Abstract
Rationale
Postpartum haemorrhage (PPH) is the leading cause of maternal mortality worldwide. Prophylactic uterotonic agents can prevent PPH. The current World Health Organization (WHO) recommendation for preventing PPH is 10 IU (international units) of intramuscular or intravenous oxytocin. Several uterotonics prevent PPH, but there remains uncertainty about the most effective agent with the fewest side effects. This is an update of a review first published in April 2018, and incorporates trustworthiness screening of eligible trials.
Objectives
To identify the most effective uterotonic agent(s) to prevent PPH with the fewest side effects, and generate a ranking according to their effectiveness and side effect profile.
Search methods
On 5 February 2024, we searched CENTRAL, MEDLINE, Embase and CINAHL in collaboration with the Cochrane Information Specialist.
Eligibility criteria
All randomised controlled trials (RCTs) or cluster‐RCTs that compared the effectiveness and side effects of uterotonic agents with other uterotonic agents, placebo or no treatment for preventing PPH were eligible for inclusion. We screened eligible trials for trustworthiness. We included randomised trials published only as abstracts if we could retrieve sufficient information; we excluded quasi‐randomised trials.
Outcomes
Primary outcomes were PPH ≥ 500 mL and PPH ≥ 1000 mL. Secondary outcomes included use of additional uterotonics, blood transfusion, vomiting, hypertension, and fever.
Risk of bias
We used RoB 1 to assess risk of bias.
Synthesis methods
At least three review authors independently assessed trials for inclusion, trustworthiness, risk of bias, and certainty of evidence using GRADE. We estimated the relative effects and rankings for the primary and secondary outcomes. We reported primary outcomes for prespecified subgroups, stratified by mode of birth (caesarean versus vaginal), setting (hospital versus community), prior risk of PPH (high versus low), dose of misoprostol (≥ 600 μg versus < 600 μg), and regimen of oxytocin (bolus versus bolus plus infusion versus infusion only). We performed pairwise meta‐analyses and network meta‐analysis to determine the relative effects and rankings of all available agents.
Included studies
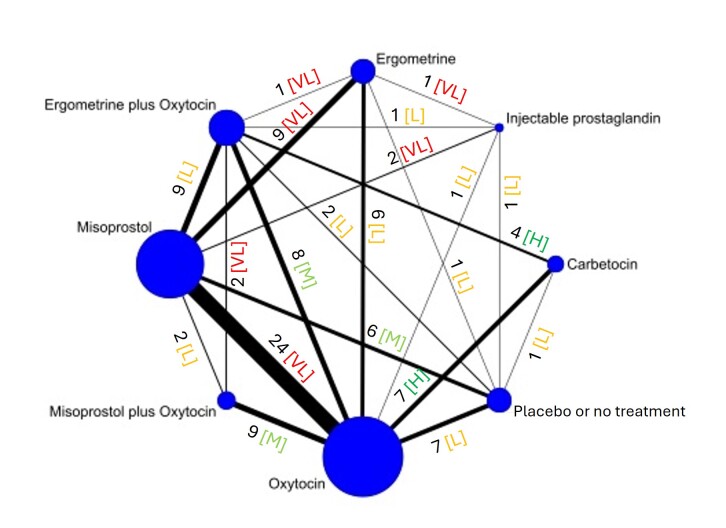
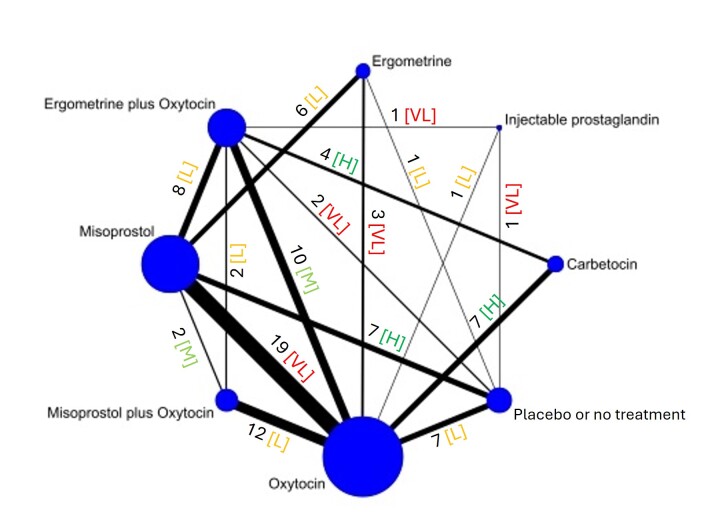
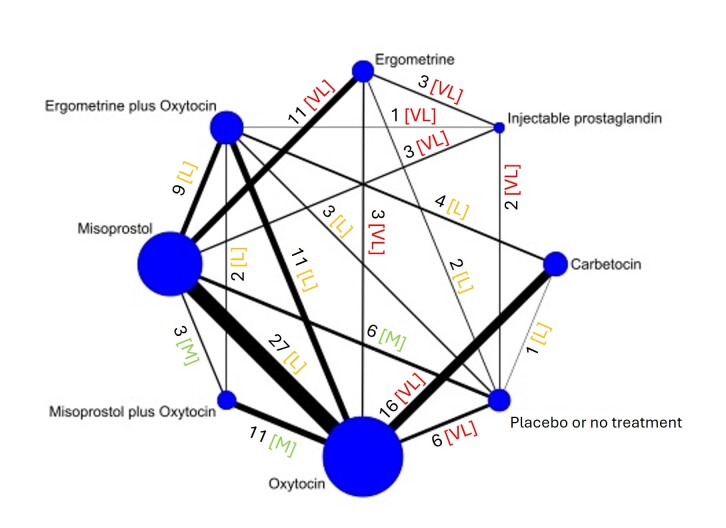
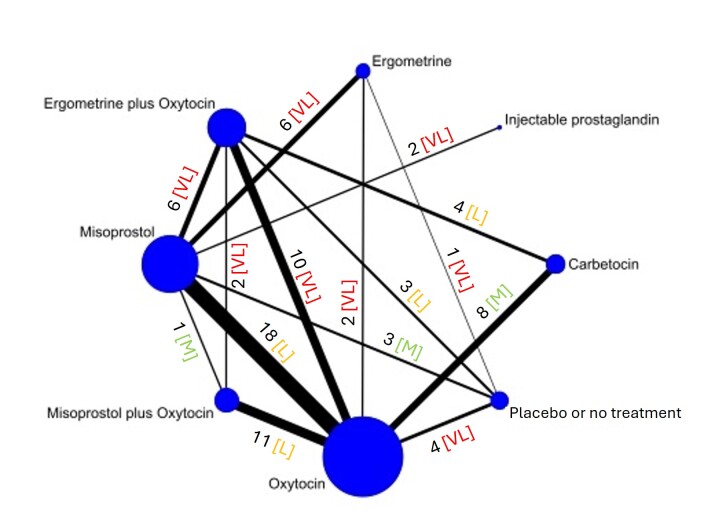
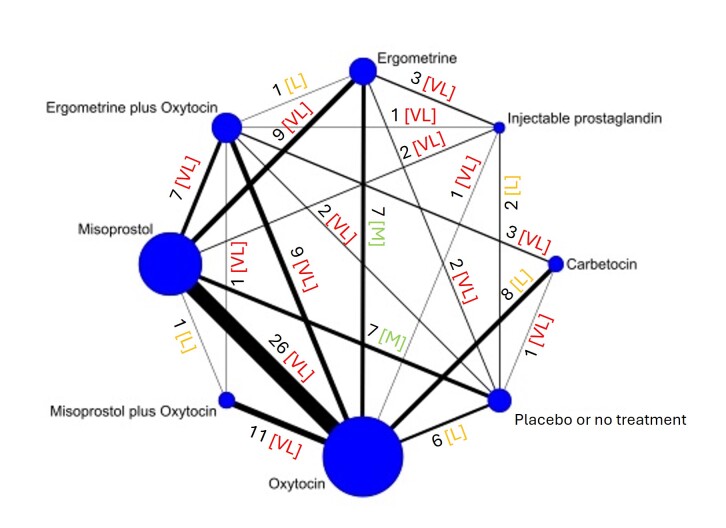
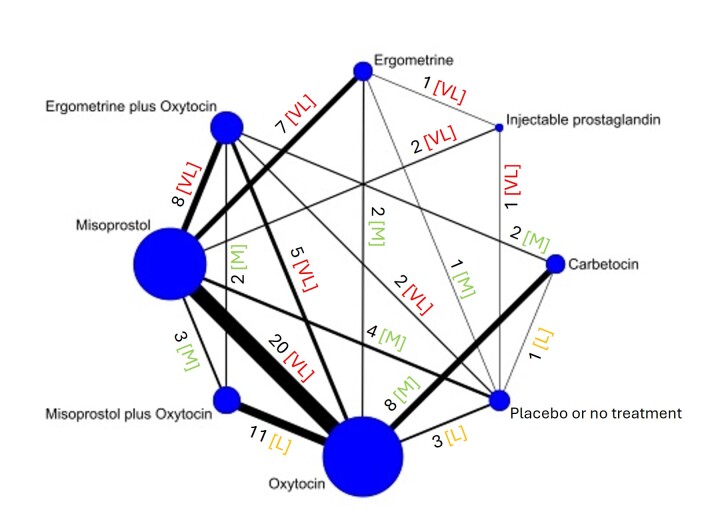
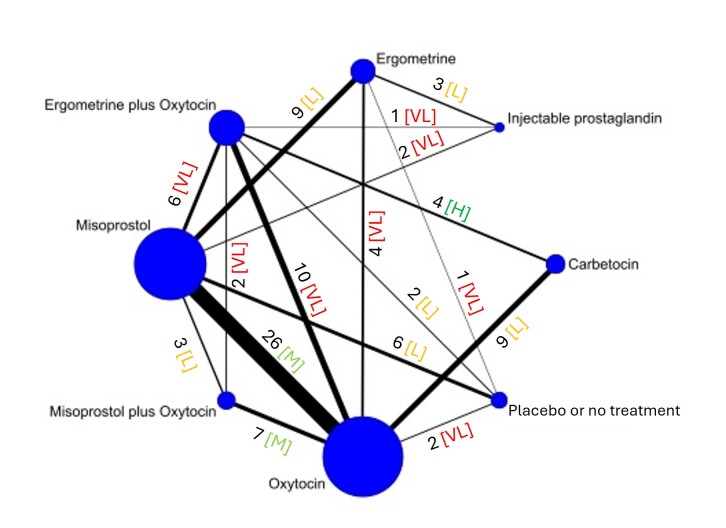
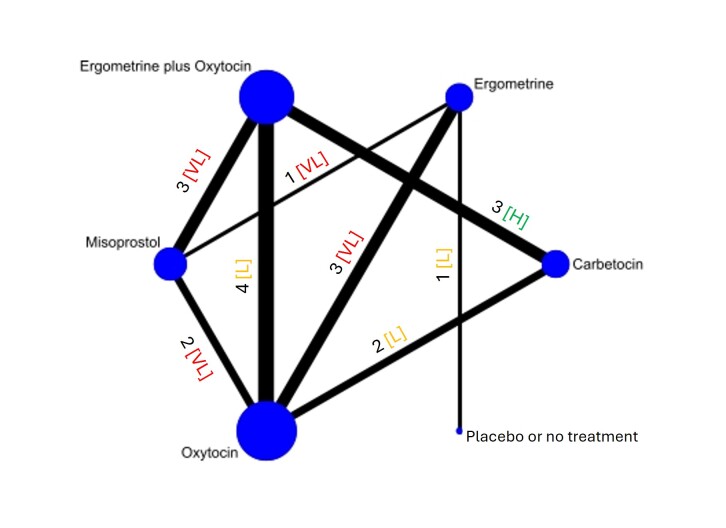
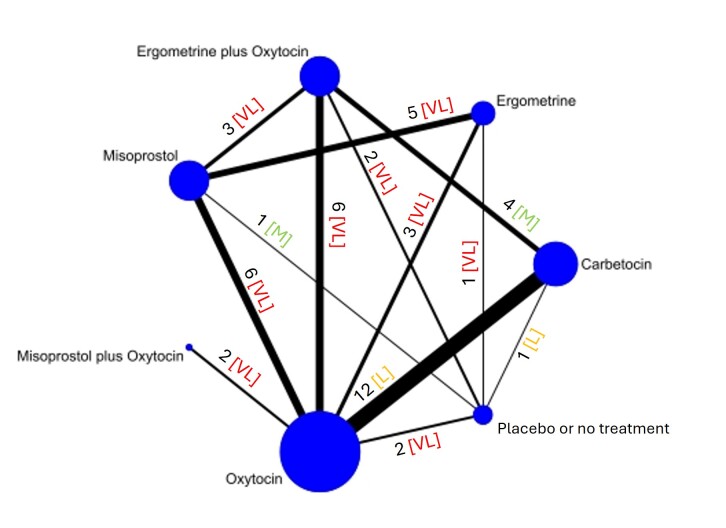
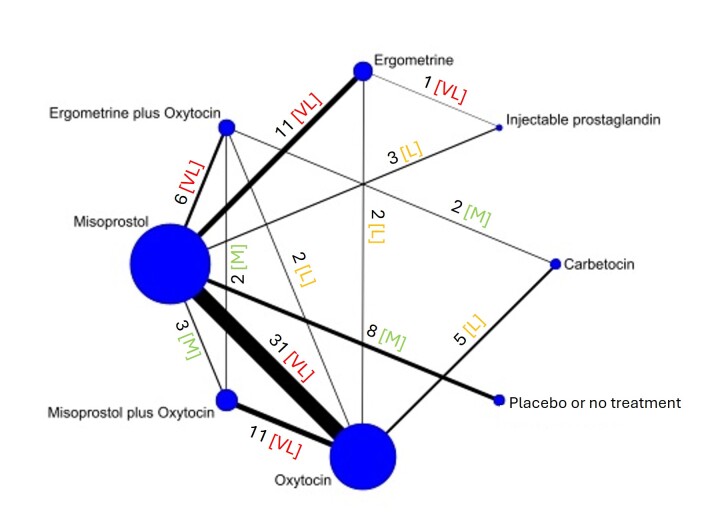
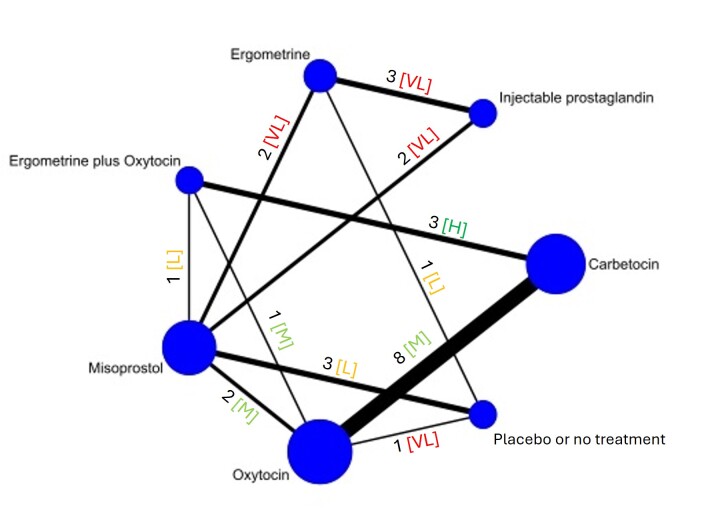
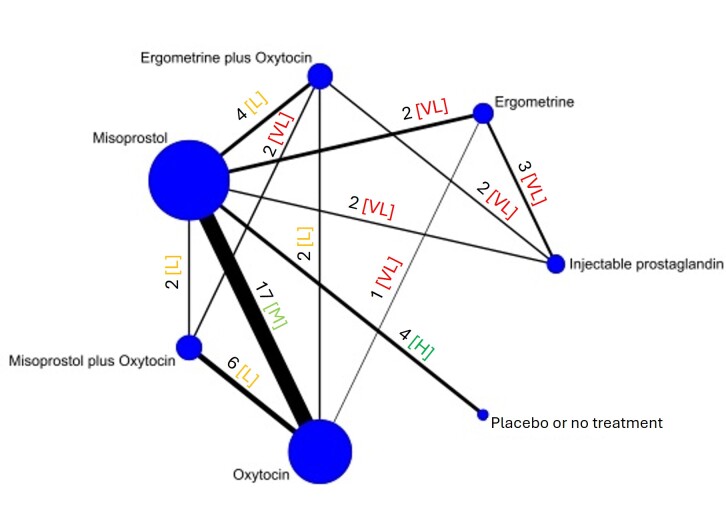
The network meta‐analysis included 122 trials (121,931 women), involving seven uterotonic agents and placebo or no treatment, conducted across 48 high‐, middle‐ and low‐income countries. Most were in a hospital setting (115/122, 94%), with women having a vaginal birth (87/122, 71%).
Synthesis of results
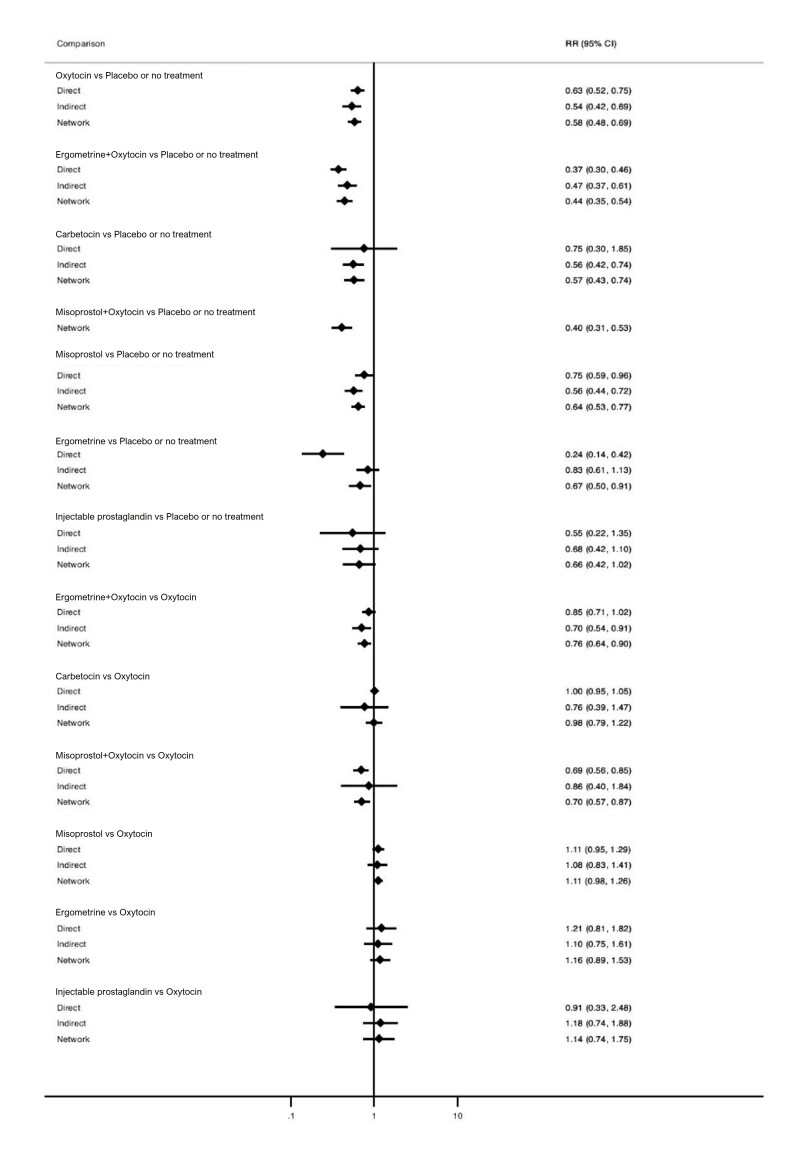
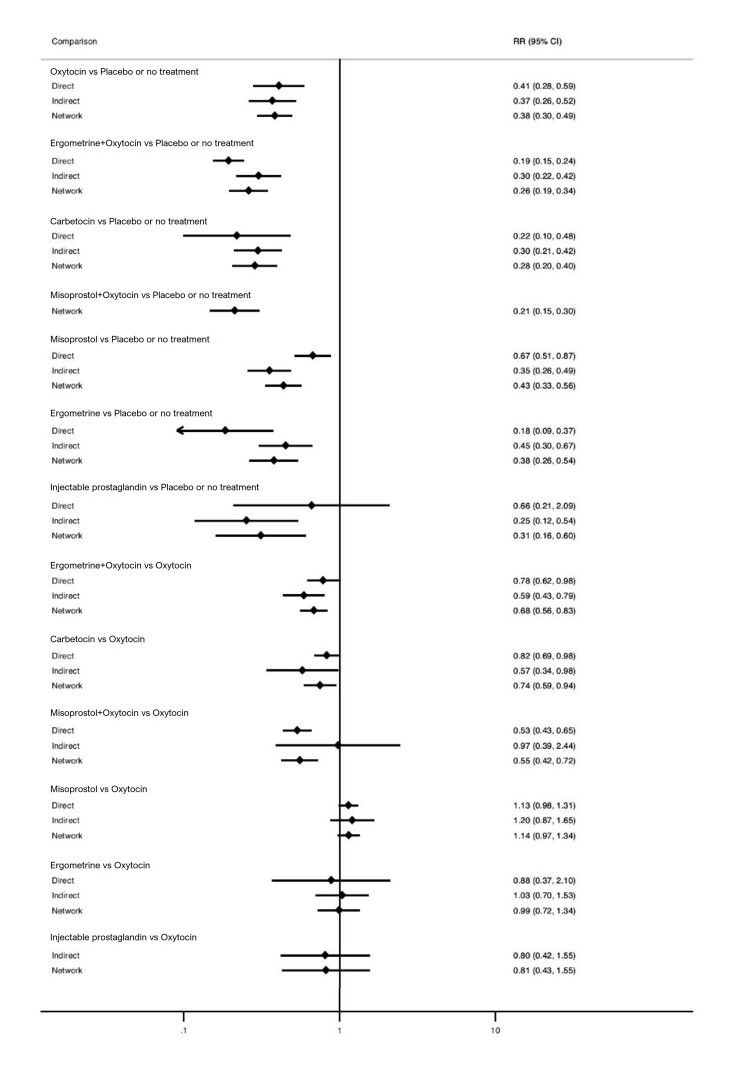
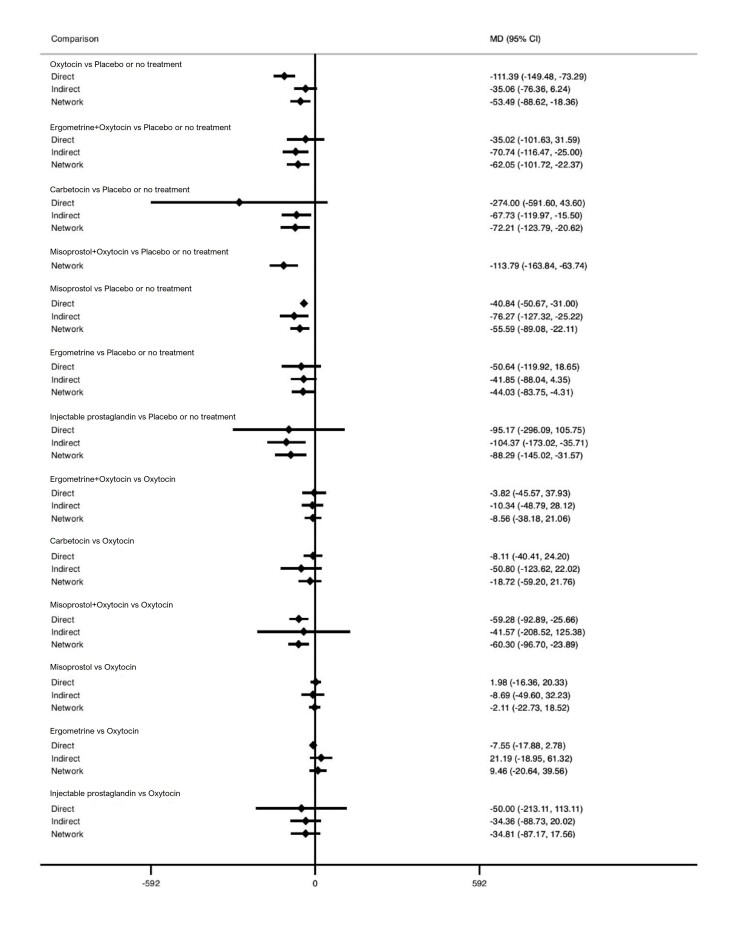
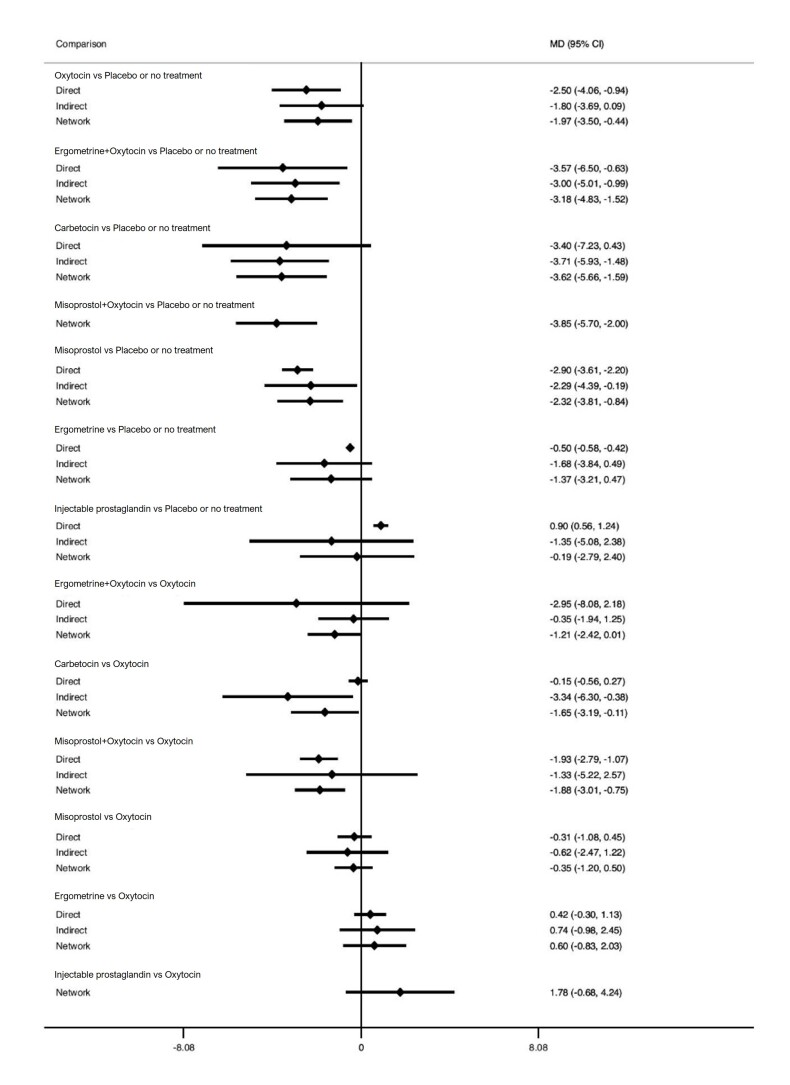
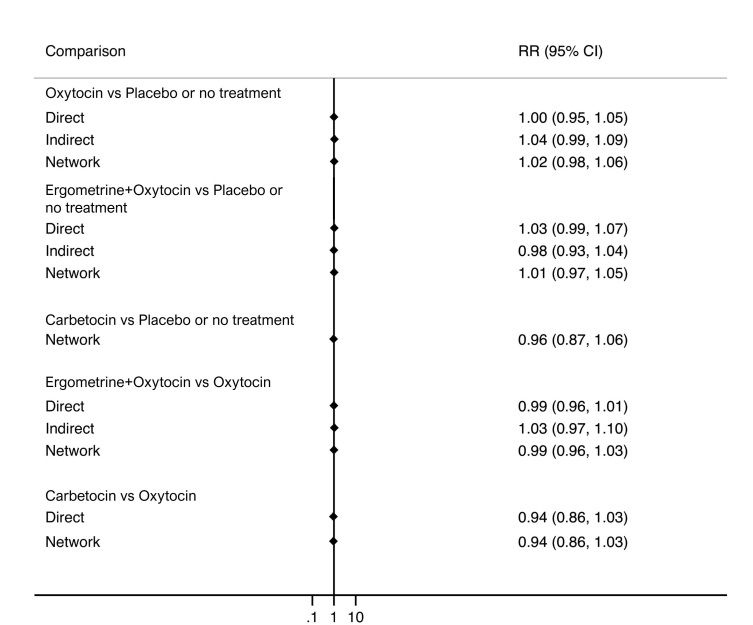
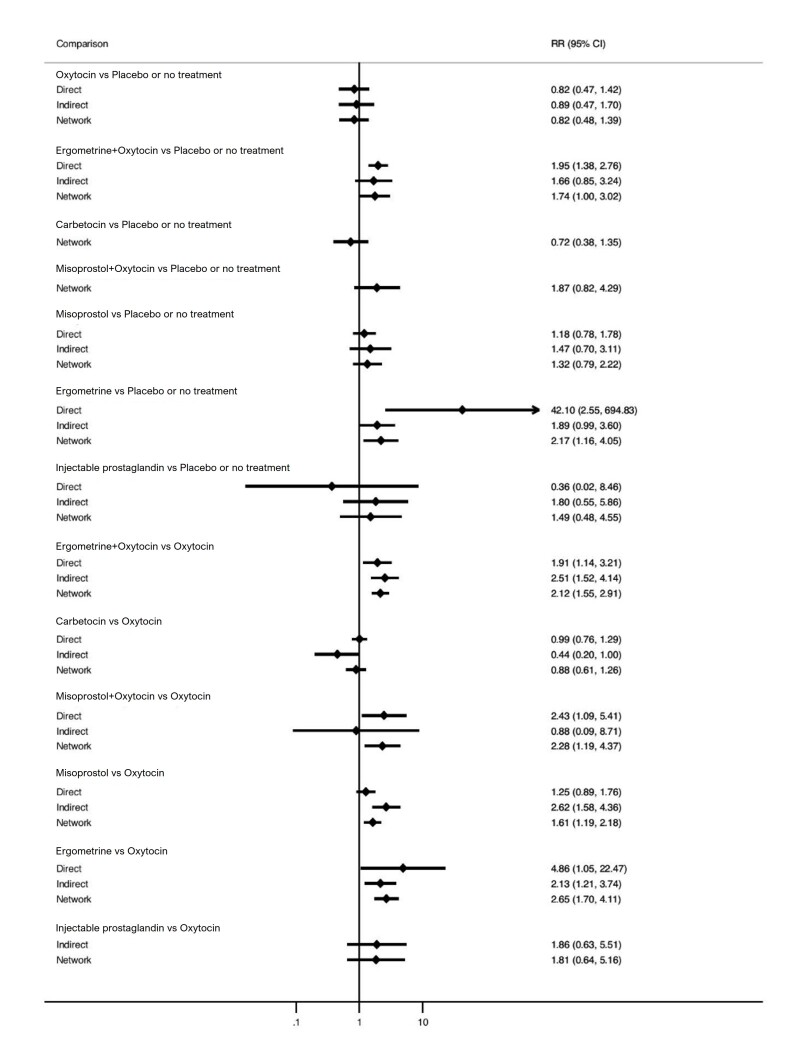
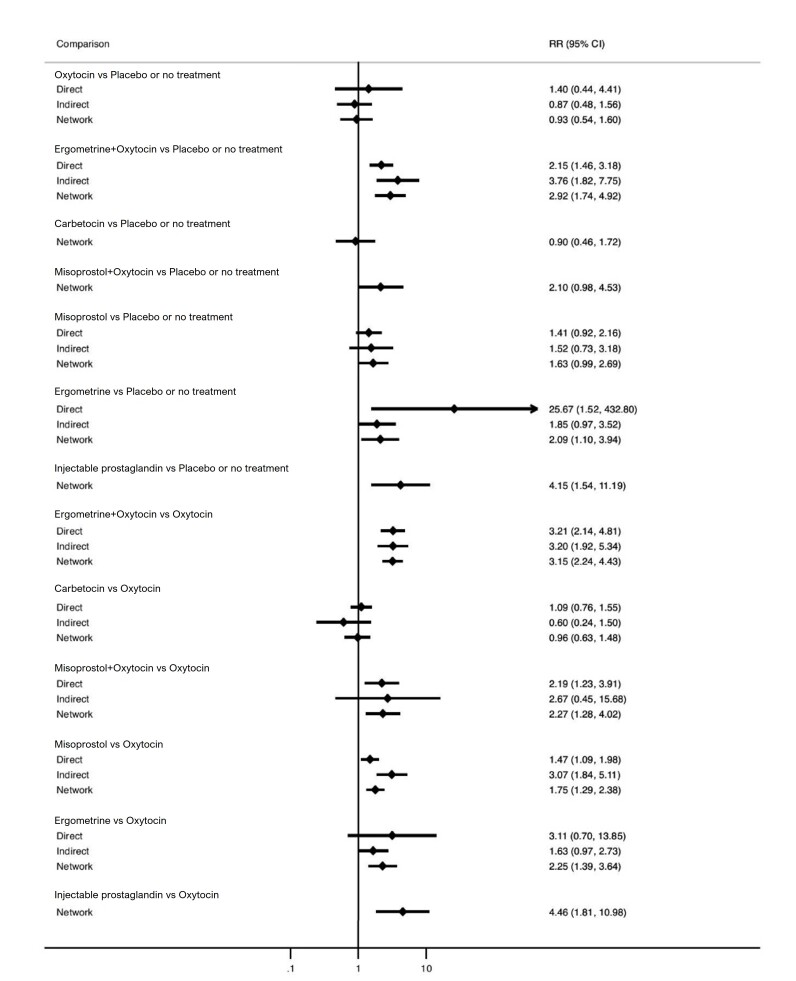
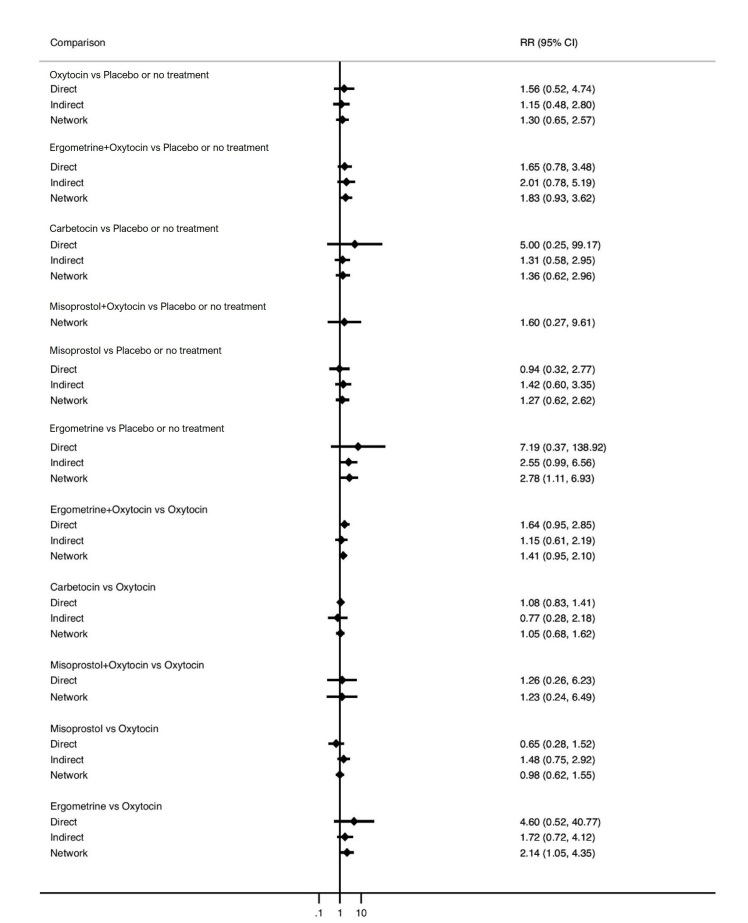
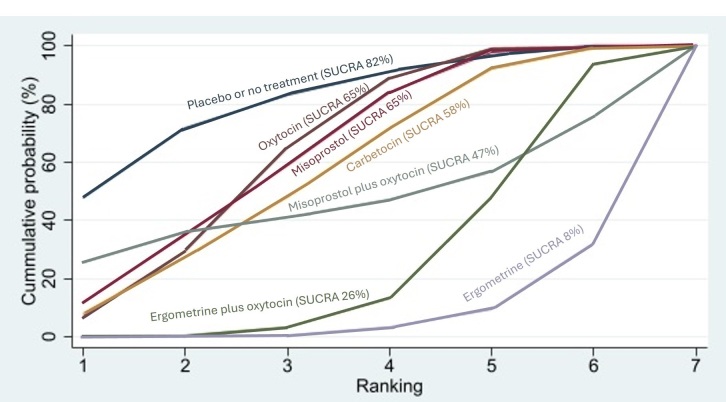
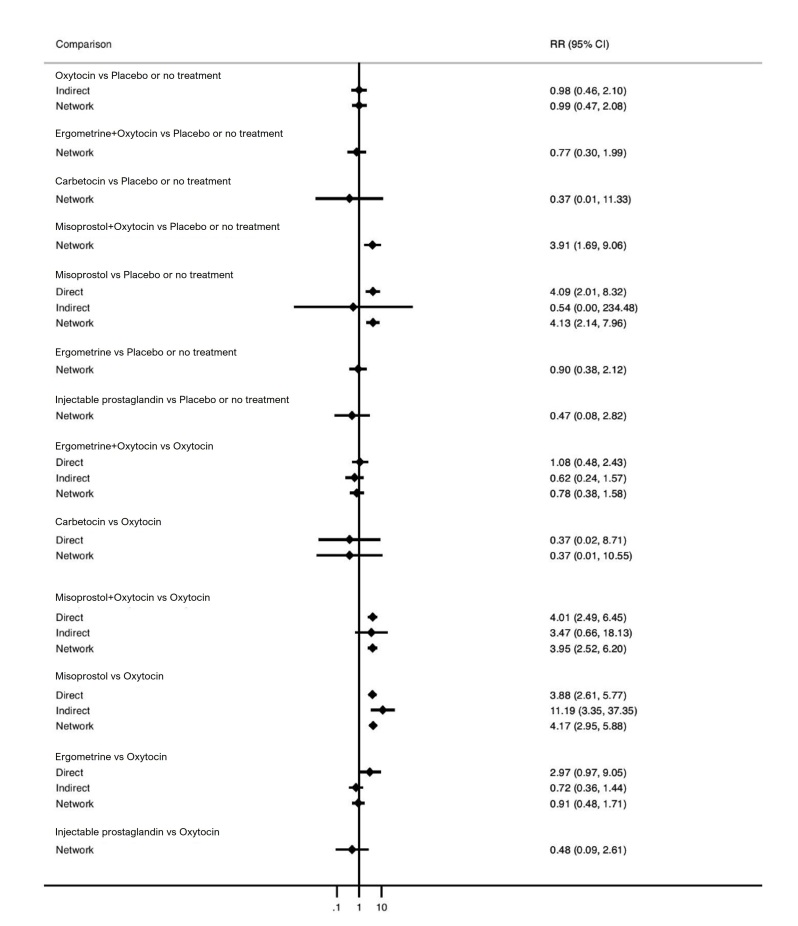
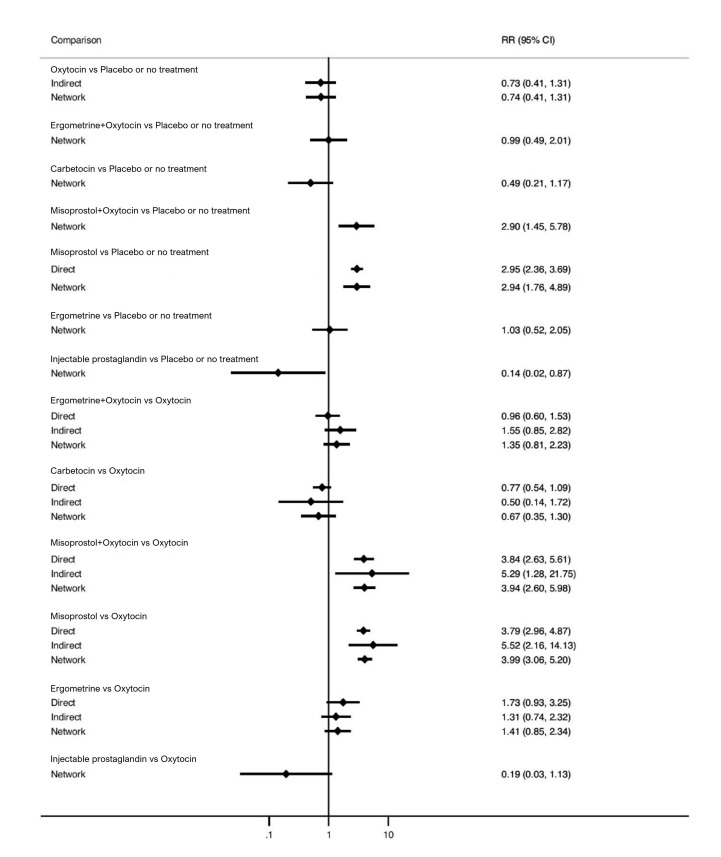
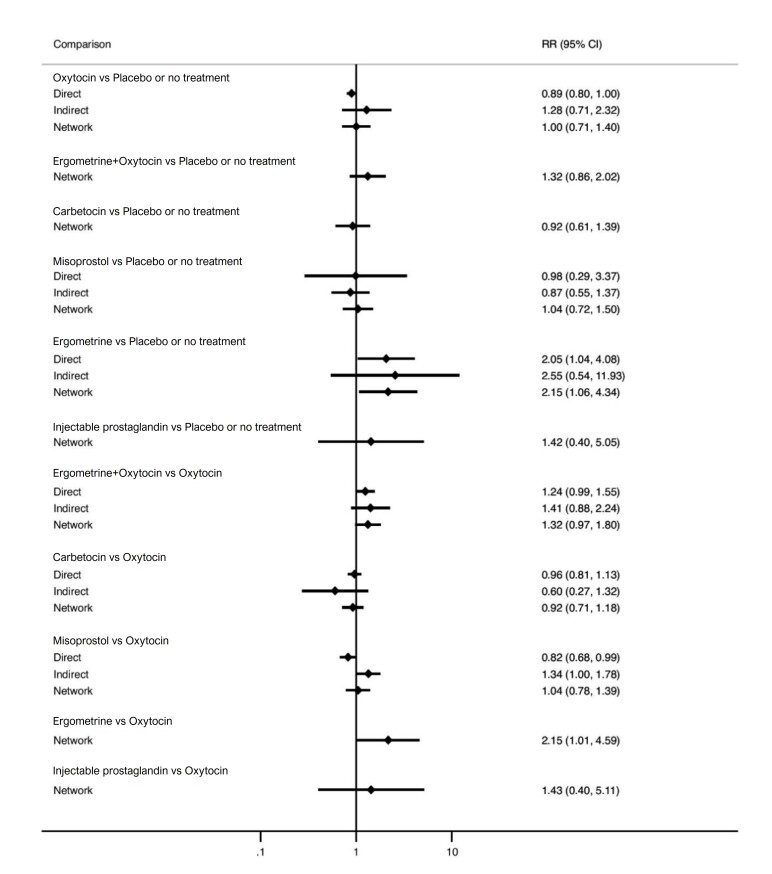
Relative effects from the network meta‐analysis suggested that all agents, except injectable prostaglandins, for which data were limited, were effective for preventing PPH ≥ 500 mL compared with placebo or no treatment. The two highest‐ranked agents were ergometrine plus oxytocin and misoprostol plus oxytocin. Compared with oxytocin, ergometrine plus oxytocin reduces PPH ≥ 500 mL (risk ratio (RR) 0.76, 95% confidence interval (CI) 0.64 to 0.90, high‐certainty evidence), and misoprostol plus oxytocin probably reduces PPH ≥ 500 mL (RR 0.70, 95% CI 0.57 to 0.87; moderate‐certainty evidence). Carbetocin (high‐), injectable prostaglandins (moderate‐) and ergometrine (low‐certainty evidence) have similar effects compared with oxytocin. The evidence for misoprostol is very low certainty.
