinical sensitivity and specificity required for an accurate diagnosis and do not always effectively discriminate Mycobacterium bovis BCG-vaccinated and purified protein derivative (PPD)-positive individuals from those with active TB (8, 36). Several notable antigens have been identified which have merit in TB diagnosis, among which is the immunodominant 38-kDa phosphate transport protein (1, 5, 9, 19). This antigen has been used in the early development of commercial assays for TB detection that have a broad range in sensitivity (1, 5, 9, 19, 36). While highly specific for TB, it lacks sensitivity, particularly in the detection of disease in smear-negative but TB-infected individuals, as well as those patients with HIV-TB coinfections. There is clearly a need to identify other antigens of diagnostic utility that can be used in combination with the 38-kDa antigen to improve sensitivity without jeopardizing specificity. To achieve this, we used sera from individuals with active pulmonary and extrapulmonary TB, 38-kDa antibody-negative sera from TB-positive individuals, and a rabbit anti-TB serum to screen genomic expression libraries of M. tuberculosis. From these studies, several novel antigens have been identified that can complement the 38-kDa protein in the serodiagnosis of active TB (4, 13, 17, 28, 43). These antigens have been combined into fusion recombinants (polyproteins) with the 38-kDa antigen. TbF6 is a fusion of the 38-kDa protein with three antigens and TbF10 with two antigens. Both polyproteins were demonstrated to fully represent individual serological reactivity of each component included in the fusion by enzyme-linked immunosorbent assay (ELISA) and a multiantigen print immunoassay (MAPIA). These fusions were used in combination with a proline-rich antigen, DPEP (also known as MPT32) (18, 27) in evaluating sera from patients with active pulmonary TB (acid-fast bacillus [AFB] positive and negative), extrapulmonary TB, or HIV-TB coinfection and PPD-positive individuals with no disease, as well as PPD-negative individuals. In addition, an antigen, Mtb81, was identified by proteomics and shown to further enhance reactivity in the HIV-TB coinfection group (22, 25, 26, 40, 41).
MATERIALS AND METHODS
Bacterial strains.
M. tuberculosis strains H37Rv, H37Ra (ATCC 25177), and Erdman were obtained from Sean Skerritt (Seattle Veterans' Administration Hospital, Seattle, Wash.).
Library preparation and serological expression cloning.
M. tuberculosis Erdman H37Ra and H37Rv genomic DNA was isolated and, in the case of Erdman H37Ra DNA, sheared by sonication to a size range of 0.5 to 4 kb while H37Rv DNA was partially digested with Sau3A1. Libraries were constructed with Lambda Zap II (Stratagene, La Jolla, Calif.) with EcoRI adapters. Expression screening was performed with Escherichia coli lysate-absorbed sera from patients with pulmonary TB or extrapulmonary TB or with a rabbit antiserum raised to M. tuberculosis culture filtrate proteins. In addition, a pool of patient sera shown to be negative for antibodies to the 38-kDa protein was also used. Approximately 3 × 105 PFU was screened for each serum pool with nitrocellulose filters (Schleicher & Schuell, Keene, N.H.) for the presence of immunoreactive proteins. Phagemids were isolated from positive plaques that had been visualized with a goat anti-human or anti-rabbit immunoglobulin alkaline phosphatase detection system (Zymed Laboratories. Inc., South San Francisco, Calif.), and the resulting DNA was sequenced. DNA and protein homology searches were performed against the GenBank database.
Recombinant antigens.
Screening of genomic expression libraries from Mtb with sera from active pulmonary or extrapulmonary TB patients, the 38-kDa protein antibody-negative sera, or with a rabbit antiserum to soluble TB proteins led to the identification of several novel genes. Three of the resulting encoded proteins, Mtb8, -11, and -48, were selected because they were independently reactive with TB sera with high specificity and complemented the immunodominant 38-kDa phosphate transport protein in serological assays for TB. Mtb11 is also known as CFP-10 or MTSA-10 (4, 13, 17, 43). Mtb48 is a proline-rich protein with a 100% match to M. tuberculosis H37Rv genomic DNA (Genbank no. AL022120) and protein sequence (GenBank no. NP218398) (28). Mtb8 shows homology to the protein sequence with GenBank accession no. NP214893. All of the genes of interest were incorporated into pET vector systems with a hexahistidine tag, and the recombinant proteins were expressed in E. coli and purified using an Ni2+-nitrilotriacetic acid affinity column. A proline-rich protein (DPEP) was also identified as necessary for seroreactivity and was also expressed and purified from E. coli. A further antigen (Mtb81) was identified from culture filtrate proteins by two-dimensional gel electrophoresis and mass spectrometry and was shown to be reactive with HIV-TB coinfection sera (22, 25, 26). Its sequence shows homology to malate synthase (22).
Based on the serological reactivity of these individual antigens, which will be described, a polyprotein (TbF6), was designed to incorporate four of the genes of interest. Mtb8, the 38-kDa protein, Mtb11, and Mtb48 were incorporated into a single expression vector to minimize the number of individual recombinant antigens necessary to formulate the serological assays (Fig. 1). This fusion construct (TbF6) was made and purified as follows. Pfu DNA polymerase (Stratagene) was used to PCR amplify each of the constructs. The Mtb8 PCR fragment was digested with NdeI and EcoRI and cloned directly into a modified pET vector with NdeI and EcoRI sites. The 38-kDa PCR fragment was digested with Sse8387I, treated with T4 DNA polymerase to make blunt ends, and then digested with EcoRI for direct cloning into the pT7Δ L2Mtb8-1 vector, which was digested with StuI and EcoRI. The Mtb11 PCR fragment was digested with Eco47III and EcoRI and directly subcloned into pT7ΔL2Mtb8/38 kDa-17 digested with the same enzymes. The whole fusion was then transferred to a modified pET vector with NdeI and EcoRI sites. The Mtb48 fragment was digested with EcoRI and ScaI and cloned directly into the pET28 Mtb8/38 kDa/Mtb11 construct that was digested with DraI and EcoRI. The fusion construct was then confirmed by DNA sequencing. The expression construct was transformed to BLRpLysS E. coli (Novagen, Madison, Wis.) and grown overnight in Luria-Bertani broth with kanamycin and chloramphenicol. This culture was then used to inoculate 2XYT medium (16 g of Tryptone/liter, 10 g of yeast extract/liter, and 5 g of NaCl/liter) with the same antibiotics and induced with IPTG (isopropyl-Â-d-thiogalactopyranoside). Induced bacteria were harvested by centrifugation and disrupted by sonication in Tris-buffered saline, and the recombinant protein was concentrated by centrifugation. The recombinant TbF6 construct was solubilized from the bacterial pellet in Tris-buffered saline with 8 M urea, after the pellet was first washed with 0.5% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) in 20 mM Tris (pH 8), and isolated by Ni-nitrilotriacetic acid metal ion affinity chromatography using increasing imidazole concentrations to elute the protein (28). Active fractions were combined and dialyzed with 10 mM Tris-HCl (pH 8). Similarly, a fusion polyprotein, TbF10, was prepared by linking Mtb8, Mtb11, and the 38-kDa antigen in a single recombinant protein. M. tuberculosis lysate was prepared as described in reference 22. Southern blot analysis of Mtb48 (28), Mtb8, Mtb11 (28), and Mtb81 was performed with a panel of DNA from Mycobacterium leprae, Mycobacterium smegmatis, Mycobacterium vaccae, Mycobacterium gordonae, Mycobacterium chelonae, Mycobacterium fortuitum, Mycobacterium scrofulaceum, and Mycobacterium avium and shown to be negative on the DNA panel for other mycobacteria while being positive for H37Rv, H37Ra, Erdman, and C strains of M. tuberculosis.
