Abstract
We developed a multiplexed indirect immunofluorescence assay for antibodies to Haemophilus influenza type b (Hib) polysaccharide and the toxoids of Clostridium tetani (Tet) and Corynebacterium diphtheriae (Dip) based on the Luminex multiple-analyte profiling system. A pooled serum standard was calibrated against World Health Organization standards for Dip and Tet and an international standard for Hib. The multiplexed Luminex assay was compared to individual enzyme-linked immunosorbent assays (ELISAs) for the same analytes. By both methods, 75 (92.6%) of 81 of random serum samples had protective levels of antibody to Tet (≥0.1 IU/ml). For Dip, 81.5% of the samples had protective antibody levels (≥0.1 IU/ml) by ELISA and 80.2% had protective antibody levels by Luminex. Protective levels (≥1.0 μg/ml) of antibody to Hib were found in 45.0% of the samples tested by ELISA and in 39.0% of the samples tested by Luminex. The correlations (R2) between ELISA and Luminex of the 81 samples were 0.96, 0.96, and 0.91 for Tet, Dip, and Hib, respectively. There was also similar agreement between Luminex and ELISA for sera collected before and 1 month after Tet, Dip, and Hib vaccine administration. Both methods detected strong postvaccination responses. The Luminex method is an attractive alternative to ELISA since it reduces labor and reagent costs, as well as assay time.
With the introduction of pneumococcal conjugate (PnC) vaccines, current recommendations are for infants to receive injections of four or five different vaccines at 2 and 4 months of age (2). To reduce the number of injections required, vaccines are under development that combine the diphtheria (Dip), tetanus (Tet), and acellular pertussis (DTaP) vaccines in various combinations with conjugate Haemophilus influenzae type b (Hib), hepatitis B (HB), and inactivated poliovirus (IPV) (5, 6).
To assess the immunogenicity of conjugate vaccines, the antibody response to each individual component of the vaccine must be determined. Additional assays must also be done to assess possible interference with other childhood vaccines that are administered concurrently.
The enzyme-linked immunosorbent assay (ELISA) has been widely used for vaccine antibody testing. Testing of antibody responses to a combination vaccine by ELISA, however, requires a separate assay for each different component of the vaccine. As more serotypes are added to the vaccine formulas, immunogenicity testing by ELISA becomes ever more laborious and time-consuming.
Luminex multiple-analyte profiling technology is a flow cytometric system that allows a single sample to be tested simultaneously for a number of different analytes. Our goal has been to determine whether the Luminex system can yield results comparable to those of ELISA. In this study, we compared a Luminex immunoassay to ELISAs for quantitation of immunoglobulin G (IgG) antibodies to H. influenzae type b and the toxoids of tetanus and diphtheria.
MATERIALS AND METHODS
Reagents.
Hib capsular polysaccharide conjugated to human serum albumin (HbO-HA) was purchased from Wyeth Lederle Vaccines, W. Henrietta, N.Y. (9). Dip and Tet toxoids were purchased from University of Massachusetts Biologic Laboratories, Jamaica Plain, Mass.
Horseradish peroxidase (HRP)- and R-phycoerythrin-conjugated goat anti-human IgGs (γ specific) were obtained from ICN Pharmaceuticals/Cappel, Costa Mesa, Calif., and Jackson ImmunoResearch Laboratories, West Grove, Pa., respectively.
StabilZyme HRP conjugate stabilizer and StabilCoat were obtained from SurModics, Eden Prairie, Minn.
N-Hydroxysulfosuccinimide and 1-ethyl-3(3-dimethylamino-propyl)carbodiimide-HCl were obtained from Pierce, Rockford, Ill. 3,3′,5,5′-Tetramethylbenzidine liquid substrate solution, polyoxyethylenesorbitan monolaurate (Tween 20), and Tris(hydroxymethyl)aminomethane (Trizma base) were purchased from Sigma, St. Louis, Mo.
Antisera.