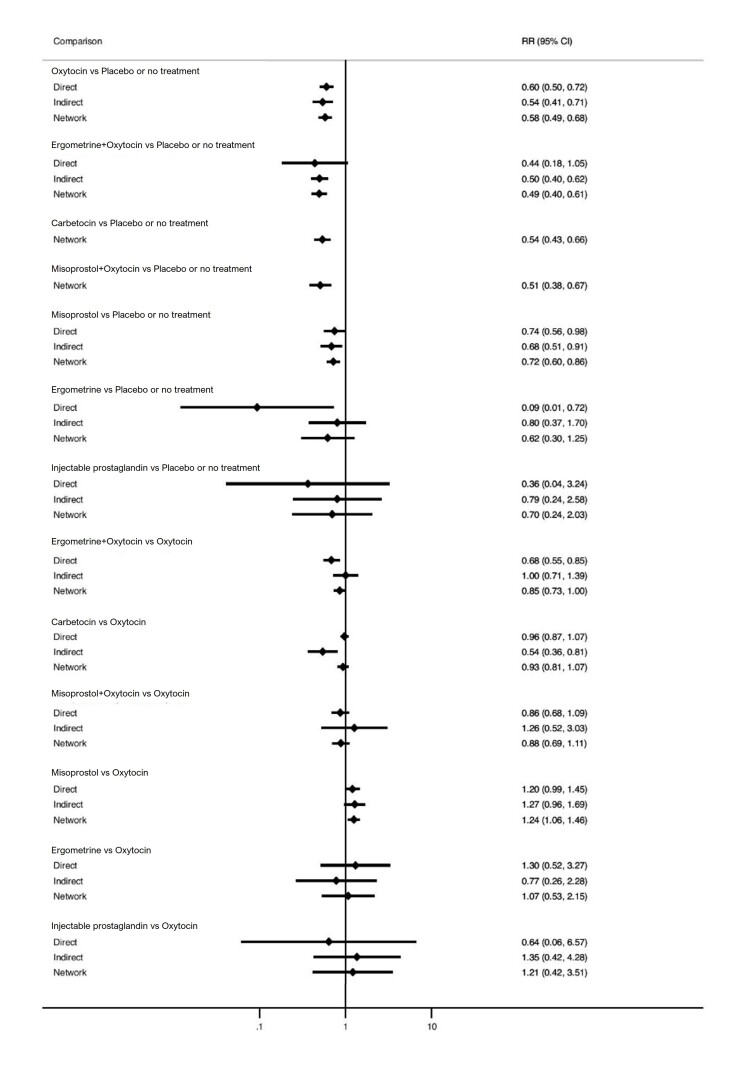
All agents, except ergometrine and injectable prostaglandins, for which data were limited, were effective for preventing PPH ≥ 1000 mL compared with placebo or no treatment. Ergometrine plus oxytocin, and misoprostol plus oxytocin were the highest‐ranked agents. Compared with oxytocin, carbetocin and injectable prostaglandins (both moderate‐certainty evidence), and misoprostol plus oxytocin (low‐certainty evidence) make little or no difference to PPH ≥ 1000 mL. Misoprostol may be less effective in preventing PPH ≥ 1000 mL compared with oxytocin (RR 1.24, 95% CI 1.06 to 1.46; low‐certainty evidence). The certainty of evidence for ergometrine and ergometrine plus oxytocin was very low.
Compared with oxytocin, misoprostol plus oxytocin probably reduces the use of additional uterotonics (RR 0.55, 95% CI 0.42 to 0.72, moderate‐certainty evidence), and carbetocin (RR 0.74, 95% CI 0.59 to 0.94; low‐certainty evidence), and ergometrine plus oxytocin may reduce the use of additional uterotonics (RR 0.68, 95% CI 0.56 to 0.83; low‐certainty evidence). Misoprostol (low‐certainty evidence) makes little or no difference to this outcome.
Misoprostol plus oxytocin probably reduces the risk of needing a blood transfusion (RR 0.40, 95% CI 0.28 to 0.58; moderate‐certainty‐evidence), and ergometrine plus oxytocin may reduce the risk of blood transfusion compared with oxytocin (RR 0.73, 95% CI 0.56 to 0.96, low‐certainty evidence). Carbetocin (moderate‐certainty evidence) and misoprostol (low‐certainty evidence) probably make little or no difference to this outcome compared with oxytocin.
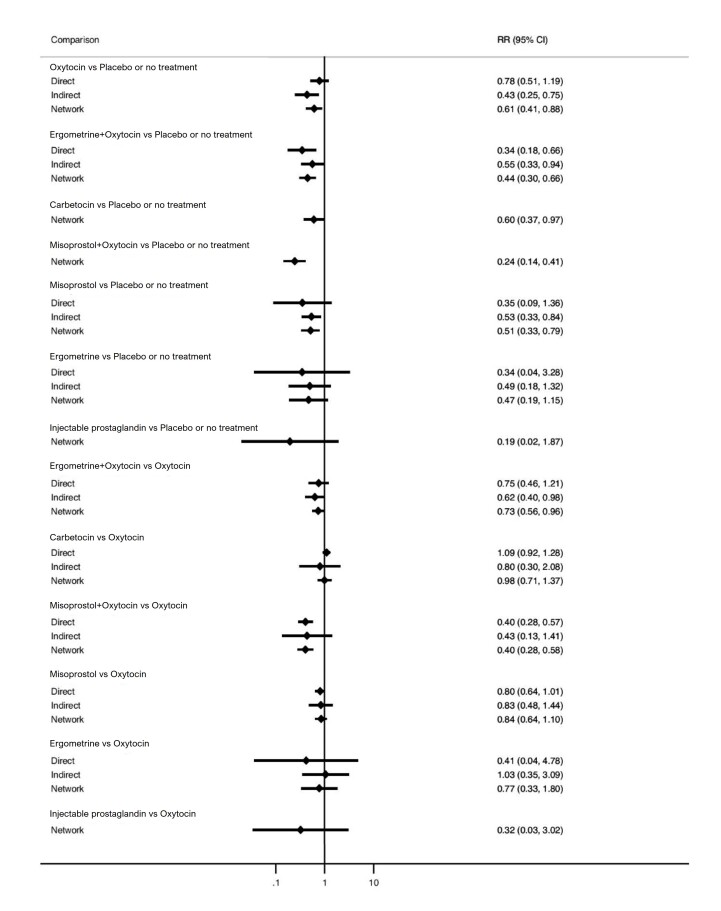
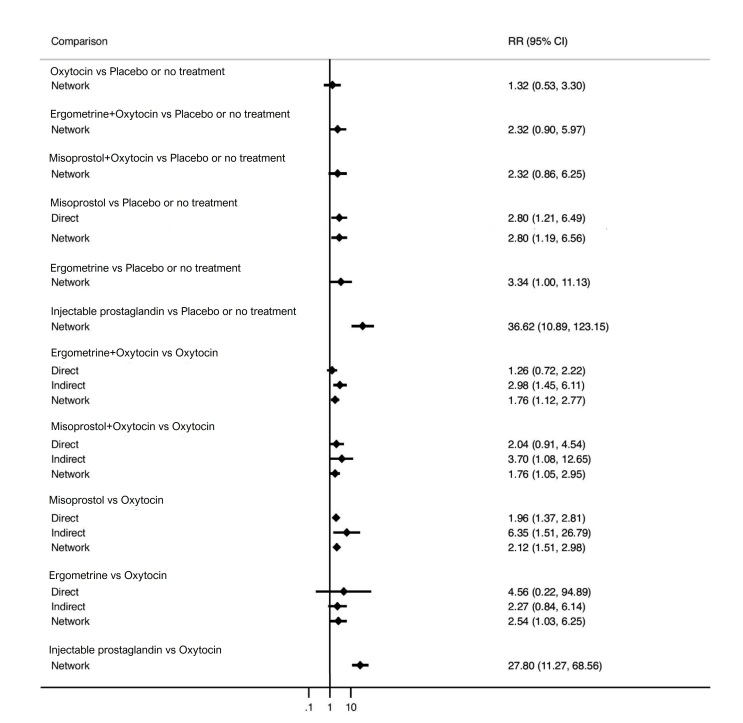
All uterotonic agents, except for carbetocin, were associated with increased risks of side effects compared with oxytocin. Misoprostol may increase the likelihood of nausea, vomiting and fever, and probably increases the risk of diarrhoea. Injectable prostaglandins may increase the likelihood of diarrhoea. Ergometrine probably increases the likelihood of nausea and vomiting, and may increase the likelihood of hypertension, headache, and diarrhoea. Ergometrine plus oxytocin may increase the likelihood of nausea, vomiting, and diarrhoea. Misoprostol plus oxytocin probably increases the likelihood of nausea, vomiting and diarrhoea, and may increase the likelihood of fever.
Analyses of the prespecified subgroups did not reveal important subgroup differences.
Evidence for outcomes not presented above but reported in the summary of findings tables was very low certainty.
Authors' conclusions
Most agents are effective for preventing PPH when compared with placebo or no treatment. Ergometrine plus oxytocin, and misoprostol plus oxytocin may be more effective than the current standard oxytocin. All agents, except for carbetocin, are associated with an increased risk of some side effects compared with oxytocin.
Funding
Supported by UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a cosponsored programme executed by the WHO (Award No. HQHRP2220228‐22.1‐74309).
Registration
Cochrane Library; Registration number: CD011689 and protocol [and previous versions] available via DOI: 10.1002/14651858.CD011689 [DOI: 10.1002/14651858.CD011689.pub3 and DOI: 10.1002/14651858.CD011689.pub2]
Plain language summary
Which medication is best for reducing excessive blood loss after childbirth and has the fewest unwanted effects?
Key messages
Most medications (uterotonics) to prevent excessive blood loss after childbirth (postpartum haemorrhage (PPH)) are more effective than no treatment.
Ergometrine plus oxytocin, and misoprostol plus oxytocin are most effective compared with oxytocin (standard treatment).
All uterotonics, except for carbetocin, are associated with an increased risk of unwanted effects compared with oxytocin.
What is postpartum haemorrhage, and how is it treated?
Postpartum haemorrhage (PPH) is excessive blood loss, of more than 500 mL, in the first 24 hours after childbirth. It is the most common reason mothers die in childbirth worldwide. Treatments, such as blood transfusion or hysterectomy, are a burden on women's health and health services. To reduce the risk of PPH, clinicians often give medication to make the uterus (womb) contract ‐ a uterotonic. Uterotonics include oxytocin, misoprostol, ergometrine, carbetocin, injectable prostaglandins and combinations of these medications. They may cause unwanted effects, including nausea, vomiting, diarrhoea and fever. Oxytocin is currently recommended as the standard medication to reduce the risk of PPH.
What did we want to find out?
We aimed to find out which uterotonic is most effective in preventing PPH and has the fewest unwanted effects. We were interested in how well they prevented blood loss (more than 500 mL and more than 1000 mL); whether additional uterotonics or a blood transfusion were needed; and unwanted effects.
What did we do?
We collected and analysed all the relevant, trustworthy studies that investigated uterotonics to prevent PPH.
What did we find?
We found 122 studies involving 121,931 women, in 48 countries. Most women gave birth normally (vaginally), in hospital.
All uterotonics are more effective in preventing blood loss than placebo (sham medicine) or no treatment. We are not sure about injectable prostaglandins and ergometrine because we did not find much evidence about them. The two most effective uterotonics were ergometrine plus oxytocin, and misoprostol plus oxytocin.
Blood loss of 500 mL or more Based on our results, for vaginal birth, 83 women in 1000 given oxytocin would experience blood loss of 500 mL or more.
Compared with oxytocin:
ergometrine plus oxytocin reduces the likelihood of blood loss of 500 mL or more. Of 1000 women having a vaginal birth, 63 given ergometrine plus oxytocin would experience blood loss of 500 mL or more;
misoprostol plus oxytocin probably reduces the likelihood of blood loss of 500 mL or more. Of 1000 women having a vaginal birth, 58 given misoprostol plus oxytocin would experience blood loss of 500 mL or more;
carbetocin makes little or no difference; injectable prostaglandins and ergometrine probably make little or no difference. We are uncertain about the effects of misoprostol.
Blood loss of 1000 mL or more Based on our results, for vaginal birth, 24 women in 1000 given oxytocin would experience blood loss of 1000 mL or more.
Compared with oxytocin:
carbetocin and injectable prostaglandins probably, and misoprostol plus oxytocin may make little to no difference;
misoprostol may be less effective than oxytocin; 30 of 1000 women having a vaginal birth given misoprostol may experience blood loss of 1000 mL or more;
we are uncertain about the effects of ergometrine, and ergometrine plus oxytocin.
Other results Compared with oxytocin:
misoprostol plus oxytocin probably reduces the need for additional uterotonics and the need for blood transfusion;
carbetocin probably makes little or no difference to the need for blood transfusion;
we are less sure about the other medications.
Unwanted effects Compared with oxytocin:
misoprostol probably increases the likelihood of diarrhoea and may increase the likelihood of nausea, vomiting and fever;
injectable prostaglandins may increase the likelihood of diarrhoea;
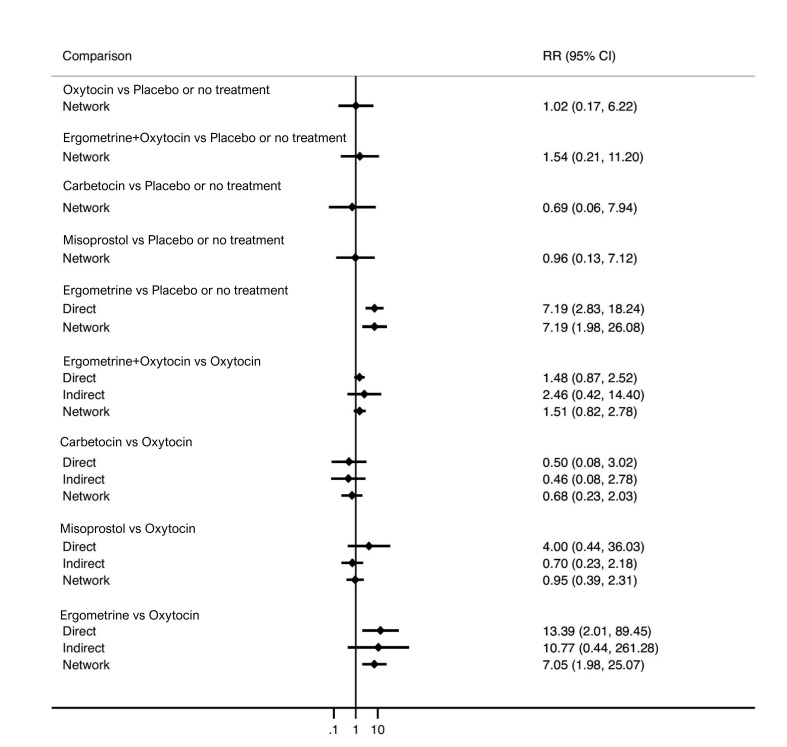
ergometrine probably increases the likelihood of nausea and vomiting, and may increase the likelihood of high blood pressure, headache and diarrhoea;
ergometrine plus oxytocin may increase the likelihood of nausea, vomiting and diarrhoea;
misoprostol plus oxytocin probably increases the likelihood of nausea, vomiting and diarrhoea, and may increase the likelihood of fever;
carbetocin was associated with few unwanted effects.
We found similar results whether women gave birth normally or by caesarean section, in hospital or in the community, were at high or low risk for PPH, received a high or low dose of misoprostol, and whether they received one single large injection or an infusion (drip) of oxytocin, or both.
What are the limitations of the evidence?
We found little information about home births or about women giving birth by caesarean section, or who had other illnesses and who were at high risk of having PPH. We did not differentiate between the different doses and methods of giving the different uterotonics. Not all the studies provided information about unwanted effects, and they used different methods to measure blood loss.
How up to date is the evidence?
This evidence is up to date until 5 February 2024.
Summary of findings
Summary of findings 1. Postpartum haemorrhage ≥ 500 mL.
| Patient or population: women in the third stage of labour Interventions: carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine), misoprostol plus oxytocin Comparison (reference): oxytocin Outcome: PPH ≥ 500 mL Setting: hospital or community setting | |||||||||
| Uterotonic agent(s) | Direct evidence | Indirect evidence | NMA evidence | Anticipated absolute effects for NMA estimate | |||||
| RR (95% CI) | Certainty of the evidence | RR (95% CI) | Certainty of the evidence | RR (95% CI) | Certainty of the evidence | Risk with oxytocin | Risk with intervention (other uterotonics) | Risk difference with intervention | |
| Carbetocin | 1.00 (0.95 to 1.05) | ⊕⊕⊕⊕ Higha |
0.76 (0.39 to 1.47) | ⊕⊕⊕⊝ Moderateb | 0.98 (0.79 to 1.22) |
⊕⊕⊕⊕ Highc |
115 per 1000 | 113 per 1000 | 2 fewer per 1000 (from 24 fewer to 25 more) |
| Vaginal birth: 83 per 1000 | Vaginal birth: 81 per 1000 | Vaginal birth: 2 fewer per 1000 (from 17 fewer to 18 more) | |||||||
| Caesarean birth: 657 per 1000 | Caesarean birth: 644 per 1000 | Caesarean birth: 13 fewer per 1000 (from 138 fewer to 145 more) | |||||||
| Misoprostol | 1.11 (0.95 to 1.29) | ⊕⊝⊝⊝ Very lowd | 1.08 (0.83 to 1.41) | ⊕⊝⊝⊝ Very lowe | 1.11 (0.98 to 1.26) | ⊕⊝⊝⊝ Very lowf | 115 per 1000 | 128 per 1000 | 13 more per 1000 (2 fewer to 30 more) |
| Vaginal birth: 83 per 1000 | Vaginal birth: 92 per 1000 | Vaginal birth: 9 more per 1000 (2 fewer to 22 more) | |||||||
| Caesarean birth: 657 per 1000 | Caesarean birth: 729 per 1000 | Caesarean birth: 72 more per 1000 (13 fewer to 171 more) | |||||||
| Injectable prostaglandins | 0.91 (0.33 to 2.48) | ⊕⊕⊝⊝ Lowg | 1.18 (0.74 to 1.88) | ⊕⊝⊝⊝ Very lowh | 1.14 (0.74 to 1.75) | ⊕⊕⊕⊝ Moderatei | 115 per 1000 | 131 per 1000 | 16 more per 1000 (30 fewer to 86 more) |
| Vaginal birth: 83 per 1000 | Vaginal birth: 95 per 1000 | Vaginal birth: 12 more per 1000 (22 fewer to 62 more) | |||||||
| Caesarean birth: 657 per 1000 | Caesarean birth: 749 per 1000 | Caesarean birth: 92 more per 1000 (171 fewer to 493 more) | |||||||
| Ergometrine | 1.21 (0.81 to 1.82) | ⊕⊕⊝⊝ Lowj | 1.10 (0.75 to 1.61) | ⊕⊝⊝⊝ Very lowh | 1.16 (0.89 to 1.53) | ⊕⊕⊝⊝ Lowk | 115 per 1000 | 133 per 1000 | 18 more per 1000 (13 fewer to 61 more) |
| Vaginal birth: 83 per 1000 | Vaginal birth: 96 per 1000 | Vaginal birth: 13 more per 1000 (9 fewer to 44 more) |
|||||||
| Caesarean birth: 657 per 1000 | Caesarean birth: 762 per 1000 | Caesarean birth: 105 more per 1000 (72 fewer to 348 more) | |||||||
| Ergometrine plus oxytocin | 0.85 (0.71 to 1.02) | ⊕⊕⊕⊝ Moderatel | 0.70 (0.54 to 0.91) | ⊕⊝⊝⊝ Very lowm | 0.76 (0.64 to 0.90) | ⊕⊕⊕⊕ Highc |
115 per 1000 | 87 per 1000 | 28 fewer per 1000 (41 fewer to 12 fewer) |
| Vaginal birth: 83 per 1000 | Vaginal birth: 63 per 1000 | Vaginal birth: 20 fewer per 1000 (30 fewer to 8 fewer) | |||||||
| Caesarean birth: 657 per 1000 | Caesarean birth: 499 per 1000 | Caesarean birth: 158 fewer per 1000 (237 fewer to 66 fewer) | |||||||
| Misoprostol plus oxytocin | 0.69 (0.56 to 0.85) | ⊕⊕⊕⊝ Moderaten | 0.86 (0.40 to 1.84) | ⊕⊝⊝⊝ Very lowh | 0.70 (0.57 to 0.87) | ⊕⊕⊕⊝ Moderateo | 115 per 1000 | 81 per 1000 | 35 fewer per 1000 (49 fewer to 15 fewer) |
| Vaginal birth: 83 per 1000 | Vaginal birth: 58 per 1000 | Vaginal birth: 25 fewer per 1000 (36 fewer to 11 fewer) | |||||||
| Caesarean birth: 657 per 1000 | Caesarean birth: 460 per 1000 | Caesarean birth: 197 fewer per 1000 (283 fewer to 85 fewer) | |||||||
|
The assumed risks in the oxytocin group are based on weighted means of baseline risks from the trials with oxytocin groups in the network meta‐analysis. The corresponding risks in the carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine), misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effect of individual uterotonic when compared with oxytocin (and its 95% CI) derived from the network meta‐analysis. CI: confidence interval; NMA: network meta‐analysis; PPH: postpartum haemorrhage; RR: risk ratio | |||||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. The starting rating for assessing the certainty of evidence for the indirect estimate is the lower of the preliminary certainty ratings of the two direct estimates forming the most dominant first‐order loop. The starting rating for assessing the certainty of evidence for the network estimate is the higher of the preliminary certainty ratings of the direct or indirect estimate or the rating of the estimate that contributes the most or the rating of the direct estimate. | |||||||||
aDirect evidence not downgraded. bIndirect evidence start rating high, then downgraded −1 due to serious imprecision. cNetwork evidence start rating high (high certainty of preliminary direct evidence). dDirect evidence downgraded −3 due to multiple limitations in trial design, severe unexplained statistical heterogeneity (I2 = 79%), publication bias and serious imprecision. eIndirect evidence start rating low (−2 due to multiple crucial limitations in trial design), then downgraded −1 due to serious imprecision. fNetwork evidence start rating low (low certainty of preliminary indirect evidence), then further downgraded −1 due to serious imprecision. gDirect evidence downgraded −2 due to very serious imprecision. hIndirect evidence start rating very low (−3 due to multiple limitations in trial design, severe unexplained statistical heterogeneity and publication bias), also serious imprecision. iNetwork evidence start rating high (high certainty of preliminary direct evidence), then downgraded −1 due to serious imprecision. jDirect evidence downgraded −2 due to multiple limitations in trial design and serious imprecision. kNetwork evidence start rating moderate (moderate certainty of preliminary direct evidence), then downgraded −1 due to serious imprecision. lDirect evidence downgraded −1 due to serious imprecision. mIndirect evidence start rating very low (−3 due to multiple limitations in trial design, severe unexplained statistical heterogeneity and publication bias). nDirect evidence downgraded −1 due to multiple limitations in trial design. oNetwork evidence start rating moderate (moderate certainty of preliminary direct evidence).
Summary of findings 2. Postpartum haemorrhage ≥ 1000 mL.
| Patient or population: women in the third stage of labour Interventions: carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine), misoprostol plus oxytocin Comparison (reference): oxytocin Outcome: PPH ≥ 1000 mL Setting: hospital or community setting | |||||||||
| Uterotonic agent(s) | Direct evidence | Indirect evidence | NMA evidence | Anticipated absolute effects for NMA estimate | |||||
| RR (95% CI) | Certainty of the evidence | RR (95% CI) | Certainty of the evidence | RR (95% CI) | Certainty of the evidence | Risk with oxytocin | Risk with intervention (other uterotonics) | Risk difference with intervention | |
| Carbetocin | 0.96 (0.87 to 1.07) |
⊕⊕⊕⊕ Higha |
0.54 (0.36 to 0.81) |
⊕⊕⊕⊝ Moderateb | 0.93 (0.81 to 1.07) |
⊕⊕⊕⊝ Moderatec | 44 per 1000 | 42 per 1000 | 3 fewer per 1000 (from 8 fewer to 3 more) |
| Vaginal birth: 24 per 1000 | Vaginal birth: 22 per 1000 | Vaginal birth: 2 fewer per 1000 (5 fewer to 2 more) | |||||||
| Caesarean birth: 143 per 1000 | Caesarean birth: 133 per 1000 | Caesarean birth: 10 fewer per 1000 (from 27 fewer to 10 more) | |||||||
| Misoprostol | 1.20 (0.99 to 1.45) |
⊕⊝⊝⊝ Very lowd | 1.27 (0.96 to 1.69) |
⊕⊝⊝⊝ Very lowe | 1.24 (1.06 to 1.46) |
⊕⊕⊝⊝ Lowf | 44 per 1000 | 55 per 1000 | 11 more per 1000 (3 more to 20 more) |
| Vaginal birth: 24 per 1000 | Vaginal birth: 30 per 1000 | Vaginal birth: 6 more per 1000 (1 more to 11 more) | |||||||
| Caesarean birth: 143 per 1000 | Caesarean birth: 177 per 1000 | Caesarean birth: 34 more per 1000 (9 more to 66 more) | |||||||
| Injectable prostaglandins | 0.64 (0.06 to 6.57) |
⊕⊕⊝⊝ Lowg | 1.35 (0.42 to 4.28) |
⊕⊝⊝⊝ Very lowe | 1.21 (0.42 to 3.51) |
⊕⊕⊕⊝ Moderateh | 44 per 1000 | 53 per 1000 | 9 more per 1000 (26 fewer to 110 more) |
| Vaginal birth: 24 per 1000 | Vaginal birth: 29 per 1000 | Vaginal birth: 5 more per 1000 (14 fewer to 60 more) | |||||||
| Caesarean birth: 143 per 1000 | Caesarean birth: 173 per 1000 | Caesarean birth: 30 more per 1000 (83 fewer to 359 more) | |||||||
| Ergometrine | 1.30 (0.52 to 3.27) |
⊕⊝⊝⊝ Very lowi | 0.77 (0.26 to 2.28) |
⊕⊝⊝⊝ Very lowj | 1.07 (0.53 to 2.15) |
⊕⊝⊝⊝ Very lowk | 44 per 1000 | 47 per 1000 | 3 more per 1000 (21 fewer to 51 more) |
| Vaginal birth: 24 per 1000 | Vaginal birth: 26 fewer per 1000 | Vaginal birth: 2 more per 1000 (11 fewer to 28 more) | |||||||
| Caesarean birth: 143 per 1000 | Caesarean birth: 153 per 1000 | Caesarean birth: 10 more per 1000 (67 fewer to 164 more) | |||||||
| Ergometrine plus oxytocin | 0.68 (0.55 to 0.85) |
⊕⊕⊕⊝ Moderatel | 1.00 (0.71 to 1.39) |
⊕⊝⊝⊝ Very lowj | 0.85 (0.73 to 1.00) |
⊕⊝⊝⊝ Very lowm | 44 per 1000 | 37 per 1000 | 7 fewer per 1000 (12 fewer to 0 more) |
| Vaginal birth: 24 per 1000 | Vaginal birth: 20 per 1000 | Vaginal birth: 4 fewer per 1000 (6 fewer to 0 more) | |||||||
| Caesarean birth: 143 per 1000 | Caesarean birth: 122 per 1000 | Caesarean birth: 21 fewer per 1000 (39 fewer to 0 more) | |||||||
| Misoprostol plus oxytocin | 0.86 (0.68 to 1.09) |
⊕⊕⊝⊝ Lown | 1.26 (0.52 to 3.03) |
⊕⊝⊝⊝ Very lowj | 0.88 (0.69 to 1.11) |
⊕⊕⊝⊝ Lowo | 44 per 1000 | 39 per 1000 | 5 fewer per 1000 (14 fewer to 5 more) |
| Vaginal birth: 24 per 1000 | Vaginal birth: 21 per 1000 | Vaginal birth: 3 fewer per 1000 (7 fewer to 3 more) | |||||||
| Caesarean birth: 143 per 1000 | Caesarean birth: 126 per 1000 | Caesarean birth: 17 fewer per 1000 (44 fewer to 16 more) | |||||||
|
The assumed risks in the oxytocin group are based on weighted means of baseline risks from the trials with oxytocin groups in the network meta‐analysis. The corresponding risks in the carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine), misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effect of individual uterotonic when compared with oxytocin (and its 95% CI) derived from the network meta‐analysis. CI: confidence interval; NMA: network meta‐analysis; PPH: postpartum haemorrhage; RR: risk ratio | |||||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. The starting rating for assessing the certainty of evidence for the indirect estimate is the lower of the preliminary certainty ratings of the two direct estimates forming the most dominant first‐order loop. The starting rating for assessing the certainty of evidence for the network estimate is the higher of the preliminary certainty ratings of the direct or indirect estimate or the rating of the estimate that contributes the most, or the rating of the direct estimate. | |||||||||
aDirect evidence not downgraded. bIndirect evidence start rating moderate (−1 due to multiple limitations in trial design). cNetwork evidence start rating high (high certainty of preliminary direct evidence), then downgraded −1 due to incoherence. dDirect evidence downgraded −3 due to multiple limitations in trial design, publication bias and serious imprecision. eIndirect evidence start rating low (−2 due to multiple crucial limitations in trial design), then downgraded −1 due to serious imprecision. fNetwork evidence start rating low (low certainty of preliminary direct evidence). gDirect evidence downgraded −2 due to very serious imprecision. hNetwork evidence start rating high (high certainty of preliminary direct evidence), then downgraded −1 due to serious imprecision. iDirect evidence downgraded −3 due to multiple crucial limitations in trial design and serious imprecision. jIndirect evidence start rating low (−2 due to multiple limitations in trial design and publication bias), then downgraded −1 due to serious imprecision. kNetwork evidence start rating low (low certainty of preliminary direct evidence), then downgraded −1 due to serious imprecision. lDirect evidence downgraded −1 due to multiple limitations in trial design. mNetwork evidence start rating moderate (moderate certainty of preliminary direct evidence), then downgraded −2 due to incoherence and serious imprecision. nDirect evidence downgraded −2 due to multiple limitations in trial design and serious imprecision. oNetwork evidence start rating moderate (moderate certainty of preliminary direct evidence), then downgraded −1 due to serious imprecision.
