Abstract
Production of proinflammatory cytokines is implicated in the pathogenesis of viridans streptococcus-induced α-streptococcal shock syndrome and infective endocarditis. Streptococcus mutans, one of the opportunistic pathogens causing infective endocarditis, was reported previously to stimulate monocytes and epithelial and endothelial cells in vitro to produce various cytokines. We found that glucosyltransferases (GTFs) GtfC and GtfD of S. mutans stimulated predominantly the production of interleukin-6 (IL-6) from T cells cultured in vitro. The level of IL-6 but not of tumor necrosis factor alpha in blood was significantly elevated when rats were injected intravenously with S. mutans GS-5, whereas IL-6 was detected at a much lower level when rats were challenged with NHS1DD, an isogenic mutant defective in the expression of GTFs. The serum IL-6 level was elevated in patients with endocarditis caused by different species of viridans streptococci which express GTF homologues. Affinity column-purified GTFs reduced the levels of detectable IL-2 of T cells stimulated by another bacterial antigen, tetanus toxoid. These results suggested that GTFs might modulate the production of Th1-type cytokines and that GTFs of S. mutans play a significant role in stimulating the production of the proinflammatory cytokine IL-6 in vivo.
Species of viridans streptococci, such as Streptococcus mutans, Streptococcus sanguis, or Streptococcus oralis, are common causes of infective endocarditis in humans (2, 13). In Taiwan, S. oralis and S. sanguis are most frequently isolated from blood cultures in endocarditis, but S. mutans, a primary etiological agent of human dental caries (14), is associated with the highest incidence of endocarditis in bacteremia-associated pyogenic infections (5). S. mutans and other oral streptococci may enter the bloodstream and cause transient bacteremia in humans following dental extractions, brushing of teeth, and chewing (9). Transient bacteremia facilitated S. mutans colonization of the valve tissues, particularly in those patients with preexisting valvular damage. The development of endocarditis depends on a balance between the abilities of the organism to adhere to vegetations and to resist the array of host responses.
S. mutans was able to stimulate in vitro the proliferation of peripheral blood mononuclear cells (PBMC), including CD4+ T cells, CD8+ T cells, and natural killer (NK) cells, in an antigen-dependent manner (16). The stimulated PBMC secreted gamma interferon (IFN-γ), tumor necrosis factor β (TNF-β), and interleukin 10 (IL-10), etc., but the level of detectable IL-2 was relatively low compared to that of the others when PBMC were stimulated by soluble factors secreted from S. mutans (16). Antigen I/II of S. mutans, an adhesin for saliva-coated surfaces, interacted directly with human monocytes or epithelial or endothelial cells through lectin-like binding and stimulated the production of proinflammatory cytokines, such as IL-1, IL-6, IL-8, and TNF-α (19, 20, 22). The induction of excess proinflammatory cytokines, especially IL-6, in response to microbial challenge was found on all occasions in patients with endocarditis (18). These results demonstrated that S. mutans antigens exerted immunomodulatory effects on human cells of different origins and might contribute to the development of immunopathological reactions.
We have been studying the human immune response to a family of immunologically and structurally related proteins named glucosyltransferases (GTFs) with molecular masses around 155 kDa. GTFs are enzymes responsible for the synthesis from sucrose of water-soluble and insoluble glucose polymers (glucans). Glucans, along with the GTFs, enhance colonization by bacteria and the formation of biofilms as well. S. mutans expresses three GTFs (1, 11, 12) with distinct functions and localization. GtfB and GtfC are cell wall associated and synthesize primarily insoluble glucan, whereas GtfD is secreted and synthesizes water-soluble glucan (21). We found that S. mutans GTFs efficiently stimulated humoral and cellular immune responses in young human adults; although the response to GtfD is higher, the response to GtfC is still robust (6, 8). In this report, we provide further evidence to indicate that GtfC and GtfD directed distinct cytokine profiles in the T cells and modulated the T-cell response to another antigen. The specificity of GTFs in the induction of IL-6 was also demonstrated in vivo.