The U.S. human anti-Hib standard reference serum, lot 1983, was obtained from the Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Bethesda, Md. (8). World Health Organization international standards for Tet and Dip, code no. 76/589 and 00/496, respectively, were obtained from the National Institute for Biological Standards and Control, Potters Bar, United Kingdom. Other sera used in this study, including paired pre- and postvaccination sera, were submitted to Associated Regional and University Pathologists Laboratories for antibody testing. All samples were deidentified in accordance with protocols approved by the University of Utah Institutional Review Board. Sera containing various concentrations of antibodies to Hib, Dip, and Tet were pooled to prepare controls and a secondary standard.
Coupling of antigens to Luminex microspheres.
HbO-HA and the Dip and Tet toxoids were coupled to a carboxylated Luminex microsphere by using a two-step carbodiimide reaction (12). Carboxylated microspheres were activated for 20 min at 6.25 × 106/ml in phosphate-buffered saline (PBS), pH 6.1, with 5 mg of 1-ethyl-3(3-dimethylamino-propyl)carbodiimide-HCl per ml and 5 mg of N-hydroxysulfosuccinimide per ml. Activated microspheres were washed with PBS, pH 7.3, by centrifugation and incubated with HbO-HA, Tet, or Dip antigens for 1 h at room temperature. Microspheres were washed and stored in PBS, pH 7.3, with 0.1% bovine serum albumin and sodium azide.
Luminex multiplexed assay for anti-Hib, -Dip, and -Tet.
A two-step indirect procedure was used for the multiplexed Luminex assay for IgG antibodies to HbO-HA and the Dip and Tet toxoids. Standard, control, and unknown sera were diluted in PBS, pH 7.2, with 0.05% Tween 20 (PBST). Serial standard dilutions were included in each assay. Unknown sera and controls were diluted 1:100. Each dilution of standard, control, and unknown sera was mixed with a set of coupled Luminex microspheres in 96-well filtration plates (Millipore Multiscreen; Millipore Corporation, Bedford, Mass.) and incubated for 20 min at room temperature with shaking. Microspheres were collected by vacuum filtration and washed with PBST. R-phycoerythrin-conjugated anti-human IgG was added to each well. Following a second 20-min incubation and a wash step, microspheres were resuspended in PBST, transferred to a 96-well round-bottom plate, and read in a Luminex 100 analyzer equipped with an XY platform. A multiplexed Luminex assay for quantitation of IgG antibodies to 14 PnPs serotypes, 1, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 12F, 14, 18C, 19F, and 23F, was previously described (10). This assay was performed essentially as described above for the Luminex Tet, Dip, and Hib assay except that pneumococcal serotype 22F was included in the PBST sample diluent at a final concentration of 2 μg/ml. The combined Luminex assay for Tet, Dip, Hib, and 14 PnPs serotypes also contained serotype 22F polysaccharide in the sample diluent as an absorbent.
ELISA for anti-Hib, Dip, and Tet.
HbO-HA and the Dip and Tet toxoids were adsorbed to Nunc Maxisorb microtiter plates. Plates were washed with Tris-buffered saline, pH 7.5, with 0.05% Tween 20 (TBST), blocked with StabilCoat, and air dried. Serial dilutions of standard serum and 1:100 dilutions of controls and unknown sera were prepared in TBST, and 100-μl volumes of each were added to separate wells of the antigen-coated microtiter plates. Following a 1-h incubation at room temperature, plates were washed with TBST and 100 μl of HRP-conjugated goat anti-human IgG was added to each well. After a second 1-h incubation at room temperature, the plates were washed again and 100 μl of 3,3′,5,5′-tetramethylbenzidine substrate solution was added to each well. The substrate reaction was stopped after a 1-h incubation at room temperature by addition of 1 N sulfuric acid.