Summary of findings 3. Additional uterotonics.
| Patient or population: women in the third stage of labour Interventions: carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine), misoprostol plus oxytocin Comparison (reference): oxytocin Outcome: use of additional uterotonics Setting: hospital or community setting | |||||||||
| Uterotonic agent(s) | Direct evidence | Indirect evidence | NMA evidence | Anticipated absolute effects for NMA estimate | |||||
| RR (95% CI) | Certainty of the evidence | RR (95% CI) | Certainty of the evidence | RR (95% CI) | Certainty of the evidence | Risk with oxytocin | Risk with intervention (other uterotonics) | Risk difference with intervention | |
| Carbetocin | 0.82 (0.69 to 0.98) |
⊕⊝⊝⊝ Very lowa | 0.57 (0.34 to 0.98) |
⊕⊕⊝⊝ Lowb | 0.74 (0.59 to 0.94) |
⊕⊕⊝⊝ Lowc | 124 per 1000 | 92 per 1000 | 32 fewer per 1000 (51 fewer to 7 fewer) |
| Vaginal birth: 89 per 1000 | Vaginal birth: 66 per 1000 | Vaginal birth: 23 fewer per 1000 (36 fewer to 5 fewer) | |||||||
| Caesarean birth: 230 per 1000 | Caesarean birth: 170 per 1000 | Caesarean birth: 60 fewer per 1000 (94 fewer to 14 fewer) | |||||||
| Misoprostol | 1.13 (0.98 to 1.31) |
⊕⊕⊝⊝ Lowd | 1.20 (0.87 to 1.65) |
⊕⊝⊝⊝ Very lowe | 1.14 (0.97 to 1.34) |
⊕⊕⊝⊝ Lowf | 124 per 1000 | 141 per 1000 | 17 more per 1000 (4 fewer to 42 more) |
| Vaginal birth: 89 per 1000 | Vaginal birth: 101 per 1000 | Vaginal birth: 12 more per 1000 (3 fewer to 30 more) | |||||||
| Caesarean birth: 230 per 1000 | Caesarean birth: 262 per 1000 | Caesarean birth: 32 more per 1000 (7 fewer to 78 more) | |||||||
| Injectable prostaglandins | Not reported by included trialsg | ‐ | 0.80 (0.42 to 1.55) |
⊕⊝⊝⊝ Very lowh | 0.81 (0.43 to 1.55) |
⊕⊝⊝⊝ Very low i | 124 per 1000 | 100 per 1000 | 24 fewer per 1000 (71 fewer to 68 more) |
| Vaginal birth: 89 per 1000 | Vaginal birth: 72 per 1000 | Vaginal birth: 17 fewer per 1000 (51 fewer to 49 more) | |||||||
| Caesarean birth: 230 per 1000 | Caesarean birth: 186 per 1000 | Caesarean birth: 44 fewer per 1000 (131 fewer to 127 more) | |||||||
| Ergometrine | 0.88 (0.37 to 2.10) |
⊕⊝⊝⊝ Very lowj | 1.03 (0.70 to 1.53) |
⊕⊝⊝⊝ Very lowk | 0.99 (0.72 to 1.34) |
⊕⊝⊝⊝ Very lowl | 124 per 1000 | 123 per 1000 | 1 fewer per 1000 (35 fewer to 42 more) |
| Vaginal birth: 89 per 1000 | Vaginal birth: 88 per 1000 | Vaginal birth: 1 fewer per 1000 (25 fewer to 30 more) | |||||||
| Caesarean birth: 230 per 1000 | Caesarean birth: 228 per 1000 | Caesarean birth: 2 fewer per 1000 (64 fewer to 78 more) | |||||||
| Ergometrine plus oxytocin | 0.78 (0.62 to 0.98) |
⊕⊕⊝⊝ Lowm | 0.59 (0.43 to 0.79) |
⊕⊕⊝⊝ Lown | 0.68 (0.56 to 0.83) |
⊕⊕⊝⊝ Lowo | 124 per 1000 | 84 per 1000 | 40 fewer per 1000 (55 fewer to 21 fewer) |
| Vaginal birth: 89 per 1000 | Vaginal birth: 61 per 1000 | Vaginal birth: 28 fewer per 1000 (39 fewer to 15 fewer) | |||||||
| Caesarean birth: 230 per 1000 | Caesarean birth: 156 per 1000 | Caesarean birth: 74 fewer per 1000 (101 fewer to 39 fewer) | |||||||
| Misoprostol plus oxytocin | 0.53 (0.43 to 0.65) |
⊕⊕⊕⊝ Moderatep |
0.97 (0.39 to 2.44) |
⊕⊕⊝⊝ Lowq | 0.55 (0.42 to 0.72) |
⊕⊕⊕⊝ Moderater |
124 per 1000 | 69 per 1000 | 55 fewer per 1000 (72 fewer to 33 fewer) |
| Vaginal birth: 89 per 1000 | Vaginal birth: 50 per 1000 | Vaginal birth: 39 fewer per 1000 (52 fewer to 24 fewer) | |||||||
| Caesarean birth: 230 per 1000 | Caesarean birth: 129 per 1000 | Caesarean birth: 101 fewer per 1000 (133 fewer to 62 fewer) | |||||||
|
The assumed risks in the oxytocin group are based on weighted means of baseline risks from the trials with oxytocin groups in the network meta‐analysis. The corresponding risks in the Carbetocin, Misoprostol, Injectable prostaglandins, Ergometrine, Ergometrine plus oxytocin (Syntometrine), Misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effect of individual uterotonic when compared with oxytocin (and its 95% CI) derived from the network meta‐analysis. CI: confidence interval; NMA: network meta‐analysis; PPH: postpartum haemorrhage; RR: risk ratio | |||||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. The starting rating for assessing the certainty of evidence for the indirect estimate is the lower of the preliminary certainty ratings of the two direct estimates forming the most dominant first‐order loop. The starting rating for assessing the certainty of evidence for the network estimate is the higher of the preliminary certainty ratings of the direct or indirect estimate or the rating of the estimate that contributes the most, or the rating of the direct estimate. | |||||||||
aDirect evidence downgraded −3 due to multiple limitations in trial design, serious statistical heterogeneity (I2 = 64%) and publication bias. bIndirect evidence start rating low (−2 due to multiple limitations in trial design and serious statistical heterogeneity). cNetwork evidence start rating low (low certainty of preliminary indirect evidence). dDirect evidence downgraded −2 due to multiple limitations in trial design and serious imprecision. eIndirect evidence start rating low (−2 due to multiple limitations in trial design and serious statistical heterogeneity), then downgraded −1 due to serious imprecision. fNetwork evidence start rating moderate (moderate certainty of preliminary direct evidence), then downgraded −1 due to serious imprecision. gNo available evidence to rate. hIndirect evidence start rating very low (−3 due to multiple crucial limitations in trial design and serious statistical heterogeneity), also serious imprecision. iNetwork evidence start rating very low (very low certainty of preliminary indirect evidence), also very serious imprecision. jDirect evidence downgraded −3 due to multiple limitations in trial design, serious statistical heterogeneity (I2 = 88%) and serious imprecision. kIndirect evidence start rating very low (−3 due to multiple crucial limitations in trial design and serious statistical heterogeneity), also very serious imprecision. lNetwork evidence start rating low (low certainty of preliminary direct evidence), then downgraded −1 due to serious imprecision. mDirect evidence downgraded −2 due to multiple limitations in trial design and serious statistical heterogeneity (I2 = 74%). nIndirect evidence start rating low (−2 due to multiple crucial limitations in trial design). oNetwork evidence start rating low (low certainty of preliminary direct evidence). pDirect evidence downgraded −1 due to multiple limitations in trial design. qIndirect evidence start rating moderate (−1 due to multiple limitations in trial design), then downgraded −1 due to serious imprecision. rNetwork evidence start rating moderate (moderate certainty of preliminary direct evidence).
Summary of findings 4. Blood transfusion.
| Patient or population: women in the third stage of labour Interventions: carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine), misoprostol plus oxytocin Comparison (reference): oxytocin Outcome: blood transfusion Setting: hospital or community setting | |||||||||
| Uterotonic agent(s) | Direct evidence | Indirect evidence | NMA evidence | Anticipated absolute effects for NMA estimate | |||||
| RR (95% CI) | Certainty of the evidence | RR (95% CI) | Certainty of the evidence | RR (95% CI) | Certainty of the evidence | Risk with oxytocin | Risk with intervention (other uterotonics) | Risk difference with intervention | |
| Carbetocin | 1.09 (0.92 to 1.28) | ⊕⊕⊕⊝ Moderatea | 0.80 (0.30 to 2.08) | ⊕⊝⊝⊝ Very lowb | 0.98 (0.71 to 1.37) | ⊕⊕⊕⊝ Moderatec | 21 per 1000 | 21 per 1000 | 0 more per 1000 (6 fewer to 8 more) |
| Vaginal birth: 15 per 1000 |
Vaginal birth: 15 per 1000 | Vaginal birth: 0 more per 1000 (4 fewer to 6 more) | |||||||
| Caesarean birth: 56 per 1000 |
Caesarean birth: 55 per 1000 | Caesarean birth:1 fewer per 1000 (16 fewer to 21 more) | |||||||
| Misoprostol | 0.80 (0.64) to 1.01) |
⊕⊕⊝⊝ Lowd | 0.83 (0.48 to 1.44) | ⊕⊝⊝⊝ Very lowb | 0.84 (0.64 to 1.10) | ⊕⊕⊝⊝ Lowe | 21 per 1000 | 18 per 1000 | 3 fewer per 1000 (8 fewer to 2 more) |
| Vaginal birth: 15 per 1000 | Vaginal birth: 13 per 1000 | Vaginal birth: 2 fewer per 1000 (5 fewer to 2 more) | |||||||
| Caesarean birth: 56 per 1000 |
Caesarean birth: 47 per 1000 | Caesarean birth: 9 fewer per 1000 (20 fewer to 6 more) | |||||||
| Injectable prostaglandins | Not reported by included trialsf | ‐ | 0.32 (0.03 to 3.02) | ⊕⊝⊝⊝ Very lowg | 0.32 (0.03 to 3.02) | ⊕⊝⊝⊝ Very lowh | 21 per 1000 | 7 per 1000 | 14 fewer per 1000 (20 fewer to 42 more) |
| Vaginal birth: 15 per 1000 | Vaginal birth: 5 per 1000 | Vaginal birth: 10 fewer per 1000 (15 fewer to 30 more) | |||||||
| Caesarean birth: 56 per 1000 |
Caesarean birth: 18 per 1000 | Caesarean birth: 38 fewer per 1000 (54 fewer to 113 more) | |||||||
| Ergometrine | 0.41 (0.04 to 4.78) | ⊕⊝⊝⊝ Very lowi | 1.03 (0.35 to 3.09) | ⊕⊝⊝⊝ Very lowj | 0.77 (0.33 to 1.80) | ⊕⊝⊝⊝ Very lowh | 21 per 1000 | 16 per 1000 | 5 fewer per 1000 (14 fewer to 17 more) |
| Vaginal birth: 15 per 1000 | Vaginal birth: 12 per 1000 | Vaginal birth: 3 fewer per 1000 (10 fewer to 12 more) | |||||||
| Caesarean birth: 56 per 1000 |
Caesarean birth: 43 per 1000 | Caesarean birth: 13 fewer per 1000 (38 fewer to 45 more) | |||||||
| Ergometrine plus oxytocin | 0.75 (0.46 to 1.21) | ⊕⊝⊝⊝ Very lowk | 0.62 (0.40 to 0.98) | ⊕⊕⊝⊝ Lowl | 0.73 (0.56 to 0.96) | ⊕⊕⊝⊝ Lowm | 21 per 1000 | 15 per 1000 | 6 fewer per 1000 (9 fewer to 1 more) |
| Vaginal birth: 15 per 1000 | Vaginal birth: 11 per 1000 | Vaginal birth: 4 fewer per 1000 (7 fewer to 1 fewer) | |||||||
| Caesarean birth: 56 per 1000 |
Caesarean birth: 41 per 1000 | Caesarean birth: 15 fewer per 1000 (25 fewer to 2 more) | |||||||
| Misoprostol plus oxytocin | 0.40 (0.28 to 0.57) | ⊕⊕⊝⊝ Lown | 0.43 (0.13 to 1.41) | ⊕⊕⊝⊝ Lowo | 0.40 (0.28 to 0.58) | ⊕⊕⊕⊝ Moderatep | 21 per 1000 | 8 per 1000 | 13 fewer per 1000 (15 fewer to 9 fewer) |
| Vaginal birth: 15 per 1000 | Vaginal birth: 6 per 1000 | Vaginal birth: 9 fewer per 1000 (11 fewer to 6 fewer) | |||||||
| Caesarean birth: 56 per 1000 | Caesarean birth: 22 per 1000 | Caesarean birth: 34 fewer per 1000 (40 fewer to 24 fewer) | |||||||
|
The assumed risks in the oxytocin group are based on weighted means of baseline risks from the trials with oxytocin groups in the network meta‐analysis. The corresponding risks in the carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine), misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effect of individual uterotonic when compared with oxytocin (and its 95% CI) derived from the network meta‐analysis. CI: confidence interval; NMA: network meta‐analysis; PPH: postpartum haemorrhage; RR: risk ratio | |||||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. The starting rating for assessing the certainty of evidence for the indirect estimate is the lower of the preliminary certainty ratings of the two direct estimates forming the most dominant first‐order loop. The starting rating for assessing the certainty of evidence for the network estimate is the higher of the preliminary certainty ratings of the direct or indirect estimate or the rating of the estimate that contributes the most, or the rating of the direct estimate. | |||||||||
aDirect evidence downgraded −1 due to serious imprecision. bIndirect evidence start rating very low (−3 due to multiple limitations in trial design, serious statistical heterogeneity and publication bias), also serious imprecision. cNetwork evidence start rating high (high certainty of preliminary direct evidence), then downgraded −1 for serious imprecision. dDirect evidence downgraded −2 due to multiple limitations in trial design and serious imprecision. eNetwork evidence start rating moderate (moderate certainty of direct evidence), then downgraded −1 for serious imprecision. fNo available evidence to rate. gIndirect evidence start rating low (−2 due to multiple crucial limitations in trial design), then downgraded −1 due to very serious imprecision. hNetwork evidence start rating low (low certainty of preliminary indirect evidence), then downgraded −1 for serious imprecision. iDirect evidence downgraded −3 due to multiple crucial limitations in trial design, serious statistical heterogeneity (I2 = 69%) and serious imprecision. jIndirect evidence start rating low (−2 due to multiple crucial limitations in trial design), then downgraded −1 for serious imprecision. kDirect evidence downgraded −3 due to multiple limitations in trial design, serious statistical heterogeneity (I2 = 62%), publication bias and serious imprecision. lIndirect evidence start rating low (−2 due to multiple crucial limitations in trial design). mNetwork evidence start rating low (low certainty of preliminary indirect evidence). nDirect evidence downgraded −2 due to multiple limitations in trial design and publication bias. oIndirect evidence start rating moderate (−1 due to multiple limitations in trial design), then downgraded −1 due to serious imprecision. pNetwork evidence start rating moderate (moderate certainty of preliminary indirect evidence).
Summary of findings 5. Vomiting.
|
Patient or population: women in the third stage of labour
Interventions: carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine), misoprostol plus oxytocin
Comparison (reference): oxytocin
Outcome: vomiting Setting: hospital or community setting | |||||||||
| Uterotonic agent(s) | Direct evidence | Indirect evidence | NMA evidence | Anticipated absolute effects for NMA estimate | |||||
| RR (95% CI) | Certainty of the evidence | RR (95% CI) | Certainty of the evidence | RR (95% CI) | Certainty of the evidence | Risk with oxytocin | Risk with intervention (other uterotonics) | Risk difference with intervention | |
| Carbetocin | 1.09 (0.76 to 1.55) | ⊕⊕⊝⊝ Lowa | 0.60 (0.24 to 1.50) | ⊕⊝⊝⊝ Very lowb | 0.96 (0.63 to 1.48) | ⊕⊕⊝⊝ Lowc | 20 per 1000 | 19 per 1000 | 1 fewer per 1000 (7 fewer to 10 more) |
| Vaginal birth: 12 per 1000 | Vaginal birth: 12 per 1000 | Vaginal birth: 0 fewer per 1000 (4 fewer to 6 more) | |||||||
| Caesarean birth: 65 per 1000 | Caesarean birth: 62 per 1000 | Caesarean birth: 3 fewer per 1000 (24 fewer to 31 more) | |||||||
| Misoprostol | 1.47 (1.09 to 1.98) | ⊕⊕⊕⊝ Moderated | 3.07 (1.84 to 5.11) | ⊕⊝⊝⊝ Very lowe | 1.75 (1.29 to 2.38) | ⊕⊕⊝⊝ Lowf | 20 per 1000 | 35 per 1000 | 15 more per 1000 (6 more to 28 more) |
| Vaginal birth: 12 per 1000 | Vaginal birth: 21 per 1000 | Vaginal birth: 9 more per 1000 (3 more to 17 more) | |||||||
| Caesarean birth: 65 per 1000 | Caesarean birth: 114 per 1000 | Caesarean birth: 49 more per 1000 (19 more to 90 more) | |||||||
| Injectable prostaglandins | Not reported by included trialsg | ‐ | 4.46 (1.81 to 10.98) | ⊕⊝⊝⊝ Very lowh | 4.46 (1.81 to 10.98) | ⊕⊝⊝⊝ Very lowi | 20 per 1000 | 89 per 1000 | 69 more per 1000 (16 more to 200 more) |
| Vaginal birth: 12 per 1000 | Vaginal birth: 54 per 1000 | Vaginal birth: 42 more per 1000 (10 more to 120 more) | |||||||
| Caesarean birth: 65 per 1000 | Caesarean birth: 290 per 1000 | Caesarean birth: 225 more per 1000 (53 more to 649 more) | |||||||
| Ergometrine | 3.11 (0.70 to 13.85) | ⊕⊝⊝⊝ Very lowj | 1.63 (0.97 to 2.73) | ⊕⊕⊝⊝ Lowk | 2.25 (1.39 to 3.64) | ⊕⊕⊕⊝ Moderatel | 20 per 1000 | 45 per 1000 | 25 more per 1000 (8 more to 53 more) |
| Vaginal birth: 12 per 1000 | Vaginal birth: 27 per 1000 | Vaginal birth: 15 more per 1000 (5 more to 32 more) | |||||||
| Caesarean birth: 65 per 1000 | Caesarean birth: 146 per 1000 | Caesarean birth: 81 more per 1000 (25 more to 172 more) | |||||||
| Ergometrine plus oxytocin | 3.21 (2.14 to 4.81) | ⊕⊝⊝⊝ Very lowm | 3.20 (1.92 to 5.34) | ⊕⊕⊝⊝ Lown | 3.15 (2.24 to 4.43) | ⊕⊕⊝⊝ Lowo | 20 per 1000 | 63 per 1000 | 43 more per 1000 (25 more to 69 more) |
| Vaginal birth: 12 per 1000 | Vaginal birth: 38 per 1000 | Vaginal birth: 26 more per 1000 (15 more to 41 more) | |||||||
| Caesarean birth: 65 per 1000 | Caesarean birth: 205 per 1000 | Caesarean birth: 140 more per 1000 (81 more to 223 more) | |||||||
| Misoprostol plus oxytocin | 2.19 (1.23 to 3.91) | ⊕⊕⊕⊝ Moderated | 2.67 (0.45 to 15.68) | ⊕⊕⊝⊝ Lowk | 2.27 (1.28 to 4.02) | ⊕⊕⊕⊝ Moderatep | 20 per 1000 | 45 per 1000 | 25 more per 1000 (6 more to 60 more) |
| Vaginal birth: 12 per 1000 | Vaginal birth: 27 per 1000 | Vaginal birth: 15 more per 1000 (3 more to 36 more) | |||||||
| Caesarean birth: 65 per 1000 | Caesarean birth: 128 per 1000 | Caesarean birth: 83 more per 1000 (18 more to 196 more) | |||||||
|
The assumed risks in the oxytocin group are based on weighted means of baseline risks from the trials with oxytocin groups in the network meta‐analysis. The corresponding risks in the carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine), misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effect of individual uterotonic when compared with oxytocin (and its 95% CI) derived from the network meta‐analysis. CI: confidence interval; NMA: network meta‐analysis; PPH: postpartum haemorrhage; RR: risk ratio | |||||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. The starting rating for assessing the certainty of evidence for the indirect estimate is the lower of the preliminary certainty ratings of the two direct estimates forming the most dominant first‐order loop. The starting rating for assessing the certainty of evidence for the network estimate is the higher of the preliminary certainty ratings of the direct or indirect estimate or the rating of the estimate that contributes the most, or the rating of the direct estimate. | |||||||||
aDirect evidence downgraded −2 due to multiple limitations in trial design and serious imprecision. bIndirect evidence start rating very low (−3 due to multiple limitations in trial design, serious statistical heterogeneity and publication bias), also serious imprecision. cNetwork evidence start rating moderate (moderate certainty of preliminary direct evidence) then downgraded −1 for serious imprecision. dDirect evidence downgraded −1 due to multiple limitations in trial design. eIndirect evidence start rating very low (−3 due to multiple limitations in trial design, serious statistical heterogeneity and publication bias). fNetwork evidence start rating moderate (moderate certainty of preliminary direct evidence), then downgraded −1 due to incoherence. gNo available evidence to rate. hIndirect evidence start rating very low (−3 due to multiple crucial limitations in trial design and serious statistical heterogeneity), also serious imprecision. iNetwork evidence start rating very low (very low certainty of preliminary indirect evidence). jDirect evidence downgraded −3 due to multiple limitations in trial design, serious statistical heterogeneity (I2 = 67%) and serious imprecision. kIndirect evidence start rating moderate (−1 due to multiple limitations in trial design), then downgraded −1 due to serious imprecision. lNetwork evidence start rating moderate (moderate certainty of preliminary indirect evidence). mDirect evidence downgraded −3 due to multiple limitations in trial design, serious statistical heterogeneity (I2 = 61%) and publication bias. nIndirect evidence start rating low (−2 due to multiple crucial limitations in trial design). oNetwork evidence start rating low (low certainty of preliminary indirect evidence). pNetwork evidence start rating moderate (moderate certainty of preliminary direct evidence).
Summary of findings 6. Hypertension.
| Patient or population: women in the third stage of labour Interventions: carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine), misoprostol plus oxytocin Comparison (reference): oxytocin Outcome: hypertension Setting: hospital or community setting | |||||||||
| Uterotonic agent(s) | Direct evidence | Indirect evidence | NMA evidence | Anticipated absolute effects for NMA estimate | |||||
| RR (95% CI) | Certainty of the evidence | RR (95% CI) | Certainty of the evidence | RR (95% CI) | Certainty of the evidence | Risk with oxytocin | Risk with intervention (other uterotonics) | Risk difference with intervention | |
| Carbetocin | 0.50 (0.08 to 3.02) | ⊕⊕⊝⊝ Lowa | 0.46 (0.08 to 2.78) |
⊕⊕⊝⊝ Lowb | 0.68 (0.23 to 2.03) |
⊕⊕⊝⊝ Lowc | 73 per 1000 | 50 per 1000 | 23 fewer per 1000 (56 fewer to 75 more) |
| Vaginal birth: 54 per 1000 | Vaginal birth: 37 per 1000 | Vaginal birth: 17 fewer per 1000 (42 fewer to 56 more) | |||||||
| Caesarean birth: 110 per 1000 | Caesarean birth: 75 per 1000 | Caesarean birth: 35 fewer per 1000 (85 fewer to 113 more) | |||||||
| Misoprostol | 4.00 (0.44 to 36.03) |
⊕⊝⊝⊝ Very lowd | 0.70 (0.23 to 2.18) |
⊕⊝⊝⊝ Very lowe | 0.95 (0.39 to 2.31) |
⊕⊝⊝⊝ Very lowf | 73 per 1000 | 69 per 1000 | 4 fewer per 1000 (45 fewer to 96 more) |
| Vaginal birth: 54 per 1000 | Vaginal birth: 51 per 1000 | Vaginal birth: 3 fewer per 1000 (33 fewer to 71 more) | |||||||
| Caesarean birth: 110 per 1000 | Caesarean birth: 105 per 1000 | Caesarean birth: 6 fewer per 1000 (67 fewer to 144 more) | |||||||
| Injectable prostaglandins | Not reported by included trialsg | ‐ | Not reported by included trialsg | ‐ | Not reported by included trialsg | ‐ | |||
| see comment* | see comment** | see comment*** | |||||||
| Ergometrine | 13.39 (2.01 to 89.44) |
⊕⊝⊝⊝ Very lowh | 10.77 (0.44 to 261.28) |
⊕⊝⊝⊝ Very lowe | 7.05 (1.98 to 25.07) |
⊕⊕⊝⊝ Lowi | 73 per 1000 | 515 per 1000 | 442 more per 1000 (72 more to 1757 more) |
| Vaginal birth: 54 per 1000 | Vaginal birth: 381 per 1000 | Vaginal birth: 327 more per 1000 (53 more to 1000 more) | |||||||
| Caesarean birth: 110 per 1000 | Caesarean birth: 776 per 1000 | Caesarean birth: 666 more per 1000 (108 more to 1000 more) | |||||||
| Ergometrine plus oxytocin | 1.48 (0.87 to 2.52) |
⊕⊕⊝⊝ Lowj | 2.46 (0.42 to 14.40) |
⊕⊝⊝⊝ Very lowe | 1.51 (0.82 to 2.78) |
⊕⊕⊝⊝ Lowc | 73 per 1000 | 110 per 1000 | 37 more per 1000 (13 fewer to 130 more) |
| Vaginal birth: 54 per 1000 | Vaginal birth: 82 per 1000 | Vaginal birth: 28 more per 1000 (10 fewer to 96 more) | |||||||
| Caesarean birth: 110 per 1000 | Caesarean birth: 166 per 1000 | Caesarean birth: 56 more per 1000 (20 fewer to 196 more) | |||||||
| Misoprostol plus oxytocin | Not reported by included trialsg | ‐ | Not reported by included trialsg | ‐ | Not reported by included trialsg | ‐ | No included trials or there are no events in included trials to estimate the baseline risk | Absolute risk with uterotonics cannot be estimated in the absence of absolute risk with oxytocin | Risk difference cannot be estimated in the absence of absolute risks with intervention and oxytocin |
|
The assumed risks in the oxytocin group are based on weighted means of baseline risks from the trials with oxytocin groups in the network meta‐analysis. The corresponding risks in the carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine), misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effect of individual uterotonic when compared with oxytocin (and its 95% CI) derived from the network meta‐analysis. CI: confidence interval; NMA: network meta‐analysis; PPH: postpartum haemorrhage; RR: risk ratio | |||||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. The starting rating for assessing the certainty of evidence for the indirect estimate is the lower of the preliminary certainty ratings of the two direct estimates forming the most dominant first‐order loop. The starting rating for assessing the certainty of evidence for the network estimate is the higher of the preliminary certainty ratings of the direct or indirect estimate or the rating of the estimate that contributes the most, or the rating of the direct estimate. | |||||||||
aDirect evidence downgraded −2 due to serious statistical heterogeneity (I2 = 69%) and serious imprecision. bIndirect evidence start rating moderate (−1 due to multiple limitations in trial design), then downgraded −1 due to serious imprecision. cNetwork evidence start rating moderate (moderate certainty of preliminary direct evidence), then downgraded −1 due to serious imprecision. dDirect evidence downgraded −3 due to multiple crucial limitations in trial design and serious imprecision. eIndirect evidence start rating low (−2 due to multiple crucial limitations in trial design), then downgraded −1 due to serious imprecision. fNetwork evidence start rating low (low certainty of preliminary direct evidence), then downgraded −1 due to very serious imprecision. gNo available evidence to rate. hDirect evidence downgraded −3 due to multiple crucial limitations in trial design, serious statistical heterogeneity (I2 = 63%) and serious imprecision. iNetwork evidence start rating low (low certainty of preliminary indirect evidence). jDirect evidence downgraded −2 due to multiple limitations in trial design and serious imprecision.
Summary of findings 7. Fever.
| Patient or population: women in the third stage of labour Interventions: carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine), misoprostol plus oxytocin Comparison (reference): oxytocin Outcome: fever Setting: hospital or community setting | |||||||||
| Uterotonic agent(s) | Direct evidence | Indirect evidence | NMA evidence | Anticipated absolute effects for NMA estimate | |||||
| RR (95% CI) | Certainty of the evidence | RR (95% CI) | Certainty of the evidence | RR (95% CI) | Certainty of the evidence | Risk with oxytocin | Risk with intervention (other uterotonics) | Risk difference with intervention | |
| Carbetocin | 0.37 (0.02 to 8.71) | ⊕⊝⊝⊝ Very lowa | Not availableb | ‐ | 0.37 (0.01 to 10.55) |
⊕⊕⊝⊝ Lowc | 23 per 1000 | 9 per 1000 | 14 fewer per 1000 (23 fewer to 220 more) |
| Vaginal birth: 20 per 1000 | Vaginal birth: 7 per 1000 | Vaginal birth: 13 fewer per 1000 (20 fewer to 191 more) | |||||||
| Caesarean birth: 34 per 1000 | Caesarean birth: 13 per 1000 | Caesarean birth: 21 fewer per 1000 (34 fewer to 325 more) | |||||||
| Misoprostol | 3.88 (2.61 to 5.77) |
⊕⊕⊝⊝ Lowd | 11.19 (3.35 to 37.35) |
⊕⊝⊝⊝ Very lowe | 4.17 (2.95 to 5.88) |
⊕⊕⊝⊝ Lowf | 23 per 1000 | 96 per 1000 | 73 more per 1000 (45 more to 112 more) |
| Vaginal birth: 20 per 1000 | Vaginal birth: 83 per 1000 | Vaginal birth: 63 more per 1000 (39 more to 98 more) | |||||||
| Caesarean birth: 34 per 1000 | Caesarean birth: 142 per 1000 | Caesarean birth: 108 more per 1000 (66 more to 166 more) | |||||||
| Injectable prostaglandins | Not reported by included trialsb | ‐ | 0.48 (0.09 to 2.61) |
⊕⊝⊝⊝ Very lowe | 0.48 (0.09 to 2.61) |
⊕⊝⊝⊝ Very lowg | 23 per 1000 | 11 per 1000 | 12 fewer per 1000 (21 fewer to 37 more) |
| Vaginal birth: 20 per 1000 | Vaginal birth: 10 per 1000 | Vaginal birth: 10 fewer per 1000 (18 fewer to 32 more) |
|||||||
| Caesarean birth: 34 per 1000 | Caesarean birth: 16 per 1000 | Caesarean birth: 18 fewer per 1000 (31 fewer to 55 more) | |||||||
| Ergometrine | 2.97 (0.97 to 9.05) |
⊕⊝⊝⊝ Very lowh | 0.72 (0.36 to 1.44) |
⊕⊝⊝⊝ Very lowe | 0.91 (0.48 to 1.71) |
⊕⊝⊝⊝ Very lowi | 23 per 1000 | 21 per 1000 | 2 fewer per 1000 (12 fewer to 16 more) |
| Vaginal birth: 20 per 1000 | Vaginal birth: 18 per 1000 | Vaginal birth: 2 fewer per 1000 (10 fewer to 14 more) | |||||||
| Caesarean birth: 34 per 1000 | Caesarean birth: 31 per 1000 | Caesarean birth: 3 fewer per 1000 (18 fewer to 24 more) | |||||||
| Ergometrine plus oxytocin | 1.08 (0.48 to 2.43) |
⊕⊕⊝⊝ Lowj | 0.62 (0.24 to 1.57) |
⊕⊝⊝⊝ Very lowe | 0.78 (0.38 to 1.58) |
⊕⊕⊝⊝ Lowc | 23 per 1000 | 18 per 1000 | 5 fewer per 1000 (14 fewer to 13 more) |
| Vaginal birth: 20 per 1000 | Vaginal birth: 16 per 1000 | Vaginal birth: 4 fewer per 1000 (12 fewer to 12 more) | |||||||
| Caesarean birth: 34 per 1000 | Caesarean birth: 27 per 1000 | Caesarean birth: 7 fewer per 1000 (21 fewer to 20 more) | |||||||
| Misoprostol plus oxytocin | 4.01 (2.49 to 6.45) |
⊕⊕⊝⊝ Lowk | 3.47 (0.66 to 18.13) |
⊕⊝⊝⊝ Very lowe | 3.95 (2.52 to 6.20) |
⊕⊕⊝⊝ Lowl | 23 per 1000 | 91 per 1000 | 68 more per 1000 (35 more to 120 more) |
| Vaginal birth: 20 per 1000 | Vaginal birth: 79 per 1000 | Vaginal birth: 59 more per 1000 (30 more to 104 more) | |||||||
| Caesarean birth: 34 per 1000 | Caesarean birth: 134 per 1000 | Caesarean birth: 100 more per 1000 (52 more to 177 more) | |||||||
|
The assumed risks in the oxytocin group are based on weighted means of baseline risks from the trials with oxytocin groups in the network meta‐analysis. The corresponding risks in the carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine), misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effect of individual uterotonic when compared with oxytocin (and its 95% CI) derived from the network meta‐analysis. CI: confidence interval; NMA: network meta‐analysis; PPH: postpartum haemorrhage; RR: risk ratio | |||||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. The starting rating for assessing the certainty of evidence for the indirect estimate is the lower of the preliminary certainty ratings of the two direct estimates forming the most dominant first‐order loop. The starting rating for assessing the certainty of evidence for the network estimate is the higher of the preliminary certainty ratings of the direct or indirect estimate or the rating of the estimate that contributes the most, or the rating of the direct estimate. | |||||||||
aDirect evidence downgraded −3 due to multiple limitations in trial design and very serious imprecision. bNo available evidence to rate. cNetwork evidence start rating moderate (moderate certainty of preliminary direct evidence), then downgraded −1 due to serious imprecision. dDirect evidence downgraded −2 due to multiple limitations in trial design and serious statistical heterogeneity (I2 = 64%). eIndirect evidence start rating low (−2 due to multiple limitations in trial design and serious statistical heterogeneity), then downgraded −1 due to serious imprecision. fNetwork evidence start rating low (low certainty of preliminary direct evidence). gNetwork evidence start rating low (low certainty of preliminary indirect evidence), then downgraded −1 due to serious imprecision. hDirect evidence downgraded −3 due to multiple crucial limitations in trial design and serious imprecision. iNetwork evidence start rating low (low certainty of preliminary direct evidence), then downgraded −1 due to incoherence and serious imprecision. jDirect evidence downgraded −2 due to multiple limitations in trial design and serious imprecision. kDirect evidence downgraded −2 due to multiple limitations in trial design and serious statistical heterogeneity (I2 = 73%). lNetwork evidence start rating low (low certainty of direct evidence).