MATERIALS AND METHODS
Subjects and specimens.
The volunteers who participated in the present study were 26 healthy students, 20 to 22 years of age, from the National Taiwan University. Umbilical blood was collected from the Gynecology Department of National Taiwan University Hospital. Blood samples of patients suffering from infective endocarditis of confirmed bacterial origin were collected from the Department of Infectious Diseases of National Taiwan University Hospital. The statement on informed consent for use of human sera and umbilical blood samples followed the regulations of the university hospital committee. The blood samples were immediately prepared for the isolation of monocytes, and plasma samples were stored frozen at −70°C until use for enzyme-linked immunosorbent assay (ELISA) or Western blot analysis, as described previously (6).
Preparation of antigens.
Recombinant GtfC and GtfD expressed in Escherichia coli were purified by chromatography on a Ni2+ affinity resin. The construction of the expression systems pRSETAgtfC and pYND72-His was recently described (8). pRSETAgtfC, expressing gtfC with a deletion of its signal sequence (amino acids 1 to 43) and an N-terminal six-His tag, was introduced into E. coli BL21(DE3) (Novagen Inc., Madison, Wis.), which contains the T7 polymerase gene on the chromosome under the control of the lacUV5 promoter. Plasmid pYND72-His, which expresses gtfD with a seven-His tag immediately C terminal to the putative signal sequence (amino acids 1 to 29) under the control of the lac promoter, was introduced in E. coli MM294. E. coli harboring pRSETAgtfC or pYND72-His was grown to an A550 of 0.4 to 0.5, and the T7 or lac promoter was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 2.0 mM. The cultures were grown for an additional 4 h and then harvested. The purification of His-GtfC and His-GtfD was performed as previously described (8). The homogeneity of the purified proteins was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by silver staining or activity staining with periodic acid-Schiff reagent (7). The bands were analyzed with an Electrophoresis Documentation and Analysis System 120 (Scientific Imaging Systems, Eastman Kodak Co., Rochester, N.Y.). Protein concentrations were determined by a modification of the method of Lowry et al., with bicinchoninic acid as the colorimetric detection reagent (BCA protein assay reagent; Pierce). GTF activity was determined by the [14C]glucose-sucrose (New England Nuclear Corp., Boston, Mass.) incorporation assay as described previously (6).
S. mutans GS-5, a laboratory strain, was grown in brain heart infusion broth (Difco Laboratories, Detroit, Mich.). The extracellular protein antigens (EXP-A) and cell wall-associated protein antigens (CWP-A) were prepared as described previously (8). Glutaraldehyde-inactivated tetanus toxoid (TT) was provided by Ming-Yi Liau of the Department of Health, Center for Disease Control, Vaccine Center, Taiwan, Republic of China. Mononuclear cells or enriched T cells were analyzed with a Becton Dickinson FACScan (San Jose, Calif.) apparatus, and data analysis was performed with the LYSYS II software program. All antigens, including purified GtfC and GtfD and reagents used for proliferation assays, exhibited undetectable endotoxin levels (<30 pg/ml) as determined by the Limulus amebocyte lysate assay (Sigma, St. Louis, Mo.).
Detection of antibodies.
Human and rat plasma samples were examined for anti-GtfC-GtfD antibody activity, by ELISA or Western blot analysis, at various dilutions as described previously (6). Rabbit anti-GtfC and anti-GtfD immunoglobulin G antibodies, PJS-2 and PJS-3, respectively (7), were used as positive controls.
Cell preparation and proliferation assay.
