Abstract
Measurement of cytomegalovirus (CMV)-specific immunoglobulin G (IgG) avidity has proven to be a powerful tool for distinguishing primary from nonprimary CMV infection. An in-house enzyme-linked immunosorbent assay (ELISA) for measuring CMV IgG avidity was validated using 84 sera from pregnant women who had recently seroconverted following primary CMV infection and 74 sera from individuals with past CMV infection (IgG-positive and IgM-negative profile). Of the 84 sera from pregnant women, 73 sera were collected within 120 days of the last IgG-negative sample, and 72 of these 73 sera (99%) exhibited an avidity index (AI) of <50%. In contrast, 71 of 74 (96%) sera from individuals with past CMV infection exhibited CMV AI values of >60%. Thus, low avidity in the in-house ELISA was defined as an AI of ⩽50%, whereas high avidity was defined as an AI of ⩾60%. In additional studies, the relationship between CMV IgG avidity and CMV IgM levels was examined using 64 CMV IgG-positive sera (time since seroconversion unknown) exhibiting equivocal or positive results in a CMV IgM capture ELISA (Diamedix). Of these 64 sera, 29 exhibited IgM index values of ⩾3.0, and 27 of these 29 (93%) exhibited low IgG avidity. A similar trend was observed when a subset of these 64 sera (n = 48) was tested in another CMV IgM capture ELISA (Trinity); of 18 sera with IgM index values of ⩾3.0, 17 (94%) exhibited low IgG avidity. These findings demonstrate the validity of an in-house ELISA for CMV IgG avidity and further show that strong reactivity of CMV IgG-positive sera in either of two CMV IgM capture assays is a reliable indicator of low CMV IgG avidity, and thus, recent CMV infection.
Congenital cytomegalovirus (CMV) infection represents the most common form of intrauterine viral infection, occurring in approximately 1% of all live births in developed countries (9). The debilitating effects of congenital CMV infection are strongly linked to maternal primary (new) infection during pregnancy rather than reinfection or reactivation (2, 5). Since most CMV infections in immunocompetent adults (including pregnant women) are asymptomatic, detection of CMV-specific antibodies is the most common approach used to identify CMV-infected individuals. If serologic evidence suggests CMV infection, the next challenge is to determine if the infection represents a newly acquired (primary) infection or, alternatively, represents reinfection or reactivation of a preexisting (nonprimary) infection. CMV immunoglobulin M (IgM) detection is a very sensitive marker for primary infection, but, unfortunately, it is not specific for primary infection (5, 7, 10). CMV IgM may be detectable for many months following primary infection and may also be produced following reinfection or reactivation. Likewise, detection of increasing CMV IgG levels over time is an unreliable approach for distinguishing primary from nonprimary CMV infection, since most seropositive patients show high IgG levels in the first serum sample collected for testing (1).
Measurement of CMV IgG avidity has proven to be a powerful tool for distinguishing primary from nonprimary CMV infection in pregnant women and solid organ transplant recipients (1-12). Defined as the strength with which the IgG attaches to antigen, IgG avidity matures with the length of time following primary infection. Thus, IgG produced within the first 3 to 5 months following primary infection exhibits low avidity, whereas IgG produced several months or years later exhibits high avidity (3-5, 9-12). Detection of low-avidity CMV IgG in a pregnant woman indicates that primary CMV infection may have occurred since conception, and the fetus may be at increased risk for congenital CMV. Alternatively, detection of high-avidity CMV IgG indicates that primary infection most likely occurred prior to conception, with little chance of debilitating congenital CMV infection (2, 7, 9). Among solid organ transplant recipients who acquire primary CMV infection at the time of transplantation, delayed maturation of CMV IgG avidity is associated with persistence of antigenemia, higher numbers of severe CMV infections, and increased organ rejection rates (7, 8).
Because a commercial CMV IgG avidity kit is not readily available in the United States, we have developed an in-house enzyme-linked immunosorbent assay (ELISA) for measuring CMV IgG avidity. In order to validate this assay for routine use, we utilized a panel of well-characterized sera collected from pregnant women who had recently undergone CMV seroconversion. In addition, we evaluated a large number of CMV IgG-positive and IgM-positive sera submitted without seroconversion data to investigate the relationship between IgM levels and IgG avidity.