FIG. 1.
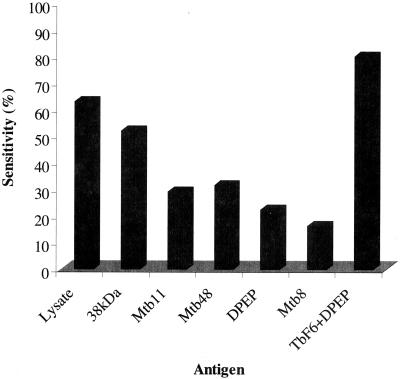
Sensitivity for individual antigens and the combination of TbF6 and DPEP of a Brazilian population of smear-positive (n = 105) and -negative (n = 39) TB sera.
ELISA.
ELISA for Mtb antigens was performed as follows. Ninety-six-well microtiter plates (Corning Easiwash) were coated overnight at 4°C with either Mtb8 (100 ng/well); Mtb48 (or the N-terminal portion of TbH4), Mtb11, the 38-kDa antigen, Mtb81, TbF10, or TbF6 at 200 ng/well; and DPEP at 500 ng/well. Plates were then aspirated, blocked with phosphate-buffered saline (PBS) containing 1% (wt/vol) bovine serum albumin for 2 h at room temperature, and then washed in PBS containing 0.1% Tween 20 (PBST). Serum (1/100) diluted in PBST was added to the wells and incubated for 30 min at room temperature. The plates were washed six times with PBST and then incubated with protein A-horseradish peroxidase conjugate at a 1/20,000 dilution for a further 30 min. The plates were washed six times with PBST and then incubated with tetramethylbenzidine substrate for a further 15 min. The reaction was stopped by the addition of 1 N sulfuric acid, and the plates were read at an optical density of 450 nm with an ELISA plate reader. The cutoff value for the assays was determined from the mean of the negative population plus 3 standard deviations of the mean.
MAPIA.
MAPIA was performed as described previously (32). Briefly, antigens were immobilized on a nitrocellulose membrane (Schleicher & Schuell) at a protein concentration of 0.05 mg/ml by using a semiautomated airbrush printing device (Linomat IV; Camag Scientific, Inc., Wilmington, Del.). The membrane was cut perpendicular to the antigen bands into 4-mm-wide strips. The strips were blocked for 1 h with 1% nonfat skim milk in PBS with 0.05% Tween 20 and then incubated for 1 h with serum samples diluted 1:50 in blocking solution. After being washed, the strips were incubated for 1 h with alkaline phosphatase-conjugated anti-human immunoglobulin G antibody (Sigma Chemical Co., St. Louis, Mo.) diluted 1:5,000 in blocking solution, followed by another washing step. Bound antibodies were visualized with 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (BCIP-NBT) (Kirkegaard & Perry Laboratories, Gaithersburg, Md.).
Study population.
Serum samples were obtained from individuals who had pulmonary tuberculosis alone prior to treatment (culture and/or AFB smear positive) or had documented coinfections with HIV as evidenced by a positive HIV-1 and/or −2 antibody ELISA. These included samples from Brazil (n = 144) (Roberto Badaro, Federal University of Bahia, Salvador, Brazil), sub-Saharan Africa (n = 66; Uganda and South Africa) (Milton Tam and Deborah Burges, PATH, Seattle, Wash., and Robert Cole, Frenchs Forest, Australia), the Philippines (n = 51) (Babi Tan, American Leprosy Foundation, Cebu City, Philippines), and Turkey (n = 11) (Nevin Turgay, Ege University Medical School, Borna-Iznir, Turkey). Samples from patients with lung cancer and pneumonia (China) (n = 44) were obtained from Robert Cole (Amrad-ICT, Frenchs Forest, Australia). To further evaluate the specificity of the antigens, we obtained sera from individuals that were (i) PPD positive (>10 mm) but culture and clinically and radiographically negative for TB or (ii) PPD negative (Charles Nolan and David Lewinsohn, King County TB Clinic, Seattle, Wash., and Oregon Health Sciences University, Portland, Oregon) (n = 86). Samples from healthy United States blood donors (n = 339) were obtained from Boston Biomedica (West Bridgewater, Mass.), and sera from individuals who were HIV seropositive alone were obtained from Robert Ackridge (Fred Hutchinson Cancer Research Institute, Seattle, Wash.).
RESULTS
M. tuberculosis antigens.
Genomic DNA expression libraries, representing the H37Ra, H37Rv, or Erdman strains were screened with either a panel of sera from TB patients (pulmonary, extrapulmonary, or 38-kDa antibody negative) or sera from rabbits immunized with Mtb culture filtrates containing antigens that were secreted or shed (17, 28, 46). The purpose was to search for antigens that could aid diagnosis of TB by augmenting the reactivity of the immunodominant 38-kDa protein. This protein has been shown to have sensitivity varying from 27 to 89% in various groups (36). Several criteria for selecting antigens of serodiagnostic utility were used. First, the gene is specific for M. tuberculosis (e.g., by Southern blot analysis). Second, by ELISA and/or Western blotting, the antigens are complementary to the 38-kDa antigen in detecting active TB. Third, they are nonreactive with sera from patients who have been BCG vaccinated or individuals who are PPD positive and do not have active TB. Fourth, they improve detection of disease from smear-negative, extrapulmonary TB, and HIV-TB coinfection samples. The aim of this strategy was to define a multiantigen cocktail that capable of differentiating active TB from that in patients with latent infection or those who were BCG vaccinated. Several recombinants were derived which could not only supplement 38-kDa antigen reactivity in serological testing of TB patients but could also be combined effectively with this antigen in a polyprotein to form the basis of a unique serological assay. Several of these antigens also detected the presence of TB in individuals who were AFB negative. Studies were also performed with samples from PPD-positive individuals who had no active disease as well as those from PPD-negative individuals to verify that the antigens were specific for active infection. Four recombinant proteins designated Mtb8, Mtb11, DPEP, and Mtb48 were initially of particular interest in combination with the 38-kDa antigen. Mtb8 reacted with the rabbit anti-soluble protein antiserum but also reacted with a small but discrete population of pulmonary TB sera. Mtb11 was isolated with a pool of TB sera that were negative for antibodies to the 38-kDa antigen. DPEP and the N-terminal portion of Mtb48 were both proline-rich proteins identified with a pool of high-titer pulmonary TB sera. In a subsequent screen with extrapulmonary TB sera, the C-terminal sequence of Mtb48 that overlapped the N-terminal portion was identified, and the full-length Mtb48 was predicted, constructed, and later expressed (28).