RESULTS
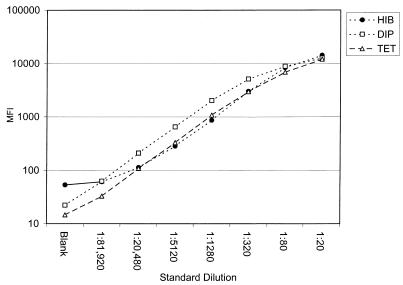
We prepared a reference standard for our multiplexed Luminex assay for Dip, Tet, and Hib (Luminex DTH) by pooling human sera with high levels of antibodies to Hib, Dip, and Tet. With the Luminex assay, the pooled standard was assigned IgG antibody concentrations in international units per milliliter for Dip and Tet by comparison to World Health Organization standards 00/496 and 76/589, respectively. Likewise, the pooled standard was assigned a value for IgG to Hib in micrograms per milliliter by comparison to international standard 1983. Eight fourfold dilutions of our reference standard were tested in the multiplexed Luminex assay (Fig. 1). The three analytes had similar parallel slopes and were linear over approximately seven fourfold dilutions of our reference standard.
FIG. 1.
Titration of the pooled serum standard against Dip-, Tet-, and Hib-coupled Luminex microspheres. The mean fluorescence intensity (MFI) for each dilution of the standard was determined simultaneously for all three analytes with a Luminex 100 analyzer. The curves were constructed by plotting the log MFI against the dilution factor for the standard serum.
Comparison of Luminex to ELISA.
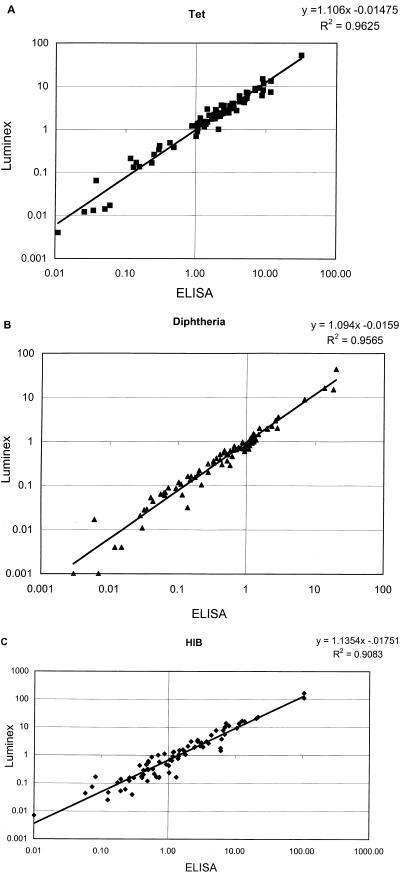
We compared the multiplexed Luminex assay for quantitation of IgG antibodies to Hib, Dip, and Tet to individual ELISA methods for the same analytes. As a basis for comparison, we used the same pooled standard, calibrated as described above with the Luminex assay, as the standard for each of the three ELISAs. We first compared the multiplex Luminex assay with ELISA by using a panel of 81 sera that were submitted for Hib, Dip, or Tet antibody testing. The 81 sera were tested simultaneously in the Luminex DTH assay and in individual ELISAs for IgG antibodies to Tet, Dip, and Hib. Antibody concentrations for the 81 sera, determined by both methods, were subjected to linear regression analysis. These results are shown in Fig. 2A, B, and C. The correlation coefficients (R2) for Tet, Dip, and Hib were 0.96, 0.96, and 0.91, respectively.
FIG. 2.
Comparison of concentrations of IgG antibodies to Dip, Tet, and Hib determined by the multiplexed Luminex assay and ELISA. A random panel of sera (n = 81) were quantitated by both methods as described in Materials and Methods. Antibody concentrations in international units per milliliter after log transformation are shown in panels A and B, respectively, and in micrograms per milliliter in panel C.
The multiplexed Luminex DTH assay determined that 75 (92.6%), 62 (76.5%), and 39 (48.2%) of the 81 samples had protective levels of IgG antibodies to Tet (≥0.1 IU/ml), Dip (≥0.1 IU/ml), and Hib (≥1.0 μg/ml), respectively. The corresponding ELISA values were 75 (92.6%), 64 (79.0%), and 45 (55.6%). With respect to determination of protective status, there were no discrepancies between the Luminex DTH assay and ELISA for Tet and only two samples for Dip. As summarized in Table 1, however, there were 10 discrepancies between the Luminex DTH assay and ELISA for Hib. Eight samples were slightly above the protective cutoff of 1.0 μg/ml by ELISA, ranging from 1.02 to 1.43 μg/ml, while testing below 1.0 μg/ml by Luminex. Likewise, two samples tested below 1.0 μg/ml by ELISA and were just above the cutoff by the Luminex DTH assay. All values were very close to the somewhat arbitrary cutoff, which is generally accepted as indicative of protection (1, 7).