Background
Description of the condition
Millions of women suffer postpartum haemorrhage (PPH) every year, resulting in an estimated 70,000 maternal deaths [1, 2]. Almost all deaths occur in low‐ or middle‐income countries. Even when death from PPH is avoided, the need for blood transfusion, hysterectomy and additional interventions place a huge burden on women's health and health services [3, 4].
The third stage of labour, defined as the period of time from birth until the delivery of the placenta, and the immediate postpartum period are the most hazardous periods of childbirth due to the risk of PPH. The World Health Organization (WHO) defines PPH as when the blood loss after birth equals or exceeds 500 mL in the first 24 hours [5]. The most common cause of PPH is uterine atony (failure of the uterus to contract after birth) [6]. Even though risk factors for adverse maternal outcomes from haemorrhage have been identified [4], often PPH is unpredictable as it occurs in the absence of identifiable clinical or historical risk factors [6, 7]. Therefore, effective prevention of PPH is advocated for all women during childbirth [5]. The administration of uterotonic agents routinely in the third stage of labour is the key intervention that prevents PPH, although there is uncertainty about which agent may be the most effective.
Description of the intervention and how it might work
The administration of uterotonic agents to prevent PPH has traditionally been part of the active management of the third stage of labour [8]. The active management of the third stage of labour refers to the administration of a uterotonic agent, early cord clamping, and controlled cord traction until delivery of the placenta. In 2018, a WHO guideline panel revisited the evidence underpinning each component of active management of the third stage of labour and considered the use of uterotonics as the main intervention within this package [5].
Several different uterotonic agents have been used for preventing PPH. These agents include ergometrine, misoprostol, carbetocin, oxytocin, injectable prostaglandins (such as carboprost and sulprostone) and the combinations of agents such as misoprostol plus oxytocin and ergometrine plus oxytocin.
Oxytocin
Oxytocin (Syntocinon) is the most widely used uterotonic agent. At low doses, it produces rhythmic uterine contractions that are indistinguishable in frequency, force and duration from those observed during spontaneous labour, but at higher dosages, it causes sustained uterine contractions [9]. It has a short half‐life, approximately three to five minutes, and can be used as an infusion to maintain uterine contraction. When used intramuscularly, the latent phase lasts three to seven minutes, but produces a longer‐lasting clinical effect of up to one hour [9]. However, oxytocin cannot be used orally. It is unstable at ambient temperatures, and it requires a cold chain through storage and transport. It should also not be given intravenously as a large bolus, because it can cause severe hypotension (Thomas 2007 [10, 11, 12]). Because of its antidiuretic effect, water intoxication can occur with prolonged infusion of oxytocin [9].
Ergometrine
Ergometrine and methylergometrine are ergot alkaloids that increase the uterine muscle tone by causing sustained uterine contractions. They have a latent phase of two to five minutes after intramuscular injection and the plasma half‐life is 30 to 120 minutes [13]. After intravenous administration, the onset of action is one minute or less and the duration of action is 45 minutes (although rhythmic contractions may persist for up to three hours). However, ergometrine and methylergometrine have an unpredictable bioavailability, which prevents oral use of the agents and requires protection from light, and storage at a temperature between 2° C and 8° C to prolong shelf life [14]. They are vasoconstrictive and are contraindicated in women with hypertensive or cardiovascular disorders [9].
Misoprostol
Misoprostol is a prostaglandin E1 analogue, which is licensed for the prevention and treatment of gastric ulcers. It is well known for its off‐label use as a uterotonic agent [15]. It is water‐soluble and heat‐stable [16]. It is absorbed nine to 15 minutes after sublingual, oral, vaginal, and rectal use. The half‐life is about 20 to 40 minutes. Oral and sublingual routes have the advantage of rapid onset of action, while the vaginal and rectal routes result in prolonged activity and greater bioavailability [17].
Injectable prostaglandins
Prostaglandin preparations are available in injectable forms, and the most commonly used agents are carboprost tromethamine (Hemabate), an analogue of 15‐methyl‐prostaglandin F2a, and sulprostone, which is a PGE2 analogue. After intramuscular administration, the time to peak plasma concentration is between 15 and 60 minutes. The half‐life is about eight minutes. They require storage at a temperature between 2° C and 8° C to prolong shelf life [9]. They both enhance uterine contractility and cause vasoconstriction in postpartum women [9]. However, they are not contraindicated in hypertensive women [9]. In the management of the third stage of labour, injectable prostaglandins have mainly been used for intractable PPH as a last resort when other measures fail. Important disadvantages of injectable prostaglandins have been their cost and availability.
Carbetocin
Carbetocin is a newer long‐acting synthetic analogue of oxytocin with agonist properties. After intravenous injection, it produces sustained uterine contractions within two minutes, lasting for approximately six minutes, followed by rhythmic contractions for 60 minutes [18]. When carbetocin is administered by an intramuscular injection, the sustained uterine contractions last for approximately 11 minutes and the rhythmic contractions for 120 minutes [18]. A heat‐stable carbetocin is now available and has been evaluated against oxytocin in a large randomised trial [19]. Carbetocin also appears to have a favourable side‐effect profile [20].
Combination agents
The use of combinations of uterotonic agents is also popular, and the most commonly used agent is ergometrine plus oxytocin (Syntometrine). This is a fixed‐combination agent containing 5 international units (IU) of oxytocin and 500 μg of ergometrine. Intramuscular injection is the recommended route [9]. When used intramuscularly, the latent period for the occurrence of the uterine response is about 2.5 minutes and the uterotonic effects last for around three hours. Another combination that has been investigated is misoprostol plus oxytocin. This combination is not in synthetic (fixed‐drug) or naturally occurring forms.
The WHO recommends that all women giving birth should be offered uterotonics during the third stage of labour for the prevention of PPH; oxytocin (intramuscular/intravenous, 10 IU) is the uterotonic agent of choice [5]. Other injectable uterotonics and misoprostol are recommended as alternatives for the prevention of PPH in settings where oxytocin is not available.
Why it is important to do this review
The individual uterotonics described above have been compared in existing Cochrane reviews and all comparisons are based on trials that directly compared one uterotonic against another uterotonic agent in head‐to‐head trials [8, 15, 20, 21, 22, 23]. The existing Cochrane reviews have variable eligibility criteria for trial inclusion, uterotonic agent comparisons and outcomes. In the absence of a single randomised controlled trial comparing all available uterotonic agents, uncertainty remains over their relative effectiveness and ranking. When multiple interventions are available, a network meta‐analysis is better placed for synthesising and interpreting the wider picture of the evidence and to understand the relative effects of all available interventions. Network meta‐analysis has advantages over conventional pairwise meta‐analysis, as the technique uses both direct and indirect evidence in a single coherent analysis to improve certainty about all possible treatment comparisons. Indirect evidence is obtained when the relative effectiveness of two competing interventions is inferred through a common comparator, even though this pair may not have been compared directly [24, 25].
This review updates the previous Cochrane review update published in 2018 and incorporates results from trials published since then [26]. It uses Cochrane Pregnancy and Childbirth’s trustworthiness screening tool (CPC‐TST) for assessment of trustworthiness and scientific integrity to screen eligible trials [27, 28]. The tool aims to minimise the inclusion of potentially untrustworthy trials and to ensure that evidence is reliable.
Objectives
To identify the most effective uterotonic agent(s) to prevent postpartum haemorrhage (PPH) with the fewest side effects, and generate a ranking according to their effectiveness and side effect profile.
Methods
A copy of the originally registered protocol is available in the Cochrane Library [29]. Any deviations from the original protocol, including amendments to the inclusion criteria or methods, are documented and justified in the final review.
The protocol adheres to Cochrane’s standards for systematic reviews and was developed in compliance with the PRISMA‐P guidelines, ensuring rigorous and transparent methodology [30]. The protocol was reviewed by a multidisciplinary team to ensure relevance and methodological quality, and we received feedback from content experts and stakeholders.
Differences between versions
Selection of studies (differences)
This review uses Cochrane Pregnancy and Childbirth’s trustworthiness screening tool (CPC‐TST) to screen eligible trials [27, 28]. This was not done in previous updates [26].
Sensitivity analysis (differences)
We did not undertake sensitivity analyses of the following criteria in the current review, as they did not affect the overall results in the last update [26].
Removing trials that also randomised participants to cointerventions such as uterine massage or controlled cord traction
Removing trials with more than 10% missing data (this is already a criterion in the risk of bias assessment)
Removing trials published before 1990 (we have already made age‐of‐trial‐related adjustments as part of the trustworthiness screening)
Randomisation unit (restricted to individually randomised trials and removing cluster‐randomised trials)
Choice of relative effect measure (risk ratio (RR) versus odds ratio (OR))
Use of a fixed‐effect model versus a random‐effects model
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) or cluster‐RCTs that compared the effectiveness and side effects of uterotonic agents with other uterotonic agents, placebo or no treatment for preventing PPH were eligible for inclusion. We included randomised trials published only as abstracts if sufficient information could be retrieved. We excluded quasi‐randomised trials.
Types of participants
The review included trials of women in the third stage of labour following a vaginal or caesarean birth in hospital or community settings.
Types of interventions
Trials were eligible if they administered uterotonic agents of any dosage, route or regimen systemically at birth for preventing PPH, and compared them with other uterotonic agents, placebo or no treatment. We excluded trials that evaluated uterotonic agents not administered systemically, such as intrauterine administration, or not immediately after birth, or that exclusively compared different dosages, routes or regimens of the same uterotonic agent. We included trials in which non‐pharmacologic co‐interventions such as controlled cord traction, cord clamping, or uterine massage were performed as a randomised intervention in all arms of the trial.
We classified agents into single agents, including:
oxytocin;
carbetocin;
injectable prostaglandins (carboprost tromethamine, sulprostone);
misoprostol; and
ergometrine (including also ergonovine, methylergonovine).
And combination agents, including:
ergometrine plus oxytocin (Syntometrine as a fixed‐combination agent containing 5 IU of oxytocin and 500 μg of ergometrine, any oxytocin dose and route when combined with any dose and route of ergometrine, ergonovine, or methylergonovine), and
misoprostol plus oxytocin (any oxytocin dose and route when combined with any dose and route of misoprostol).
For this review, we assumed that any woman who meets the inclusion criteria is, in principle, equally likely to be randomised to any of the eligible uterotonic agents.
Outcome measures
We estimated the relative effects and rankings of the competing interventions according to the following outcomes.
Critical outcomes
The primary outcomes of the review were the following.
PPH ≥ 500 mL
PPH ≥ 1000 mL
Important outcomes
The secondary outcomes of the review were as follows.
Maternal deaths
Severe maternal morbidity: intensive care admissions
Severe maternal morbidity: shock (as defined by the trial authors)
Use of additional uterotonics
Blood transfusion
Mean volume of blood loss (mL)
Change in haemoglobin measurements before versus after birth (g/L)
Breastfeeding at hospital discharge
Nausea
Vomiting
Hypertension
Headache
Fever (≥ 38° C)
Shivering
Abdominal pain
Diarrhoea
Maternal sense of well‐being (as defined by the trial authors)
Maternal satisfaction (as defined by the trial authors)
Search methods for identification of studies
The search methods are described in Supplementary material 1.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase and CINAHL (Cumulative Index to Nursing and Allied Health Literature) in collaboration with the Cochrane Information Specialist (5 February 2024) using the terms given in Supplementary material 1.
CENTRAL (2024, Issue 2) in the Cochrane Library (searched 5 February 2024)
MEDLINE Ovid (24 May 2018 to 5 February 2024)
Embase Ovid (24 May 2018 to 5 February 2024)
CINAHL EBSCOhost (24 May 2018 to 5 February 2024)
Date limits were applied to cover the period from the last review search on 24 May 2018 until 5 February 2024. Filters were applied to identify RCTs in the MEDLINE, Embase and CINAHL databases. Details of the search methods conducted in the original review are described in the previously published review [26].
Searching other resources
We retrieved additional relevant references cited in papers identified through the above search strategy, and we searched for the full texts of trials initially identified as abstracts. For randomised trials published only as abstracts, we sought information from primary authors to investigate whether these trials met our eligibility criteria before including them. Trials that compared at least two of the agents were eligible, and we searched for all possible comparisons.
Data collection and analysis
Authors of this review, who are authors of any potentially eligible trials, did not make any decisions about trial eligibility, extract data, carry out risk of bias assessments, or perform GRADE assessments of that trial.
Selection of studies
Three review authors retrieved and independently assessed for inclusion all the potential trials we identified (IY, AD, MP). We resolved any disagreements through discussion or, if required, in consultation with a third person (IDG). We created a trial flow diagram to map out the number of records identified, included and excluded (Figure 1) [31].
1.

Diagram showing the flow of trials in the review
Trustworthiness screening of eligible studies
At least two authors (IY, AD, MP) independently undertook trustworthiness assessment of all eligible trials according to Cochrane Pregnancy and Childbirth editorial guidelines, using the trustworthiness screening tool [27]. The trustworthiness screening tool was composed of the following four domains: governance, baseline data, feasibility and results. We adapted the tool for each domain to account for historical differences in expectation of reporting and research methodology. We resolved any disagreements through consensus discussion or input from another review author. We included data from abstracts only if the trial authors confirmed that it came from the final analysis and would not change subsequently.
Governance
Are there retraction notices or expressions of concern on the Retraction Watch Database [32]? Was the trial prospectively registered (post 2010)? If not, is there a satisfactory reason and did the authors provide a copy of the protocol? Did the authors provide details of ethics approval and patient consent? Are there details about the trial dates and recruitment of participants? Did the authors correspond with requests for further information in time? Did the authors provide individual patient data (IPD) if requested? Are there fewer than three authors and the reason for this?
Baseline characteristics
Are baseline characteristics of the participants available? Are the characteristics too similar (distribution of mean)? Are the recalculated standard deviations (SD) and P values accurate?
Feasibility
Are the characteristics implausible, for example, a large number of participants recruited in a short timeframe in a single centre? Are details about randomisation provided? Is there less than a six‐month period between the trial ending and publication and why? How was the placebo sourced without industry sponsorship?
Results
Are the results implausible, for example, a large risk reduction with a small sample size or no complications? Are the results very different to the results of the other included trials? Are the recalculated SDs and P values accurate?
Historical differences in expectation of reporting and research methodology
We did not contact authors of trials published before 1980 due to the age of the publications and authors. We made trustworthiness screening assessments based on the available information. Trials published before 1990 were deemed trustworthy even if trial dates, ethics and consent information and randomisation and blinding details were not explicitly detailed. Trials published before 2010 did not require prospective registration, and either the trial dates, ethics information or consent details could be omitted from the manuscript.
Data extraction and management
We designed electronic forms on Microsoft Excel to extract data [33]. For eligible trials, at least three review authors independently extracted the data using a blank electronic form (IY, AD, MP, IDG). We resolved discrepancies through discussion or, if required, we consulted another person (AC). We entered data into STATA [34], and Review Manager [35], and checked for accuracy. When information was unclear, we attempted to contact the authors of the original reports to provide further details. We extracted the following data.
Outcome data
From each included trial we extracted the number of participants, the gestational age and the parity of participants, and any exclusion criteria. We also extracted the interventions being compared, and their respective primary and secondary outcomes. We extracted all relevant arm‐level data (e.g. number of events and number of participants for binary outcomes, and means and SDs per trial arm for continuous outcomes).
Data on potential effect modifiers
From each included trial we extracted the following intervention and population characteristics that may act as effect modifiers.
Mode of delivery (vaginal or caesarean birth)
Prior risk of PPH (as defined by trial authors and categorised as low, high, mixed or not stated)
Dosage, regimen, and route of administration (sublingual, subcutaneous, intramuscular, rectal, oral, intravenous bolus and/or infusion)
Setting of the trial (community or hospital)
Other data
From each included trial we extracted the following additional information.
Country or countries in which the trial was performed
Date of publication and dates of recruitment
Type of publication (full‐text publication, abstract publication, unpublished data)
Trial registration reference
Risk of bias assessment in included studies
At least three review authors (IY, AD, IDG) independently assessed the risk of bias for each trial using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions [36]. We resolved any disagreements by discussion or by involving another assessor (AC).
1. Random sequence generation (checking for possible selection bias)
Trials were excluded if found to be at high risk of bias for random sequence generation (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number). We described for each included trial the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the methods as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator); or
unclear risk of bias.
2. Allocation concealment (checking for possible selection bias)
We described for each included trial the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
3.1 Blinding of participants and personnel (checking for possible performance bias)
We described for each included trial the methods used, if any, to blind trial participants and personnel from knowledge of which intervention a participant received. We considered that trials were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to have affected the results.
We assessed the methods as:
low, high or unclear risk of bias for participants; and
low, high or unclear risk of bias for personnel.
3.2 Blinding of outcome assessment (checking for possible detection bias)
We described for each included trial the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
4. Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included trial the completeness of data, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we re‐included missing data in the analyses. We assessed methods to handle incomplete outcome data as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups and less than 10% of missing outcome data);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation or more than 10% of missing outcome data); or
unclear risk of bias.
5. Selective reporting (checking for reporting bias)
We described for each included trial how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the trial’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the trial’s prespecified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; trial fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias.
6. Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described, for each included trial, any important concerns about other possible sources of bias, such as intention‐to‐treat bias.
We assessed these biases as:
low risk of other bias (analysed participants in the intervention groups to which they were randomised, regardless of the interventions they actually received);
high risk of other bias (not all randomised participants were analysed according to the intervention group they were assigned); or
unclear risk of other bias.
Another source of bias was related to the source of funding and potential conflicts of interest.
We assessed these interests as:
low risk of other bias (public funding or no funding and no significant conflicts of interest identified);
high risk of other bias (industry funding or significant conflicts of interest identified); or
unclear risk of other bias.
Another source of bias was generated by the method of measuring blood loss. We assessed the method described in each trial and classified it at:
low risk of other bias (objective measurements such as weighing sponges, measurements in drapes, volumetric assessment, tagged red cells, etc.);
high risk of other bias (subjective measurements such as clinical or visual estimates); or
unclear risk of other bias (unspecified methods of measurement).
7. Overall risk of bias
We made explicit judgements about whether trials were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions [36]. For our primary outcomes, we combined risk of bias domains and judged trials as 'low risk of bias' if they were double‐blinded, had allocation concealment and with little loss to follow‐up (less than 10%). We judged trials as 'intermediate risk of bias' if they demonstrated adequate allocation concealment, with assessor blinding and little loss to follow‐up (less than 10%). Alternatively, we considered trials to be at 'high risk of bias. We explored the impact of the level of bias through undertaking sensitivity analyses. See Sensitivity analysis for information about how we incorporated the risk of bias in the sensitivity analysis. When undertaking the GRADE assessments, we judged the overall risk of bias for trials for all outcomes in the same way as for the primary outcomes.
Measures of treatment effect
Relative treatment effects
We summarised relative treatment effects for dichotomous outcomes as risk ratios (RR) and for continuous outcomes as mean difference (MD) with 95% CIs [37]. These are summarised in forest plots displaying the results from pairwise, indirect and network (combining direct and indirect) analyses for the comparisons of uterotonic agents versus placebo or no treatment and the comparisons of uterotonic agents versus oxytocin. All other comparisons are available from Supplementary material 8.
Relative treatment ranking
We estimated the cumulative probabilities for each uterotonic agent being at each possible rank and obtained a treatment hierarchy using the surface under the cumulative ranking curve (SUCRA); the larger the SUCRA, the higher its rank among all available agents [38]. The probabilities to rank the treatments are estimated under a Bayesian model with flat priors, assuming that the posterior distribution of the parameter estimates is approximated by a normal distribution with mean and variance equal to the frequentist estimates and variance–covariance matrix. Rankings are constructed by drawing 1000 samples from their approximate posterior density. For each draw, the linear predictor is evaluated for each trial, and the largest linear predictor is noted [39].
Unit of analysis issues
Cluster‐randomised trials
For one cluster‐randomised trial included in this review (Stanton 2013 [40, 41, 42]), we used the intracluster correlation coefficient (ICC = 0.012) to reduce it to its effective sample size, taking into account the design effects as described in the Cochrane Handbook for Systematic Reviews of Interventions [43]. Another cluster‐randomised trial (Chandhiok 2006 [44]), did not report the ICC, so we used the ICC from Stanton 2013. We considered it reasonable to combine the results from the cluster‐randomised and the individually‐randomised trials as there was little heterogeneity between the trial designs and any interaction between the relative effects of agents and the choice of randomisation unit was considered to be unlikely. The effect of the unit of randomisation was also assessed in sensitivity analysis [43].
Multi‐arm trials
Multi‐arm trials were included, and we accounted for the correlation between the effect estimates in the network meta‐analysis. We treated multi‐arm trials as multiple independent comparisons in pairwise meta‐analyses and did not combine them in any analysis.
Dealing with missing data
For included trials, we noted the levels of attrition. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we included all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. We used the number randomised minus any participants whose outcomes were known to be missing as the denominator for each outcome in each trial.
Reporting bias assessment
If there were 10 or more trials in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots of pairwise meta‐analyses. We visually assessed the funnel plots for asymmetry. We also assessed potential reporting bias for the primary outcomes by assessing the sensitivity of results to the exclusion of trials with fewer than 400 participants.
Synthesis methods
Methods for direct treatment comparisons
Initially, we performed pairwise meta‐analyses for every treatment comparison with at least two trials [45], using a random‐effects model in Stata [34], and Review Manager software [35]. However, we did not pool results for an outcome if clinical judgment on the level of heterogeneity suggested that the pooled results would be of little clinical value and potentially misleading.
Assessment of clinical and methodological heterogeneity within treatment comparisons
To evaluate the presence of clinical heterogeneity, we described the trial population characteristics across all included trials, including potential effect modifiers: mode of delivery (vaginal or caesarean birth); prior risk of PPH (as defined by trial authors, and categorised as low, high, mixed or not stated); dosage, regimen, and route of administration (sublingual, subcutaneous, intramuscular, rectal, oral, intravenous bolus and/or infusion); and setting of the trial. We assessed the presence of clinical heterogeneity by comparing these characteristics.
Methods for indirect and network comparisons
We initially generated and assessed the network diagrams to determine if a network meta‐analysis was feasible. Then we performed the network meta‐analysis within a frequentist framework for multivariate meta‐analysis using the restricted maximum likelihood (REML) estimator for heterogeneity. We carried out all analyses using Stata statistical software, release 18 (StataCorp, College Station, TX). We used the network suite of Stata commands designed for this purpose [46, 47].
We present the results from the network diagrams, the forest plots with the pairwise, indirect and network (combining direct and indirect) effect estimates and the cumulative rankograms for all the outcomes with available data.
Assessment of intransitivity across treatment comparisons
In this context, we expect that the transitivity assumption holds, assuming the following.
The common treatment used to compare different uterotonics indirectly is similar when it appears in different trials (e.g. oxytocin is administered in a similar way in oxytocin versus misoprostol trials and in oxytocin versus oxytocin plus ergometrine trials).
All pairwise comparisons do not differ with respect to the distribution of effect modifiers (e.g. the design and trial characteristics of oxytocin versus misoprostol trials are similar to oxytocin versus oxytocin plus ergometrine trials).
We evaluated the assumption of transitivity epidemiologically by comparing the clinical and methodological characteristics of sets of trials from the various treatment comparisons.
Investigation of heterogeneity and subgroup analysis
Assessment of statistical heterogeneity
Assumptions when estimating the heterogeneity
In pairwise meta‐analyses, we estimated the heterogeneity for each comparison using Der Simonian and Laird Estimator fit [45], using the Review Manager package [35]. In network meta‐analysis, we assumed a common estimate for the heterogeneity variance across all the different comparisons using the REML Estimator in STATA version 18 [34].
Measures and tests for heterogeneity
We assessed, statistically, the presence of heterogeneity within each pairwise comparison for the primary outcomes using the I2 statistic, which measures the percentage of variability that cannot be attributed to random error [48]. We downgraded the certainty of the evidence for inconsistency where the I2 statistic was equal to or greater than 60%. The assessment of statistical heterogeneity in the entire network was based on the magnitude of the heterogeneity variance parameter estimated from the multivariate meta‐analysis.
Assessment of statistical inconsistency
We used global and local approaches to evaluate the statistical agreement between the various sources of evidence in a network of interventions (consistency) to complement the evaluation of transitivity. To evaluate the presence of inconsistency locally, we used the loop‐specific approach. This method evaluates the consistency assumption in each closed loop of the network separately, as the difference between direct and indirect estimates for a specific comparison in the loop (inconsistency factor). Then, the magnitude of the inconsistency factors and their 95% CIs was used to infer the presence of inconsistency in each loop. We assumed a common heterogeneity estimate within each loop. To check the assumption of consistency in the entire network, we used the 'design‐by‐treatment' interaction model as described by Higgins [49]. This method accounts for a different source of inconsistency that can occur when trials with different designs (two‐arm trials versus three‐arm trials) give different results, as well as disagreement between direct and indirect evidence. Using this approach, we inferred the presence of inconsistency from any source in the entire network based on a Chi2 test.
Investigation of heterogeneity and inconsistency
Where we found important heterogeneity or inconsistency, or both, we explored the possible sources for primary outcomes. Where sufficient trials were available, we performed multivariate meta‐analyses for subgroups and sensitivity analyses by using potential effect modifiers as possible sources of inconsistency or heterogeneity.
Subgroup analysis
For the primary outcomes, we carried out the following prespecified subgroup analyses.
Population: prior risk of PPH (high versus low), mode of delivery (vaginal versus caesarean birth), setting (hospital versus community)
Intervention: dose of misoprostol (≥ 600 μg versus < 600 μg), and regimen of oxytocin (bolus versus bolus plus infusion versus infusion only).
We assessed subgroup differences by first comparing the network diagram for each subgroup. Next, we performed a pairwise and network meta‐analysis for each subgroup, and we compared their relative treatment effects and their relative treatment ranking. We examined the subgroups for qualitative interactions where the direction of effect could be reversed, that is, if an intervention was beneficial in one subgroup but harmful in another.
Equity‐related assessment
In this review, we have considered the potential effects of the intervention(s) on health equity by examining the applicability and differential impacts in a hospital and community setting.
Sensitivity analysis
For the primary outcomes, we performed sensitivity analysis for the following.
Risk of bias (restricted to trials at low risk of bias only): trials are ranked as 'low risk of bias' if they are double‐blinded, and have allocation concealment with little loss to follow‐up (less than 10%). The concealed trials with assessor blinding and little loss to follow‐up (less than 10%) are ranked as 'intermediate risk of bias' and the rest as 'high risk of bias'. We considered that assessor blinding was likely to be very important, in order to eliminate any risk of bias in subjective measurements or estimates of blood loss (not all trials measure this outcome objectively). We considered protocol publication in advance of the results to be an unsuitable criterion for sensitivity analyses, because protocol publication only became widespread in recent years.
Funding source (restricted to trials with funding source at low risk of bias (public or no funding))
Whether an objective method of outcome assessment was employed (restricted to trials with an objective method of measuring blood loss). Objective methods of blood loss measurement were considered to be all methods that employed a measurement of blood loss. This is in contrast to subjective methods where a healthcare professional estimates blood loss, usually visually.
Trial size (restricted to large trials (> 400 participants), in recognition of the greater likelihood for small trials than large or multicentre trials to suffer publication bias). In terms of trial size, there is evidence that smaller trials can exaggerate estimated benefits [50]. However, the cut‐off for deciding the definition of a small trial can vary between research topics. For this topic, it appears that trials with more than 400 participants are more likely to be of higher quality, prospectively registered and overall at low risk of bias.
We assessed differences by evaluating the relative effects and assessment of model fit.
Certainty of the evidence assessment
The summary of findings tables present evidence comparing all other uterotonic agents with a reference comparator, oxytocin. Each table describes key features of the evidence relating to a single outcome, and there is one table for each of our seven most important outcomes in accordance with the GRADE approach. These include PPH ≥ 500 mL, PPH ≥ 1000 mL, blood transfusion, additional uterotonics, vomiting, hypertension, and fever.
We used the GRADE Working Group’s approach for rating the certainty of the network meta‐analysis effect estimates for all the comparisons and all outcomes [51, 52]. We appraised the certainty of the direct, indirect, and network evidence sequentially (in this order).
First, we assessed the certainty of the preliminary direct evidence (where available) for a given outcome, and rated the evidence using the standard GRADE approach based on consideration of: trial design limitations (risk of bias); inconsistency; indirectness; and publication bias [43]. This preliminary certainty assessment was used to inform subsequent indirect and network certainty ratings as appropriate. The final certainty assessment for the direct evidence was reported in the summary of findings tables following incorporation of the imprecision assessment. On the network diagram, we display the certainty of the final direct evidence for all the comparisons and all outcomes.
Then we rated the preliminary certainty of the indirect evidence for the same outcome, and this was determined based on the lower of the preliminary direct certainty ratings of the two arms forming the dominant ‘first‐order’ loop in the network diagram for this outcome, and also assessing intransitivity. The final certainty assessment for the indirect evidence was reported in the summary of findings tables following incorporation of the imprecision assessment.
Our final step was to determine the certainty of the network evidence based on: (i) the higher certainty rating of the preliminary direct or indirect estimate, or the preliminary rating of the estimate that contributes the most, or the preliminary rating of the direct estimate, (ii) consideration of coherence between direct and indirect effect estimates, and (iii) precision of the network effect estimate.
We followed the Puhan 2014 approach for assessing certainty in the network estimates [52]. However, in the summary of findings tables, we also considered imprecision in relation to the direct and indirect estimates. We did not carry these judgements forward to the rating of the network estimate. For these assessments, we considered the preliminary judgements of the direct and indirect evidence (before considering imprecision), as well as incoherence and the imprecision of the network estimate. At each of these stages, at least two review authors (IY, AD, MP) independently appraised the certainty ratings for the direct, indirect and network evidence. Disagreements between authors were resolved through discussion and consultation with a third review author (IG, AC) where necessary.
The certainty of network evidence for each outcome was rated as ‘high’, ‘moderate’, ‘low’ or ‘very low’ in accordance with the GRADE approach.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect;
Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different;
Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect; and
Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
For ease of comparison when interpreting the relative effects of all uterotonic agents, the summary of findings tables include the effect estimate and certainty judgements for each of the direct evidence, indirect evidence and the network meta‐analysis, describing all the findings for a single outcome in each table. The anticipated absolute effects are also included, based on the network effect estimate for each agent/agent combination in comparison with oxytocin. The assumed risks in the oxytocin group are based on weighted means of baseline risks from the trials with oxytocin arms in the network meta‐analysis. The corresponding risks in the carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine), misoprostol plus oxytocin groups (and their 95% CI) are based on the assumed risk in the oxytocin group and the relative effect of the individual uterotonic when compared with oxytocin (and its 95% CI) as derived from the network meta‐analysis. The baseline risks differed significantly by the mode of birth subgroups, so the anticipated absolute effects are presented separately for vaginal and caesarean births based on the weighted means of baseline risks according to these modes of birth.
Consumer involvement
We sought involvement at the plain language summary stage from a nonmedical consumer with a personal interest in PPH, and who had expertise in writing (see Acknowledgements). The consumer provided critical feedback on the plain language summary, particularly on the language used to make it accessible and easy to understand for non‐specialist readers.
Results
Description of studies
Results of the search
We conducted an updated search for this review on 5 February 2024. The results of the search are summarised in the PRISMA flow diagram (Figure 1).
We identified 1896 records through database searches and 193 additional records from other sources. Of these 2089 records, 140 were duplicates and 1148 were deemed ineligible on title and abstract screening. We reviewed 801 full‐text records for inclusion. We excluded 300 trials (reported in 339 publications) as they did not meet the inclusion criteria (Supplementary material 3). We classified 142 trials (reported in 167 publications) as ‘awaiting classification’ (Supplementary material 4), and we identified 88 trials as ‘ongoing studies’ (Supplementary material 5).
We included 122 trials in the analysis following screening for eligibility and trustworthiness, details of which are described below. We contacted the authors of 66 trials for additional data or clarification. We obtained additional data for 25 trials (Supplementary material 2).
Trustworthiness assessment of eligible studies
We used the CPC‐TST to assess 196 trials from the previous version of the review [26], and 54 new trials identified by this update.
For the assessment of the 196 trials from the previous version of the review, 103 were deemed trustworthy on first screening with the CPC‐TST. We identified 93 trials as having trustworthiness concerns on the first screen. We were unable to contact 33 of these trials' authors due to missing or inactive contact details. Despite two attempts at contact, we had no response from the authors of 46 trials with trustworthiness concerns. Nine authors replied to our correspondence about concerns, and we were satisfied with their responses. In the case of three trials, we still had concerns after responses were received from the authors. Two trials were by an author who had published concerns raised, and we moved them to ‘awaiting classification’ without contacting the author [53]. Following trustworthiness assessment, we included 112 trials from the previous version of the review.
For the 54 new trials identified by this updated review, we assessed 10 as trustworthy on the first screen using the CPC‐TST. We identified 44 trials as having trustworthiness concerns (the most common reason being no prospective trial registration). We were unable to contact 12 of these trials' authors due to missing or inactive contact details. Despite two attempts at contact, we had no response from the authors of 28 trials with trustworthiness concerns. In the case of two trials, we still had concerns after responses were received from the authors. Two trial records were by an author who had published concerns raised, and we moved them to ‘awaiting classification’ without contacting the author [53]. Following trustworthiness assessment, we included 10 new trials for our review update.
Our updated review contains a total of 122 trials assessed as trustworthy by application of the CPC‐TST. We moved trials with remaining trustworthiness concerns and those whose authors we were unable to contact to ‘awaiting classification’. Details of the screening decisions for individual trials are described in Supplementary material 2; Supplementary material 4.