ELISA evaluation using multiple antigens.
Initial evaluation of the novel recombinant antigens by ELISA indicated that while many TB sera reacted with several antigens, there were sera that were more restricted in their response and only reacted with discrete antigens. The complexity of the immune response to TB antigens has been described by others for known TB antigens but not for these novel proteins (26, 29-32, 39-41). The diversity is demonstrated in Table 1 for representative sera and their reactivity with individual antigens (the 38-kDa antigen, Mtb11, Mtb8, Mtb48, and DPEP. Clearly, some sera primarily reacted with individual antigens, e.g., serum samples 387004 (the 38-kDa antigen), 300004 (Mtb48), 274004 (Mtb8), 308004 (Mtb11), and 528004 (DPEP). In general, however, most sera reacted with a variety of different antigens (e.g., serum samples 254004, 258004,and 263004). Previous evaluations of serological responses indicate a heterogeneous response to TB antigens (29-32). More recently, there have been indications that the responses are more homogeneous in that there is a response to a distinct repertoire of antigens, but in varying degrees depending on the extent of disease (39). With the antigens described in Table 1, we can clearly identify sera that react almost exclusively with a single antigen. However, the majority of the sera tested react with multiple antigens. The reactivity with the fusion recombinants TbF6 and TbF10 in the presence and absence of DPEP that are also listed in Table 1 will be discussed later.
TABLE 1.
Evaluation of serum samples from healthy donors and patients with TB (smear positive and negative)a
| Serum sample no. | Patient status | Reactivity by:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smear | ELISA (OD450)
|
|||||||||||
| Individual antigen
|
Fusion
|
|||||||||||
| Tb Lysate | 38 kDa | Mtb11 | Mtb8 | Mtb48 | DPEP | TbF10 | TbF6 | F10-DPEP-Mtb48 | TbF6 + DPEP | |||
| 254004 | TB | − | 2.139 | 0.965 | 0.102 | 0.173 | 0.296 | 1.591 | 0.482 | 1.01 | 1.666 | 2.859 |
| 258004 | TB | ++ | 2.225 | 3.154 | 0.197 | 2.865 | 0.568 | 0.045 | 2.500 | 3.592 | 2.587 | 3.619 |
| 263004 | TB | +++ | 2.543 | 1.444 | 1.155 | 0.097 | 0.469 | 1.117 | 2.017 | 2.337 | 2.136 | 3.04 |
| 264004 | TB | + | 2.147 | 0.615 | 0.242 | 0.059 | 0.708 | 0.233 | 0.318 | 1.028 | 0.699 | 1.716 |
| 266004 | TB | ++ | 1.369 | 2.395 | 0.096 | 2.727 | 0.065 | 0.231 | 2.391 | 3.506 | 2.390 | 3.552 |
| 301004 | TB | +++ | 0.661 | 2.565 | 0.049 | 0.02 | 0.397 | 0.135 | 1.781 | 3.184 | 1.718 | 3.227 |
| 322004 | TB | − | 1.12 | 0.781 | 0.046 | 0.024 | 0.692 | 0.152 | 0.632 | 1.409 | 1.109 | 2.234 |
| 546004 | TB | + | 1.88 | 2.191 | 0.013 | 2.117 | 0.195 | 0.228 | 1.924 | 3.028 | 1.903 | 3.398 |
| 269004 | TB | − | 1.518 | 2.217 | 0.017 | 0.014 | 0.336 | 0.051 | 0.875 | 0.783 | 1.203 | 2.316 |
| 305004 | TB | − | 1.755 | 1.201 | 0.073 | 0.068 | 0.123 | 0.142 | 0.494 | 0.957 | 0.212 | 0.678 |
| 330004 | TB | − | 2.047 | 1.87 | 0.021 | 0.056 | 0.147 | 0.12 | 1.303 | 2.21 | 1.443 | 2.626 |
| 387004 | TB | − | 0.612 | 2.09 | 0.089 | 0.023 | 0.023 | 0.04 | 1.228 | 2.051 | 1.231 | 2.395 |
| 261004 | TB | − | 2.565 | 0.073 | 0.034 | 0.018 | 1.101 | 0.233 | 0.040 | 0.556 | 0.640 | 1.102 |
| 283004 | TB | − | 0.601 | 0.239 | 0.073 | 0.015 | 0.604 | 0.344 | 0.048 | 1.059 | 0.791 | 1.658 |
| 298004 | TB | + | 1.4 | 0.233 | 0.06 | 0.028 | 1.973 | 0.141 | 0.063 | 2.463 | 1.138 | 2.802 |
| 300004 | TB | +++ | 1.048 | 0.082 | 0.045 | −0.004 | 0.624 | 0.106 | 0.688 | 1.54 | 0.955 | 1.714 |
| 320004 | TB | ++ | 0.452 | 0.114 | 0.033 | −0.025 | 0.757 | 0.179 | 0.056 | 1.034 | 0.744 | 1.291 |
| 445004 | TB | ++ | 0.621 | 0.238 | 0.182 | 0.078 | 2.129 | 0.086 | 0.127 | 1.657 | 1.433 | 1.918 |
| 274004 | TB | − | 1.794 | 0.101 | 0.012 | 3.054 | 0.035 | 0.354 | 2.376 | 3.483 | 2.599 | 3.573 |
| 312004 | TB | − | 1.327 | 0.076 | 0.011 | 0.763 | 0.271 | 0.118 | 1.010 | 1.802 | 1.088 | 1.981 |
| 420004 | TB | − | 2.133 | 0.121 | 0.008 | 1.511 | 0.121 | 0.041 | 1.906 | 3.214 | 2.051 | 3.322 |
| 276004 | TB | + | 2.253 | 0.063 | 2.094 | 0.023 | 0.454 | 0.078 | 2.013 | 2.457 | 2.049 | 2.094 |
| 308004 | TB | − | 1.433 | 0.072 | 2.859 | 0.036 | 0.242 | 0.142 | 2.509 | 3.067 | 2.522 | 2.898 |
| 416004 | TB | − | 1.459 | 0.451 | 1.014 | 0.036 | 0.689 | 0.291 | 1.261 | 1.745 | 1.532 | 2.239 |
| 424004 | TB | − | 0.992 | 0.113 | 1.561 | 0.033 | 0.113 | 0.17 | 1.858 | 2.807 | 1.937 | 2.942 |
| 464004 | TB | ++ | 0.876 | 0.234 | 0.753 | 0.021 | 0.318 | 0.098 | 0.914 | 1.524 | 1.127 | 1.887 |
| 495004 | TB | ++ | 0.685 | 0.054 | 1.236 | 0.03 | 0.063 | 0.052 | 1.276 | 1.616 | 1.434 | 1.702 |
| 270004 | TB | − | 1.269 | 0.058 | 0.022 | 0.046 | 0.082 | 0.672 | 0.021 | 0.132 | 0.168 | 0.694 |
| 303004 | TB | +++ | 1.297 | 0.057 | 0.021 | −0.004 | 0.81 | 0.604 | 0.011 | 0.923 | 1.167 | 1.508 |
| 326004 | TB | − | 2.116 | 0.077 | 0.042 | −0.019 | 0.386 | 0.634 | 0.122 | 0.975 | 0.572 | 1.564 |
| 410004 | TB | + | 0.614 | 0.225 | 0.063 | 0.03 | 0.198 | 1.135 | 0.133 | 0.392 | 1.671 | 2.483 |
| 411004 | TB | − | 1.667 | 0.096 | 0.008 | 0.111 | 0.042 | 0.433 | 0.103 | 0.241 | 0.566 | 1.053 |
| 421004 | TB | − | 0.896 | 0.068 | 0.029 | 0.222 | 0.062 | 0.78 | 0.176 | 0.307 | 0.989 | 1.522 |
| 528004 | TB | − | 0.163 | 0 | 0.037 | −0.051 | 0.074 | 1.359 | 0.004 | 0.025 | 0.225 | 1.044 |
| A6-1 | Donor | 0.329 | 0.042 | 0.059 | 0.024 | 0.171 | 0.025 | 0.029 | 0.147 | 0.111 | 0.123 | |
| A6-2 | Donor | 0.159 | 0.055 | 0.057 | 0.101 | 0.106 | 0.032 | 0.012 | 0.104 | 0.086 | 0.122 | |
| A6-3 | Donor | 0.678 | 0.033 | 0.037 | 0.03 | 0.208 | 0.032 | 0.013 | 0.095 | 0.098 | 0.11 | |
| A6-4 | Donor | 0.108 | 0.04 | 0.018 | 0.031 | 0.062 | 0.024 | 0.005 | 0.056 | 0.037 | 0.079 | |
| A6-5 | Donor | 0.272 | 0.066 | 0.066 | 0.091 | 0.154 | 0.051 | 0.031 | 0.163 | 0.062 | 0.285 | |