TABLE 1.
Summary of discrepant results between ELISA and Luminex assays for 81 serum samples
| Dip (IU/ml)
|
Hib (μg/ml)
|
||||
|---|---|---|---|---|---|
| ELISA | Luminex DTH assay | Luminex DTH/14-PnPs assay | ELISA | Luminex DTH assay | Luminex DTH/14-PnPs assay |
| 0.22 | 0.11 | 0.07 | 1.43 | 0.91 | 0.68 |
| 0.14 | 0.03 | 0.02 | 1.39 | 0.75 | 0.44 |
| 0.12 | 0.06 | 0.05 | 1.32 | 0.16 | 0.07 |
| 0.10 | 0.12 | 0.07 | 1.18 | 1.27 | 0.95 |
| 1.44 | 1.07 | 0.93 | 1.17 | 0.89 | 0.75 |
| 1.13 | 0.64 | 0.54 | |||
| 1.06 | 0.70 | 0.45 | |||
| 1.03 | 0.24 | 0.09 | |||
| 1.02 | 0.42 | 0.30 | |||
| 0.88 | 1.12 | 0.96 | |||
| 0.70 | 1.00 | 0.88 | |||
We next tested prevaccination and 1-month postvaccination serum samples for IgG antibody to Tet, Dip, and Hib by the multiplexed Luminex DTH assay and by ELISA. These serum samples were submitted to Associated Regional and University Pathologists Laboratories for testing as paired sera. Mean values for pre- and postvaccination samples and postvaccination/prevaccination ratios are summarized in Table 2. There was good overall agreement between the Luminex DTH assay and ELISA with respect to mean values for pre- and postvaccination samples. Most of the sera tested had strong postvaccination responses when tested by either ELISA or the Luminex DTH assay.
TABLE 2.
Summary of ELISA and Luminex results of pre- and postvaccination serum samples for IgG antibodies to Tet, Dip, and Hib
| Samples and test | Tet (n = 31) (mean IU/ml) | Dip (n = 19) (mean IU/ml) | Hib (n = 9) (mean μg/ml) | Mean postvaccination/prevaccination ratio (range)
|
||
|---|---|---|---|---|---|---|
| Tet | Dip | Hib | ||||
| Prevaccination | ||||||
| ELISA | 1.72 | 0.83 | 1.11 | 30.5 (2.5-179.2) | 32.3 (3.7-158.7) | 100.4 (36.2-397) |
| Luminex DTH | 1.52 | 0.62 | 0.62 | 65.6 (2.6-643.3) | 31.6 (3.8-68.3) | 87.2 (42.1-989) |
| Postvaccination | ||||||
| ELISA | 19.99 | 14.62 | 111.55 | |||
| Luminex DTH | 21.81 | 14.92 | 53.88 | |||
We investigated the possibility of quantitating IgG antibodies to PnPs, in addition to Dip, Tet, and Hib, in the same Luminex assay. We recently described a multiplexed Luminex assay for simultaneous detection of IgG antibodies to 14 PnPs serotypes (10). We combined the above-described Luminex DTH assay with the 14-PnPs serotype assay to simultaneously quantitate IgG antibodies to all 17 analytes in a single dilution of serum. Purified polysaccharide from pneumococcal serotype 22F is used as an absorbent in the Luminex PnPs assay (10) and was also included in the combined assay. We retested the panel of 81 serum samples described above for IgG antibodies to Dip, Tet, and Hib with the 17-analyte Luminex assay. These results were compared to those obtained by ELISA and with the Luminex DTH assay without the 14 PnPs serotypes. The 17-analyte Luminex assay, composed of Dip, Tet, Hib, and the 14 PnPs serotypes, had correlation coefficients (R2) of 0.95, 0.95, and 0.88 for Dip, Tet, and Hib, respectively, compared to the individual ELISAs.