MATERIALS AND METHODS
Patient sera.
Three panels of sera were utilized to evaluate the CMV IgG avidity ELISA. Panel 1, generously donated by M. Bodeus (Universite Catholique du Louvain, Brussels, Belgium), consisted of 84 sera (representing 55 individuals) from pregnant women with documented seroconversion within the preceding 8 months (3). Seroconversion was defined as the appearance of CMV-specific IgG, together with CMV-specific IgM, in a previously seronegative patient. Each serum was collected at a known time point (expressed in days) after the last IgG-negative sample (3). Panel 2 consisted of 74 sera submitted for CMV antibody testing and found to exhibit a CMV IgG-positive and IgM-negative profile. The length of time since seroconversion was unknown; these sera were presumed to represent past infection based on the absence of CMV-specific IgM. Panel 3 consisted of 64 sera submitted for CMV antibody testing and found to exhibit an IgG-positive and IgM-positive profile. The length of time since seroconversion was unknown; these sera were presumed to represent either recent infection, past infection with long-lasting IgM, or CMV reinfection or reactivation with IgM production.
CMV IgM capture ELISA.
CMV IgM levels for sera in all three panels were measured using the CMV IgM capture ELISA manufactured by Diamedix Corporation (Miami, Fla.). The assay was performed according to the manufacturer's instructions. All panel 1 sera and some panel 3 sera were also tested using the CMV IgM capture ELISA manufactured by Trinity Biotech (Wicklow, Ireland); the assay was performed according to the manufacturer's instructions. For both CMV IgM capture assays, index values of <0.90 were considered negative, 0.90 to 1.1 were considered equivocal, and >1.1 were considered positive.
In-house CMV IgG ELISA.
Microtiter wells (Polysorb; Nunc, Copenhagen, Denmark) were coated with CMV antigen (Microbix Biosystems, Toronto, Canada) in phosphate-buffered saline (PBS), blocked with PBS containing 0.1% bovine serum albumin (Sigma, St. Louis, Mo.), air dried, and stored at 4°C. Prior to assay setup, the microwell strips were washed with 0.25 ml of wash buffer (PBS containing 0.1% Tween 20 [PBST]; Sigma). Patient sera, assay control sera, and a calibrator serum were diluted 1:100 in PBST containing 0.1% bovine serum albumin and added to assigned microtiter wells (0.1 ml per well). After an hour at room temperature, the well contents were discarded. All wells were washed three times with wash buffer and then received 0.1 ml of appropriately diluted horseradish peroxidase-conjugated goat anti-human IgG (Fc specific; Jackson Immunoresearch, West Grove, Pa.). After 30 min at room temperature, the well contents were discarded and all wells were washed three times with wash buffer. Substrate reagent (tetramethylbenzidine; Moss Inc., Pasadena, Mo.) was then added to all wells (0.1 ml per well); after 10 min at room temperature, the reaction was stopped by the addition of 0.1 ml of 1 N sulfuric acid (Ricca, Arlington, Tex.). Optical density (OD) values (at 450 nm) were determined using an ELISA plate reader (Bio-Tek Instruments, Winooski, Vt.). Results were expressed as an index using the following formula: patient or assay control serum OD/(calibrator serum OD × 0.63). Index values of <0.90 were considered negative, 0.90 to 1.1 were considered equivocal, and >1.1 were considered positive. This assay was validated using the CMV/herpes simplex virus evaluation panel purchased from the Centers for Disease Control (Atlanta, Ga.); 100% qualitative agreement with expected results was observed.
In-house CMV IgG avidity ELISA.
CMV-coated microtiter strips (see CMV IgG ELISA description) were washed once with 0.25 ml of wash buffer as described for the CMV IgG ELISA. Patient sera were diluted 1:100 and 1:400 in PBST containing 0.1% bovine serum albumin, and each dilution was added to duplicate microtiter wells (0.1 ml per well). After an hour at room temperature, the well contents were discarded. Wash buffer (hereafter referred to as control wash buffer) was then added to one of each pair of duplicate wells; dissociating buffer (control wash buffer containing 6 M urea [ICN, Aurora, Ohio]) was added to the other duplicate well. After 5 min at room temperature, the well contents were discarded and the wash procedure repeated (including the 5-min incubation step). All wells were washed once more with control wash buffer; the assay was then completed as described for the CMV IgG ELISA, and the OD values were determined. For a given patient serum dilution, the avidity index (AI) was calculated using the following formula: (OD for the well washed with dissociating buffer/OD for the well washed with control buffer) × 100, expressed as a percentage. Results for the 1:400 serum dilution were used only when the 1:100 dilution well washed with control buffer exhibited an OD value of >3.0.