| A6-6 | Donor | 0.079 | 0.031 | 0.016 | 0.015 | 0.025 | 0.061 | 0.001 | 0.078 | 0.010 | 0.089 | |
| A6-7 | Donor | 0.151 | 0.019 | 0.023 | 0.002 | 0.044 | 0.056 | 0.017 | 0.099 | 0.048 | 0.13 | |
| A6-8 | Donor | 0.336 | 0.056 | 0.061 | 0.039 | 0.029 | 0.149 | 0.034 | 0.116 | 0.055 | 0.214 | |
| A6-9 | Donor | 0.167 | 0.158 | 0.043 | 0.038 | 0.031 | 0.026 | 0.177 | 0.153 | 0.108 | 0.121 | |
| A6-10 | Donor | 0.077 | 0.022 | 0.031 | 0.016 | 0.045 | 0.041 | 0.012 | 0.051 | 0.084 | 0.096 | |
A subset of TbF6-DPEP-positive sera was assayed with the recombinant antigens and lysate to demonstrate the restricted reactivity of some sera to discrete antigens. Values in italic and boldface type represent sera positive for a particular antigen. Boldface numbers outline examples of sera that are restricted in some cases to an individual antigen.
It is also evident from these studies that a cocktail of the antigens would improve the overall sensitivity of the detection of antibodies in TB-infected individuals. Similar reactivity profiles were seen in Western blot analyses with the individual antigens of interest (data not shown). These studies also indicated the need to include DPEP in a cocktail because of its reactivity with extrapulmonary sera. Typical sensitivities seen with each of the antigens in serum samples from a Brazilian TB cohort prior to treatment or in early stages of treatment (<1 month) for pulmonary TB is shown in Fig. 1. This group includes 39 smear-negative and 105 smear-positive samples. While the 38-kDa antigen was the most immunodominant, detecting 51.7% of sera (lysate, 62.7%), the other antigens detected discrete subpopulations of TB antibodies in sera, as seen in Fig. 1. Mtb8, -11, and -48 and DPEP reacted with 16.2, 28.9, 31.3, and 22.2% of TB-positive sera, respectively. As indicated in Table 1, the theoretical combination of such antigens should lead to incremental improvements in the sensitivity of serological assays for TB. To best achieve this goal and to facilitate serological assay development, a polyprotein was constructed that combined three of the novel antigens (Mtb8, Mtb11, and Mtb48) together with the 38-kDa antigen in a single recombinant protein (TbF6). An additional polyprotein, TbF10, was also prepared that only incorporated Mtb8, Mtb11, and the 38-kDa antigen. All attempts to incorporate a fully active DPEP into the polyprotein were unsuccessful. The data presented below describe extensive studies with TbF6 as well as data substantiating the functionality of TbF10.
Performance of multiantigen polyproteins by ELISA-MAPIA. (i) ELISA sensitivity.
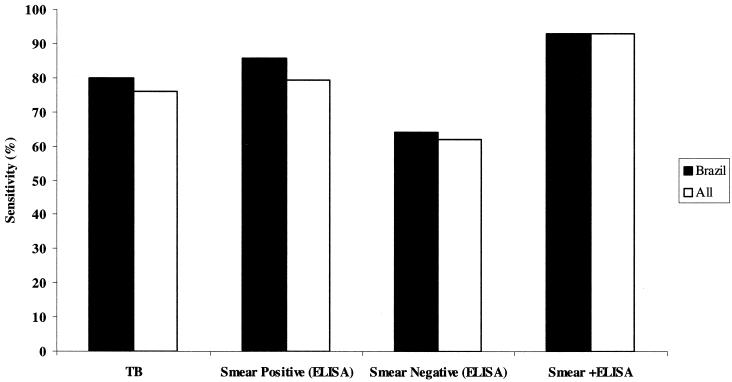
The fusion polyproteins TbF6 and TbF10, containing three or all of the four antigens Mtb8, the 38-kDa antigen, Mtb11, and Mtb 48, were constructed (Fig. 2), expressed, and protein purified as described in Materials and Methods. The resulting polyproteins had molecular weights of 102 (TbF6) and 55 (TbF10) kDa and included an N-terminal hexahistidine tag for ease of purification. Extensive serological studies were then performed with TbF6 alone and in combination with DPEP to determine sensitivity and specificity compared to the individual antigens. TbF10 was also evaluated to confirm reactivity of the individual components. Table 1 shows a subset of samples used to describe the additive effects of the four independent antigens and the ability of the polyproteins to detect the same samples as the individual antigens that they include. It is evident TbF6 has all of the attributes of the individual proteins Mtb8, Mtb11, Mtb48, and 38 kDa included in the polyprotein, whereas TbF10 only reflects the activity of Mtb8, Mtb11, and the 38-kDa protein antigens. To demonstrate the additive effects of the different antigens in this particular subset of sera, Mtb8, Mtb11, Mtb48, and the 38-kDa antigen detected 8, 10, 18, and 18 of 34 serum samples, respectively, whereas TbF10 detected 23 of 34 and TbF6 detected 32 of 34 samples. A combination of the individual activities would predict detection of 31of 34 samples. The inclusion of DPEP in the mixture allowed the detection of a further 2 sera for a total of 34 of 34 samples. A combination of TbF10 with DPEP and Mtb48 (33 of 34 serum samples) had reactivity similar to that of TbF6 plus DPEP. For ELISA, however, it was decided to pursue the TbF6-DPEP combination. A summary of the overall sensitivity and specificity of this antigen combination in sera of TB smear-positive and -negative individuals as well as PPD-positive and PPD-negative individuals from wide geographical areas and its activity relationship to the immunodominant 38-kDa antigen is shown in Table 2 and Fig. 3. The sensitivity of the antigen combination (TbF6-DPEP) in ELISA to detect smear-positive and -negative sera indicated that ∼86% of Brazilian, 75% of Phillipines, 82% of Turkish and 71.2% of sub-Saharan African smear-positive samples were detected; in all cases, >60% of smear-negative samples (Brazil and Philippines) were detected. This might indicate geographical differences in the antibody responses to the antigens used in the ELISA, due to strain differences, the overall health of the population (HIV infections and socioeconomic factors), or accurate recording of HIV status or stage of treatment. A combination of TbF6-DPEP ELISA and smear analysis, however, for all the HIV-negative TB-positive individuals had a sensitivity of 93.0% (253 of 272 samples) compared to 81.6% (222 of 272 samples) for smear alone, demonstrating the improved sensitivity when both tests are used adjunctively. The data also clearly demonstrate that a combination of the four antigens in TbF6 in conjunction with DPEP in a cocktail provides additive increases in sensitivity beyond that seen with the antigens when used individually (see Fig. 1).