With respect to determination of protective levels of IgG antibody to Tet, there was no difference between the 17-analyte Luminex DTH/14-PnPs assay and ELISA. Both assays indicated that 75 (92.6%) of the 81 samples had protective concentrations of IgG antibodies to Tet. These results were identical to those obtained as described above when ELISA was compared with the Luminex DTH assay without inclusion of the PnPs serotypes. For Dip, the 17-analyte Luminex DTH/14-PnPs assay predicted that 60 (74.1%) of 81 samples had protective levels of IgG (≥0.1 IU/ml). Four samples that were found to be protective by ELISA were slightly below the cutoff of ≥0.1 IU/ml in the Luminex DTH/14-PnPs assay (Table 1). The Luminex DTH/14-PnPs assay predicted that 35 (43.2%) of the 81 samples had protective concentrations of ≥1.0 μg/ml for Hib, compared to 45 (55.6%) for ELISA and 39 (48.2%) for the Luminex DTH assay.
Paired prevaccination and 1-month postvaccination serum samples (n = 5) were tested for IgG antibodies to Dip, Tet, and Hib with the 17-analyte Luminex DTH/14-PnPs assay. These results were compared to those reported above that were obtained by testing the same samples in the Luminex DTH assay, which did not include the 14 PnPs serotypes. Both Luminex assays, either with or without inclusion of the 14 PnPs serotypes, gave similar mean values for prevaccination and postvaccination samples (Table 3).
TABLE 3.
Summary of IgG concentrations in pre- and postvaccination samples for Dip, Tet, and Hib determined by Luminex multiplexed assays with and without 14 PnPs serotypes
| Sample and test | Dip (n = 5), mean IU/ml (range) | Tet (n = 5), mean IU/ml (range) | Hib (n = 5), mean μg/ml (range) |
|---|---|---|---|
| Prevaccination | |||
| Luminex DTH | 0.45 (0.12-1.37) | 0.15 (0.01-0.31) | 0.33 (0.06-0.88) |
| Luminex DTH/14-PnPs | 0.35 (0.16-0.62) | 0.23 (0.06-0.40) | 0.40 (0.06-1.09) |
| Postvaccination | |||
| Luminex DTH | 12.32 (2.70-23.57) | 28.16 (2.58-65.35) | 58.32 (17.61-147.47) |
| Luminex DTH/14-PnPs | 17.35 (3.61-32.29) | 29.20 (3.46-75.88) | 55.45 (17.17-151.42) |
Finally, we investigated whether adding Dip, Tet, and Hib to our Luminex DTH/14-PnPs serotype assay interfered with detection of antibodies to PnPs serotypes. Paired prevaccination and 1-month postvaccination sera (n = 5) were tested by the Luminex assay for the 14 PnPs serotypes with and without inclusion of Dip, Tet, and Hib. As shown in Table 4, inclusion of Dip, Tet, and Hib had little effect on the assay results. The mean concentrations of IgG antibodies for all 14 serotypes in prevaccination and postvaccination serum samples were very similar in both assays.
TABLE 4.