Included studies
The network meta‐analysis includes 122 randomised trials involving 121,931 women (Abdel‐Aleem 1993 [54]; Abdel‐Aleem 2010 [55, 56]; Acharya 2001 [57]; Amant 1999 [58]; Amornpetchakul 2018 [59, 60, 61]; Askar 2011 [62]; Attilakos 2010 [63, 64, 65, 66, 67]; Atukunda 2014 [68, 69]; Balki 2008 [70, 71]; Balki 2021 [72, 73]; Bamigboye 1998a [74, 75]; Bamigboye 1998b [76]; Baskett 2007 [77, 78, 79, 80]; Begley 1990 [81, 82, 83]; Bellad 2012 [84, 85, 86]; Benchimol 2001 [87]; Bhullar 2004 [88]; Boucher 1998 [89]; Boucher 2004 [90, 91]; Bugalho 2001 [92]; Butwick 2010 [93, 94]; Caliskan 2002 [95]; Caliskan 2003 [96]; Carbonell 2009 [97]; Cayan 2010 [98]; Chandhiok 2006; Chaudhuri 2010 [99, 100]; Chaudhuri 2012 [101]; Chaudhuri 2015 [102, 103]; Chaudhuri 2016 [104, 105]; Chhabra 2008 [106]; Choy 2002 [107]; Chua 1995 [108]; Cook 1999 [109]; Dansereau 1999 [110, 111, 112, 113, 114, 115]; Dasuki 2002 [116]; Dawoud 2023 [117]; De Groot 1996 [118]; Derman 2006 [119, 120, 121, 122, 123, 124, 125, 126, 127]; Diop 2016 [128, 129]; Docherty 1981 [130]; El‐Refaey 2000 [131]; Enakpene 2007 [132]; Ezeama 2014 [133, 134]; Fawole 2011 [135]; Fekih 2009 [136]; Fu 2003 [137]; Gulmezoglu 2001 [138, 139, 140]; Gupta 2006 [141]; Hamm 2005 [142]; Harriott 2009 [143]; Hernandez‐Castro 2016 [144, 145]; Hofmeyr 1998 [146, 147, 148]; Hofmeyr 2001 [149, 150]; Hofmeyr 2011 [151, 152]; Hoj 2005 [153, 154]; Jago 2007 [155]; Jangsten 2011 [156]; Jans 2017 [157]; Karkanis 2002 [158]; Kerekes 1979 [159]; Khan 1995 [160, 161]; Khurshid 2010 [162]; Koen 2016 [163, 164]; Kumar 2021 [165]; Kundodyiwa 2001 [166]; Lam 2004 [167]; Lamont 2001 [168]; Lapaire 2006 [169]; Leung 2006 [170]; Liu 2020 [171, 172]; Lokugamage 2001 [173, 174, 175]; Lumbiganon 1999 [176]; Mannaerts 2018 [177]; Masse 2022 [178, 179]; McDonagh 2022 [180, 181]; McDonald 1993 [182, 183, 184, 185, 186]; Mobeen 2011 [187, 188, 189, 190]; Moertl 2011 [191, 192, 193]; Moir 1979 [194]; Moodie 1976 [195]; Musa 2015 [196]; Nasr 2009 [197]; Nellore 2006 [198]; Ng 2001 [199, 200]; Ng 2007 [201, 202]; Oboro 2003 [203]; Ogunbode 1979 [204]; Orji 2008 [205]; Ortiz‐Gomez 2013 [206]; Othman 2016 [207, 208]; Owonikoko 2011 [209]; Pakniat 2015 [210, 211, 212]; Parsons 2006 [213]; Parsons 2007 [214, 215]; Penaranda 2002 [216]; Poeschmann 1991 [217, 218, 219]; Prendiville 1988 [220]; Quibel 2016 [221, 222, 223]; Rabow 2023 [224]; Rashid 2009 [225]; Ray 2001 [226, 227]; Reyes 2011 [228]; Reyes, Gonzalez 2011 [229]; Rogers 1998 [230]; Rosseland 2013 [231, 232]; Sadeghi Afkham 2022 [233]; Soltan 2007 [234]; Stanton 2013; Su 2009 [235]; Supe 2016 [236]; Surbek 1999 [237, 238]; Thilaganathan 1993 [239]; Vaid 2009 [240]; Van Der Nelson 2020 [241, 242, 243, 244]; Verma 2006 [245]; Vimala 2004 [246]; Vimala 2006 [247]; Walley 2000 [248]; Whigham 2016 [249, 250]; Widmer 2018 [19, 251, 252, 253]; Zachariah 2006 [254]). Most trials were reported in English; we obtained seven translations (four Spanish, two French and one Chinese). The trials were conducted across 48 countries (including high‐, low‐ and middle‐income countries) and sometimes involved more than one country. Several multi‐arm trials were identified: one five‐arm trial; six four‐arm trials; and 12 three‐arm trials. The median size of the trials was 356 participants (interquartile range (IQR) 141 to 742).
Most trials (94%, 115/122) were performed in a hospital setting, six were performed in a community setting (1%), and one (1%) in an unspecified setting. Most of the trials included women having a vaginal birth (71%, 87/122), and 31 trials (26%) involved women having an elective or emergency caesarean. Only four (3%) trials included women having either a vaginal or caesarean birth. Women included in the trials were judged to be at high risk for PPH in 23 of 122 trials (19%), low risk in 51 trials (42%), and 38 trials (31%) included women at both high or low risk for PPH. The risk for PPH was not specified in 10 trials (8%).
Women with a singleton pregnancy only were recruited in 76 trials (62%), and 22 trials (18%) included women with either singleton or multiple pregnancies. Twenty‐four trials (20%) did not specify. One trial (1%) included only nulliparous or primigravida women, one trial (1%) included only multiparous women, 63 trials (51%) included both nulliparous and multiparous women of all parities, and 57 trials (47%) did not specify the parity of the women included in the trials. Exclusion criteria varied significantly and usually encompassed women with significant medical comorbidities.
Across all 122 trials (271 trial arms) in the network meta‐analysis, trials used the following agents, either as intervention or comparison:
oxytocin: 87 trial arms (32%);
misoprostol: 61 trial arms (23%);
ergometrine plus oxytocin: 28 trial arms (10%);
ergometrine: 23 trial arms (8%);
placebo or no treatment: 23 trial arms (8%);
carbetocin: 21 trial arms (8%);
misoprostol plus oxytocin: 20 trial arms (7%);
injectables: 8 trial arms (3%).
See Supplementary material 2 for details.
Excluded studies
We excluded 300 trials (Supplementary material 3). The most common reasons for exclusion were because trials exclusively compared doses or routes of the same uterotonic agents, were quasi‐randomised or investigated ineligible interventions such as tranexamic acid.
Risk of bias in included studies
We present summaries of the methodological quality of the included trials for each of the domains we assessed across all trials (Figure 2) and for each included trial (Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We excluded trials with evidence of inadequate random sequence generation from this review. We found 98 of 122 included trials (80%) to have used an adequate method of random sequence generation and were at low risk of bias. However, 24 trials (20%) did not report the method used in sufficient detail, and we judged the risk of bias to be unclear.
Adequate methods of allocation concealment were reported in 83 of 122 trials (68%) and we judged them to be at low risk of bias. Only two trials (2%) showed evidence of inadequate allocation concealment, and 37 trials (30%) did not provide enough information to assess allocation concealment, so we judged the risk of bias to be unclear.
Blinding
Adequate methods of blinding for both participants and personnel to treatment allocation were reported in 60 of 122 trials (49%) and we judged them to be at low risk of bias. We judged 39 trials (32%) at high risk of bias for blinding of participants and personnel, and 23 trials (19%) did not provide enough information to assess the blinding of participants and personnel, so we judged the risk of bias to be unclear.
Adequate methods of blinding the assessment of the primary outcomes were reported in 60 of 122 trials (49%) and so we deemed the risk of bias to be low. We judged 14 trials (11%) to be at high risk of bias for blinding the assessment of the primary outcomes. Forty‐eight trials (39%) did not provide enough information, so we judged the risk of bias to be unclear.
Incomplete outcome data
We judged 102 of 122 trials (84%) to be at low risk of bias for the incomplete outcome data domain. In these trials, missing outcome data were less than 10% for the primary outcomes of the review and balanced in numbers across intervention groups with similar reasons for missing data across groups. In nine trials (7%), more than 10% of patients dropped out following randomisation, indicating a high risk of bias. Eleven trials (9%) did not provide enough information to assess this domain, so it was uncertain whether or not the handling of incomplete data was appropriate. We judged the risk of bias to be unclear in these trials.
Selective reporting
Only 24 of 122 trials (20%) prespecified all outcomes in publicly available trial protocols; we judged them to be at low risk of bias. Twelve trials (10%) did not report all prespecified outcomes as reported in their published protocols or methodology within the main report, and we judged them to be at high risk of bias for selective reporting. We were unable to identify a published protocol for most trials (70%, 86/122), and we judged the risk of bias to be unclear.
Other potential sources of bias
Sixty‐six of 122 trials (54%) analysed data by the intention‐to‐treat principle, and we judged them to be at low risk of bias. Thirty‐eight trials (31%) did not analyse data by the intention‐to‐treat principle, and we judged them to be at high risk of bias. We were unable to identify whether the remaining 18 trials (15%) analysed data by the intention‐to‐treat principle, and we judged the risk of bias to be unclear.
We found that 50 of 122 trials (41%) were either conducted with public or no funding, and declared that they had no potential conflicts of interest. We judged nine trials (7%) to be at high risk of bias, as the trial or authors were funded directly by the manufacturer of the drug under investigation. Sixty‐three trials (52%) did not provide enough information to assess the source of funding or potential conflicts of interest, and we judged the risk of bias to be unclear.
Among all the trials, we judged 67 of 122 trials (55%) at low risk of bias for objective assessment of blood loss as they reported objective methods for measuring blood loss, such as weighing sponges, measurements in drapes or volumetric assessment or did not measure blood loss as this was not an outcome of interest. We judged 28 trials (23%) to be at high risk of bias for measuring blood loss as they used subjective measurements such as clinical or visual estimates. Twenty‐seven trials (22%) did not provide enough information to assess the method for measuring blood loss, and we judged the risk of bias to be unclear.
We analysed how many trials we had judged to be at low, intermediate or high overall risk of bias for the sensitivity analysis. For PPH ≥ 500 mL, we found 32 of 81 trials (40%) to be at low overall risk of bias. We judged 49 of 81 trials (60%) to be at high risk of bias. We did not judge any trials as intermediate risk of bias. For PPH ≥ 1000 mL, we found 39 of 85 trials (46%) to be at low overall risk of bias. We judged 46 of 85 trials (54%) to be at high risk of bias. We did not judge any trials as intermediate risk of bias. See Supplementary material 8 for information about how this risk of bias has impacted the results.
Publication bias
In cases where 10 or more trials contributed to the pairwise meta‐analyses, we used a funnel plot to assess the potential presence of publication bias (Supplementary material 8). This information was used to inform the GRADE assessments (Supplementary material 8).
Synthesis of results
Please note that all of the analyses presented in the Supplementary material 6 section relate to the 'direct evidence' and were used as per our methods to grade the evidence. The results from Supplementary material 6 were also used to check the direction of effect in the subgroups and not to formally check for subgroup effects using the interaction test. These results are not described.
The following section presents the results as reported in all of the figures (Figure 4 to Figure 5). The figures present the results from the network diagrams, the forest plots with the pairwise, indirect and network (combining direct and indirect) effect estimates and the cumulative rankograms for all the outcomes with available data. The figures present the results for different uterotonics in comparison to placebo or no treatment and different uterotonics in comparison to the reference uterotonic agent oxytocin. All other comparisons are available from Supplementary material 8.
4.
Network Diagram for PPH ≥ 500 mL. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [L] low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
5.
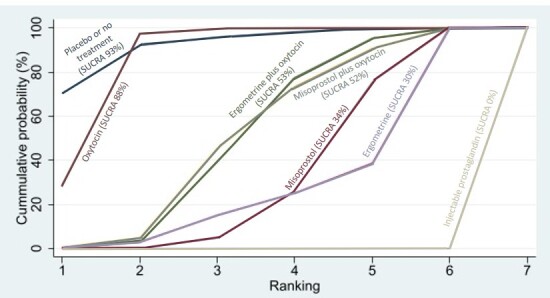
Cumulative rankograms comparing each of the uterotonic agents for diarrhoea. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Primary outcomes
Postpartum haemorrhage ≥ 500 mL
The network diagram for PPH ≥ 500 mL is presented in Figure 4. Oxytocin was the most frequently investigated uterotonic agent (53 of 81 trials, 65%) for this outcome (Figure 4).
Relative effects from the network meta‐analysis of 81 trials suggested that all agents, with the exception of injectable prostaglandins (limited available data), were effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment (Figure 6).
6.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for prevention of PPH ≥ 500 mL
When compared with oxytocin, ergometrine plus oxytocin combination and misoprostol plus oxytocin combination were more effective in preventing PPH ≥ 500 mL. When compared with oxytocin, high‐certainty evidence for the network meta‐analysis evidence suggests that ergometrine plus oxytocin (RR 0.76, 95% CI 0.64 to 0.90) reduces PPH ≥ 500 mL. Moderate‐certainty evidence suggests misoprostol plus oxytocin (RR 0.70, 95% CI 0.57 to 0.87) probably reduces PPH ≥ 500 mL. Based on these results, about 83 per 1000 women, given oxytocin for a vaginal birth, would experience a PPH of ≥ 500 mL compared with 63 given ergometrine plus oxytocin combination and 58 given misoprostol plus oxytocin combination (Table 1). High‐certainty evidence suggests that carbetocin makes little or no difference to this outcome compared with oxytocin. Moderate‐certainty evidence suggests injectable prostaglandins probably make little or no difference to this outcome compared with oxytocin. Low‐certainty evidence suggests ergometrine may make little or no difference to this outcome compared with oxytocin. The evidence for misoprostol was of very low certainty for this outcome (Table 1).
The cumulative probabilities for each agent being at each possible rank for preventing PPH ≥ 500 mL are shown in Figure 7. The highest‐ranked agents were misoprostol plus oxytocin combination (SUCRA 95%) and ergometrine plus oxytocin combination (SUCRA 89%). Carbetocin (SUCRA 57%) and oxytocin (56%) closely ranked together at third and fourth, respectively, followed by injectable prostaglandins (SUCRA 37%), misoprostol (SUCRA 35%), ergometrine (SUCRA 30%), and placebo or no treatment (SUCRA 1%).
7.
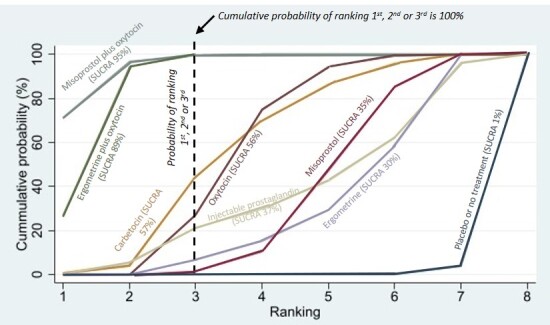
Cumulative rankograms comparing each of the uterotonic agents for prevention of PPH ≥ 500 mL. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Postpartum haemorrhage ≥ 1000 mL
The network diagram for PPH ≥ 1000 mL is presented in Figure 8. Oxytocin was the most frequently investigated uterotonic agent (36 of 85 trials, 42%) for this outcome (Figure 8).
8.
Network Diagram for PPH ≥ 1000 mL. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [L] low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
Relative effects from the network meta‐analysis of 85 trials suggested that all agents except ergometrine and injectable prostaglandins, due to limited data, were effective for preventing PPH ≥ 1000 mL when compared with placebo or no treatment (Figure 9).
9.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for prevention of PPH ≥ 1000 mL.
Carbetocin (RR 0.93, 95% CI 0.81 to 1.07, moderate‐certainty evidence) and injectable prostaglandins (RR 1.21, 95% CI 0.42 to 3.51, moderate‐certainty evidence) probably make little or no difference, and misoprostol plus oxytocin combination (RR 0.88, 95% CI 0.69 to 1.11, low‐certainty evidence) may make little or no difference in the outcome of PPH ≥ 1000 mL compared with oxytocin. The certainty of evidence for ergometrine plus oxytocin (RR 0.85, 95% CI 0.73 to 1.00), and ergometrine (RR 1.07, 95% CI 0.53 to 2.15) with the reference uterotonic agent oxytocin for PPH ≥ 1000 mL was very low (Table 2).
Low‐certainty evidence suggests that misoprostol may be less effective in preventing PPH ≥ 1000 mL when compared with oxytocin (RR 1.24, 95% CI 1.06 to 1.46) Table 2. In absolute terms, this result suggests that about 24 per 1000 women given oxytocin for a vaginal birth would experience PPH ≥ 1000 mL, compared with 30 given misoprostol (Table 2).
The cumulative probabilities for each agent being at each possible rank for preventing PPH ≥ 1000 mL are shown in Figure 10. Ergometrine plus oxytocin (SUCRA 84%), misoprostol plus oxytocin (SUCRA 77%) combinations and carbetocin (SUCRA 68%) were the highest‐ranked agents. Oxytocin ranked fourth (50%), followed by ergometrine (SUCRA 49%) and injectable prostaglandins (SUCRA 42%). Misoprostol was seventh (SUCRA 26%), ranking higher than placebo or no treatment (5%).
10.
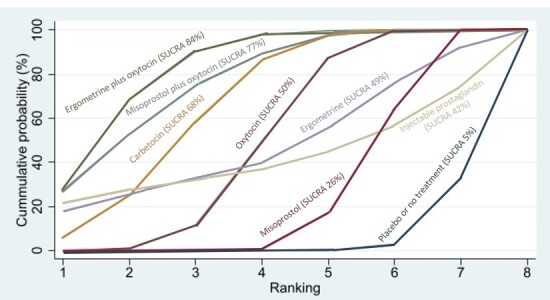
Cumulative rankograms comparing each of the uterotonic agents for prevention of PPH ≥ 1000 mL. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Secondary outcomes
Maternal death
The network diagram for maternal death is presented in Figure 11. Relative effects from the network meta‐analysis of five trials suggested that no meaningful differences could be detected between all uterotonic agents for maternal deaths as this outcome was rare (13 deaths across all trials were reported; Figure 12). When compared with oxytocin, carbetocin (RR 2.00, 95% CI 0.37 to 10.92, moderate‐certainty evidence) and misoprostol (RR 0.74, 95% CI 0.14 to 3.95, low‐certainty evidence) make little or no difference to maternal death.
11.
Network Diagram for maternal death. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
12.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for prevention of maternal death.
Figure 13 shows the cumulative probabilities for each agent being at each possible rank for maternal death. No reliable ranking could be derived for this outcome because of the rarity of maternal deaths.
13.
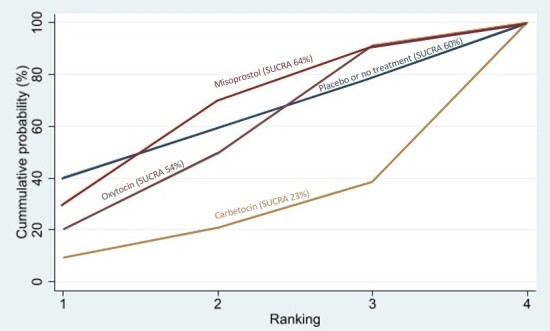
Cumulative rankograms comparing each of the uterotonic agents for prevention of maternal death. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Severe maternal morbidity: intensive care admissions
The network diagram for intensive care admissions as an outcome of severe morbidity is presented in Figure 14. Relative effects from the network meta‐analysis of seven trials for the various comparisons suggested that there were no detectable differences among uterotonic agents for intensive care admissions as this outcome was rare. This outcome was not reported for any trial involving injectable prostaglandins or ergometrine plus oxytocin combination (Figure 15).
14.
Network Diagram for severe maternal morbidity: intensive care admissions. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [L] low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
15.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for prevention of severe maternal morbidity: intensive care admissions.
Figure 16 shows the cumulative probabilities for each agent being at each possible rank for intensive care admissions. The ranking for all agents was not clear for this outcome due to limited data.
16.
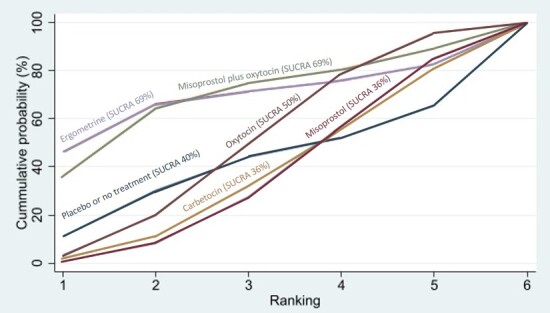
Cumulative rankograms comparing each of the uterotonic agents for prevention of severe maternal morbidity: intensive care admissions. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking. We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Severe maternal morbidity: shock
No trials reported shock as an outcome of severe maternal morbidity.
Additional uterotonics
The network diagram for the use of additional uterotonics is presented in Figure 17. Relative effects from the network meta‐analysis of 98 trials suggested that all agents were effective at reducing the use of additional uterotonics when compared with placebo or no treatment (Figure 18).
17.
Network Diagram for additional uterotonics. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [L] low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
18.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for additional uterotonics.
Moderate‐certainty evidence suggests that misoprostol plus oxytocin combination (RR 0.55, 95% CI 0.42 to 0.72) probably reduces the use of additional uterotonics when compared with oxytocin. Low‐certainty evidence suggests carbetocin (RR 0.74, 95% CI 0.59 to 0.94) and ergometrine plus oxytocin combination (RR 0.68, 95% CI 0.56 to 0.83) may reduce the use of additional uterotonics when compared with oxytocin. Based on these results, about 89 per 1000 women given oxytocin for a vaginal birth would require the administration of additional uterotonic agents, compared with 50 given misoprostol plus oxytocin, 66 given carbetocin and 61 given ergometrine plus oxytocin (Table 3).
Low‐certainty evidence suggests misoprostol (RR 1.14, 95% CI 0.97 to 1.34) may make little or no difference to the use of additional uterotonics when compared with oxytocin.
There was very low‐certainty evidence for the effects of ergometrine (RR 0.99, 95% CI 0.72 to 1.34) and injectable prostaglandins (RR 0.81, 95% CI 0.43 to 1.55) compared with the reference uterotonic agent oxytocin for the use of additional uterotonics. (Table 3).
Figure 19 shows the cumulative probabilities for each agent being at each possible rank for the use of additional uterotonics. The highest‐ranked agents were misoprostol plus oxytocin (SUCRA 96%), ergometrine plus oxytocin (SUCRA 80%), carbetocin (SUCRA 69%) and injectable prostaglandins (59%). Oxytocin (SUCRA 38%) was ranked with ergometrine (SUCRA 38%). The lowest‐ranked agents were misoprostol (SUCRA 20%) and placebo or no treatment (SUCRA 0%).
19.
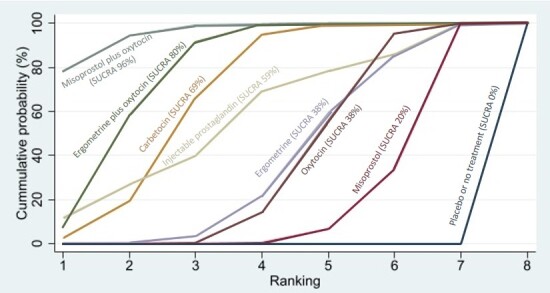
Cumulative rankograms comparing each of the uterotonic agents for additional uterotonics. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Blood transfusion
The network diagram for blood transfusion is presented in Figure 20. Relative effects from the network meta‐analysis of 80 trials suggested that all agents except ergometrine and injectable prostaglandins were effective for preventing blood transfusion when compared with placebo or no treatment (Figure 21).
20.
Network Diagram for blood transfusion. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [L] low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
21.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for blood transfusion.
Moderate‐certainty evidence suggests that misoprostol plus oxytocin probably prevents the need for blood transfusion when compared with oxytocin (RR 0.40, 95% CI 0.28 to 0.58). This suggests that whilst around 15 per 1000 women would require a blood transfusion when given oxytocin for a vaginal birth, about 6 per 1000 women would need a transfusion with misoprostol plus oxytocin. Low‐certainty evidence suggests that ergometrine plus oxytocin may prevent the need for blood transfusion when compared with oxytocin (RR 0.73, 95% CI 0.56 to 0.96). This means 11 per 1000 women would need a transfusion with ergometrine plus oxytocin for a vaginal birth compared with 15 per 1000 women given oxytocin alone (Table 4).
Carbetocin (RR 0.98, 95% CI 0.71 to 1.37, moderate‐certainty evidence) and misoprostol (RR 0.84, 95% CI 0.64 to 1.10, low‐certainty evidence) make little or no difference to the need for blood transfusion compared with oxytocin. There was very low‐certainty evidence for injectable prostaglandins and ergometrine (Table 4).
Figure 22 shows the cumulative probabilities for each agent being at each possible rank for preventing blood transfusion. The highest‐ranked agents were misoprostol plus oxytocin (SUCRA 91%), injectable prostaglandins (SUCRA 79%) and ergometrine plus oxytocin (SUCRA 65%). Oxytocin was ranked seventh (SUCRA 29%) behind ergometrine (SUCRA 53%), misoprostol (SUCRA 50%) and carbetocin (SUCRA 31%), but higher than placebo or no treatment (SUCRA 2%).
22.
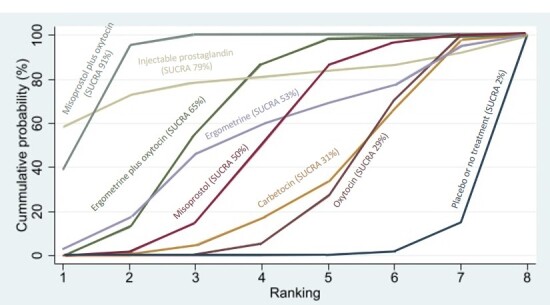
Cumulative rankograms comparing each of the uterotonic agents for blood transfusion. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Mean volumes of blood loss
The network diagram for blood loss (mL) as a continuous outcome is presented in Figure 23. Relative effects from the network meta‐analysis of 88 trials suggested that all agents are effective for reducing blood loss as a continuous outcome when compared with placebo or no treatment (Figure 24).
23.
Network Diagram for mean blood loss (mL). The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [L] low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
24.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for mean blood loss (mL).
When compared with oxytocin, moderate‐certainty evidence suggests that little or no difference was observed in blood loss among women receiving ergometrine (mean difference (MD) 9.46 mL higher, 95% CI 20.64 mL lower to 39.56 mL higher). When compared with oxytocin, low‐certainty evidence suggests that little or no difference was observed on blood loss among women receiving carbetocin (MD 18.72 mL lower, 95% CI 59.20 mL lower to 21.76 mL higher) and injectable prostaglandins (MD 34.81 mL lower, 95% CI 87.17 mL lower to 17.56 mL higher). The effects of misoprostol, ergometrine plus oxytocin, and misoprostol plus oxytocin were unclear because the certainty of the evidence was very low (Figure 24).
Figure 25 shows the cumulative probabilities for each agent being at each possible rank for preventing blood loss (mL) as a continuous outcome. The highest‐ranked agents were misoprostol plus oxytocin (SUCRA 96%), injectable prostaglandins (SUCRA 79%), carbetocin (SUCRA 65%), and ergometrine plus oxytocin (SUCRA 53%). Oxytocin was ranked sixth (SUCRA 38%), behind misoprostol (SUCRA 43%). The lowest‐ranked agents were ergometrine (SUCRA 26%) and placebo or no treatment (SUCRA 0%).
25.
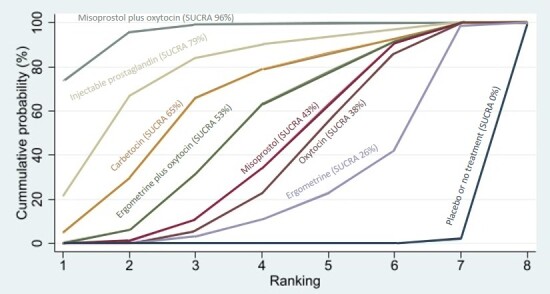
Cumulative rankograms comparing each of the uterotonic agents for mean blood loss (mL). Ranking indicates the cumulative probability of being the best, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Change in haemoglobin
The network diagram for the change in haemoglobin measurements before versus after birth (g/L) is presented in Figure 26. Relative effects from the network meta‐analysis of 58 trials suggested that all agents except ergometrine and the injectable prostaglandins were effective for reducing the change in haemoglobin measurements when compared with placebo or no treatment (Figure 27).
26.
Network Diagram for change in haemoglobin measurements before and after birth (g/L). The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [L] low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
27.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for change in haemoglobin measurements before and after birth (g/L).
There is low‐certainty evidence to suggest that the mean change in haemoglobin level before versus after birth may be lower among women receiving carbetocin (MD 1.65 g/L lower, 95% CI from 3.19 g/L lower to 0.11 g/L lower), and misoprostol plus oxytocin (MD 1.88 g/L lower, 95% CI from 3.01 g/L lower to 0.75 g/L lower), compared with those receiving oxytocin. There is low‐certainty evidence to suggest that the mean change in haemoglobin level before versus after birth may not be different among women receiving ergometrine (MD 0.60 g/L higher, 95% CI from 0.83 g/L lower to 2.03 g/L higher) compared with those receiving oxytocin. The effects of misoprostol, injectable prostaglandins and ergometrine plus oxytocin were unclear because the certainty of the evidence was very low (Figure 27).
Figure 28 shows the cumulative probabilities for each agent being at each possible rank for change in haemoglobin measurements before versus after birth (g/L). The highest‐ranked agents were misoprostol plus oxytocin (91%), carbetocin (85%), and ergometrine plus oxytocin (77%). Oxytocin ranked fifth (SUCRA 42%), behind misoprostol (SUCRA 55%), but ranked better than ergometrine (SUCRA 29%), injectable prostaglandins (SUCRA 14%), and placebo or no treatment (SUCRA 7%).
28.
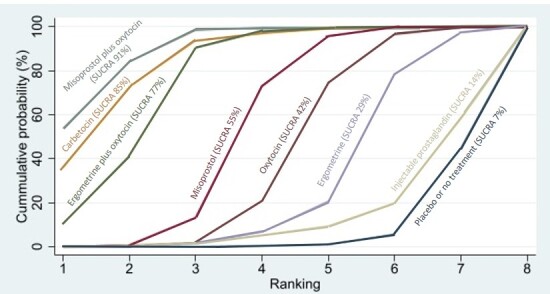
Cumulative rankograms comparing each of the uterotonic s for change in haemoglobin measurements before and after birth (g/L). Ranking indicates the cumulative probability of being the best, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Breastfeeding at hospital discharge
The network diagram for breastfeeding at hospital discharge is presented in Figure 29. Relative effects from the network meta‐analysis of six trials suggested that there were no detectable differences between oxytocin, carbetocin and ergometrine plus oxytocin for breastfeeding at hospital discharge when compared with placebo or no treatment.
29.
Network Diagram for breastfeeding at discharge. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [L] low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
High‐certainty evidence suggests that ergometrine plus oxytocin (RR 0.99, 95% CI 0.96 to 1.03) makes little or no difference to the proportion of women who are breastfeeding at the time of discharge from hospital when compared with oxytocin. In absolute terms, these results suggest that about 849 per 1000 women given oxytocin would be breastfeeding at discharge, compared to 841 per 1000 women with ergometrine plus oxytocin. Moderate‐certainty evidence suggests that carbetocin (RR 0.94, 95% CI 0.86 to 1.03) probably makes little or no difference to the proportion of women who are breastfeeding at the time of discharge from hospital when compared with oxytocin.
There were no clear findings relating to any other uterotonics as the outcome was not reported in any of the included trials involving misoprostol, injectable prostaglandins, ergometrine and misoprostol plus oxytocin (Figure 30).
30.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for breastfeeding at discharge.
Figure 31 shows the cumulative probabilities for each agent being at each possible rank for breastfeeding at hospital discharge. The ranking for all agents was not clear for this outcome due to limited data.
31.
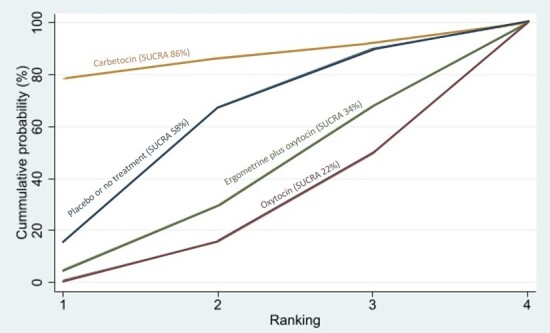
Cumulative rankograms comparing each of the uterotonic agents for breastfeeding at discharge. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Side effects
Nausea
The network diagram for nausea is presented in Figure 32. Relative effects from the network meta‐analysis of 69 trials suggest that ergometrine and ergometrine plus oxytocin are more likely to cause nausea than placebo or no treatment (Figure 33).
32.
Network Diagram for nausea. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [L] low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
33.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for nausea.
When compared with oxytocin, there is moderate‐certainty evidence to suggest that women receiving ergometrine (RR 2.65, 95% CI 1.70 to 4.11) and misoprostol plus oxytocin combination (RR 2.28, 95% CI 1.19 to 4.37) are probably more likely to experience nausea. Low‐certainty evidence suggests that women receiving misoprostol (RR 1.61, 95% CI 1.19 to 2.18) and ergometrine plus oxytocin combination (RR 2.12, 95% CI 1.55 to 2.91) may be more likely to experience nausea than women receiving oxytocin alone (Figure 33). Based on these results, about 44 per 1000 women given oxytocin would experience nausea, compared with 117 given ergometrine, 100 given misoprostol plus oxytocin, 71 given misoprostol, and 93 given ergometrine plus oxytocin. The findings for carbetocin and injectable prostaglandins were unclear, because we found the evidence to be of very low certainty.
Figure 34 shows the cumulative probabilities for each agent being at each possible rank for causing nausea. The highest‐ranked agents with which women are less likely to experience nausea are carbetocin (SUCRA 93%), oxytocin (SUCRA 84%), and placebo or no treatment (SUCRA 69%). These are followed by misoprostol (SUCRA 50%), injectable prostaglandins (SUCRA 42%), ergometrine plus oxytocin (SUCRA 27%) and misoprostol plus oxytocin (SUCRA 24%). The lowest‐ranked agent is ergometrine (SUCRA 12%).
34.
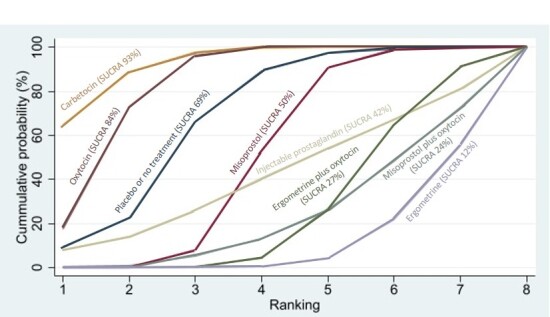
Cumulative rankograms comparing each of the uterotonic agents for nausea. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Vomiting
The network diagram for vomiting is presented in Figure 35. Relative effects from the network meta‐analysis of 77 trials suggest that ergometrine, injectable prostaglandins, and ergometrine plus oxytocin are more likely to cause vomiting than placebo or no treatment (Figure 36).
35.
Network Diagram for vomiting. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
36.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for vomiting.
Moderate‐certainty evidence suggests ergometrine (RR 2.25, 95% CI 1.39 to 3.64) and misoprostol plus oxytocin (RR 2.27, 95% CI 1.28 to 4.02) probably increase the likelihood of vomiting when compared to oxytocin. These results suggest that 12 per 1000 women given oxytocin for vaginal birth experience vomiting, compared with 27 per 1000 with ergometrine and 27 per 1000 with misoprostol plus oxytocin. Low‐certainty evidence suggests misoprostol (RR 1.75, 95% CI 1.29 to 2.38) and ergometrine plus oxytocin combination (RR 3.15, 95% CI 2.24 to 4.43) may increase the likelihood of vomiting compared to oxytocin. Low‐certainty evidence suggests carbetocin (RR 0.96, 95% CI 0.63 to 1.48) may make little or no difference to the likelihood of vomiting compared to oxytocin. The certainty of evidence for injectable prostaglandins was very low (Table 5).
Figure 37 shows the cumulative probabilities for each agent being at each possible rank for causing vomiting. The highest‐ranked agents were carbetocin (SUCRA 89%), oxytocin (SUCRA 87%) and placebo or no treatment (SUCRA 81%). These are followed by misoprostol (SUCRA 53%), misoprostol plus oxytocin (SUCRA 35%), and ergometrine (SUCRA 35%). The lowest‐ranked agents were ergometrine plus oxytocin (SUCRA 14%) and injectable prostaglandins (SUCRA 6%).
37.
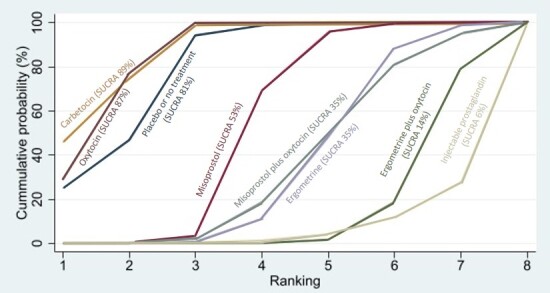
Cumulative rankograms comparing each of the uterotonic agents for vomiting. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Hypertension
The network diagram for hypertension is presented in Figure 38. Relative effects from the network meta‐analysis of 17 trials suggest that ergometrine is more likely to cause hypertension than placebo or no treatment (Figure 39).
38.
Network Diagram for hypertension. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
39.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for hypertension.
Low‐certainty evidence suggests that ergometrine (RR 7.05, 95% CI 1.98 to 25.07) may increase the risk of hypertension when compared to oxytocin. These results suggest that 54 per 1000 women given oxytocin for vaginal birth experience hypertension, compared with 381 per 1000 with ergometrine. Low‐certainty evidence suggests that carbetocin (RR 0.68, 95% CI 0.23 to 2.03) and ergometrine plus oxytocin combination (RR 1.51, 95% CI 0.82 to 2.78) may make little or no difference to the risk of hypertension when compared with oxytocin. The certainty of evidence for misoprostol was very low (Table 6).
Figure 40 shows the cumulative probabilities for each agent being at each possible rank for causing hypertension. The lowest‐ranked agents were ergometrine (SUCRA 1%) and ergometrine plus oxytocin (32%). The rest of the agents were of comparable ranking except carbetocin, which was ranked first (SUCRA 81%). No trials involved misoprostol plus oxytocin or injectable prostaglandins, so we could not rank these agents.