RESULTS
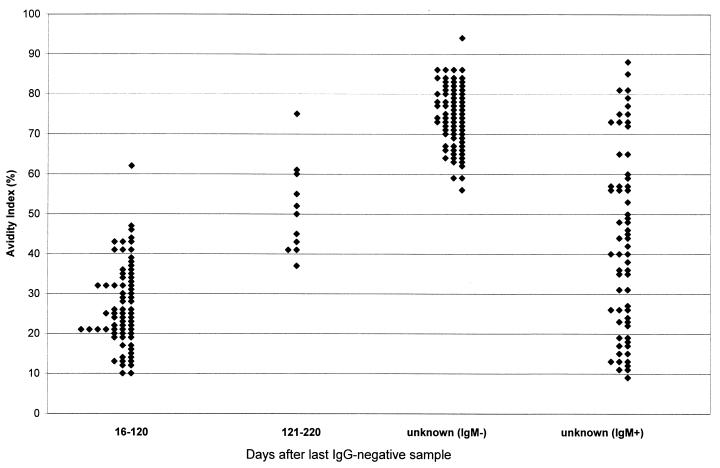
Shown in Fig. 1 are CMV IgG AI values for all 222 sera included in the three serum panels evaluated. Of the 84 sera collected from pregnant women with documented seroconversion (i.e., panel 1), 73 were collected between 16 and 120 days after the last IgG-negative sample; 72 of these sera (99%) exhibited CMV IgG AI values of <50%. The remaining 11 sera from panel 1 were collected between 121 and 220 days (4 to 8 months) after the last IgG-negative sample. These sera exhibited a broad range of AI values; six exhibited AI values of ⩽50%, two exhibited AI values between 51 and 59%, and three exhibited AI values of ⩾60%. For the entire group of 84 sera in the first panel, significant correlation was observed between AI values and the number of days after the last IgG-negative sample (correlation coefficient = 0.72; P < 0.001).
FIG. 1.
CMV IgG AI values in various groups of sera. The two groups of sera collected at a known number of days after the last IgG-negative sample were part of panel 1, the unknown (IgM−) group represents panel 2, and the unknown (IgM+) group represents panel 3 (see text for panel descriptions).
Also shown in Fig. 1 are AI values for the sera from panel 2, consisting of 74 sera for which the length of time since seroconversion was unknown and which were presumed to represent past CMV infection based on the absence of CMV-specific IgM. Of 74 sera, 71 (96%) exhibited CMV IgG AI values of >60%. The three sera with AI values of <60% exhibited values between 51 and 59%; none of these 74 sera exhibited AI values of <50%. Based on the findings for panels 1 and 2, CMV IgG AI values of ⩽50% were defined as low avidity, AI values of 51 to 59% were defined as intermediate avidity, and AI values of ⩾60% were defined as high avidity.
Panel 3, consisting of 64 CMV IgG-positive and IgM-positive sera for which the length of time since seroconversion was unknown, was tested in the CMV IgG avidity assay (Fig. 1). As expected, AI values were broadly distributed; 41 of 64 sera (64%) exhibited low avidity, whereas 8 of 64 sera (13%) exhibited intermediate avidity and 15 of 64 sera (23%) exhibited high avidity. This serum panel was further used to investigate the relationship between CMV IgG avidity and CMV IgM levels, as represented by index values generated using the CMV IgM capture ELISA from Diamedix. As shown in Table 1, nearly all sera with Diamedix IgM index values of ⩾3.0 exhibited low IgG avidity (27 of 29 [93%]). Of the 35 sera in panel 3 with Diamedix IgM index values of <3.0, a broad range of AI values was observed; 14 (40%) exhibited low IgG avidity, 8 (23%) exhibited intermediate avidity, and 13 (37%) exhibited high avidity.