Summary of IgG concentrations in pre- and postpneumococcal vaccination samples for 14 pneumococcal serotypes determined by Luminex multiplexed assays with and without Dip, Tet, and Hib
| Serotype | Mean μg of IgG/ml
|
|||
|---|---|---|---|---|
| Prevaccination
|
Postvaccination
|
|||
| Luminex 14-PnPs | Luminex DTH/14-PnPs | Luminex 14-PnPs | Luminex DTH/14-PnPs | |
| 1 | 0.59 | 0.69 | 20.68 | 15.03 |
| 3 | 0.11 | 0.12 | 4.01 | 4.46 |
| 4 | 0.13 | 0.15 | 5.24 | 4.95 |
| 5 | 0.94 | 1.05 | 9.39 | 8.38 |
| 6B | 0.88 | 0.96 | 6.84 | 7.01 |
| 7F | 0.31 | 0.27 | 17.70 | 15.21 |
| 8 | 2.01 | 2.15 | 13.38 | 12.71 |
| 9N | 0.88 | 0.66 | 2.09 | 1.89 |
| 9V | 0.59 | 0.67 | 2.05 | 2.09 |
| 12F | 0.70 | 0.81 | 12.86 | 11.70 |
| 14 | 0.47 | 0.42 | 20.95 | 20.63 |
| 18C | 0.21 | 0.22 | 3.58 | 3.76 |
| 19F | 0.58 | 0.62 | 3.17 | 3.41 |
| 23F | 1.17 | 1.09 | 23.18 | 21.31 |
DISCUSSION
The Luminex multiple-analyte profiling technology has a number of potential advantages over ELISA for determining vaccine responses. Instead of separate individual assays for each antigen in the vaccine, antibody concentrations for all analytes of interest can be determined simultaneously with a single serum dilution. This not only greatly reduces the time and labor required but also conserves sample volumes, which, in the case of pediatric samples, are often limited. In addition, the reaction kinetics of the liquid phase, particle-based Luminex system is more rapid than that of the solid-phase ELISA system. Incubation times and total assay times, therefore, are much shorter with the Luminex system, thus allowing more assays to be completed within an allotted time period.
The multiplex Luminex assay for Dip, Tet, and Hib showed excellent agreement with individual ELISAs for the same analytes. The correlation coefficients (R2) were 0.96, 0.96, and 0.91, respectively. When 14 PnPs serotype assays were combined with Dip, Tet, and Hib, the correlation with ELISA was only slightly reduced. The correlation coefficients were 0.95, 0.95, and 0.88 for Dip, Tet, and Hib, respectively.
When used to assign protective IgG concentrations of ≥0.1 IU/ml for Tet, there was no difference between ELISA and either the Luminex DTH assay or the Luminex DTH/14-PnPs assay. For Dip, only 2 of 81 samples were discrepant between ELISA and the Luminex DTH assay and 4 samples were discrepant between ELISA and the Luminex DTH/14-PnPs assay (Table 1). There was slightly less agreement between ELISA and the Luminex DTH/14-PnPs assay for Hib. Eight of 81 samples that tested above the ≥1.0-μg/ml protective concentration by ELISA tested below 1.0 μg/ml by the Luminex DTH assay (Table 1). Results were similar for the Luminex DTH/14-PnPs assay. Ten samples that tested above the protective cutoff by ELISA tested below the cutoff by the Luminex DTH/14-PnPs assay. However, two other samples that were discrepant between ELISA and the Luminex DTH assay were resolved in favor of ELISA by the Luminex DTH/14-PnPs assay. The lower level of agreement between the Luminex assay and ELISA for Hib was due, in part, to a larger number of samples in the panel near the cutoff. The protective cutoffs of ≥1.0 μg/ml for Hib and ≥0.1 IU/ml for Dip and Tet are somewhat arbitrary standards so that some variability around these values can be expected. It is probably better to slightly underestimate rather than overestimate protective status. The real clinical usefulness of any of these assays is to determine the prevaccination-to-postvaccination response rather than to determine protective status.
Compared with the Luminex DTH assay, the Luminex DTH/14-PnPs assay for anti-Hib IgG produced lower values for most of the samples at the low end of the range below the cutoff of ≥1.0 μg/ml (Table 1). Purified PnPs type 22F was added as an absorbent in the Luminex DTH/14-PnPs assay but was not used in the Luminex DTH assay. Inclusion of 22F polysaccharide as an absorbent improves the specificity of the assay for pneumococcal antibodies by removing some low-titer, nonspecific antibodies (3, 10). The 22F absorbent may have had a similar effect on the anti-Hib assay by removing some low-titer, nonspecific antipolysaccharide antibodies. The specificity of the assay, however, was little affected by the absorbent since there were only four discrepant samples between the Luminex DTH assay without 22F and the Luminex DTH/14-PnPs assay with 22F.