FIG. 2.
Schematic for the structure of the polyproteins TbF6 and TbF10.
TABLE 2.
Serological reactivity of TB antigen combinations in smear-positive and smear-negative TB (HIV-negative) patients and healthy PPD-positive and PPD-negative individuals
| Patient status and origin | n | Smear | No. of samples positive by ELISAa
|
||
|---|---|---|---|---|---|
| 38 kDa | TbF6 | TbF6 + DPEP (%) | |||
| TB | |||||
| Brazil | 105 | + | 60 | 82 | 90 (85.7) |
| 39 | − | 14 | 21 | 25 (64.1) | |
| Total | 144 | 74 | 103 | 115 (79.8) | |
| Philippines | 40 | + | 14 | 21 | 30 (75.0) |
| 11 | − | 4 | 5 | 6 (54.6) | |
| Total | 51 | 18 | 26 | 36 (70.6) | |
| Turkey | 11 | + | 9 | 9 | 9 (81.8) |
| Sub-Saharan Africa | 66 | + | 27 | 44 | 47 (71.2) |
| Class II TB (PPD positive) | |||||
| Africa | 3 | − | 0 | 0 | 0 |
| Europe-Asiab | 6 | − | 0 | 0 | 0 |
| Southeast Asia | 7 | − | 2 | 1 | 0 |
| United States-Latin America | 41 | − | 3 | 2 | 3c |
| Total | 57 | 5 | 3 | 3 | |
| Class 0 or I (PPD negative) | |||||
| Africa | 1 | − | 0 | 0 | 0 |
| Europe-Asiab | 3 | − | 0 | 0 | 0 |
| Southeast Asia | 3 | − | 0 | 0 | 0 |
| United States-Latin America | 22 | − | 1 | 1 | 1c |
| Total | 29 | 1 | 1 | 1 | |
| Healthy donor, United States | 7/339 | 5/299 | 5/299 | ||
Positive values were greater than the mean + 3 SD of the healthy PPD-negative sera.
Primarily from Russia and its satellite regions endemic for TB.
At or near cutoff for ELISA.
FIG. 3.
Sensitivity of TbF6-DPEP ELISA for detection of smear-positive and -negative individuals and a combination of ELISA and smear analysis for Brazilian sera and samples from all geographical locations combined.
(ii) ELISA specificity.
Table 2 also shows the specificity of TbF6 alone or mixed with DPEP in ELISA of samples from PPD-positive and -negative individuals as well as samples from a group of healthy blood donors. For the group of healthy blood donors, the overall specificity was 98.3%. Within the small group of PPD-positive individuals presenting at a TB clinic, the specificity was 94.7%; in the PPD-negative group, specificity was 96.6%. In all cases, the reactivity was borderline, based on a cutoff of the mean plus 3 standard deviations.
(iii) MAPIA.
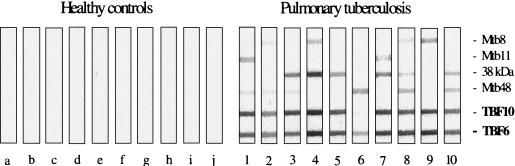
TbF6 and TbF10 were tested in MAPIA to (i) further establish the functionality of each of the component antigens and (ii) demonstrate that they work on nitrocellulose as a prelude to potentially configuring a rapid lateral-flow test. Figure 4 demonstrates the functionality of each of the antigens in the polyproteins TbF6 and TbF10 when compared to the individual antigens and further demonstrates the heterogeneity of responses to individual antigens. From the intensity of band patterns, it is also indicated that in several sera the response of the polyprotein represents the additive effect of the individual antigens. In serum sample 6, Mtb48 (as predicted) was most reactive with TbF6, as this antigen is absent in TbF10. The data also clearly show the utility of these polyproteins in nitrocellulose membrane assays.
FIG. 4.
MAPIA of TbF6 and TbF10 and their component antigens with sera from healthy individuals (n = 10) (lanes a to j) and those with pulmonary TB (n = 10) (lanes 1 to 10).
Antigen detecting TB in HIV coinfections.
A gene encoding a high-molecular-weight antigen, Mtb81, with homology to malate synthase was identified by serological proteome analysis (22). Briefly, two-dimensional gel electrophoresis coupled with mass spectrometry was used to identify peptide sequences in a protein complex binding to the monoclonal antibody IT57 (22). This, in turn, enabled the open reading frame for the protein to be identified from the TB genome. The encoded protein of an ∼81-kDa molecular mass was expressed in E. coli, and the resulting protein, which did not react with IT57, was evaluated by ELISA. This protein was clearly superior to all the other recombinants in its ability to detect TB in HIV-infected individuals and will be included in the panel of antigens for the development of the rapid test (22). The ELISA reactivity of the Mtb81 antigen is shown in Table 3. This antigen detected TB antibodies in samples from patients from Africa with HIV-TB coinfections, a sample population that typically had low sensitivity to other commercial antibody tests. The data shown in Table 3 with the Mtb 81 antigen clearly indicate that this antigen reacts better with HIV-TB coinfection sera than the TbF6-DPEP mixture and will clearly improve ELISA reactivity in detection of TB in this population, especially when used in combination. The theoretical sensitivity of the combination of antigens in the TbF6-DPEP cocktail and Mtb81 is 84.4% (54 of 64 samples) in the HIV sera tested and 84.9% (56 of 66 samples) in the HIV-negative TB sera.