Mononuclear cells were isolated from peripheral blood or umbilical blood specimens from healthy children or adult volunteers by Ficoll-Hypaque centrifugation. Irradiated human PBMC were used as accessory cells. Suspensions (2 × 105 cells per 50 μl) of PBMC in RPMI 1640 medium (Gibco BRL Laboratories, Grand Island, N.Y.) supplemented with 10% fetal calf serum (Gibco BRL) (complete RPMI medium) were irradiated at 4,500 rads with an X-ray irradiator (Hitachi Medical Co., Tokyo, Japan) to inhibit proliferation and used as accessory cells in T-cell proliferation assays. T cells were enriched directly from whole blood by antibody-mediated separation with RosetteSep (StemCell Technologies Inc.). The enriched T-cell fractions were collected and used in the proliferation assays.
Enriched T cells (105 cells per well) were cultured in the presence of irradiated autologous PBMC (2 × 105 cells per well) in RPMI 1640, supplemented with 2% fetal calf serum, 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, penicillin (100 μg/ml), streptomycin sulfate (100 μg/ml), and 2% thiophene-2-carboxylic acid hydrazide (Celox). Three replicates of each culture were incubated with recombinant GtfC or GtfD (10 μg/ml), crude extracts of CWP and EXP, TT (10 μg/ml), and S. mutans whole cells (2 × 105 CFU) or were unsupplemented controls. To determine the modulation effects of GTFs on cytokines, S. mutans or GTFs were added along with TT. Incubation was at 37°C in a humidified atmosphere with 5% CO2 for 4 days. Culture supernatant was collected on day 5 following addition of antigens and then frozen at −20°C for future analysis.
Detection of cytokines.
Cytokines were quantitated by ELISA kits (Quantikine; R & D Systems Inc., Minneapolis, Minn.) used according to the manufacturer's instructions. Standard curves were constructed according to the manufacturer's instructions. The minimum detectable cytokine concentrations were estimated to be 3.9 pg/ml for IL-2, 7.8 pg/ml for IFN-γ, 1.6 pg/ml for IL-6, 3.9 pg/ml for IL-10, and 7.8 pg/ml for TNF-α. Results are expressed as means ± standard errors.
In vivo induction of cytokines.
Wistar rats (150 to 200 g) from the animal center of College of Medicine, National Taiwan University, were given an ordinary diet (MF; 22% energy; Purina Mills Inc., Richmond, Ind.) and deionized water containing tetracycline (40 mg/ml of water). Oral swabs were taken from all animals for bacteriological surveys of preexisting viridans streptococci. Serum samples were taken to determine the titers of antibody to S. mutans or GTFs and cytokine levels. Rats with serum anti-S. mutans antibody titers lower than 1/100 and IL-6 levels lower than 20 pg/ml were selected and randomly divided into two groups. The rats in the treatment group were injected intravenously with 108 CFU of S. mutans GS-5 and NHS1DD (GS-5-derived mutant lacking the expression of GTFs) in saline. Rats in the noninfected control group were injected with saline. Serum samples were taken periodically over the 48-h period after injection. Cytokine levels were monitored by ELISA (DuoSet; rat IL-6; R & D Systems Inc.). The serum cytokine levels in the control group or in the treatment group before infection were below the minimum detection level (<250 pg/ml).
RESULTS
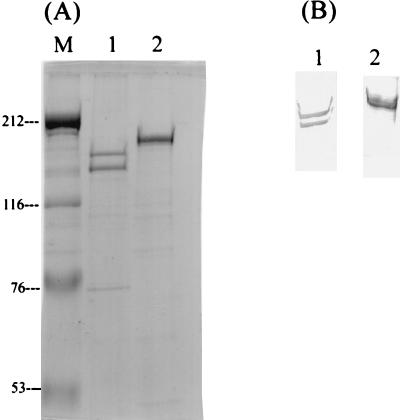
S. mutans produces three GTFs, GtfB, GtfC, and GtfD, sharing similar characteristics and a high molecular mass (∼150 kDa). To stimulate T-cell response, recombinant GtfC and GtfD expressed in E. coli were purified by affinity chromatography with His-tagged expression and purification systems. Only minor bands from the E. coli host were observed in the final purified GtfC and GtfD, following prolonged exposure of sodium dodecyl sulfate-polyacrylamide gels to silver staining reagent (Fig. 1A). The authenticity of GtfC and GtfD was confirmed by Western blot analysis (Fig. 1B), and their biological activities were determined by glucan synthesis assay. We have previously demonstrated that, although GtfC-GtfD preparations do not contain mitogenic or superantigenic components, both antigens stimulated the proliferation of populations of PBMC other than T cells nonspecifically (8). To delineate the effects specifically exerted on T cells by GTFs, T cells were enriched from PBMC with a purity over 95%.