TABLE 1.
Distribution of CMV IgG avidity values in relation to Diamedix CMV IgM capture ELISA index values for 64 CMV IgG-positive and IgM-positive sera (panel 3)
| Diamedix IgM index | n | No. of sera with indicated CMV IgG avidity
|
||
|---|---|---|---|---|
| Low | Intermediate | High | ||
| 0.9-1.9 | 27 | 9 | 7 | 11 |
| 2.0-2.9 | 8 | 5 | 1 | 2 |
| 3.0-3.9 | 9 | 7 | 0 | 2 |
| ≥4.0 | 20 | 20 | 0 | 0 |
We next sought to determine if the marked association between high IgM index values in the Diamedix IgM assay and low IgG avidity was a phenomenon unique to the Diamedix IgM kit. Thus, 48 of 64 sera from panel 3 (i.e., those sera with sufficient volume for additional testing) were evaluated using the Trinity CMV IgM capture ELISA. Table 2 demonstrates the good overall agreement between index values in the Diamedix and Trinity IgM capture assays. Most (26 of 27 [96%]) sera exhibiting a Diamedix index of <3.0 also exhibited a Trinity index of <3.0, and most (17 of 21 [81%]) sera exhibiting a Diamedix index of ⩾3.0 also exhibited a Trinity index of ⩾3.0. Although 6 of 48 sera gave negative results in the Trinity assay, 5 of these 6 sera showed low positive index values (0.9 to 1.9) in the Diamedix assay. Table 3 presents data on the relationship between Trinity IgM index values and IgG avidity. A trend similar to that seen using the Diamedix kit (Table 1) was observed using the Trinity kit; the majority of sera with Trinity CMV IgM values of ⩾3.0 exhibited low IgG avidity (17 of 18 [94%]). Of the 30 sera with Trinity IgM index values of <3.0, 10 (33%) exhibited low IgG avidity, 6 (20%) exhibited intermediate avidity, and 14 (47%) exhibited high avidity, again representing a broad range of AI values.
TABLE 2.
Relationship between index values in Diamedix and Trinity IgM capture assays for 48 sera from panel 3
| Trinity index | No. of sera with indicated Diamedix index
|
|||
|---|---|---|---|---|
| 0.9-1.9 | 2.0-2.9 | 3.0-3.9 | ≥4.0 | |
| <0.9 | 5 | 1 | 0 | 0 |
| 0.9-1.9 | 13 | 2 | 0 | 0 |
| 2.0-2.9 | 2 | 3 | 2 | 2 |
| 3.0-3.9 | 0 | 0 | 4 | 7 |
| ≥4.0 | 1 | 0 | 1 | 5 |
TABLE 3.
Distribution of CMV IgG avidity values in relation to Trinity CMV IgM capture ELISA index values for 48 sera from panel 3
| Trinity IgM index | n | No. of sera with indicated CMV IgG avidity
|
||
|---|---|---|---|---|
| Low | Intermediate | High | ||
| <0.90 | 6 | 1 | 0 | 5 |
| 0.9-1.9 | 15 | 3 | 5 | 7 |
| 2.0-2.9 | 9 | 6 | 1 | 2 |
| 3.0-3.9 | 11 | 11 | 0 | 0 |
| ≥4.0 | 7 | 6 | 1 | 0 |
Based on the observation that about one-third of the panel 3 sera with low IgG avidity exhibited IgM index values of <3.0 (see Tables 1 and 3), we determined the distributions of IgM index values in both capture assays for sera from patients with documented seroconversion (panel 1). As shown in Table 4, 40 of 84 (48%) sera exhibited Diamedix IgM index values of <3.0, including 34 of 78 (44%) sera with low IgG avidity. Similarly, 50 of 84 (60%) sera exhibited Trinity IgM index values of <3.0, including 44 of 78 (56%) sera with low IgG avidity. Also of note was the finding that seven sera exhibited negative results in the Diamedix IgM assay, but six of these seven nevertheless exhibited low IgG avidity; these same seven sera, plus one additional serum sample, also exhibited negative results in the Trinity IgM assay.
TABLE 4.