TABLE 3.
Sensitivity of TbF6 in combinations with Mtb81 and DPEP with HIV-TB sera (pretreatment) and sera from other disease categories
| Patient statusa | Source | n | Smear | No. (%) positive
|
||
|---|---|---|---|---|---|---|
| TbF6 + DPEP | Mtb81 | TbF6 + DPEP + Mtb81 | ||||
| HIV+-TB+ | Sub-Saharan Africac | 59 | + | 29 | 46 | 49 |
| 5 | − | 1 | 5 | 5 | ||
| Total | 64 | 30 (46.9) | 51 (79.7) | 54 (84.4) | ||
| HIV−-TB+ | Sub-Saharan Africa | 66 | + | 47 (71.2) | 38 (57.6) | 56 (84.8) |
| HIV+-TB− | United States | 11 | 0 | 0 | 0 | |
| PPD+b | Africa-Europe-Asia-Americas | 57 | 3 | 2 | 4 | |
| PPD−b | Africa-Europe-Asia-Americas | 29 | 1 | 0 | 1 | |
| Lung cancer | China | 13 | 0 | 0 | 0 | |
| Bone cancer | China | 4 | 0 | 0 | 0 | |
| Non-TB lung infections | China/Caucasian | 18 | 0 | 1 | 1 | |
| Healthy | China/Caucasian | 9 | 0 | 0 | 0 | |
+, positive; −, negative.
See Table 2 for geographical distribution.
Includes 2 extrapulmonary TB sera and 27 sera previously shown to be negative to the 38-kDa antigen.
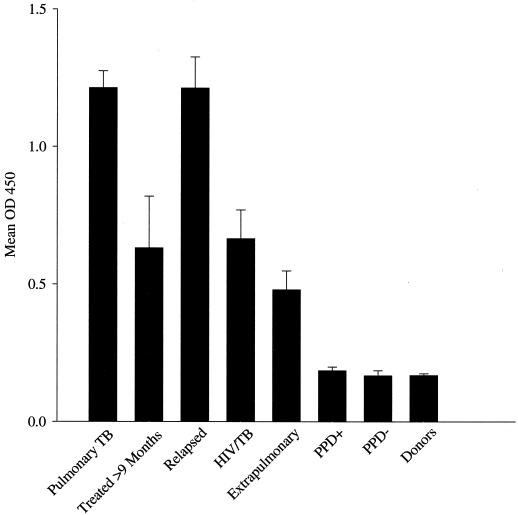
Figure 5 shows the mean ELISA signal for TbF6-DPEP seen in sera from individuals with pulmonary TB prior to treatment, posttreatment, and during relapse. The data are indicative of decreasing antibody titers upon treatment but with rapid increase upon relapse. Figure 5 also indicates that the ELISA signals for the combination of TbF6 and DPEP in the HIV-TB and extrapulmonary sera tested are lower than those seen in individuals with pulmonary TB alone, indicating lower but detectable antibody titers in samples from these individuals than in the PPD-positive and -negative samples.
FIG. 5.
TbF6-DPEP ELISA activity of different patient populations. Data are presented as the mean optical densities at 450 nm (OD450) ± the standard errors of the mean (error bars) for sera from patients as follows, from left to right: with pulmonary TB before treatment, after >9 months of treatment, or at relapse; with HIV-TB coinfections; with extrapulmonary TB; PPD-positive individuals without TB; PPD-negative individuals; and healthy donors in the United States.
DISCUSSION
The data presented show that we have identified several antigens that augment the 38-kDa antigen in the serodiagnosis of TB. This was achieved by screening genomic expression libraries with pulmonary and extrapulmonary TB sera, 38-kDa antibody-negative sera, and a rabbit antibody to TB-soluble proteins. In particular, four antigens (Mtb8, Mtb11, Mtb48, and DPEP) were identified and subsequently expressed as recombinant proteins. These antigens were extensively tested with a large panel of sera. A fifth antigen, Mtb81, was subsequently identified by two-dimensional gel electrophoresis in combination with mass spectrometry and also expressed as a recombinant. This antigen was found to be particularly effective in the serodiagnosis of HIV-TB coinfections. The reactivity of each of these antigens with smear-negative and smear-positive pulmonary TB sera and extrapulmonary sera is presented.
Mtb8, Mtb11, and Mtb48 were each shown to react by ELISA with subpopulations of sera from TB patients, consistent with other observations that antibody responses in TB patients are heterogeneous (29). In addition, all three were shown to provide an incremental increase in sensitivity for TB detection when used in conjunction with the 38-kDa antigen (Table 1), indicating the need for appropriate multiantigen cocktails to increase sensitivity (30). Further increases in clinical sensitivity were achievable with the addition of the proline-rich antigen DPEP.
To facilitate assay development and cost when using multiple antigens, we have combined several antigens into multiepitope polyproteins. Once such polyprotein including three of these antigens (Mtb8, Mtb11, and Mtb48) in combination with the 38-kDa antigen (TbF6) was constructed and expressed in E. coli. This multiepitope polyprotein was evaluated by ELISA alone and in combination with the proline-rich protein DPEP to determine if the sensitivity was increased by the combination of these antigens. The ELISA data (Table 1) showed that while TbF6 provided the sensitivity expected based on its four component antigens, the 38-kDa antigen, Mtb11, Mtb8, and Mtb48, a further increase in sensitivity could be observed with the addition of DPEP into the ELISA well with TbF6. Similar sensitivity was achievable with TbF10 (the 38-kDa antigen and Mtb11 and Mtb8) in a cocktail together with Mtb48 and DPEP (Table 1). The functionality of the individual antigens in the polyproteins TbF6 and TbF10 was also demonstrated in the membrane-based MAPIA assay, indicating their potential use in developing rapid lateral-flow formats (Fig. 4). In addition, the specificity of the TbF6 and TbF10 antigens was tested by MAPIA with 12 sera from mycobacteria other than TB and shown to be negative (data not shown).
Further increases in sensitivity are predicted if Mtb81 was used in cocktails with the polyproteins. The theoretical inclusion of Mtb81 in the antigen composition (which was tested by ELISA alone) predicted improved sensitivity with the HIV-TB population from 46.9% (30 of 64 samples) with TbF6 and DPEP to 79.7% (51 of 64 samples) with Mtb81 and 84.4% (54 of 64 samples) for TbF6-DPEP-Mtb81. The above studies have served to identify antigens that, when used together, predict a high degree of clinical sensitivity in the detection of active TB. In addition, these antigens provide the basis for cocktails that maintain a high degree of specificity in TB diagnosis.