Current vaccine recommendations are for Hib, DTaP, IPV, and PnC vaccine to be given to infants at 2, 4, and 6 months of age (2). Two doses of HB are also recommended before 2 months of age. In an effort to reduce the number of injections required for infants, vaccines that combine Hib with DTaP, HB, or PnC are under development (5, 6). Vaccine immunogenicity studies must include all vaccine components that are given concurrently to assess all possible component interactions. For example, reduced antibody responses to Hib have been reported when the Hib conjugate vaccine is administered in combination with DTaP or concurrently with PnC vaccines that use the same carrier protein (4). In another study, reduced anti-Hib responses resulted when IPV was given concurrently with a DTaP-Hib combination vaccine (11). Our results indicate that a number of different vaccine components can be tested simultaneously by using the Luminex technology, which should increase our ability to accurately assess the immune responses to these combination vaccines.
In conclusion, the Luminex assay is rapid and sensitive and shows good correlation with ELISA for testing of IgG antibody responses to Hib, Dip, and Tet. As we have demonstrated, additional analytes, such as the 14 different PnPs serotypes, can be multiplexed to simultaneously detect the antibody response to an even larger number of different vaccine components. The multiplexed Luminex format should simplify immunogenicity testing of combination vaccines by reducing the number of serologic assays required, the volume of serum required, the amount of technician time required, and the cost of testing.
REFERENCES
- 1.Anderson, P. 1984. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenza type b. J. Infect. Dis. 149:1034-1035. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention.2002. Recommended childhood immunization schedule—United States. Morb. Mortal. Wkly. Rep. 50:7-10. [PubMed] [Google Scholar]
- 3.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dagan, R., J. Eskola, C. Leclerc, and O. Leroy. 1998. Reduced response to multiple vaccines sharing common protein epitopes that are administered simultaneously to infants. Infect. Immun. 66:2093-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decker, M. D., and K. M. Edwards. 2000. Combination vaccines: problems and promise. J. Pediatr. 137:291-295. [DOI] [PubMed] [Google Scholar]
- 6.Edwards, K. M., and M. D. Decker. 2001. Combination vaccines. Infect. Dis. Clin. N. Am. 15:209-230. [DOI] [PubMed] [Google Scholar]
- 7.Kayhty, H., H. Peltola, V. Karanko, and P. H. Makela. 1983. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenza type b. J. Infect. Dis. 147:1109. [DOI] [PubMed] [Google Scholar]
- 8.Madore, D. V., P. Anderson, B. D. Baxter, G. M. Carlone, K. M. Edwards, R. G. Hamilton, P. Holder, H. Kayhty, D. C. Phipps, C. C. A. Peeters, R. Achneerson, G. R. Siber, J. I. Ward, and C. E. Frasch. 1996. Interlaboratory study evaluating quantitation of antibodies to Haemophilus influenza type b polysaccharide by enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 3: 84-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phipps, D. C., J. West, R. Eby, M. Koster, D. V. Madore, and S. A. Quataert. 1990. An ELISA employing a Haemophilus influenza type b oligosaccharide-human serum albumin conjugate correlate with the radioantigen binding assay. J. Immunol. Methods 135:121-128. [DOI] [PubMed] [Google Scholar]
- 10.Pickering, J. W., T. M. Martins, R. W. Greer, M. C. Schroder, M. E. Astill, C. M. Litwin, S. W. Hildreth, H. R. Hill. 2002. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol., 117:589-596. [DOI] [PubMed]
- 11.Rennels, M. B., J. A. Englund, D. I. Bernstein, G. A. Losonsky, E. L. Anderson, M. E. Pichichero, F. M. Munoz, and M. C. Wolff. 2000. Diminution of the anti-polyribosylribitol phosphate response to a combined diphtheria-tetanus-acellular pertussis/Haemophilus influenzae type b vaccine by concurrent inactivated poliovirus vaccination. Pediatr. Infect. Dis. J. 19:417-423. [DOI] [PubMed] [Google Scholar]
- 12.Staros, J. V., R. W. Wright, and D. M. Swingle. 1986. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal. Biochem. 156:220-222. [DOI] [PubMed] [Google Scholar]