Distribution of IgM index values in Diamedix and Trinity CMV IgM capture assays for panel 1 sera
| IgM index | Diamedix IgM capture ELISA
|
Trinity IgM capture ELISA
|
||
|---|---|---|---|---|
| n | No. with low IgG avidity | n | No. with low IgG avidity | |
| <0.9 | 7 | 6 | 8 | 6 |
| 0.9-1.9 | 23 | 19 | 19 | 16 |
| 2.0-2.9 | 10 | 9 | 23 | 22 |
| 3.0-3.9 | 10 | 10 | 27 | 27 |
| ≥4.0 | 34 | 34 | 7 | 7 |
DISCUSSION
Our findings demonstrate that an in-house ELISA measuring CMV IgG avidity can effectively distinguish recent (primary) from past (nonprimary) CMV infection. With a single exception, all 73 sera collected from pregnant women within 4 months of seroconversion exhibited low CMV IgG avidity, whereas none of 74 sera with a profile indicative of past CMV infection (CMV IgG positive and CMV IgM negative) exhibited low CMV IgG avidity.
The AI values we selected for discriminating low versus high CMV IgG avidity in our in-house avidity assay are consistent with values published by other investigators. Bodeus et al., utilizing 8 M urea as dissociating buffer, defined high avidity as an AI of ⩾65%, a value similar to our value of 60% (2, 3). Grangeot-Keros et al. also used 8 M urea as dissociating buffer, and they defined low avidity as an AI of <50% and high avidity as an AI of >60% (5). Similarly, Eggers et al., utilizing, as we did, 6 M urea as dissociating buffer, defined low avidity as an AI of <40% and high avidity as an AI of >60% (4).
In additional analyses, we demonstrated that strong serum reactivity (index of ⩾3.0) in either of two CMV IgM capture ELISAs reliably predicts low CMV IgG avidity (and thus primary infection) with >90% accuracy (Diamedix, 93%; Trinity, 94%). These findings suggest that, in some clinical settings, a serum specimen that is CMV IgG positive and exhibits a CMV IgM capture ELISA index of ⩾3.0 may not need to be tested for CMV IgG avidity in order to reliably identify primary infection.
The vast majority of CMV IgM-positive sera with intermediate or high IgG avidity, presumed to represent either long-lasting IgM produced during primary infection many months earlier or IgM produced following reinfection or reactivation, exhibited IgM index values of <3.0. It is important to remember, however, that a CMV capture IgM index of <3.0 does not rule out low IgG avidity and thus does not rule out recent primary infection. Indeed, roughly half of all low-avidity sera from pregnant women with documented recent seroconversion exhibited IgM index values of <3.0 (Table 4). Thus, a CMV IgM capture index of <3.0 may be associated with the entire spectrum of IgG avidity values.
Direct comparison of Diamedix and Trinity CMV IgM capture assays was not a specific aim of our study. Because equivocal or positive reactivity in the Diamedix IgM ELISA was a criterion for inclusion in panel 3, those panel 3 sera tested in the Trinity IgM ELISA were biased toward equivocal or positive reactivity, thus precluding an objective comparison of the two kits. It is noteworthy, however, that both IgM capture assays failed to detect IgM in a small number of panel 1 sera that were positive in the CMV IgM assay (Axsym; Abbott Diagnostics, Wiesbaden, Germany) used by other investigators to define seroconversion (3). Thus, as emphasized by Lazzarotto et al. (12), testing algorithms that call for CMV avidity testing only on CMV IgM-positive sera may miss some patients with primary CMV infection, depending on the sensitivity of the IgM assay used.
Resource limitations did not allow us to evaluate other commercially available CMV IgM assays, including noncapture ELISAs, for relationships to CMV IgG avidity. In light of the strong association we found between high capture ELISA index values and low IgG avidity, such studies appear warranted.
Due to its superior clinical utility for distinguishing primary from nonprimary infection, CMV IgG avidity testing should be a major component of testing regimens designed to manage patients with increased risk of debilitating CMV disease. Further, the accurate prediction of low CMV IgG avidity by strong reactivity in CMV IgM capture assays may allow streamlining of these testing regimens in some clinical settings.
Acknowledgments
We thank Alli Priela and Jane Filamore for expert technical assistance with IgM assays.