The six antigens described here (the 38-kDa antigen, Mtb8, Mtb11, Mtb48, DPEP, and Mtb81), several of which are incorporated into the polyproteins TbF6 or TbF10, provide the basis for the development of sensitive and specific tests for evaluating TB infections, particularly as an adjunctive test to the AFB smear typically used for TB diagnosis. By combining multiple antigens into polyproteins, this provides a potentially cost-effective way of developing tests for use in developing countries where cost per test is important. Efforts are now under way to provide the optimal combinations of antigens and/or polyproteins for the development of commercially viable rapid lateral-flow tests and ELISA for TB serodiagnosis that can be used alone or in combination with smear testing. In this context, the combination of TbF6 and DPEP seems more amenable to ELISA development and that of TbF10 with DPEP and Mtb48 to rapid lateral-flow test development. Incorporation of Mtb81 into these formats to increase sensitivity in HIV-TB population will also be necessary and could require further polyprotein engineering. In this context, we are epitope mapping Mtb81 to identify a smaller more antigenic fragment that would be more amenable to incorporation into a polyprotein. The incremental increases in sensitivity demonstrated with the described antigens, along with incorporation into polyproteins, should facilitate the development of ELISA and rapid tests with improved characteristics over current technology. Algorithms to incorporate such tests into TB diagnosis will also need to be evaluated. Rapid formats, providing they are sensitive and specific enough, would have field utility and, since they detect many AFB-negative but TB-positive individuals, would complement AFB smear analyses to increase clinical sensitivity.
Acknowledgments
We thank Steve Johnson and John Webb for M. tuberculosis H37Ra and H37Rv genomic libraries. We thank Dan Hoppe for sequencing efforts and Karen Kinch for assistance in manuscript and grant preparation.
This work was partially supported by National Institutes of Health SBIR grants AI-39879 (R.L.H.) and AI-43781 (K.P.L.).
REFERENCES
- 1.Andersen, A. B., and E. B. Hansen. 1989. Structure and mapping of antigen domains of protein antigen b, a 38,000 molecular weight protein of Mycobacterium tuberculosis. Infect. Immun. 57:2481-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes, P. F., V. Mehra, B. Rivoire, S-J. Fong, et al. 1992. Immunoreactivity of a 10-kDa antigen of Mycobacterium tuberculosis. J. Immunol. 148:1835-1840. [PubMed] [Google Scholar]
- 3.Benjamin, R. G., S. M. Debanne, Y. Ma, and T. M. Daniel. 1984. Evaluation of mycobacterial antigens in an enzyme-linked immunoabsorbent assay (ELISA) for the serodiagnosis of tuberculosis. J. Med. Microbiol. 18:309-318. [DOI] [PubMed] [Google Scholar]
- 4.Berthet, F. X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low molecular mass culture filtrate protein (CFP-10). Microbiology 144:3195-3203. [DOI] [PubMed] [Google Scholar]
- 5.Bothamley, G. H., and R. M. Rudd. 1994. Clinical evaluation of a serological assay using a monoclonal antibody (TB72) to the 38kDa antigen of Mycobacterium tuberculosis. Eur. Respir. J. 7:240-246. [DOI] [PubMed] [Google Scholar]
- 6.Bothamley, G. H., P. Udani, R. Rudd, et al. 1988. Humoral response to defined epitopes of tubercle bacilli in adult pulmonary and child tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 7:639-645. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control. 1993. Estimates of future global tuberculosis morbidity and mortality—United States. JAMA 272: 265-266. [Google Scholar]
- 8.Chan, E. D., L. Heifets, and M. D. Iseman. 2000. Immunologic diagnosis of tuberculosis: a review. Tuber. Lung Dis. 80:131-140. [DOI] [PubMed] [Google Scholar]
- 9.Chang, Z., A. Choudhary, R. Lithigra, and F. A. Quiocho. 1994. The immunodominant 38-kDa lipoprotein antigen of Mycobacterium tuberculosis is a phosphate-binding protein. J. Biol. Chem. 269:1956-1958. [PubMed] [Google Scholar]
- 10.Coates, A. R., H. Nicolai, M. J. Pallen, et al. 1989. The 45 kilodalton molecule of Mycobacterium tuberculosis identified by immunoblotting and monoclonal antibodies as antigenic in patients with tuberculosis. Br. J. Exp. Pathol. 70:215-225. [PMC free article] [PubMed] [Google Scholar]
- 11.Cocito, C. G. 1991. Properties of the mycobacterial antigen complex 60 and its applications to the diagnosis and prognosis of tuberculosis. Chest 100:1687-1692. [DOI] [PubMed] [Google Scholar]
- 12.Cohen, M. L., L. W. Mayer, H. S. Rumschlag, M. A. Yakrus, W. D. Jones, and R. C. Good. 1987. Expression of proteins of Mycobacterium tuberculosis in Escherichia coli and potential of recombinant genes and proteins for development of diagnostic reagents. J. Clin. Microbiol. 25:1176-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colangeli, R., J. S. Spencer, P. Bifani, A. Williams, K. Lyashchenko, M. A. Keen, P. J. Hill, J. Belisle, and M. L. Gennaro. 2000. MTSA-10, the product of the Rv3874 gene of Mycobacterium tuberculosis, elicits tuberculosis-specific, delayed-type hypersensitivity in guinea pigs. Infect. Immun. 68:990-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cousins, D. V., S. D. Wilton, B. R. Francis, and B. L. Gow. 1992. Use of polymerase chain reaction for rapid diagnosis of tuberculosis. J. Clin. Microbiol. 30:255-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniel, T. M., and S. M. Debanne. 1987. The serodiagnosis of tuberculosis and other mycobacterial diseases by enzyme linked immunoabsorbent assay. Am. Rev. Respir. Dis. 135:1137-1151. [DOI] [PubMed] [Google Scholar]
- 16.De Kesel, M., P. Gilot, M.-C. Misonne, M. Coene, and C. Cocito. 1993. Cloning and expression of portions of the 34-kilodalton protein gene of Mycobacterium paratuberculosis: its application to serological analysis of Johne's disease. J. Clin. Microbiol. 31:947-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. W. Skeiky, R. Badaro, S. G. Reed, and R. L. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espitia, C., R. Espinosa, R. Saavedra, R. Mancilla, F. Romain, A. Laqueyrerie, and C. Moreno. 1995. Antigenic and structural similarities between Mycobacterium tuberculosis 50- to 55-kilodalton and Mycobacterium bovis BCG 45- to 47 kilodalton antigens. Infect. Immun. 63:580-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Espitia, C., L. Cervera, R. Gonzalea, and R. Mancilla. 1989. A 38kDa Mycobacterium tuberculosis antigen associated with infection. Its isolation and serological evaluation. Clin. Exp. Immunol. 77:373-377. [PMC free article] [PubMed] [Google Scholar]
- 20.Foulds, J., and R. O'Brien. 1998. New tools for the diagnosis of tuberculosis: the perspective of developing countries. Int. J. Tuberc. Lung Dis. 2:778-783. [PubMed] [Google Scholar]