40.
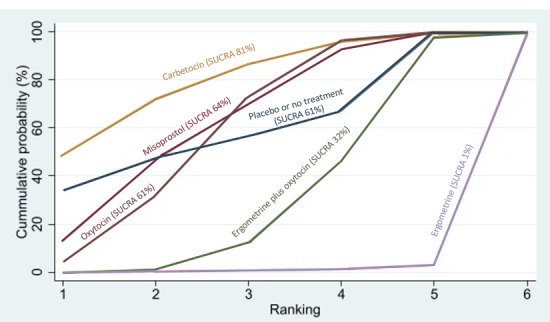
Cumulative rankograms comparing each of the uterotonic agents for hypertension. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Headache
The network diagram for headache is presented in Figure 41. Relative effects from the network meta‐analysis of 42 trials suggested that ergometrine is more likely to cause headache than placebo or no treatment (Figure 42).
41.
Network Diagram for headache. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [L] low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
42.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for headache.
When compared with oxytocin, there is low‐certainty evidence to suggest that women receiving ergometrine (RR 2.14, 95% CI 1.05 to 4.35) may be more likely to experience headache (Figure 42), with 36 per 1000 women given oxytocin experiencing headache compared to 77 with ergometrine. Low‐certainty evidence suggests that carbetocin (RR 1.05, 95% CI 0.68 to 1.62) and ergometrine plus oxytocin combination (RR 1.41, 95% CI 0.95 to 2.10) may make little or no difference to the experience of headache when compared with oxytocin. It is uncertain whether misoprostol, and misoprostol plus oxytocin impact women’s experience of headache because the certainty of evidence was very low.
Figure 43 shows the cumulative probabilities for each agent being at each possible rank for causing headache. The highest‐ranked intervention was placebo or no treatment (SUCRA 82%), oxytocin (SUCRA 65%), misoprostol (SUCRA 65%), carbetocin (SUCRA 58%), and misoprostol plus oxytocin (SUCRA 47%). The lowest‐ranked agents were ergometrine plus oxytocin (SUCRA 26%), and ergometrine (SUCRA 8%).
43.
Cumulative rankograms comparing each of the uterotonic agents for headache. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Fever
The network diagram for fever is presented in Figure 44. Relative effects from the network meta‐analysis of 53 trials suggested that misoprostol and misoprostol plus oxytocin are more likely to cause fever than placebo or no treatment (Figure 45).
44.
Network Diagram for fever. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [L] low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
45.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for fever.
When compared with oxytocin, low‐certainty evidence suggests that women receiving misoprostol (RR 4.17, 95% CI 2.95 to 5.88) and misoprostol plus oxytocin (RR 3.95, 95% CI 2.52 to 6.20) may be more likely to experience fever. These results suggest that 20 per 1000 women given oxytocin for vaginal birth would experience fever, compared to 83 with misoprostol and 79 with misoprostol plus oxytocin. Low‐certainty evidence suggests that carbetocin (RR 0.37, 95% CI 0.01 to 10.55) and ergometrine plus oxytocin (RR 0.78, 95% CI 0.38 to 1.58) probably make little or no difference to women having fever when compared to oxytocin. The certainty of evidence for ergometrine and injectable prostaglandins was very low (Table 7).
Figure 46 shows the cumulative probabilities for each agent being at each possible rank for causing fever. The lowest‐ranked agents were misoprostol (SUCRA 7%) and misoprostol plus oxytocin (SUCRA 10%). The rest of the agents were ranked as follows: injectable prostaglandins (SUCRA 79%), carbetocin (SUCRA 74%), ergometrine plus oxytocin combination (SUCRA 67%), ergometrine (SUCRA 58%), placebo or no treatment (SUCRA 53%) and oxytocin (SUCRA 52%).
46.
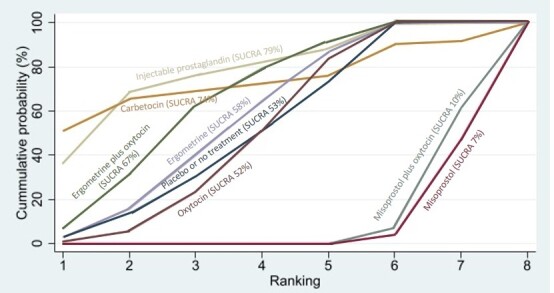
Cumulative rankograms comparing each of the uterotonic agents for fever. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Shivering
The network diagram for shivering is presented in Figure 47. Relative effects from the network meta‐analysis of 69 trials suggested that misoprostol and misoprostol plus oxytocin are more likely to cause shivering than placebo or no treatment (Figure 48).
47.
Network Diagram for shivering. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [L] low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
48.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for shivering.
When compared with oxytocin, there is low‐certainty evidence to suggest that carbetocin (RR 0.67, 95% CI 0.35 to 1.30), ergometrine (RR 1.41, 95% CI 0.85 to 2.34), and ergometrine plus oxytocin (RR 1.35, 95% CI 0.81 to 2.23) may have little or no impact on women's experience of shivering. When compared with oxytocin, it is uncertain whether misoprostol, injectable prostaglandins, and misoprostol plus oxytocin impact on women’s experience of shivering because the certainty of evidence was very low (Figure 48).
Figure 49 shows the cumulative probabilities for each agent being at each possible rank for causing shivering. The highest‐ranked agents are injectable prostaglandins (SUCRA 98%), carbetocin (SUCRA 84%) and oxytocin (SUCRA 68%). These are followed by ergometrine plus oxytocin and placebo or no treatment (SUCRA 46%) and ergometrine (SUCRA 44%). The lowest‐ranked agents were misoprostol plus oxytocin, and misoprostol (SUCRA 7%).
49.
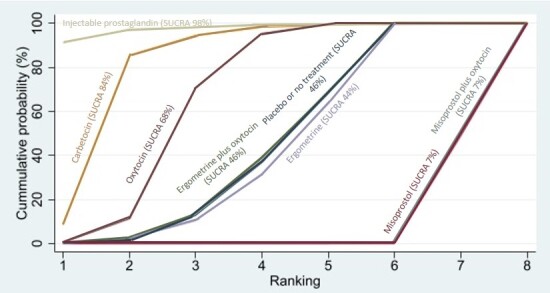
Cumulative rankograms comparing each of the uterotonic agents for shivering. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Abdominal pain
The network diagram for abdominal pain is presented in Figure 50. Relative effects from the network meta‐analysis of 22 trials suggested that ergometrine is more likely to cause abdominal pain than placebo or no treatment (Figure 51).
50.
Network Diagram for abdominal pain. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [L] low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
51.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for abdominal pain.
When compared with oxytocin, there is moderate‐certainty evidence to suggest that carbetocin (RR 0.92, 95% CI 0.71 to 1.18) and ergometrine plus oxytocin (RR 1.32, 95% CI 0.97 to 1.80) probably make little or no difference to women's experience of abdominal pain. When compared with oxytocin, it is uncertain whether misoprostol, injectable prostaglandins and ergometrine impact on women’s experience of abdominal pain because the certainty of evidence was very low (Figure 51).
Figure 52 shows the cumulative probabilities for each agent being at each possible rank for causing abdominal pain. The highest‐ranked agent was carbetocin (SUCRA 80%), followed by placebo or no treatment (SUCRA 68%), oxytocin (SUCRA 66%), and misoprostol (SUCRA 60%). These were followed by injectable prostaglandins (SUCRA 42%) and ergometrine plus oxytocin (SUCRA 27%). The lowest‐ranked agent was ergometrine (SUCRA 7%).
52.
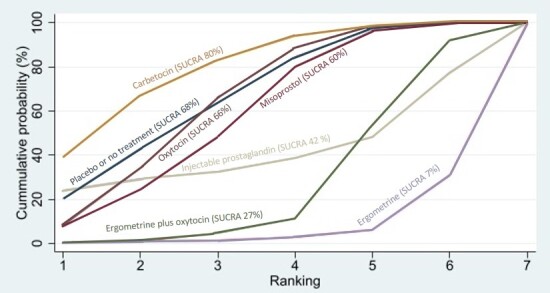
Cumulative rankograms comparing each of the uterotonic agents for abdominal pain. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Diarrhoea
The network diagram for diarrhoea is presented in Figure 53. Relative effects from the network meta‐analysis of 38 trials suggested that misoprostol, ergometrine and injectable prostaglandins are more likely to cause diarrhoea than placebo or no treatment (Figure 54).
53.
Network Diagram for diarrhoea. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials for each comparison. [VL] very low‐certainty evidence; [L] low‐certainty evidence; [M] moderate‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
54.
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for diarrhoea.
Moderate‐certainty evidence shows that misoprostol (RR 2.12, 95% CI 1.51 to 2.98) and misoprostol plus oxytocin (RR 1.76, 95% CI 1.05 to 2.95) probably increase the likelihood of diarrhoea when compared with oxytocin (Figure 54). Low‐certainty evidence suggests that injectable prostaglandins (RR 27.80, 95% CI 11.27 to 68.56), ergometrine (RR 2.54, 95% CI 1.03 to 6.25), and ergometrine plus oxytocin (RR 1.76, 95% CI 1.12 to 2.77) may increase the likelihood of diarrhoea when compared with oxytocin (Figure 54).
Figure 5 shows the cumulative probabilities for each agent being at each possible rank for causing diarrhoea. The highest‐ranked agents were placebo or no treatment (SUCRA 93%) and oxytocin (SUCRA 88%). These were followed by ergometrine plus oxytocin (SUCRA 53%), and misoprostol plus oxytocin (SUCRA 52%). The lowest‐ranked agents are misoprostol (SUCRA 34%), ergometrine (SUCRA 30.3%) and injectable prostaglandins (SUCRA 0%).
Maternal sense of well‐being
In total, two trials reported outcomes relevant to maternal sense of well‐being. However, because of the heterogeneous ways these trials defined maternal sense of well‐being, we decided not to perform a meta‐analysis.
One trial, which compared oxytocin with no treatment, reported this outcome in two different ways (Jans 2017; Analysis 1.19)).
Low‐certainty evidence suggests that the use of prophylactic oxytocin may make little or no difference to women’s experience of having less energy than before birth at three months postpartum (RR 1.02, 95% CI 0.93 to 1.13; 1 trial, 1543 women), or to the experience of fatigue at three months postpartum (RR 0.99, 95% CI 0.95 to 1.04; 1 trial, 1571 women).
Another trial, which compared ergometrine plus oxytocin with no treatment, reported this outcome in eight different ways (Rogers 1998; Analysis 6.19). Low‐certainty evidence suggests that prophylactic ergometrine plus oxytocin may make little or no difference to women’s general health at six weeks postpartum when compared to no treatment (RR 0.99, 95% CI 0.71 to 1.37; 1 trial, 1447 women). Moderate‐certainty evidence suggests that prophylactic ergometrine plus oxytocin probably makes little or no difference to women's exhaustion after giving birth when compared to no treatment (RR 0.95, 95% CI 0.79 to 1.15; 1 trial, 1447 women). Low‐certainty evidence suggests that prophylactic ergometrine plus oxytocin may make little or no difference to women's exhaustion at six weeks postpartum when compared to no treatment (RR 0.95, 95% CI 0.74 to 1.21; 1 trial, 1447 women). Moderate‐certainty evidence suggests that prophylactic ergometrine plus oxytocin probably makes little or no difference to women's 'blues' (temporary lower mood and feelings of sadness) at six weeks postpartum when compared to no treatment (RR 0.93, 95% CI 0.83 to 1.04; 1 trial, 1447 women). Low‐certainty evidence suggests that prophylactic ergometrine plus oxytocin may make little or no difference to women experiencing depression at six weeks postpartum when compared to no treatment (RR 1.22, 95% CI 0.84 to 1.78; 1 trial, 1447 women). Low‐certainty evidence suggests that prophylactic ergometrine plus oxytocin may make little or no difference to women looking for help for depression at six weeks postpartum when compared to no treatment (RR 1.05, 95% CI 0.82 to 1.35; 1 trial, 1447 women). It is uncertain whether prophylactic ergometrine plus oxytocin reduces admissions to hospital for depression at six weeks postpartum when compared to no treatment because the certainty of this evidence is very low (RR 3.06, 95% CI 0.12 to 75.06; 1 trial, 1447 women). Moderate‐certainty evidence suggests that prophylactic ergometrine plus oxytocin probably makes little or no difference to women reporting health problems at six weeks postpartum when compared to no treatment (RR 0.95, 95% CI 0.90 to 1.01; 1 trial, 1447 women).
Maternal satisfaction
In total, four trials reported outcomes relevant to maternal satisfaction. However, because of the heterogeneous ways these trials defined maternal satisfaction, we decided not to perform a meta‐analysis.
One trial, which compared oxytocin with no treatment, reported this outcome in three different ways (Jangsten 2011; Analysis 1.20). Moderate‐certainty evidence suggests that the use of prophylactic oxytocin may make little or no difference to women’s perception of whether management of the birth positively influenced the childbirth experience for mothers (RR 1.01, 95% CI 0.89 to 1.15; 1 trial, 1631 women); or made little or no difference to the mother’s childbirth experience (RR 1.02, 95% CI 0.90 to 1.15; 1 trial, 1631 women). Low‐certainty evidence suggests that the use of prophylactic oxytocin may make little or no difference to the extent to which women perceive that management of the birth negatively influences the childbirth experience for mothers (RR 0.73, 95% CI 0.47 to 1.13; 1 trial, 1631 women).
One trial, which compared ergometrine plus oxytocin with no treatment, reported this outcome in two different ways (Rogers 1998; Analysis 6.20). Moderate‐certainty evidence suggests that prophylactic ergometrine plus oxytocin probably makes little or no difference to satisfaction with third‐stage management when compared to no treatment (RR 1.03, 95% CI from 1.00 to 1.05; 1 trial, 1507 women). Moderate‐certainty evidence suggests that prophylactic ergometrine plus oxytocin probably decreased women's feeling of being in control during third‐stage labour when compared to no treatment (RR 0.95, 95% CI 0.91 to 0.99; 1 trial, 1507 women).
One trial, which compared misoprostol with oxytocin, reported this outcome in four different ways (Diop 2016; Analysis 8.20). Moderate‐certainty evidence suggests that prophylactic misoprostol, when compared to oxytocin, probably makes little or no difference to women being satisfied or very satisfied with the uterotonic agent they received (RR 1.01, 95% CI 1.00 to 1.02; 1 trial, 1338 women), or that women would take the specific uterotonic agent again after subsequent deliveries (RR 1.01, 95% CI 1.00 to 1.02; 1 trial, 1295 women), or that women would recommend the specific uterotonic agent to a friend (RR 1.01, 95% CI 1.00 to 1.02; 1 trial, 1266 women). Women were less likely to make a complaint about, or have a problem with, misoprostol compared with oxytocin (RR 0.36, 95% CI 0.20 to 0.64; 1 trial, 1339 women).
One trial, which compared ergometrine plus oxytocin with misoprostol reported this outcome using an eight‐item Client Satisfaction Questionnaire (Ng 2007; Analysis 17.20). Moderate‐certainty evidence suggests that prophylactic ergometrine plus oxytocin, when compared to misoprostol, probably makes little or no difference to women being satisfied with the uterotonic agent they received (MD 0.6 lower, 95% CI 1.22 lower to 0.02 higher; 1 trial, 355 women).
Subgroup analyses
We carried out subgroup analyses for the outcomes of PPH ≥ 500 mL and PPH ≥ 1000 mL, by mode of birth (caesarean versus vaginal birth), setting (hospital versus community), risk of PPH (high versus low risk for PPH), dose of misoprostol (≥ 600 μg versus < 600 μg), and regimen of oxytocin (bolus versus bolus plus infusion versus infusion only).
The network diagrams for all subgroups are available from Supplementary material 8. Relative effects and cumulative probabilities for each agent being at each possible rank for each subgroup are also available from Supplementary material 8. Subgroup analyses did not reveal important subgroup differences for any of the subgroups.
Subgroup analysis for high risk of PPH for the primary outcomes showed that, for PPH ≥ 500 mL, the combination treatment of misoprostol plus oxytocin was the most effective, and for PPH ≥ 1000 mL, the combination treatment of ergometrine plus oxytocin was the most effective. High risk of PPH, as defined by the trial authors, included women with the following: multiple pregnancy, previous antepartum or postpartum haemorrhage, uterine fibroids, abnormal placentation, pre‐eclampsia, bleeding disorders, asthma, diabetes, epilepsy, severe anaemia, and cardiovascular, liver and renal disease, as well as other co‐morbidities.
Sensitivity analyses
We carried out prespecified sensitivity analyses by restricting our analyses to trials at low risk of bias, trials at low risk of bias in terms of funding sources, trials that used an objective method of measuring blood loss, and large trials with more than 400 participants. Details of these analyses are available from Supplementary material 8.
The sensitivity analysis with the outcome of PPH ≥ 500 mL restricted to trials at low overall risk of bias, showed ergometrine to be the highest‐ranking agent (SUCRA 96%). Other than this, the sensitivity analyses showed that the overall results are not affected by the above‐mentioned criteria or decisions.
Discussion
Summary of main results
Relative effects from our network meta‐analysis of 122 trials (121,931 women) suggested that all agents, except injectable prostaglandins, for which data were limited, were effective for preventing PPH ≥ 500 mL compared with placebo or no treatment. The two highest‐ranked agents were ergometrine plus oxytocin, and misoprostol plus oxytocin. Evidence suggests that ergometrine plus oxytocin (high‐certainty evidence) reduces PPH ≥ 500 mL compared with oxytocin alone, and misoprostol plus oxytocin (moderate‐certainty evidence) probably reduces PPH ≥ 500 mL compared with oxytocin. Carbetocin (high‐certainty evidence), injectable prostaglandins (moderate‐certainty evidence), and ergometrine (low‐certainty evidence) have similar effects for PPH ≥ 500 mL compared with oxytocin. In the last review update, carbetocin was ranked alongside ergometrine plus oxytocin, and misoprostol plus oxytocin for its increased effectiveness in preventing PPH ≥ 500 mL compared with oxytocin [26], but in this current update it is as effective as oxytocin. The evidence for misoprostol was of very low certainty.
All agents, except ergometrine and injectable prostaglandins, for which data were limited, were effective for preventing PPH ≥ 1000 mL compared with placebo or no treatment. Ergometrine plus oxytocin, and misoprostol plus oxytocin were the highest‐ranked agents. Little or no differences were observed in the effects of carbetocin (moderate‐certainty evidence), injectable prostaglandins (moderate‐certainty evidence), and misoprostol plus oxytocin (low‐certainty evidence) compared with oxytocin for PPH ≥ 1000 mL. Low‐certainty evidence suggests that misoprostol may be less effective in preventing PPH ≥ 1000 mL compared with oxytocin. The certainty of evidence for ergometrine and ergometrine plus oxytocin was very low.
Misoprostol plus oxytocin (moderate‐certainty evidence) probably reduces the use of additional uterotonics compared with oxytocin, and carbetocin (low‐certainty evidence), and ergometrine plus oxytocin (low‐certainty evidence) may reduce the use of additional uterotonics compared with oxytocin. Misoprostol (low‐certainty evidence) may make little or no difference for this outcome compared with oxytocin.
Misoprostol plus oxytocin (moderate‐certainty evidence) probably reduces the risk of blood transfusion compared with oxytocin, and ergometrine plus oxytocin (low‐certainty evidence) may reduce the risk of blood transfusion compared with oxytocin. Carbetocin (moderate‐certainty evidence), and misoprostol (low‐certainty evidence) make little or no difference for this outcome compared with oxytocin.
All uterotonic agents, except for carbetocin, were associated with increased risks of some side effects compared with oxytocin. Misoprostol increased the likelihood of nausea (low‐certainty evidence), vomiting (low‐certainty evidence), fever (low‐certainty evidence), and diarrhoea (moderate‐certainty evidence). Injectable prostaglandins increased the likelihood of diarrhoea (low‐certainty evidence). Ergometrine increased the likelihood of nausea (moderate‐certainty evidence), vomiting (moderate‐certainty evidence), hypertension (low‐certainty evidence), headache (low‐certainty evidence) and diarrhoea (low‐certainty evidence). Ergometrine plus oxytocin increased the likelihood of nausea (low‐certainty evidence), vomiting (low‐certainty evidence), and diarrhoea (low‐certainty evidence). Misoprostol plus oxytocin increased the likelihood of nausea (moderate‐certainty evidence), vomiting (moderate‐certainty evidence), fever (low‐certainty evidence), and diarrhoea (moderate‐certainty evidence).
Subgroup analyses did not reveal important subgroup differences by mode of birth (caesarean versus vaginal birth), setting (hospital versus community), risk of PPH (high versus low risk for PPH), dose of misoprostol (≥ 600 μg versus < 600 μg) and regimen of oxytocin (bolus versus bolus plus infusion versus infusion only). Subgroup analysis for high risk of PPH for the primary outcome of PPH ≥ 500 mL showed that the combination treatment of misoprostol plus oxytocin was the most effective, and for the primary outcome of PPH ≥ 1000 mL, the combination treatment of ergometrine plus oxytocin was the most effective.
Limitations of the evidence included in the review
We recognise that there is no single established approach for assessing the certainty of the effect estimates generated by the network meta‐analysis. We applied the rigorous method for appraising certainty of network evidence as proposed by the GRADE Working Group. Overall, the evidence presented varied widely in quality, and our confidence in the effect estimates ranged from very low to high certainty. When we compared oxytocin with all the other uterotonic agents and agent combinations, most individual outcomes included a range in quality of evidence across the different interventions, and this was equally true for our most important outcomes. Our reasons for downgrading the evidence also varied across comparisons and outcomes.
To summarise the certainty of the evidence for the seven most important outcomes (also described in the summary of findings), for PPH ≥ 500 mL, high‐certainty evidence pointed to the superiority of ergometrine plus oxytocin compared with oxytocin alone. Moderate‐certainty evidence pointed to the probable superiority of misoprostol plus oxytocin when compared with oxytocin. In this case, we downgraded the evidence certainty due to concerns regarding risk of bias in the direct comparison that contributed most weight to the network estimate.
For PPH ≥ 1000 mL, low‐certainty evidence showed that misoprostol may be inferior to oxytocin alone. We downgraded the evidence due to concerns regarding risk of bias and publication bias in the direct comparison that contributed most weight to the network estimate. Moderate‐certainty evidence showed that carbetocin and injectable prostaglandins made little difference compared with oxytocin for this outcome. We downgraded the evidence for carbetocin due to incoherence, and the evidence for injectable prostaglandins due to serious imprecision.
Misoprostol plus oxytocin probably reduces the use of additional uterotonics compared with oxytocin. We downgraded the evidence to moderate certainty due to concerns regarding risk of bias in the direct comparison that contributed most weight to the network estimate. Carbetocin may reduce the use of additional uterotonics. We downgraded the evidence to low certainty due to concerns about risk of bias and statistical heterogeneity in the indirect comparison that contributed most weight to the network estimate. Ergometrine plus oxytocin may reduce the use of additional uterotonics. We downgraded the evidence to low certainty due to concerns about risk of bias and statistical heterogeneity in the direct comparison that contributed most weight to the network estimate.
Moderate‐certainty evidence suggested that misoprostol plus oxytocin probably reduces the risk of blood transfusion compared with oxytocin. We downgraded the evidence due to concerns about risk of bias in the indirect comparison that contributed most weight to the network estimate. Low‐certainty evidence suggested that ergometrine plus oxytocin may reduce the risk of blood transfusion compared with oxytocin. We downgraded the evidence due to multiple concerns about risk of bias in the indirect comparison that contributed most weight to the network estimate.
The certainty of the evidence on side effects also varied. Moderate‐certainty evidence suggested that ergometrine and misoprostol plus oxytocin probably increase the likelihood of vomiting compared with oxytocin. For ergometrine, we downgraded the evidence due to concerns about risk of bias in the indirect comparison that contributed most weight to the network estimate, and for misoprostol plus oxytocin, we downgraded the evidence due to concerns about risk of bias in the direct comparison that contributed most weight to the network estimate.
Low‐certainty evidence suggested that ergometrine may increase the risk of hypertension when compared with oxytocin. We downgraded this evidence due to multiple concerns about risk of bias in the indirect comparison that contributed most weight to the network estimate.
Low‐certainty evidence suggested misoprostol, and misoprostol plus oxytocin increased the likelihood of fever compared with oxytocin. In both cases, we downgraded the evidence due to concerns about risk of bias and statistical heterogeneity in the direct comparisons that contributed most weight to the network estimates.
This network meta‐analysis provides the relative effectiveness of all agents used for the prevention of PPH in a coherent and methodologically robust way across important clinical outcomes by combining both direct and indirect evidence, thus increasing the statistical power and confidence in the results. We found that most of the included trials reported our primary outcomes and many of the secondary outcomes. This increased the power across most of our analyses and contributed to the consistency in the ranking across all blood loss outcomes. We were thorough in our evaluation of the important potential treatment effect modifiers (mode of birth, prior risk of PPH, healthcare setting, dose, route and regimen of the agents). We did not encounter major differences in the distribution of the effect modifiers between the different comparisons. In addition, the ranking of the agents in each of the subgroups was comparable with the overall ranking. The results of the network meta‐analyses were mostly consistent and where there was significant inconsistency, it was likely due to unstable estimates from single trials.
Many trials excluded women with significant co‐morbidities and who were at very high risk for PPH. Other factors that may have altered the risk of PPH include anaemia. We did not stratify the results by the presence of antenatal anaemia.
Most of the trials were carried out in the hospital setting and with women having a vaginal birth. For women having a vaginal birth, uterotonic agent administration was a component of the active management of the third stage of labour, along with controlled cord traction and early cord clamping. The most up‐to‐date guidelines from the WHO place emphasis on the administration of a uterotonic agent as the main aspect within this package for prevention of PPH [5]. These guidelines state that early cord clamping is generally not advised, whilst controlled cord traction is an option if skilled birth attendants are present [5]. Uterine tone plays a major role in PPH at caesarean section, with a relative reduction of PPH ≥ 500 mL similar to the reduction seen in women undergoing vaginal births when more effective agents are used. The ranking is relevant to women at either high or low risk for PPH in hospital settings. There were not enough trials to be able to recommend a ranking in community settings, even though a similar ranking in terms of effectiveness can be expected.
The dosages, regimens and routes of administration for many of the uterotonic agents varied. In most of the trials that investigated carbetocin, it was administered as a single intravenous bolus of 100 μg or intramuscularly. The combination of ergometrine plus oxytocin was usually administered intramuscularly combining 500 μg of ergometrine plus 5 IU (international units) of oxytocin. Misoprostol plus oxytocin combinations varied greatly, with some trials administering an intravenous infusion of 20 IU of oxytocin and 400 μg of misoprostol sublingually, or 200 μg of misoprostol sublingually, others administering an intravenous bolus of oxytocin of 10 IU plus 400 μg misoprostol sublingually, while others administered an intravenous infusion of 10 IU of oxytocin and 400 μg of misoprostol rectally. There were also several other ways of administering the misoprostol plus oxytocin combination described (see Supplementary material 2). Global guidelines, which standardise the doses, regimens and routes of uterotonic agents and combination agents, may allow for more meaningful comparisons.
Limitations of the review processes
Some authors have been involved in one or more previous or ongoing trials related to the use of uterotonics for the prevention of PPH that could be eligible for inclusion in this review. They did not participate in any decisions regarding these trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction) for the purposes of this review – these tasks were carried out by other members of the team who were not directly involved in the trials.
We acknowledge that using the CPC‐TST to screen eligible trials for trustworthiness required adjustments for historical changes in methodological and reporting expectations. Inevitably, the criteria for older trials were less stringent, particularly with respect to requirements such as prospective trial registration. We tried to minimise bias in this process by using the tool developed by Cochrane and applied it in a manner similar to previous Cochrane reviews [28].
A team of review authors assessed the certainty of the evidence using judgements as per the GRADE approach. One review author made the initial assessments, which were then re‐assessed independently by another review author, in consultation with other authors where additional decision‐making was required.
Over the years, objective methods of measuring blood loss have become increasingly available as the evidence for the poor accuracy of subjective visual estimation has been highlighted [255]. This could have led to apparent changes in reported blood loss. These temporal changes could have contributed to heterogeneity and increased the uncertainty of findings.
The trials included in the review recruited women with varied clinical characteristics, and it is important to consider this when interpreting results. The inclusion criteria were not always reported in detail and, when they were, they varied across trials.
Further heterogeneity may also be present in the overall analysis related to the dose, route or regimen of the uterotonic agents. Even though we did not observe subgroup effects when we examined the dose of misoprostol or regimen of oxytocin administration, we were not able to perform subgroup analyses for every single increment in dosage or route of administration.
Not all trials reported data on side effects, hence these analyses were often underpowered. There was also limited data on important secondary outcomes such as intensive care unit admission, maternal shock, and maternal satisfaction and sense of well‐being.
Agreements and disagreements with other studies or reviews
In this latest update of the review first published in April 2018, we have screened previously included trials for trustworthiness concerns as well as new trials identified as eligible for inclusion. The results for the primary outcome of PPH ≥ 500 mL now suggest that the combination agent, ergometrine plus oxytocin, is more effective than oxytocin alone, and misoprostol plus oxytocin is probably more effective than oxytocin. In contrast to what the last update found [26], carbetocin has an effect similar to that of oxytocin.
Our results generally agree with other Cochrane reviews that focus on the comparison of one uterotonic agent versus another (direct comparisons) [8, 15, 21, 22, 23]. However, this network meta‐analysis includes more trials than these previous reviews as it compares all available uterotonic agents in one single analysis, and so some estimates differ.
Authors' conclusions
Implications for practice
The current World Health Organization (WHO) recommendation on the choice of uterotonics for preventing postpartum haemorrhage (PPH) is 10 IU of intramuscular or intravenous oxytocin [5]. We found that oxytocin has positive effects compared with placebo or no treatment and minor side effects. A limitation of oxytocin is that it needs to be refrigerated (2 °C to 8 °C) to maintain its potency. Several trials have shown that oxytocin loses potency if stored at room temperature or higher temperatures for long periods, making its use difficult in low‐resource settings [256, 257].
Our review update found that ergometrine plus oxytocin (Syntometrine), and misoprostol plus oxytocin combination have additional desirable effects compared with oxytocin, whereas carbetocin, misoprostol, injectable prostaglandins and ergometrine have no additional benefits compared with oxytocin. Apart from carbetocin, all these uterotonic agents have some undesirable effects as they increase the likelihood of some side effects compared with oxytocin.
While the combination of ergometrine plus oxytocin may be more effective than oxytocin alone for some desirable outcomes, this combination may also increase some side effects. Caution should be exercised when using ergot derivatives for PPH prevention in unscreened populations, as these drugs are contraindicated in women with underlying hypertensive or cardiovascular disorders.
Although the combination of misoprostol plus oxytocin may be more effective than oxytocin for some desirable outcomes, this combination also increases some side effects such as nausea, vomiting, fever and diarrhoea. Misoprostol plus oxytocin is not available as a fixed drug combination like Syntometrine. The lack of a combination preparation and the requirement for different administration routes (parenteral and oral/rectal) for the two components make its clinical use more challenging, particularly in low‐resource settings where there may be only one birth attendant present.
There is evidence that carbetocin is as effective as oxytocin for some desirable outcomes and has a comparable side‐effect profile. However, carbetocin is more expensive and currently not widely available. A room‐temperature‐stable formulation of carbetocin is available, which could make it an attractive option for settings where maintaining the cold chain for storage and transport of oxytocin and oxytocin‐containing combination treatments is problematic, provided cost limitations can be addressed. WHO guidelines for the prevention of PPH suggest that carbetocin can be used where its cost is comparable to that of other effective uterotonics [258].
Policymakers would need to balance the desirable and undesirable effects of the uterotonics presented, considering available resources. Consideration needs to be given to cost‐effectiveness, impacts on health equity, acceptability to key stakeholders and feasibility of using these agents in routine clinical practice.
Implications for research
There is still uncertainty around the optimal doses and routes of administration of each of the uterotonic agents. For oxytocin, for example, there are uncertainties around the optimal dose at caesarean section, whether it should be administered intravenously or intramuscularly, and whether it should be administered as an intravenous bolus or infusion. Our review analyses the effectiveness and side effects of the various agents at an aggregate level, grouping together all doses and routes of the different agents. The current network meta‐analysis excludes trials that compared different doses or routes of the same agent, so we are unable to present conclusions on a preferred dose or route of administration for any of the agents. Future updates of the network meta‐analysis should consider adding evidence from all trials that compare the various doses and routes of administration of the agents. This approach may elucidate preferred doses and routes for each of the agents, and identify research gaps in the evidence base.
Previous consultation with our consumer group highlighted the need for more research into outcomes identified as priorities for women and their families, such as women’s views regarding the agents used, severe maternal morbidity such as shock, and breastfeeding at discharge. Data on these outcomes remain limited and very few new data were found by this review update. Consumers also considered the side effects of uterotonic agents to be important, but these remain under‐reported.
Supporting Information
Supplementary materials are available with the online version of this article: 10.1002/14651858.CD011689.pub3.
Supplementary materials are published alongside the article and contain additional data and information that support or enhance the article. Supplementary materials may not be subject to the same editorial scrutiny as the content of the article and Cochrane has not copyedited, typeset or proofread these materials. The material in these sections has been supplied by the author(s) for publication under a Licence for Publication and the author(s) are solely responsible for the material. Cochrane accordingly gives no representations or warranties of any kind in relation to, and accepts no liability for any reliance on or use of, such material.
Supplementary material 1 Search strategies
Supplementary material 2 Characteristics of included studies
Supplementary material 3 Characteristics of excluded studies
Supplementary material 4 Characteristics of studies awaiting classification
Supplementary material 5 Characteristics of ongoing studies
Supplementary material 6 Analyses
Supplementary material 7 Data package
Supplementary material 8 Supplementary Appendix
These authors should be considered joint first author
New search for studies and content updated (conclusions changed)
Additional information
Acknowledgements
The first published version of this review [259], was supported by Ammalife (www.ammalife.org), and was undertaken as part of independent research funded by the UK National Institute for Health Research (Health Technology Assessment Project 14/139/17 Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis). The views expressed in this publication are those of the review authors and not necessarily those of the National Health Service (NHS), the National Institute for Health Research or the Department of Health.
This work was supported by UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a cosponsored programme executed by the WHO (Award No. HQHRP2220228‐22.1‐74309).
The World Health Organization (WHO) and Ioannis D Gallos, Idnan Yunas, Adam Devall, Marcelina Podesek, Aurelio Tobias, Malcolm J Price, Olufemi T Oladapo and Arri Coomarasamy retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication.
We are grateful to Jo Weeks for her advice on using the CPC‐TST. We would also like to thank Jennifer Harrison for her feedback on the plain language summary (consumer involvement).
Some authors involved in previous published versions of this review in 2018 are no longer included on the author byline: Argyro Papadopoulou, Rebecca Man, Nikolaos Athanasopoulos, Myfanwy J Williams, Virginia Diaz, Julia Pasquale, Monica Chamillard, Mariana Widmer, Özge Tunçalp, Justus Hofmeyr, Fernando Althabe, Ahmet Metin Gülmezoglu, Joshua P Vogel. Some of the content retained in this review reflects their contributions.
Editorial and peer‐reviewer contributions
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Philippa Middleton, Women and Kids, South Australian Health and Medical Research Institute;
Managing Editor (selected peer reviewers, provided editorial guidance to authors, edited the article): Sam Hinsley, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks, collated peer‐reviewer comments and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy editing and production): Denise Mitchell, Cochrane Central Production Service;
Peer‐reviewers (provided comments and recommended an editorial decision): Rachel Richardson, Cochrane Methods Support Unit (methods), Jo Platt, Central Editorial Information Specialist (search), Maria Fernanda Escobar Vidarte ‐ Global Health Equity Unit in Fundacion Valle del Lili Cali – Colombia (clinical), Prof SR Fawcus, Dept OBS/GYN, University Cape Town (clinical), and Thalía Alejandra Vargas Buitrago, Enfermera en formación de la Univerdad Nacional de Colombia (consumer).