REFERENCES
- 1.Baccard-Longere, M., F. Freymuth, D. Conte, J. M. Seigneurin, and L. Grangeot-Keros. 2001. Multicenter evaluation of a rapid and convenient method for determination of cytomegalovirus immunoglobulin G avidity. Clin. Diagn. Lab. Immunol. 8:429-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodeus, M., S. Feyder, and P. Goubau. 1998. Avidity of IgG antibodies distinguishes primary from nonprimary cytomegalovirus infection in pregnant women. Clin. Diagn. Virol. 9:9-16. [DOI] [PubMed] [Google Scholar]
- 3.Bodeus, M., D. Beulne, and P. Goubau. 2001. Ability of three IgG-avidity assays to exclude recent cytomegalovirus infection. Eur. J. Clin. Microbiol. Infect. Dis. 20:248-252. [DOI] [PubMed] [Google Scholar]
- 4.Eggers, M., U. Bader, and G. Enders. 2000. Combination of microneutralization and avidity assays: improved diagnosis of recent primary human cytomegalovirus infection in single serum sample of second trimester pregnancy. J. Med. Virol. 60:324-330. [DOI] [PubMed] [Google Scholar]
- 5.Grangeot-Keros, L., M. J. Mayaux, P. Lebon, F. Freymuth, G. Eugene, R. Stricker, and E. Dussaix. 1997. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection in pregnant women. J. Infect. Dis. 175:944-946. [DOI] [PubMed] [Google Scholar]
- 6.Landini, M. P., and M. Mach. 1995. Searching for antibodies specific for human cytomegalovirus: is it diagnostically useful? When and how. Scand. J. Infect. Dis. Suppl. 99:18-23. [PubMed] [Google Scholar]
- 7.Lazzarotto, T., P. Spezzacatena, P. Pradelli, D. A. Abate, S. Varani, and M. P. Landini. 1997. Avidity of immunoglobulin G directed against human cytomegalovirus during primary and secondary infections in immunocompetent and immunocompromised subjects. Clin. Diagn. Lab. Immunol. 4:469-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazzarotto, T., S. Varani, P. Spezzacatena, P. Pradelli, L. Potena, A. Lombardi, V. Ghisetti, L. Gabrielli, D. A. Abate, C. Magelli, and M. P. Landini. 1998. Delayed acquisition of high-avidity anti-cytomegalovirus antibody is correlated with prolonged antigenemia in solid organ transplant recipients. J. Infect. Dis. 178:1145-1149. [DOI] [PubMed] [Google Scholar]
- 9.Lazzarotto, T., P. Spezzacatena, S. Varani, L. Gabrielli, P. Pradelli, B. Guerra, and M. P. Landini. 1999. Anticytomegalovirus (anti-CMV) immunoglobulin G avidity in identification of pregnant women at risk of transmitting congenital CMV infection. Clin. Diagn. Lab. Immunol. 6:127-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazzarotto, T., S. Varani, B. Guerra, A. Nicolosi, M. Lanari, and M. P. Landini. 2000. Prenatal indicators of congenital cytomegalovirus infection. J. Pediatr. 137:90-95. [DOI] [PubMed] [Google Scholar]
- 11.Lazzarotto, T., S. Varani, P. Spezzacatena, L. Gabrielli, P. Pradelli, B. Guerra, and M. P. Landini. 2000. Maternal IgG avidity and IgM detected by blot as diagnostic tools to identify pregnant women at risk of transmitting cytomegalovirus. Viral Immunol. 13:137-141. [DOI] [PubMed] [Google Scholar]
- 12.Lazzarotto, T., C. Galli, R. Pulvirenti, R. Rescaldani, R. Vezzo, A. La Gioia, C. Martinelli, S. La Rocca, G. Agresti, L. Grillner, M. Nordin, M. Van Ranst, B. Combs, G. T. Maine, and M. P. Landini. 2001. Evaluation of the Abbott AxSYM cytomegalovirus (CMV) immunoglobulin M (IgM) assay in conjunction with other CMV IgM tests and a CMV IgG avidity assay. Clin. Diagn. Lab. Immunol. 8:196-198. [DOI] [PMC free article] [PubMed] [Google Scholar]