- 21.Harries, A. D., N. J. Hargreaves, J. Kemp, A. Jindani, D. A. Enarson, I. Maher, and F. M. Salaniponi. 2001. Deaths from tuberculosis in sub-Saharan African countries with a high prevalence of HIV-1. Lancet 357:1519-1523. [DOI] [PubMed] [Google Scholar]
- 22.Hendrickson, R. C., J. F. Douglass, L. D. Reynolds, P. D. McNeill, D. Carter, S. G. Reed, and R. L. Houghton. 2000. Mass spectrometric identification of Mtb81: a novel serological marker for tuberculosis. J. Clin. Microbiol. 38:2354-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussain Qadri, S. M., and K. K. Smith. 1992. Non-specificity of the Anda A60tb ELISA test for the serodiagnosis of mycobacterial disease. Can. J. Microbiol. 38:804-806. [DOI] [PubMed] [Google Scholar]
- 24.Jackett, P. S., G. H. Bothamley, H. V. Batra, et al. 1988. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J. Clin. Microbiol. 26:2313-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laal, S., K. M. Samanich, M. G. Sonnenberg, J. T. Belisle, J. O'Leary, M. S. Simberkoff, and S. Zolla-Pazner. 1997. Surrogate marker of preclinical tuberculosis in human immunodeficiency virus infection: antibodies to an 88-kDa secreted antigen of Mycobacterium tuberculosis. J. Infect. Dis. 176:133-143. [DOI] [PubMed] [Google Scholar]
- 26.Laal, S., K. M. Samanich, M. G. Sonnenberg, S. Zolla-Pazner, J. M. Phadtare, and J. T. Belisle. 1997. Human humoral responses to antigens of Mycobacterium tuberculosis: immunodominance of high-molecular-mass antigens. Clin. Diagn. Lab. Immunol. 4:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laqueyrerie, A., P. Militzer, F. Romain, K. Eiglmeier, S. Cole, and G. Marchal. 1995. Cloning, sequencing and expression of the apa gene encoding for the Mycobacterium tuberculosis 45/47-kilodalton secreted antigen complex. Infect. Immun. 63:4003-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodes, M. J., D. C. Dillon, R. Mohamoth, C. H. Day, D. R. Benson, L. D. Reynolds, P. D. McNeill, D. P. Sampaio, Y. A. W. Skeiky, R. Badaro, D. H. Persing, S. G. Reed, and R. L. Houghton. 2001. Serological expression cloning and immunological evaluation of Mtb48, a novel Mycobacterium tuberculosis antigen. J. Clin. Microbiol. 39:2485-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyashchenko, K., R. Colangeli, M. Houde, H. Al Jahdahli, D. Menzies, and M. L. Gennaro. 1998. Heterogeneous responses to tuberculosis. Infect. Immun. 66:3936-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyashchenko, K., C. Manca, R. Colangeli, A. Heijbel, A. Williams, and M. L. Gennaro. 1998. Use of a Mycobacterium tuberculosis complex-specific antigen cocktails for a skin test specific for tuberculosis. Infect. Immun. 66:3606-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyashchenko, K. P., J. M. Pollock, R. Colangeli, and M. L. Gennaro. 1998. Diversity of antigen recognition by serum antibodies in experimental bovine tuberculosis. Infect. Immun. 66:5344-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyashchenko, K. P., M. Singh, R. Colangeli, and M. L. Gennaro. 2000. A multi-antigen print immunoassay for the serological diagnosis of infectious diseases. J. Immunol. Methods 242:91-100. [DOI] [PubMed] [Google Scholar]
- 33.Miller, N., S. G. Hernandez, and T. J. Cleary. 1994. Evaluation of Gen-Probe Amplified Mycobacterium tuberculosis Direct Test and PCR for direct detection of Mycobacterium tuberculosis in clinical specimens. J. Clin. Microbiol. 32:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noordhoek, G. T., A. H. Kolk, G. Bjune, et al. 1994. Sensitivity and specificity of PCR for detection of Mycobacterium tuberculosis: a blind study among seven laboratories. J. Clin. Microbiol. 32:277-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunn, P. 2001. The global control of tuberculosis: what are the prospects? Scand. J. Infect. Dis. 33:329-332. [DOI] [PubMed] [Google Scholar]
- 36.Pottumarthy, S., V. C. Wells, and A. J. Morris. 2000. A comparison of seven tests for serological diagnosis of tuberculosis. J. Clin. Microbiol. 38:2227-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sada, E., D. Aguilar, M. Torres, and T. Herrera. 1992. Detection of lipoarabinomannan as a diagnostic test for tuberculosis. J. Clin. Microbiol. 30:2415-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sada, E., P. J. Brennan, T. Herrera, and M. Torres. 1990. Evaluation of lipoarabinomannan for the serological diagnosis of tuberculosis. J. Clin. Microbiol. 28:2587-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samanich, K., J. T. Belisle, and S. Laal. 2001. Homogeneity of antibody responses in tuberculosis patients. Infect. Immun. 69:4600-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samanich, K. M., J. T. Belisle, M. G. Sonnenberg, M. A. Keen, S. Zolla-Pazner, and S. Laal. 1998. Delineation of human antibody responses to culture filtrate antigens of Mycobacterium tuberculosis. J. Infect. Dis. 178:1534-1538. [DOI] [PubMed] [Google Scholar]
- 41.Samanich, K. M., M. A. Keen, V. D. Vissa, J. D. Harder, J. S. Spencer, J. T. Belisle, S. Zolla-Pazner, and S. Laal. 2000. Serodiagnostic potential of culture filtrate antigens of Mycobacterium tuberculosis. Clin. Diagn. Lab. Immunol. 7:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shawar, R. M., F. A. K. el-Zaatari, A. Nataraj, and J. E. Clarridge. 1993. Detection of Mycobacterium tuberculosis in clinical samples by two-step polymerase chain reaction and nonisotopic hybridization methods. J. Clin. Microbiol. 31:61-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skjøt, R. L. V., T. Oettinger, I. Rosenkrands, P. Ravn, I. Brock, S. Jacobsen, and P. Andersen. 2000. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect. Immun. 68:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vera-Cabrera, L., V. Handzel, and A. Laszlo. 1994. Development of an enzyme-linked immunoabsorbent assay (ELISA) combined with a streptavidin-biotin and enzyme amplification method to detect anti-2, 3-di-o-acyltrehalose (DAT) antibodies in patients with tuberculosis. J. Immunol. Methods 177:69-77. [DOI] [PubMed] [Google Scholar]
- 45.Wadee, A. A., L. Boting, and S. G. Reddy. 1990. Antigen capture assay for detection of a 43-kilodalton Mycobacterium tuberculosis antigen. J. Clin. Microbiol. 28:2786-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webb, J. R., T. S. Vedvick, M. R. Alderson, J. A. Guderian, S. S. Jen, P. J. Ovendale, S. M. Johnson, S. G. Reed, and Y. A. W. Skeiky. 1998. Molecular cloning, expression, and immunogenicity of MTB12, a novel low-molecular-weight antigen secreted by Mycobacterium tuberculosis. Infect and Immun. 66:4208-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilkins, E. G. L. 1994. The serodiagnosis of tuberculosis, p. 367-369. In P. D. O. Davies (ed.), Clinical tuberculosis. Chapman and Hall, London, England.