Contributions of authors
Idnan Yunas (IY) and Ioannis D Gallo (IDG) drafted the manuscript. IDG and Arri Coomarasamy (AC) conceived the idea for this trial. IDG drafted the protocol. IY, Adam Devall (AD), Marcelina Podesek (MP) and IDG screened trials and extracted data. IY, AD, MP and IDG assessed trials for trustworthiness. MJP (Malcolm Price) and AT (Aurelio Tobias) provided statistical advice and input. IY, AD and MP graded the evidence and created the summary of findings tables. All authors, including Olufemi Oladapo (OO), edited and revised the review.
Declarations of interest
Ioannis D Gallos (IDG): was a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis. He has been involved in one or more previous or ongoing trials related to the use of uterotonics for the prevention of PPH that were eligible for inclusion in this review. Ferring Pharmaceuticals and Novartis supplied carbetocin and oxytocin for these trials. IDG did not participate in any decisions regarding these trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction) for the purposes of this review (and will not for future updates) – these tasks were carried out by other members of the team who were not directly involved in the trials.
Idnan Yunas (IY): none known
Adam Devall (AD): none known
Marecelina Podesek (MP): none known
Malcolm J Price (MJP): was a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis.
Aurelio Tobias: none known
Olufemi T Oladapo (OTO): led the updating of WHO recommendations on uterotonics for the prevention of postpartum haemorrhage based on the findings of this review update.
Arri Coomarasamy (AC): was the Chief Investigator of UK National Institute for Health Research HTA Project Award 14/139/17 entitled Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis. He has been involved in one or more previous or ongoing trials related to the use of uterotonics for the prevention of PPH that were eligible for inclusion in this review. Ferring Pharmaceuticals and Novartis supplied carbetocin and oxytocin for these trials and another trial is supported by WHO/Merck for Mothers. AC did not participate in any decisions regarding these trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction) for the purposes of this review or future updates – these tasks have been carried out by other members of the team who were not directly involved in the trials. AC is a member of the Executive Board of Ammalife (UK registered charity 1120236). He does not receive any payment for this relationship.
Sources of support
Internal sources
-
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Switzerland
This work was supported by UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP),
a cosponsored programme executed by the WHO (Award No. HQHRP2220228‐22.1‐74309).
-
World Health Organization, Switzerland
The authors of this review are employed by the institutions indicated by their respective affiliations except where otherwise stated.
-
University of Birmingham, UK
The authors of this review are employed by the institutions indicated by their respective affiliations except where otherwise stated.
External sources
-
National Institute for Health Research, UK
This review was initially undertaken as part of independent research funded by the UK National Institute for Health Research (Health Technology Assessment Project 14/139/17 Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis).
Registration and protocol
Protocol (2015) DOI: 10.1002/14651858.CD011689 Original Review (2018) DOI: 10.1002/14651858.CD011689.pub2 Review Update (2018) DOI: 10.1002/14651858.CD011689.pub3
Data, code and other materials
As part of the published Cochrane review, the following is made available for download for users of the Cochrane Library: the data extracted for this review, along with the associated analysis code and supplementary materials, are available in accordance with the Cochrane Data Sharing Policy Supplementary material 7.
What's new
| Date | Event | Description |
|---|---|---|
| 16 April 2025 | New search has been performed | This is an update of the last review update undertaken in 2018. The major change is the introduction of trustworthiness screening of originally included studies and new studies identified as eligible for inclusion. After screening and trustworthiness screening we included a total of 122 trials (121,931 women). All agents were generally effective for preventing PPH ( ≥ 500 mL) when compared with placebo or no treatment. Ergometrine plus oxytocin, and misoprostol plus oxytocin may be more effective than the current standard oxytocin. All regimens, except for carbetocin, are associated with an increased risk of some side‐effects compared with oxytocin. |
| 16 April 2025 | New citation required and conclusions have changed | After screening and trustworthiness screening we included a total of 122 trials (121,931 women). All agents were generally effective for preventing PPH ( ≥ 500 mL) when compared with placebo or no treatment. Ergometrine plus oxytocin, and misoprostol plus oxytocin may be more effective than the current standard oxytocin. All regimens, except for carbetocin, are associated with an increased risk of some side‐effects compared with oxytocin. |
History
Protocol first published: Issue 5, 2015 Review first published: Issue 4, 2018
| Date | Event | Description |
|---|---|---|
| 24 May 2018 | New citation required but conclusions have not changed | With the addition of 56 new trials (46,612 women), the update now includes a total of 196 trials (135,559 women). The conclusions remain largely the same. The results for the primary outcome of postpartum haemorrhage (PPH) ≥ 500 mL were similar to the previously published review [259], although the quality of the evidence for carbetocin has changed from ‘very low‐certainly’ to ‘moderate‐certainty evidence’ for this outcome, due to the addition of data from three studies including approximately 30,000 women. For the primary outcome of PPH ≥ 1000 mL, none of the agents is significantly more effective when compared with the reference uterotonic agent oxytocin. In the previous version of the review, high‐quality evidence suggested that ergometrine plus oxytocin was more effective in reducing PPH ≥ 1000 mL in comparison to oxytocin. For all other outcomes (blood transfusion; additional uterotonics; and side effects), the results are largely the same. |
| 24 May 2018 | New search has been performed | Search updated. We have included 56 new trials in this update involving 46,612 women. We have updated the methods ‐ all changes are summarised in detail in 'Differences between protocol and review'. Six authors have stepped down from the team and 10 new authors have joined the review team. |
References
- 1.Say L, Chou D, Gemmill A, Tunçalp O, Moller A, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health 2014;2(6):e323-e333. [DOI] [PubMed] [Google Scholar]
- 2.Cresswell J. Trends in Maternal Mortality 2000 to 2020 : Estimates by WHO, UNICEF, UNFPA, World Bank Group and UNDESA/Population Division. WHO, 2023. [Google Scholar]
- 3.Penney G, Brace V. Near miss audit in obstetrics. Current Opinion in Obstetrics & Gynecology 2007;19:145-50. [DOI] [PubMed] [Google Scholar]
- 4.Souza JP, Gülmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. Lancet 2013;381:1747-55. [DOI] [PubMed] [Google Scholar]
- 5.WHO. WHO Recommendations: Uterotonics for the Prevention of Postpartum Haemorrhage. Geneva: Department of Reproductive Health, World Health Organization, 2018. [PubMed] [Google Scholar]
- 6.Yunas I, Islam MA, Sindhu KN, Devall AJ, Podesek M, Alam SS, et al. Causes of and risk factors for postpartum haemorrhage: a systematic review and meta-analysis. The Lancet 2025;Published online April 3. https://doi.org/10.1016/S0140-6736(25)00448-9. Available at: https://www.sciencedirect.com/science/article/pii/S0140673625004489 [accessed on 3rd April 2025]. [DOI: 10.1016/S0140-6736(25)00448-9] [DOI] [PubMed]
- 7.Combs CA, Murphy EL, Laros RK Jr. Factors associated with postpartum hemorrhage with vaginal birth. Obstetrics and Gynecology 1991;77:69-76. [PubMed] [Google Scholar]
- 8.Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD007412. [DOI: 10.1002/14651858.CD007412.pub4] [DOI] [PubMed] [Google Scholar]
- 9.Medicinesorguk. Syntocinon Ampoules 5 IU/ml - Summary of Product Characteristics (SPC) - (eMC). Available from: https://www.medicines.org.uk/emc/product/9736/smpc (last updated on emc: 25 Oct 2023, cited 8 February 2024).
- 10.Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing caesarean section. British Journal of Anaesthesia 2007;98(1):116-9. [DOI] [PubMed] [Google Scholar]
- 11.Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of intravenous bolus or infusion of oxytocin in women undergoing caesarean section [abstract]. International Journal of Obstetric Anesthesia 2006;15 Suppl 1:S13. [Google Scholar]
- 12.ISRCTN07452238. A study to determine the cardiovascular effects of different methods of administering the oxytocic drug syntocinon. isrctn.com/ISRCTN07452238 (first posted: 12 September 2003).
- 13.Groot AN, Dongen PW, Vree TB, Hekster YA, Roosmalen J. Ergot alkaloids. Current status and review of clinical pharmacology and therapeutic use compared with other oxytocics in obstetrics and gynaecology. Drugs 1998;56:523-35. [DOI] [PubMed] [Google Scholar]
- 14.Groot AN. The role of oral (methyl) ergometrine in the prevention of postpartum haemorrhage. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1996;69:31-6. [DOI] [PubMed] [Google Scholar]
- 15.Tuncalp O, Hofmeyr GJ, Gulmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD000494. [DOI: 10.1002/14651858.CD000494.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies NM, Longstreth J, Jamali F. Misoprostol therapeutics revisited. Pharmacotherapy 2001;21:60-73. [DOI] [PubMed] [Google Scholar]
- 17.Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception 2005;71(1):22-5. [DOI] [PubMed] [Google Scholar]
- 18.Hunter DJ, Schulz P, Wassenaar W. Effect of carbetocin, a long-acting oxytocin analog on the postpartum uterus. Clinical Pharmacology and Therapeutics 1992;52:60-7. [DOI] [PubMed] [Google Scholar]
- 19.Widmer M, Piaggio G, Nguyen TM, Osoti A, Owa OO, Misra S, et al. Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. New England Journal of Medicine 2018;379(8):743-52. [DOI] [PubMed] [Google Scholar]
- 20.Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No: CD005457. [DOI: 10.1002/14651858.CD005457.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam QM. Prophylactic use of ergot alkaloids in the third stage of labour. Cochrane Database of Systematic Reviews 2018, Issue 6. Art. No: CD005456. [DOI: 10.1002/14651858.CD005456.pub2] [DOI] [PubMed] [Google Scholar]
- 22.McDonald SJ, Abbott JM, Higgins SP. Prophylactic ergometrine-oxytocin versus oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No: CD000201. [DOI: 10.1002/14651858.CD000201.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD001808. [DOI: 10.1002/14651858.CD001808.pub2] [DOI] [PubMed] [Google Scholar]
- 24.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumley T. Network meta-analysis for indirect treatment comparisons. Statistics in Medicine 2002;21(16):2313-24. [DOI] [PubMed] [Google Scholar]
- 26.Gallos ID, Papadopoulou A, Man R, Athanasopoulos N, Tobias A, Price MJ, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis [Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis]. Cochrane Database of Systematic Reviews 2018;12(12):CD011689. [DOI: 10.1002/14651858.CD011689.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alfirevic Z, Kellie F, Weeks J, Stewart F, Jones J, Hampson L. Identifying and handling potentially untrustworthy trials - Trustworthiness Screening Tool (TST) developed by the Cochrane Pregnancy and Childbirth Group, version 3.0. Cochrane Evidence Synthesis and Methods 2023.
- 28.Weeks J, Cuthbert A, Alfirevic Z. Trustworthiness assessment as an inclusion criterion for systematic reviews - what is the impact on results? Cochrane Evidence Synthesis and Methods 2023;1(10):e12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallos ID, Williams HM, Price MJ, Merriel A, Gee H, Lissauer D, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD011689. [DOI: 10.1002/14651858.CD011689] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Mark Petticrew M, et al, PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews 2015;4:1. [DOI: 10.1186/2046-4053-4-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The retraction watch database. https://retractiondatabase.org version: 1.0.8.0, ISSN 2692-465X.
- 33.Microsoft Excel. Version 2405. Microsoft Corporation, 2024. Available from https://office.microsoft.com/excel.
- 34.Stata. Version 18. College Station, TX, USA: StataCorp, 2023. Available from https://www.stata.com.
- 35.Review Manager (RevMan). Version 8.1.1. The Cochrane Collaboration, 2024. Available at revman.cochrane.org.
- 36.Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from https://training.cochrane.org/handbook/archive/v5.1/.
- 37.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Medical Decision Making 2013;33(5):607-17. [DOI] [PMC free article] [PubMed]
- 38.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163-71. [DOI] [PubMed] [Google Scholar]
- 39.White I. Multivariate random-effects meta-regression: updates to mvmeta. Stata Journal 2011;11(2):255-70. [Google Scholar]
- 40.Stanton CK, Newton S, Mullany LC, Cofie P, Tawiah Agyemang C, Adiibokah E, et al. Effect on postpartum hemorrhage of prophylactic oxytocin (10 IU) by injection by community health officers in Ghana: a community-based, cluster-randomized trial. PLoS Medicine 2013;10(10):e1001524. [DOI] [PMC free article] [PubMed]
- 41.NCT01108302. Effectiveness, safety and feasibility of auxiliary nurse midwives' (ANM) use of oxytocin in uniject™ to prevent postpartum hemorrhage in India. https://clinicaltrials.gov/ct2/show/NCT01108302 (first received: 2 April 2010).
- 42.Stanton CK, Newton S, Mullany LC, Cofie P, Agyemang CT, Adiibokah E, et al. Impact on postpartum hemorrhage of prophylactic administration of oxytocin 10 IU via Uniject by peripheral health care providers at home births: design of a community-based cluster-randomized trial. BMC Pregnancy and Childbirth 2012;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higgins JPT, Eldridge S, Li T. Chapter 23: Including variants on randomized trials [last updated October 2019]. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.5. Cochrane, 2024. Available from https://www.training.cochrane.org/handbook.
- 44.Chandhiok N, Dhillon BS, Datey S, Mathur A, Saxena NC. Oral misoprostol for prevention of postpartum hemorrhage by paramedical workers in India. International Journal of Gynaecology and Obstetrics 2006;92(2):170-5. [DOI] [PubMed] [Google Scholar]
- 45.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
- 46.White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network metaanalysis: model estimation using multivariate meta-regression. Research Synthesis Methods 2012;3(2):111-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White IR. Network meta-analysis. Stata Journal 2015;15(4):951-85. [Google Scholar]
- 48.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI] [PubMed] [Google Scholar]
- 49.Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency innetwork meta-analysis: Concepts and models for multi-arm studies. Research Synthesis Methods 2012;3(2):98-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. Journal of Clinical Epidemiology 2018;93:36-44. [DOI] [PubMed] [Google Scholar]
- 52.Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Thornton JG, Li W, Wely M, Mol BW. Concerns about data integrity of 22 randomized controlled trials in women's health. American Journal of Perinatology 2023;40(3):279-89. [DOI: 10.1055/s-0041-1727280] [DOI] [PubMed] [Google Scholar]
- 54.Abdel-Aleem H, Abol-Oyoun EM, Moustafa SA, Kamel HS, Abdel-Wahab HA. Carboprost trometamol in the management of the third stage of labor. International Journal of Gynaecology and Obstetrics 1993;42:247-50. [DOI: 10.1016/0020-7292(93)90219-m] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 55.Abdel-Aleem H, Singata M, Abdel-Aleem M, Mshweshwe N, Williams X, Hofmeyr GJ. Uterine massage to reduce postpartum hemorrhage after vaginal delivery. International Journal of Gynaecology and Obstetrics 2010;111(1):32-6. [DOI: 10.1016/j.ijgo.2010.04.036] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 56.ACTRN12609000372280. Uterine massage to reduce postpartum hemorrhage in women expected to deliver normally: A randomised trial. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609000372280 (first posted: 27 May 2009).
- 57.Acharya G, Al-Sammarai MT, Patel N, Al-Habib A, Kiserud T. A randomized, controlled trial comparing effect of oral misoprostol and intravenous syntocinon on intra-operative blood loss during cesarean section. Acta Obstetricia et Gynecologica Scandinavica 2001;80(3):245-50. [DOI: 10.1034/j.1600-0412.2001.080003245.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 58.Amant F, Spitz B, Timmerman D, Corresmans A, Van Assche FA. Misoprostol compared with methylergometrine for the prevention of postpartum haemorrhage: a double-blind randomised trial. British Journal of Obstetrics and Gynaecology 1999;106:1066-70. [DOI] [PubMed] [Google Scholar]
- 59.Amornpetchakul P, Lertbunnaphong T, Boriboonhiransarn D, Leetheeragul J, Sirisomboon R, Jiraprasertwong R. Intravenous carbetocin versus intravenous oxytocin for preventing atonic postpartum hemorrhage after normal vaginal delivery in high-risk singleton pregnancies: a triple-blind randomized controlled trial. Archives of Gynecology and Obstetrics 2018;298(2):319-27. [DOI: 10.1007/s00404-018-4806-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 60.Amornpetchakul P, Lertbunnaphong T, Boriboonhiransarn D, Leetheeragul J, Sirisomboon R, Jiraprasertwong R. Efficacy of intravenous 100 mcg carbetocin versus intravenous 5 units oxytocin for prevention of immediate postpartum hemorrhage after normal vaginal delivery among high risk pregnancies; a triple-blinded randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2017;43:24-5. [Google Scholar]
- 61.TCTR20160715004. Efficacy of intravenous 100 mcg carbetocin versus intravenous 5 units oxytocin for prevention of immediate postpartum hemorrhage after normal vagina delivery among high risk pregnancies; a triple-blinded randomized controlled trial. https://www.thaiclinicaltrials.org/# (first posted 15 July 2016).
- 62.Askar AA, Ismail MT, El-Ezz AA, Rabie NH. Carbetocin versus Syntometrine in the management of third stage of labor following vaginal delivery. Archives of Gynecology and Obstetrics 2011;284(6):1359-65. [DOI] [PubMed] [Google Scholar]
- 63.Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, et al. Carbetocin versus oxytocin for the prevention of postpartum haemorrhage following caesarean section: the results of a double-blind randomised trial. BJOG 2010;117(8):929-36. [DOI] [PubMed] [Google Scholar]
- 64.Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, et al. The haemodynamic effects of oxytocin and carbetocin following caesarean section: the results of a double-blind randomised study. BJOG 2008;115(s1):140-1. [DOI] [PubMed] [Google Scholar]
- 65.Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, et al. Can a new oxytocin analogue reduce the need for additional oxytocics after caesarean section? The results of a double-blind randomised trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93 Suppl 1:Fa51. [Google Scholar]
- 66.EUCTR2005-002812-94-GB. Randomised trial of carbetocin versus oxytocin for the prevention of postpartum haemorrhage after caesarean section - LOXY Trial. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2005-002812-94 (first posted: 2005). [CENTRAL: CN-01880247] 10779996
- 67.Attilakos G, Psaroudakis D, Ash J, Buchanen R, Winter C, Draycott T. Low recruitment rate for a drug trial in obstetrics: an effect of the publicity following the TGN1412 clinical trial at the PAREXEL Research Unit in Northwick Park Hospital? [Abstract]. In: 31st British International Congress of Obstetrics and Gynaecology; 2007 July 4-6; London, UK. 2007:110.
- 68.Atukunda EC, Siedner MJ, Obua C, Mugyenyi GR, Twagirumukiza M, Agaba AG. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in Uganda: a double-blind randomized non-inferiority trial. PLoS Medicine 2014;11(11):e1001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atukunda EC, Siedner MJ, Obua C, Mugyenyi GR, Twagirumukiza M, Agaba AG. Sublingual misoprostol versus intramuscular oxytocin for prevention of post-partum haemorrhage in Uganda: a randomised, controlled, non-inferiority trial. Lancet 2014;384(Suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balki M, Dhumne S, Kasodekar S, Kingdom J, Windrim R, Carvalho JC. Oxytocin-ergometrine co-administration does not reduce blood loss at caesarean delivery for labour arrest. BJOG 2008;115(5):579-84. [DOI: 10.1111/j.1471-0528.2007.01658.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 71.NCT00481533. Ergot and oxytocin during cesarean delivery following failure to progress in labour. https://clinicaltrials.gov/ct2/show/NCT00481533 (first posted: 1 June 2007).
- 72.Balki M, Downey K, Walker A, Seaward G, Carvalho JC. Prophylactic administration of uterotonics to prevent postpartum hemorrhage in women undergoing cesarean delivery for arrest of labor: a randomized controlled trial. Obstetrics and Gynecology 2021;137(3):505-13. [DOI: 10.1097/AOG.0000000000004288] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 73.NCT01869556. Comparison of IV ergonovine with IM carboprost, with oxytocin IV, during cesarean section for failure to progress. https://clinicaltrials.gov/ct2/show/NCT01869556 (first posted: 5 June 2013).
- 74.Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum hemorrhage: a placebo-controlled trial. American Journal of Obstetrics and Gynecology 1998;179(4):1043-6. [DOI] [PubMed] [Google Scholar]
- 75.Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum haemorrhage: a placebo controlled trial. In: 17th Conference on Priorities in Perinatal Care; 1998; South Africa. 1998:49-52. [DOI] [PubMed]
- 76.Bamigboye AA, Merrell DA, Hofmeyr GJ, Mitchell R. Randomized comparison of rectal misoprostol with Syntometrine for management of third stage of labor. Acta Obstetricia et Gynecologica Scandinavica 1998;77:178-81. [PubMed] [Google Scholar]
- 77.Baskett TF, Persad VL, Clough HJ, Young DC. Misoprostol versus oxytocin for the reduction of postpartum blood loss. International Journal of Gynaecology and Obstetrics 2007;97(1):2-5. [DOI] [PubMed] [Google Scholar]
- 78.Baskett TF, Persad V, Clough H, Young D. Prophylactic use of misoprostol in the third stage of labor [abstract]. Obstetrics and Gynecology 2005;105(4 Suppl):39S. [Google Scholar]
- 79.DJ Rouse. Misoprostol versus oxytocin for the reduction of postpartum blood loss. Commentary. Obstetrical & Gynecological Survey 2007;62(9):563?564. [Google Scholar]
- 80.Chandra S, Persad V, Young D, Baskett T. A preliminary study of cutaneous blood flow associated with postpartum use of oral misoprostol. Journal of Obstetrics & Gynaecology Canada: JOGC 2004;26(12):1073-6. [DOI] [PubMed] [Google Scholar]
- 81.Begley CM. A comparison of 'active' and 'physiological' management of the third stage of labour. Midwifery 1990;6:3-17. [DOI] [PubMed] [Google Scholar]
- 82.Begley CM. Comparative Studies in the Third Stage of Labour [MSc Thesis]. Dublin: Trinity College, University of Dublin, 1990. [Google Scholar]
- 83.Begley CM. The effect of ergometrine on breast feeding. Midwifery 1990;6:60-72. [DOI] [PubMed] [Google Scholar]
- 84.Bellad MB, Tara D, Ganachari MS, Mallapur MD, Goudar SS, Kodkany BS, et al. Prevention of postpartum haemorrhage with sublingual misoprostol or oxytocin: a double-blind randomised controlled trial. BJOG 2012;119(8):975-86. [DOI] [PubMed] [Google Scholar]
- 85.Bellad M, Ganachari TD, Mallapur M. Sublingual (SL) powdered misoprostol (400 mcg) vs IM oxytocin (10 IU) for prevention of postpartum blood loss - a randomized controlled trial. International Journal of Gynaecology and Obstetrics 2009;107(Suppl 2):S124-5. [Google Scholar]
- 86.Winikoff B, Durocher J. Postpartum bleeding is reduced with sublingual powdered misoprostol when compared with oxytocin injection, but a new formulation of misoprostol is unlikely to revolutionise postpartum haemorrhage care. Evidence-based Medicine 2013;18(4):143-4. [DOI: 10.1136/eb-2012-100966] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 87.Benchimol M, Gondry J, Mention JE, Gagneur O, Boulanger JC. Role of misoprostol in the delivery outcome [Place du misoprostol dans la direction de la delivrance]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2001;30(6):576-83. [PubMed] [Google Scholar]
- 88.Bhullar A, Carlan SJ, Hamm J, Lamberty N, White L, Richichi K. Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstetrics and Gynecology 2004;104(6):1282-8. [DOI: 10.1097/01.AOG.0000144119.94565] [DOI] [PubMed] [Google Scholar]
- 89.Boucher M, Horbay GL, Griffin P, Deschamps Y, Desjardins C, Schulz M, et al. Double-blind, randomized comparison of the effect of carbetocin and oxytocin on intraoperative blood loss and uterine tone of patients undergoing cesarean section. Journal of Perinatology 1998;18(3):202-7. [PubMed] [Google Scholar]
- 90.Boucher M, Nimrod CA, Tawagi GF, Meeker TA, Rennicks White RE, Varin J. Comparison of carbetocin and oxytocin for the prevention of postpartum hemorrhage following vaginal delivery:a double-blind randomized trial. Journal of Obstetrics & Gynaecology Canada: JOGC 2004;26(5):481-8. [DOI] [PubMed] [Google Scholar]
- 91.Boucher M, Nimrod C, Tawagi G. Carbetocin IM injection vs oxytocin IV infusion for prevention of postpartum hemorrhage in women at risk following vaginal delivery. American Journal of Obstetrics and Gynecology 2001;185(6 Pt 2):Abstract no: 494. [Google Scholar]
- 92.Bugalho A, Daniel A, Foundes A, Cunha M. Misoprostol for the prevention of postpartum hemorrhage. International Journal of Gynaecology and Obstetrics 2001;73:1-6. [DOI] [PubMed] [Google Scholar]
- 93.Butwick AJ, Coleman L, Cohen SE, Riley ET, Carvalho B. Minimum effective bolus dose of oxytocin during elective caesarean delivery. British Journal of Anaesthesia 2010;104(3):338-43. [DOI] [PubMed] [Google Scholar]
- 94.Butwick AJ, Coleman L, Cohen SE, Riley ET, Carvalho B. A study of the minimum effective dose of oxytocin in patients undergoing elective cesarean delivery. In: American Society of Anaesthesiologists Annual Meeting; 2009 Oct 17-21; New Orleans, USA. 2009.
- 95.Caliskan E, Meydanli M, Dilbaz B, Aykan B, Sonmezer M, Haberal A. Is rectal misoprostol really effective in the treatment of third stage of labor? A randomized controlled trial. American Journal of Obstetrics and Gynecology 2002;187:1038-45. [DOI] [PubMed] [Google Scholar]
- 96.Caliskan E, Dilbaz B, Meydanli MM, Ozturk N, Narin MA, Haberal A. Oral misoprostol for the third stage of labor: a randomized controlled trial. Obstetrics and Gynecology 2003;101(5 Pt 1):921-8. [DOI] [PubMed] [Google Scholar]
- 97.Carbonell i Esteve JL, Hernandez JM, Piloto M, Setien SA, Texido CS, Tomasi G, et al. Active management of the third phase of labour plus 400 mug of sublingual misoprostol and 200 mug of rectal misoprostol versus active management only in the prevention of post-partum haemorrhage. A randomised clinical trial [Manejo activo de la tercera fase del parto mas 400 mug de misoprostol sublingual y 200 mug de misoprostol rectal frente a manejo activo solo en la prevencion de la hemorragia posparto. Ensayo clinico aleatorizado]. Progresos de Obstetricia y Ginecologia 2009;52(10):543-51. [Google Scholar]
- 98.Cayan F, Doruk A, Sungur MA, Dilek S. Comparison of the different dosages of rectal misoprostol on intestinal motility and pain score in high risk cesarean delivery. Turkiye Klinikleri Journal of Medical Sciences 2010;30(4):1154-9. [Google Scholar]
- 99.Chaudhuri P, Banerjee GB, Mandal A. Rectally administered misoprostol versus intravenous oxytocin infusion during cesarean delivery to reduce intraoperative and postoperative blood loss. International Journal of Gynaecology and Obstetrics 2010;109(1):25-9. [DOI] [PubMed] [Google Scholar]
- 100.CTRI/2009/091/000075. A randomised double- blind well controlled clinical trial to compare the efficacy and safety of rectally administered Misoprostol and Intravenous Oxytocin infusion in preventing intraoperative and postpartum blood loss in women undergoing caesarean section. https://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=347 (first posted: 6 April 2009). 15572964
- 101.Chaudhuri P, Biswas J, Mandal A. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in low-risk women. International Journal of Gynaecology and Obstetrics 2012;116(2):138-42. [DOI] [PubMed] [Google Scholar]
- 102.Chaudhuri P, Majumdar A. Sublingual misoprostol as an adjunct to oxytocin during cesarean delivery in women at risk of postpartum hemorrhage. International Journal of Gynaecology and Obstetrics 2015;128:48-52. [DOI] [PubMed] [Google Scholar]
- 103.CTRI/2013/05/003645. Misoprostol by sublingual route in addition to standard oxytocin injection to reduce blood loss during and after cesarean delivery in women prone to excessive blood loss [400 microgram misoprostol by sublingual route in addition to oxytocin to reduce intraoperative and post operative blood loss during cesarean delivery in high risk women]. https://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2013/05/003645 (first posted: 17 May 2013). [CENTRAL: CN-01882072] 10781819
- 104.Chaudhuri P, Majumdar A. A randomized trial of sublingual misoprostol to augment routine third-stage management among women at risk of postpartum hemorrhage. International Journal of Gynaecology and Obstetrics 2016;132:191-5. [DOI] [PubMed] [Google Scholar]
- 105.CTRI/2014/03/004491. Addition of 400 microgram misoprotol to routine management of third stage of labour in women at risk for bleeding after delivery [Efficacy and safety of 400 microgram misoprostol by sublingual route to augment routine management of third stage of labour in high risk women following vaginal delivery]. https://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2014/03/004491 (first received 2014). [CENTRAL: CN-01822763] 10722685
- 106.Chhabra S, Tickoo C. Low-dose sublingual misoprostol versus methylergometrine for active management of the third stage of labor. Journal of Obstetrics and Gynaecology Research 2008;34(5):820-3. [DOI] [PubMed] [Google Scholar]
- 107.Choy CM, Lau WC, Tam WH, Yuen PM. A randomised controlled trial of intramuscular Syntometrine and intravenous oxytocin in the management of the third stage of labour. BJOG 2002;109:173-7. [DOI] [PubMed] [Google Scholar]
- 108.Chua S, Chew SL, Yeoh CL, Roy AC, Ho LM, Selamat N, et al. A randomized controlled study of prostaglandin 15-Methyl F2 alpha compared with Syntometrine for prophylactic use in the third stage of labour. Australian & New Zealand Journal of Obstetrics & Gynaecology 1995;35:413-6. [DOI] [PubMed] [Google Scholar]
- 109.Cook CM, Spurrett B, Murray H. A randomized clinical trial comparing oral misoprostol with synthetic oxytocin or Syntometrine in the third stage of labour. Australian & New Zealand Journal of Obstetrics & Gynaecology 1999;39(4):414-9. [DOI] [PubMed] [Google Scholar]
- 110.Dansereau J, Joshi AK, Helewa ME, Doran TA, Lange IR, Luther ER, et al. Double blind comparison of carbetocin versus oxytocin in prevention of uterine atony after cesarean section. American Journal of Obstetrics and Gynecology 1999;18(3 Pt 1):670-6. [DOI] [PubMed] [Google Scholar]
- 111.Gambling DR, Dansereau J, Wassenaar W, Schulz M, Horbay GL. Double-blind randomized comparison of a single dose of carbetocin versus 8 hours oxytocin infusion after cesarean delivery: safety data. Anesthesia and Analgesia 1994;78 Suppl:S127. [Google Scholar]
- 112.Dansereau J, Gambling D, Joshi A, Helewa M, Doran T, Lange I, et al. Double-blind comparison of carbetocin vs oxytocin in preventing uterine atony post cesarean section. International Journal of Gynaecology and Obstetrics 1994;46 Suppl:77. [Google Scholar]
- 113.Dansereau J, Joshi AK, Helewa ME, Doran TA, Lange IR, Luther ER, et al. Double-blind comparison of carbetocin vs oxytocin in preventing uterine atony post cesarean section. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;69:37. [DOI] [PubMed] [Google Scholar]
- 114.Dansereau J. Comparison of carbetocin vs. oxytocin in prevention of uterine atony post cesarean section. Prenatal and Neonatal Medicine 1996;1 Suppl 1:80. [Google Scholar]
- 115.Gambling D, Dansereau J, Schulz M, Horbay GL, Waasenaar W. Double-blind, randomized comparison of a single dose of carbetocin vs 8 hours oxytocin infusion after cesarean delivery: safety data. A Canadian multi-center trial [abstract]. International Journal of Obstetric Anesthesia 1994;3:113-4. [Google Scholar]
- 116.Dasuki D, Emilia O, Harini S. Randomized clinical trial: the effectiveness of oral misoprostol versus oxytocin in prevention of postpartum hemorrhage [abstract]. Journal of Obstetrics and Gynaecology Research 2002;28(1):46. [Google Scholar]
- 117.Dawoud M, Al-Husseiny M, Helal O, Elsherbini M, Abdel-Rasheed M, Sediek M. Intravenous tranexamic acid vs. sublingual misoprostol in high-risk women for postpartum haemorrhage following cesarean delivery; a randomised clinical trial. BMC Pregnancy and Childbirth 2023;23(1):611. [DOI: 10.1186/s12884-023-05935-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Groot AN, Van Roosmalen J, Van Dongen PW, Borm GF. A placebo-controlled trial of oral ergometrine to reduce postpartum hemorrhage. Acta Obstetricia et Gynecologica Scandinavica 1996;75:464-8. [DOI] [PubMed] [Google Scholar]
- 119.Derman RJ, Kodkany BS, Goudar SS, Geller SE, Naik VA, Bellad MB, et al. Oral misoprostol in preventing postpartum haemorrhage in resource-poor communities: a randomised controlled trial. Lancet 2006;368(9543):1248-53. [DOI] [PubMed] [Google Scholar]
- 120.Geller SE, Goudar SS, Adams MG, Naik VA, Patel A, Bellad MB, et al. Factors associated with acute postpartum hemorrhage in low-risk women delivering in rural India. International Journal of Gynaecology and Obstetrics 2008;101(1):94-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Geller SE, Patel A, Niak VA, Goudar SS, Edlavitch SA, Kodkany BS, et al. Conducting international collaborative research in developing nations. International Journal of Gynaecology and Obstetrics 2004;87(3):267-71. [DOI] [PubMed] [Google Scholar]
- 122.NCT00097123. RCT of misoprostol for postpartum hemorrhage in India. https://clinicaltrials.gov/ct2/show/NCT00097123 (first received 18 November 2004).
- 123.Goudar SS, Chakraborty H, Edlavitch SA, Naik VA, Bellad MB, Patted SS, et al. Variation in the postpartum hemorrhage rate in a clinical trial of oral misoprostol. Journal of Maternal-Fetal & Neonatal Medicine 2008;21(8):559-64. [DOI] [PubMed] [Google Scholar]
- 124.Kodkany BS, Derman RJ, Goudar SS, Geller SE, Edlavitch SA, Naik VA, et al. Initiating a novel therapy in preventing postpartum hemorrhage in rural India: a joint collaboration between the United States and India. International Journal of Fertility & Womens Medicine 2004;49(2):91-6. [PubMed] [Google Scholar]
- 125.Kodkany BS, Goudar SS, Derman RJ. The efficacy of oral misoprostol in preventing postpartum hemorrhage in a community setting: a randomized double-blind placebo-controlled trial. International Journal of Gynaecology and Obstetrics 2006;94(Suppl 2):S141-S142. [DOI] [PubMed] [Google Scholar]
- 126.Wenstrom KD. Oral misoprostol in preventing postpartum hemorrhage in resource-poor communities: a randomized controlled trial. Commentary. Obstetrical & Gynecological Survey 2007;62(2):95-6. [Google Scholar]
- 127.Patted SS, Goudar SS, Naik VA, Bellad MB, Edlavitch SA, Kodkany BS, et al. Side effects of oral misoprostol for the prevention of postpartum hemorrhage: results of a community-based randomised controlled trial in rural India. Journal of Maternal-Fetal & Neonatal Medicine 2009;22(1):24-8. [DOI] [PubMed] [Google Scholar]
- 128.Diop A, Daff B, Sow M, Blum J, Diagne M, Sloan NL, et al. Oxytocin via Uniject (a prefilled single-use injection) versus oral misoprostol for prevention of postpartum haemorrhage at the community level: a cluster-randomised controlled trial. Lancet. Global Health 2016;4(1):e37-44. [DOI] [PubMed] [Google Scholar]
- 129.NCT01713153. Comparing misoprostol and oxytocin in uniject for postpartum hemorrhage (PPH) prevention in Senegal. https://clinicaltrials.gov/ct2/show/NCT01713153 (first received: 22 October 2012).
- 130.Docherty PW, Hooper M. Choice of an oxytocic agent for routine use at delivery. Journal of Obstetrics and Gynaecology 1981;2:60. [Google Scholar]
- 131.El-Refaey H, Nooh R, O'Brien P, Abdalla M, Geary M, Walder J, et al. The misoprostol third stage of labour study: a randomised controlled comparison between orally administered misoprostol and standard management. BJOG 2000;107:1104-10. [DOI] [PubMed] [Google Scholar]
- 132.Enakpene CA, Morhason-Bello IO, Enakpene EO, Arowojolu AO, Omigbodun AO. Oral misoprostol for the prevention of primary post-partum hemorrhage during third stage of labor. Journal of Obstetrics and Gynaecology Research 2007;33(6):810-7. [DOI] [PubMed] [Google Scholar]
- 133.Ezeama CO, Eleje GU, Ezeama NN, Igwegbe AO, Ikechebelu JI, Ugboaja JO, et al. A comparison of prophylactic intramuscular ergometrine and oxytocin for women in the third stage of labor. International Journal of Gynaecology and Obstetrics 2014;124(1):67-71. [DOI] [PubMed] [Google Scholar]
- 134.PACTR201105000292708. Prophylactic ergometrine versus oxytocin for women in the third stage of labour: a double blind, randomized trial. https://pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201105000292708 (first received: 2 May 2011).
- 135.Fawole AO, Sotiloye OS, Hunyinbo KI, Umezulike AC, Okunlola MA, Adekanle DA, et al. A double-blind, randomized, placebo-controlled trial of misoprostol and routine uterotonics for the prevention of postpartum hemorrhage. International Journal of Gynaecology and Obstetrics 2011;112(2):107-11. [DOI] [PubMed] [Google Scholar]
- 136.Fekih M, Jnifene A, Fathallah K, Ben Regaya L, Memmi A, Bouguizene S, et al. Benefit of misoprostol for prevention of postpartum hemorrhage in cesarean section: a randomized controlled trial. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2009;38(7):588-93. [DOI] [PubMed] [Google Scholar]
- 137.Fu YX, Ran KQ, Wang M. Prevention of early postpartum hemorrhage by way of oral misoprostol. Journal of Nursing Science 2003;18(12):910-1. [Google Scholar]
- 138.Gulmezoglu AM, Villar J, Ngoc NT, Piaggio G, Carroli G, Adetoro L, et al. WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet 2001;358:689-95. [DOI] [PubMed] [Google Scholar]
- 139.Lumbiganon P, Villar J, Piaggio G, Gulmezoglu AM, Adetoro L, Carroli G. Side effects of oral misoprostol during the first 24 hours after administration in the third stage of labour. BJOG 2002;109:1222-6. [DOI] [PubMed] [Google Scholar]
- 140.Lumbiganon P, Hofmeyr J, Gulmezoglu AM, Pinol A, Villar J. Misoprostol dose-related shivering and pyrexia in the third stage of labour. WHO collaborative trial of misoprostol in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1999;106(4):304-8. [DOI] [PubMed] [Google Scholar]
- 141.Gupta B, Jain V, Aggarwal N. Rectal misoprostol versus oxytocin in the prevention of postpartum hemorrhage - a pilot study. International Journal of Gynaecology and Obstetrics 2006;94(Suppl 2):S139-S140. [DOI] [PubMed] [Google Scholar]
- 142.Hamm J, Russell Z, Botha T, Carlan SJ, Richichi K. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. American Journal of Obstetrics and Gynecology 2005;192:1404-6. [DOI] [PubMed] [Google Scholar]
- 143.Harriott J, Christie L, Wynter S, DaCosta V, Fletcher H, Reid M. A randomized comparison of rectal misoprostol with Syntometrine on blood loss in the third stage of labour. West Indian Medical Journal 2009;58(3):201-6. [PubMed] [Google Scholar]
- 144.Hernandez-Castro F, Lopez-Serna N, Trevino-Salinas EM, Soria-Lopez JA, Sordia-Hernandez LH, Cardenas-Estrada E. Randomized double-blind placebo-controlled trial of buccal misoprostol to reduce the need for additional uterotonic drugs during cesarean delivery. International Journal of Gynaecology and Obstetrics 2016;132(2):184-7. [DOI] [PubMed] [Google Scholar]
- 145.NCT01733329. Buccal misoprostol during cesarean section for preventing postpartum hemorrhage in women with risk factors for uterine atony. https://clinicaltrials.gov/ct2/show/NCT01733329 (first received 16 November 2012).
- 146.Hofmeyr GJ, Nikodem VC, Jager M, Gelbart BR. A randomised placebo controlled trial of oral misoprostol in the third stage of labour. British Journal of Obstetrics and Gynaecology 1998;105(9):971-5. [DOI] [PubMed] [Google Scholar]
- 147.Hofmeyr GJ, Nikodem C, Jager M, Drakely A, Gilbart B. Oral misoprostol for labour third stage management: randomised assessment of side effects. In: 17th Conference on Priorities in Perinatal Care; 1998; South Africa. 1998:53-4.
- 148.Hofmeyr GJ, Jager M, Rose L, Nikodem VC, Lawrie T. Misoprostol for third stage of labour management: a double blind, placebo controlled clinical trial. In: 16th Conference on Priorities in Perinatal Care; 1997; South Africa. 1997:29-31.
- 149.Hofmeyr GJ, Nikodem VC, Jager M, Drakely A. Side-effects of oral misoprostol in the third stage of labour--a randomised placebo-controlled trial. South African Medical Journal 2001;91(5):432-5. [PubMed] [Google Scholar]
- 150.Hofmeyr GJ, Nikodem VC, De Jager M, Drakely AJ. Side effects of oral misoprostol in the third stage of labour: a random allocation placebo controlled trial. Journal of Obstetrics and Gynaecology 2000;20 Suppl 1:S40-1. [Google Scholar]
- 151.Hofmeyr GJ, Fawole B, Mugerwa K, Godi NP, Blignaut Q, Mangesi L, et al. Administration of 400mug of misoprostol to augment routine active management of the third stage of labor. International Journal of Gynaecology and Obstetrics 2011;112(2):98-102. [DOI] [PubMed] [Google Scholar]
- 152.NCT00124540. Misoprostol for preventing postpartum hemorrhage. https://clinicaltrials.gov/ct2/show/NCT00124540 (first received: 26 July 2005).
- 153.Hoj L, Cardoso P, Nielsen BB, Hvidman L, Nielsen J, Aaby P. Effect of sublingual misoprostol on severe postpartum haemorrhage in a primary health centre in Guinea-Bissau: randomised double blind clinical trial. BMJ 2005;331:723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nielsen BB, Hoj L, Hvidman LE, Nielsen J, Cardoso P, Aaby P. Reduced post-partum bleeding after treatment with sublingual misoprostol: a randomized double-blind clinical study in a developing country - secondary publication [Reduceret post partum-blodning efter sublingval misoprostol: et randomiseret dobbeltblindt klinisk studie i et udviklingsland - sekundaerpublikation]. Ugeskrift for Laeger 2006;168(13):1341-3. [PubMed] [Google Scholar]
- 155.Jago AA, Ezechi OC, Achinge GI, Okunlola MA. Effect of oxytocics on the blood pressure of normotensive Nigerian parturients. Journal of Maternal-Fetal & Neonatal Medicine 2007;20(9):703-5. [DOI] [PubMed] [Google Scholar]
- 156.Jangsten E, Mattsson L, Lyckestam I, Hellstrom A, Berg M. A comparison of active management and expectant management of the third stage of labour: a Swedish randomised controlled trial. BJOG 2011;118:362-9. [DOI] [PubMed] [Google Scholar]
- 157.Jans S, Herschderfer KC, Van Diem MT, Aitink M, Rijnders M, Van der Pal-Bruin K, et al. LENTE study: effectiveness of prophylactic intramuscular oxytocin during third stage of labour amongst low risk women. A randomized controlled trial. In: 31st International Confederation of Midwives Triennial Congress. Midwives - Making a Difference in the World; 2017 June 18-22; Toronto, Canada. 2017:Abstract no: P1.063.
- 158.Karkanis SG, Caloia D, Salenieks ME, Kingdom J, Walker M, Meffe F, et al. Randomized controlled trial of rectal misoprostol versus oxytocin in third stage management. Journal of Obstetrics & Gynaecology Canada: JOGC 2002;24(2):149-54. [DOI] [PubMed] [Google Scholar]
- 159.Kerekes L, Domokos N. The effect of prostaglandin F2alpha on third stage labour. Prostaglandins 1979;18:161-6. [DOI] [PubMed] [Google Scholar]
- 160.Khan GQ, John IS, Chan T, Wani S, Hughes AO, Stirrat GM. Abu Dhabi third stage trial: oxytocin vs Syntometrine in the active management of the third stage of labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 1995;58:147-51. [DOI] [PubMed] [Google Scholar]
- 161.Khan GQ, Susheela JI, Chan T, Wani S, Hughes AO, Stirrat GM. Abu Dhabi third stage trial: oxytocin versus Syntometrine in the active management of the third stage of labour. In: 27th British Congress of Obstetrics and Gynaecology; 1995 July 4-7; Dublin, Ireland. 1995:Abstract no: 212. [DOI] [PubMed]
- 162.Khurshid R, Fatima K, Parveen S, Ul Shamas I, Salman R. A comparison between intramuscular PGF2 a125 mG and intravenous methyl ergometrine 0.2 mg in the active management of third stage labor. Internet Journal of Gynecology & Obstetrics 2010;14:1. [Google Scholar]
- 163.Koen S, Snyman LC, Pattinson RC, Makin JA. A randomised controlled trial comparing oxytocin and oxytocin + ergometrine for prevention of postpartum haemorrhage at caesarean section. South African Medical Journal 2016;106(4):399-402. [DOI] [PubMed] [Google Scholar]
- 164.NCT02046499. A randomized trial comparing oxytocin and oxytocin + ergometrine for prevention of postpartum haemorrhage at caesarean section. https://clinicaltrials.gov/show/NCT02046499 2014. [DOI] [PubMed]
- 165.Kumar Burman S, Samanta R, Lata KK, Mukherjee J, Dey TK. Prophylactic administration of per rectal misoprostol vs intramuscular injection of oxytocin in third-stage of labour for prevention of postpartum Haemorrhage: a randomised controlled trial. Journal of Clinical and Diagnostic Research 2021;15(9):QC09-13. [CENTRAL: CN-02338556] 19201235 [EMBASE: 2014766388] [Google Scholar]
- 166.Kundodyiwa TW, Majoko F, Rusakaniko S. Misoprostol versus oxytocin in the third stage of labor. International Journal of Gynaecology and Obstetrics 2001;75:235-41. [DOI] [PubMed] [Google Scholar]
- 167.Lam H, Tang OS, Lee CP, Ho PC. A pilot-randomized comparison of sublingual misoprostol with Syntometrine on the blood loss in third stage of labor. Acta Obstetricia et Gynecologica Scandinavica 2004;83:647-50. [DOI] [PubMed] [Google Scholar]
- 168.Lamont RF, Morgan DJ, Logue M, Gordon H. A prospective randomised trial to compare the efficacy and safety of hemabate and Syntometrine for the prevention of primary postpartum haemorrhage. Prostaglandins & Other Lipid Mediators 2001;66(3):203-10. [DOI] [PubMed] [Google Scholar]
- 169.Lapaire O, Schneider MC, Stotz M, Surbek DV, Holzgreve W, Hoesli IM. Oral misoprostol vs. intravenous oxytocin in reducing blood loss after emergency cesarean delivery. International Journal of Gynaecology and Obstetrics 2006;95(1):2-7. [DOI] [PubMed] [Google Scholar]
- 170.Leung SW, Ng PS, Wong WY, Cheung TH. A randomised trial of carbetocin versus Syntometrine in the management of the third stage of labour. BJOG 2006;113:1459-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu H, Xu XY, Gu N, Ye XD, Wang ZQ, Hu YL, et al. Intravenous administration of carbetocin versus oxytocin for preventing postpartum hemorrhage after vaginal delivery in high risk women: a double-blind, randomized controlled trial. Maternal-Fetal Medicine 2020;2(2):72-9. [DOI: 10.1097/FM9.0000000000000048] [DOI] [Google Scholar]
- 172.ChiCTR1800015040. Short-infusion carbetocin versus oxytocin for prevention of postpartum hemorrhage after vaginal delivery. https://www.chictr.org.cn/hvshowproject.aspx?id=12765. [DOI] [PubMed]
- 173.Lokugamage AU, Paine M, Bassaw-Balroop K, Sullivan KR, El-Refaey H, Rodeck CH. Active management of the third stage at caesarean section: a randomised controlled trial of misoprostol versus syntocinon. Australian & New Zealand Journal of Obstetrics & Gynaecology 2001;41(4):411-4. [DOI] [PubMed] [Google Scholar]
- 174.Lokugamage AU, Moodley J, Sullivan K, Rodeck CH, Niculescu L, Tigere P. The Durban primary postpartum haemorrhage study. In: Women's Health - into the New Millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3-6; Cape Town South Africa. RCOG, 1999:77-8.
- 175.Lokugamage AU, Paine M, Bassau-Balroop H, El-Refaey K, Sullivan K, Rodek C. Active management of the third stage at caesarean section: misoprostol vs syntocinon. In: XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3-8; Washington DC, USA. Vol. Book 2. 2000:54.
- 176.Lumbiganon P, Hofmeyr J, Gülmezoglu AM, Pinol A, Villar J. Misoprostol dose-related shivering and pyrexia in the third stage of labour. British Journal of Obstetrics and Gynaecology 1999;106:304-8. [DOI] [PubMed] [Google Scholar]
- 177.Mannaerts D, Van der Veeken L, Coppejans H, Jacquemyn Y. Adverse effects of carbetocin versus oxytocin in the prevention of postpartum haemorrhage after caesarean section: a randomized controlled trial. Journal of Pregnancy 2018;2018:1374150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Masse N, Dexter F, Wong CA. Prophylactic methylergonovine and oxytocin compared with oxytocin alone in patients undergoing intrapartum cesarean birth: a randomized controlled trial. Obstetrics and Gynecology 2022;140(2):181-6. [CENTRAL: CN-02425689] 21311092 [EMBASE: 638532767] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 179.NCT03904446. Multimodal uterotonics at the time of cesarean section in laboring patients. https://clinicaltrials.gov/show/NCT03904446.
- 180.McDonagh F, Carvalho JC, Abdulla S, Cordovani D, Downey K, Ye XY, et al. Carbetocin vs. oxytocin at elective caesarean delivery: a double-blind, randomised, controlled, non-inferiority trial of low- and high-dose regimens. Anaesthesia 2022;77(8):892-900. [CENTRAL: CN-02389382] 20583207 [EMBASE: 2015417814] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 181.NCT03168698. Carbetocin vs. oxytocin at elective cesarean section [Carbetocin vs. oxytocin at elective cesarean section: a double-blind, randomized controlled non-inferiority trial of high and low dose regimens]. https://clinicaltrials.gov/ct2/show/NCT03168698 2017;[CRS ID: 9738863]:-. [Google Scholar]
- 182.McDonald SJ, Prendiville WJ, Blair E. Randomised controlled trial of oxytocin alone vs oxytocin and ergometrine in active management of third stage of labour. BMJ 1993;307:1167-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McDonald SJ. Management in the Third Stage of Labour [Thesis]. University of Western Australia, 1996. [Google Scholar]
- 184.McDonald S, Prendiville WJ, Blair E. A randomised controlled trial of syntocinon vs Syntometrine as part of the active management of the third stage of labour. In: 26th British Congress of Obstetrics and Gynaecology; 1992 July 7-10; Manchester, UK. 1992:87.
- 185.McDonald S, Prendiville WJ. A randomized controlled trial of syntocinon vs Syntometrine as part of the active management of the third stage of labour. Journal of Perinatal Medicine 1992;20 Suppl 1:97. [Google Scholar]
- 186.McDonald S. The Perth third stage oxytocic trial. In: National Conference on Research in Midwifery; 1992; Reading, UK. 1992.
- 187.Mobeen N, Durocher J, Zuberi N, Jahan N, Blum J, Wasim S, et al. Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: a randomised placebo-controlled trial. BJOG 2011;118:353-61. [DOI: 10.1111/j.1471-0528.2010.02807.x] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mobeen N, Durocher J, Zuberi NF, Jahan N, Blum J, Wasim S, et al. Use of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage during home deliveries in Pakistan: a randomised placebo-controlled trial. International Journal of Gynaecology and Obstetrics 2009;107 Suppl 2:S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.NCT00120237. Misoprostol for the prevention of postpartum hemorrhage in rural Pakistan. https://clinicaltrials.gov/ct2/show/NCT00120237 (first posted: 7 July 2005).
- 190.Hofmeyr GJ. Oral misoprostol reduces the risk of postpartum haemorrhage in home births assisted by trained traditional birth attendants in Pakistan. Evidence Based Medicine 2011;16(6):180-1. [DOI: 10.1136/ebm1403] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 191.Moertl MG, Friedrich S, Kraschl J, Wadsack C, Lang U, Schlembach D. Haemodynamic effects of carbetocin and oxytocin given as intravenous bolus on women undergoing caesarean delivery: a randomised trial. BJOG 2011;118(11):1349-56. [DOI] [PubMed] [Google Scholar]
- 192.Moertl M, Kraschl J, Friedrich S, Pickel K, Ulrich D, Eder M, et al. Hemodynamic changes of carbetocin and oxytocin in women undergoing cesarean section. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S112. [Google Scholar]
- 193.Mortl M, Pickel K, Friedrich S, Ulrich D, Lang U, Schlembach D. Hemodynamic changes of carbetocin and oxytocin given as I.V. bolus on women undergoing cesarean section. Geburtshilfe und Frauenheilkunde 2008;68:S46. [Google Scholar]
- 194.Moir DD, Amoa AB. Ergometrine or oxytocin? Blood loss and side-effects at spontaneous vertex delivery. British Journal of Anaesthesia 1979;51(2):113-7. [DOI] [PubMed] [Google Scholar]
- 195.Moodie JE, Moir DD. Ergometrine, oxytocin and extradural analgesia. British Journal of Anaesthesia 1976;48:571-4. [DOI] [PubMed] [Google Scholar]
- 196.Musa AO, Ijaiya MA, Saidu R, Aboyeji AP, Jimoh AA, Adesina KT, et al. Double-blind randomized controlled trial comparing misoprostol and oxytocin for management of the third stage of labor in a Nigerian hospital. International Journal of Gynaecology and Obstetrics 2015;129(3):227-30. [DOI] [PubMed]
- 197.Nasr A, Shahin AY, Elsamman AM, Zakherah MS, Shaaban OM. Rectal misoprostol versus intravenous oxytocin for prevention of postpartum hemorrhage. International Journal of Gynaecology and Obstetrics 2009;105(3):244-7. [DOI] [PubMed] [Google Scholar]
- 198.Nellore V, Mittal S, Dadhwal V. Rectal misoprostol vs. 15-methyl prostaglandin F2alpha for the prevention of postpartum hemorrhage. International Journal of Gynaecology and Obstetrics 2006;94(1):45-6. [DOI] [PubMed] [Google Scholar]
- 199.Ng PS, Chan AS, Sin WK, Tang LC, Cheung KB, Yuen PM. A multicentre randomized controlled trial of oral misoprostol and IM Syntometrine in the management of the third stage of labour. Human Reproduction 2001;16(1):31-5. [DOI] [PubMed] [Google Scholar]
- 200.Ng PS, Chan AS, Sin WK, Tang LC, Cheung KB, Yuen PM. Comparison of oral misoprostol and intramuscular Syntometrine in the management of the third stage of labor - a multicenter randomised controlled trial. In: XVI FIGO World Congress of Obstetrics & Gynecology. Book 4; 2000 Sept 3-8; Washington DC, USA. 2000:29.
- 201.Ng PS, Lai CY, Sahota DS, Yuen PM. A double-blind randomized controlled trial of oral misoprostol and intramuscular Syntometrine in the management of the third stage of labor. Gynecologic and Obstetric Investigation 2007;63(1):55-60. [DOI] [PubMed] [Google Scholar]
- 202.Yuen PM, Ng PS, Sahota DS. A double-blind randomised controlled trial of oral misoprostol in addition to intra-muscular Syntometrine in the management of the third stage of labour. In: 30th British Congress of Obstetrics and Gynaecology; 2004 July 7-9; Glasgow, UK. 2004:62.
- 203.Oboro VO, Tabowei TO. A randomised controlled trial of misoprostol versus oxytocin in the active management of the third stage of labour. Journal of Obstetrics and Gynaecology 2003;23(1):13-6. [DOI] [PubMed] [Google Scholar]
- 204.Ogunbode O, Obisesan K, Ayeni O. Methergin in the management of the third stage of labor: a comparative clinical trial with Syntometrine and ergometrine. Current Therapeutic Research, Clinical and Experimental 1979;26:460-5. [Google Scholar]
- 205.Orji E, Agwu F, Loto O, Olaleye O. A randomized comparative study of prophylactic oxytocin versus ergometrine in the third stage of labor. International Journal of Gynaecology and Obstetrics 2008;101(2):129-32. [DOI] [PubMed] [Google Scholar]
- 206.Ortiz-Gomez JR, Morillas-Ramirez F, Fornet-Ruiz I, Palacio-Abizanda FJ, Bermejo-Albares L. Clinical and pharmacological study of the efficacy of carbetocin in elective caesareans compared to low and usual doses of oxytocin. Revista Espanola de Anestesiologia y Reanimacion 2013;60(1):7-15. [DOI] [PubMed] [Google Scholar]
- 207.Othman ER, Fayez MF, El Aal D, El-Dine Mohamed HS, Abbas AM, Ali MK. Sublingual misoprostol versus intravenous oxytocin in reducing bleeding during and after cesarean delivery: a randomized clinical trial. Taiwanese Journal of Obstetrics & Gynecology 2016;55(6):791-5. [DOI] [PubMed] [Google Scholar]
- 208.NCT02562300. Uterotonics using to reduce bleeding at cesarean section [Comparative study of sublingual misoprostol versus oxytocin in reducing bleeding at cesarean section]. https://clinicaltrials.gov/show/NCT02562300 2015.
- 209.Owonikoko KM, Arowojolu AO, Okunlola MA. Effect of sublingual misoprostol versus intravenous oxytocin on reducing blood loss at cesarean section in Nigeria: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2011;37(7):715-21. [DOI] [PubMed] [Google Scholar]
- 210.Pakniat H, Khezri MB. The effect of combined oxytocin-misoprostol versus oxytocin and misoprostol alone in reducing blood loss at cesarean delivery: a prospective randomized double-blind study. Journal of Obstetrics and Gynaecology of India 2015;65(6):376-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.NCT01571323. Combined use of oxytocin and misoprostol versus oxytocin infusion and misoprostol alone to reduce blood loss at cesarean section. https://clinicaltrials.gov/ct2/show/NCT01571323 (first posted: 5 April 2012).
- 212.ACTRN12612000095864. Efficacy of combined use of oxytocin and misoprostol in reducing blood loss at cesarean section [Combined use of oxytocin and misoprostol versus oxytocin infusion and misoprostol alone to reduce blood loss at cesarean section]. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335994 (first posted: 19 January 2012).
- 213.Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Oral misoprostol versus oxytocin in the management of the third stage of labour. Journal of Obstetrics and Gynaecology Canada 2006;28(1):20-6. [DOI] [PubMed] [Google Scholar]
- 214.Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Rectal misoprostol versus oxytocin in the management of the third stage of labour. Journal of Obstetrics and Gynaecology Canada 2007;29(9):711-8. [DOI] [PubMed] [Google Scholar]
- 215.Parsons S, Ntumy YM, Walley RL, Wilson JB, Crane JM, Matthews K, et al. Rectal misoprostol vs intramuscular oxytocin in the routine management of the third stage of labour. In: 30th British Congress of Obstetrics and Gynaecology; 2004 July 7-9; Glasgow, UK. 2004:18.
- 216.Penaranda WA, Arrieta OB, Yances BR. Active management of childbirth with sublingual misoprostol: a controlled clinical trial in the Hospital de Maternidad Rafael Calvo [Manejo activo del alumbramiento con misoprostol sublingual: un estudio clinico controlado en al Hospital de Maternidad Rafael Calvo de Cartagena]. Revista Colombiana de Obstetricia y Ginecologia 2002;53(1):87-92. [Google Scholar]
- 217.Poeschmann RP, Doesburg WH, Eskes TK. A randomized comparison of oxytocin, sulprostone and placebo in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1991;98:528-30. [DOI] [PubMed] [Google Scholar]
- 218.Poeschmann RP, Eskes TK, Doesburg WH. Oxytocin and sulprostone reduce post partum blood loss and shorten the third stage in low risk term women. International Journal of Gynaecology and Obstetrics 1991;36 Suppl:312. [Google Scholar]
- 219.Poeschmann RP, Eskes TK, Doesburg WH, Lemmens WA, Benneker JC. Oxytocin and sulprostone reduce postpartum blood loss in low risk term women compared to saline. In: 1st European Congress on Prostaglandins in Reproduction; 1988 July 6-9; Vienna, Austria. 1988:176.
- 220.Prendiville WJ, Harding JE, Elbourne DR, Stirrat GM. The Bristol third stage trial: active versus physiological management of third stage of labour. BMJ 1988;297(6659):1295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Quibel T, Ghout I, Goffinet F, Salomon LJ, Fort J, Javoise S, et al. Active management of the third stage of labor with a combination of oxytocin and misoprostol to prevent postpartum hemorrhage: a randomized controlled trial. Obstetrics and Gynecology 2016;128(4):805-11. [DOI: 10.1097/AOG.0000000000001626] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 222.Rozenberg P, Quibel T, Ghout I, Salomon L, Bussiere L, Goffinet F. Active management of the third stage of labor with routine oxytocin and misoprostol for the prevention of postpartum hemorrhage: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S18. [DOI] [PubMed] [Google Scholar]
- 223.NCT01113229. Combined use of oxytocin and misoprostol in the prevention of post partum haemorrhage (CYTOCINON). https://clinicaltrials.gov/ct2/show/NCT01113229 (first posted: 29 April 2010).
- 224.Rabow S, Jonsson H, Bro E, Olofsson P. Cardiovascular effects of oxytocin and carbetocin at cesarean section. A prospective double-blind randomized study using noninvasive pulse wave analysis. Journal of Maternal-Fetal & Neonatal Medicine 2023;36(1):2208252. [DOI: 10.1080/14767058.2023.2208252] [DOI] [PubMed] [Google Scholar]
- 225.Rashid M, Clark A, Rashid MH. A randomised controlled trial comparing the efficacy of intramuscular Syntometrine and intravenous syntocinon, in preventing postpartum haemorrhage. Journal of Obstetrics and Gynaecology 2009;29(5):396-401. [DOI] [PubMed] [Google Scholar]
- 226.Ray A, Mukherjee P, Basu G, Chatterjee A. Misoprostol and third stage of labour. Journal of Obstetrics and Gynaecology of India 2001;51(6):53-4. [Google Scholar]
- 227.Chatterjee A. Misoprostol and the 3rd stage. In: XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3-8; Washington DC, USA. Vol. Book 4. 2000:29.
- 228.Reyes OA. Carbetocin vs oxytocin for the prevention of postpartum hemorrhage in grand multiparous patients: A randomized controlled trial. Clinica e Investigacion en Ginecologia y Obstetricia 2011;38(1):2-7.
- 229.Reyes OA, Gonzalez GM. Carbetocin versus oxytocin for prevention of postpartum hemorrhage in patients with severe preeclampsia: a double-blind randomized controlled trial. Journal of Obstetrics & Gynaecology Canada: JOGC 2011;33(11):1099-104. [DOI] [PubMed] [Google Scholar]
- 230.Rogers J, Wood J, McCandlish R, Ayers S, Truesdale A, Elbourne D. Active versus expectant management of third stage of labour: the Hinchingbrooke randomised controlled trial. Lancet 1998;351(9104):693-9. [DOI] [PubMed] [Google Scholar]
- 231.Rosseland LA, Hauge TH, Grindheim G, Stubhaug A, Langesaeter E. Changes in blood pressure and cardiac output during cesarean delivery: the effects of oxytocin and carbetocin compared with placebo. Anesthesiology 2013;119(3):541-51. [DOI] [PubMed] [Google Scholar]
- 232.Gawecka E, Rosseland LA. A secondary analysis of a randomized placebo-controlled trial comparing the analgesic effects of oxytocin with carbetocin: postcesarean delivery morphine equivalents. Anesthesia and Analgesia 2014;119(4):1004. [DOI] [PubMed] [Google Scholar]
- 233.Sadeghi Afkham M, Hashemnejad M, Esmaelzadeh Saeieh S, Ataei M, Valizadeh R. Prophylactic effect of rectal and sublingual misoprostol on postpartum hemorrhage in mothers with preeclampsia following cesarean section surgery; a double-blind randomized controlled trial. Annals of Medicine and Surgery 2022;20:104175. [DOI: 10.1016/j.amsu.2022.104175] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Soltan MH, El-Gendi E, Imam HH, Fathi O. Different doses of sublingual misoprostol versus methylergometrine for the prevention of atonic postpartum haemorrhage. International Journal of Health Sciences 2007;1(2):229-36. [PMC free article] [PubMed] [Google Scholar]
- 235.Su LL, Rauff M, Chan YH, Mohamad Suphan N, Lau TP, Biswas A, et al. Carbetocin versus Syntometrine for the third stage of labour following vaginal delivery--a double-blind randomised controlled trial. BJOG 2009;116(11):1461-6. [DOI] [PubMed] [Google Scholar]
- 236.Supe PA, Kore SJ, Nandanwar YS. A comparative study of efficacy of misoprostol with methyl ergometrine and carboprost in active management of third stage of labour. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2016;5(5):1525-31. [Google Scholar]
- 237.Surbek DV, Fehr PM, Hoesli I, Holzgreve W. Oral misoprostol vs placebo for third stage of labour [Orales misoprostol reduziert den postpartalen blutverlust]. Gynakologisch Geburtshilfliche Rundschau 1999;39:144. [Google Scholar]
- 238.Surbek DV, Fehr PM, Hoesli I, Holzgreve W. Misoprostol for prevention of postpartum hemorrhage: a randomized controlled trial. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3-8; Washington DC, USA 2000;Book 1:33. [Google Scholar]
- 239.Thilaganathan B, Cutner A, Latimer J, Beard R. Management of the third stage of labour in women at low risk of postpartum haemorrhage. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;48:19-22. [DOI] [PubMed] [Google Scholar]
- 240.Vaid A, Dadhwal V, Mittal S, Deka D, Misra R, Sharma JB, et al. A randomized controlled trial of prophylactic sublingual misoprostol versus intramuscular methyl-ergometrine versus intramuscular 15-methyl PGF2alpha in active management of third stage of labor. Archives of Gynecology and Obstetrics 2009;280(6):893-7. [DOI] [PubMed] [Google Scholar]
- 241.Van der Nelson H, O'Brien S, Burnard S, Mayer M, Alvarez M, Knowlden J, et al. Intramuscular oxytocin versus Syntometrine® versus carbetocin for prevention of primary postpartum haemorrhage after vaginal birth: a randomised double-blinded clinical trial of effectiveness, side effects and quality of life. BJOG 2021;128(7):1236-46. [DOI: 10.1111/1471-0528.16622] [DOI] [PubMed] [Google Scholar]
- 242.Van Der Nelson H, O'Brien S, Lenguerrand E, Marques E, Alvarez M, Mayer M, et al. Intramuscular oxytocin versus oxytocin/ergometrine versus carbetocin for prevention of primary postpartum haemorrhage after vaginal birth: study protocol for a randomised controlled trial (the IMox study). Trials 2019;20(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.NCT02216383. Intramuscular oxytocics: a comparison study of intramuscular carbetocin, syntocinon and Syntometrine for the third stage of labour following vaginal birth (IMox). https://clinicaltrials.gov/ct2/show/NCT02216383 (first received 15 August 2014).
- 244.ISRCTN10232550. A study comparing three medicines used for the active management of the third stage of labour (to help deliver the placenta after your baby has been born) [Intramuscular oxytocics: a multi-centre randomised comparison study of intramuscular carbetocin, syntocinon and Syntometrine for the third stage of labour following vaginal birth]. https://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN10232550 (first posted: 2018). [CENTRAL: CN-01897326] 10797032
- 245.Verma P, Aggarwal N, Jain V, Suri V. A double-blind randomized controlled trial to compare sublingual misoprostol with methylergometrine for prevention of postpartum hemorrhage. International Journal of Gynaecology and Obstetrics 2006;94 Suppl 2:S137-S138. [DOI] [PubMed] [Google Scholar]
- 246.Vimala N, Mittal S, Kumar S, Dadhwal V, Mehta S. Sublingual misoprostol versus methylergometrine for active management of third stage of labor. International Journal of Gynaecology and Obstetrics 2004;87:1-5. [DOI] [PubMed] [Google Scholar]
- 247.Vimala N, Mittal S, Kumar S. Sublingual misoprostol versus oxytocin infusion to reduce blood loss at cesarean section. International Journal of Gynaecology and Obstetrics 2006;92(2):106-10. [DOI] [PubMed] [Google Scholar]
- 248.Walley RL, Wilson JB, Crane JM, Matthews K, Sawyer E, Hutchens D. A double-blind placebo controlled randomised trial of misoprostol and oxytocin in the management of the third stage of labour. BJOG 2000;107(9):1111-5. [DOI] [PubMed] [Google Scholar]
- 249.Whigham CA, Gorelik A, Loughnan TE, Trivedi A. Carbetocin versus oxytocin to reduce additional uterotonic use at non-elective caesarean section: a double-blind, randomised trial. Journal of Maternal-Fetal & Neonatal Medicine 2016;29(23):3866-9. [DOI] [PubMed] [Google Scholar]
- 250.Whigham CA, Gorelik A, Loughnan T, Trivedi A. Carbetocin versus oxytocin in active labour. BJOG 2014;121 Suppl 2:88. [Google Scholar]
- 251.Gulmezoglu M. The WHO Champion trial. International Journal of Gynaecology and Obstetrics 2015;131 Suppl 5:E29-30. [Google Scholar]
- 252.Widmer M, Piaggio G, Abdel-Aleem H, Carroli G, Chong Y, Coomarasamy A, et al. Room temperature stable carbetocin for the prevention of postpartum haemorrhage during the third stage of labour in women delivering vaginally: study protocol for a randomized controlled trial. Trials 2016;17:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.CTRI/2016/06/006996. Effect of carbetocin RTS vs oxytocin for preventing postpartum hemorrhage on post-delivery hemoglobin: a randomized controlled trial. https://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=10619 (first received 6 June 2016).
- 254.Zachariah ES, Naidu M, Seshadri L. Oral misoprostol in the third stage of labor. International Journal of Gynaecology and Obstetrics 2006;92(1):23-6. [DOI] [PubMed] [Google Scholar]
- 255.Yunas I, Gallos ID, Devall AJ, Podesek M, Allotey J, Takwoingi Y, et al. Tests for diagnosis of postpartum haemorrhage at vaginal birth. Cochrane Database of Systematic Reviews 2025, Issue 1. Art. No: CD016134. [DOI: 10.1002/14651858.CD016134] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Hogerzeil HV, Walker GJ, Goeje MJ. Stability of Injectable Oxytocics in Tropical Climates. Results of Field Surveys and Simulation Studies on Ergometrine, Methylergometrine and Oxytocin. Geneva: World Health Organization (WHO), 1993. [Google Scholar]
- 257.WHO. Stability of Injectable Oxytocics in Tropical Climates: Results of Field Surveys and Simulation Studies on Ergometrine, Methylergometrine, and Oxytocin. Geneva: World Health Organization, 1993. [Google Scholar]
- 258.listessentialmedsorg. Carbetocin. Available from: https://list.essentialmeds.org/recommendations/906 (first added in 2019, cited 8 June 2024).
- 259.Gallos ID, Williams HM, Price MJ, Merriel A, Gee H, Lissauer D, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Systematic Reviews 2018, Issue 4. Art. No: CD011689. [DOI: 10.1002/14651858.CD011689.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 Search strategies
Supplementary material 2 Characteristics of included studies
Supplementary material 3 Characteristics of excluded studies
Supplementary material 4 Characteristics of studies awaiting classification
Supplementary material 5 Characteristics of ongoing studies
Supplementary material 6 Analyses
Supplementary material 7 Data package
Supplementary material 8 Supplementary Appendix
Data Availability Statement
As part of the published Cochrane review, the following is made available for download for users of the Cochrane Library: the data extracted for this review, along with the associated analysis code and supplementary materials, are available in accordance with the Cochrane Data Sharing Policy Supplementary material 7.