FIG. 1.
Affinity purification and identification of recombinant His-tagged GtfC and GtfD fusion proteins. (A) Purified proteins were resolved on a 7.5% polyacrylamide gel stained with silver stain. Lane 1, 1.5 μg of purified GtfC; lane 2, 1.2 μg of purified GtfD; lane M, prestained Bio-Rad high-range protein molecular mass markers. Numbers at left are molecular masses in kilodaltons. (B) Western blot analysis of GtfC (lane 1) and GtfD (lane 2) with purified anti-GtfC or GtfD rabbit immunoglobulin G (PJS-2 and PJS-3). A biologically active degradation product of GtfC was always present in the purified fractions of GtfC.
All individuals tested exhibited plasma antibody and T-cell proliferative responses to whole S. mutans, GtfC, GtfD, and TT, as previously described (data not shown). The cytokine profiles stimulated by specific antigens are summarized in Table 1. The choice of cytokines included examples of cytokines that play important roles in modulation of immune responses, including IL-2, IFN-γ, and IL-10, and in inflammatory responses, such as IL-6 and TNF-α. As shown in Table 1, S. mutans whole cells, GtfC, and GtfD all exhibited similar abilities to induce the production of IFN-γ, IL-6, IL-10, and TNF-α in T cells. Compared with another bacterial product, TT, at a similar dose, S. mutans and GtfC-GtfD induced significantly lower levels of IL-2 and significantly higher levels of proinflammatory cytokines IL-6, in particular, and TNF-α. In addition, GtfC or GtfD alone could stimulate the production of IL-6 in T cells to a level comparable to that stimulated by S. mutans whole cells. These results indicated that GTFs of S. mutans preferentially induced the production of IL-6 from T cells cultured in vitro.
TABLE 1.
Cytokine profile of T cells induced in vitro by S. mutans, GtfC-GtfD, and TT
| Antigen (μg/ml) | Level of cytokine (pg/ml [mean ± SD; n = 26])a
|
||||
|---|---|---|---|---|---|
| IL-2 | IFN-γ | IL-6 | IL-10 | TNF-α | |
| GtfC (10) | 14.53 ± 3.10b | 235.86 ± 193.44 | 10,198.43 ± 2,582.1b | 21.08 ± 16.9 | 265.51 ± 133.15b |
| GtfD (10) | 6.09 ± 4.17b | 212.47 ± 196.91 | 10,268.21 ± 3,240.2b | 62.38 ± 39.36 | 367.41 ± 126.71b |
| TT (10) | 30.85 ± 18.47 | 340.21 ± 210.10 | 827.28 ± 702.03 | 38.86 ± 27.39 | 24.62 ± 16.23 |
| S. mutans | 7.42 ± 3.20b | 92.50 ± 72.05 | 10,329.90 ± 2,195.17b | 56.14 ± 31.72 | 436.18 ± 129.88b |
Results represent average cytokine levels in supernatants, generated for 5 days by T cells (2 × 105/well) in the presence of irradiated PBMC as antigen-presenting cells and S. mutans (2 × 105 CFU) or various antigens. All antigens, including purified GtfC-GtfD and reagents, exhibited undetectable endotoxin levels (<30 pg/ml) as determined by the Limulus amebocyte lysate assay (Sigma). The cytokine level in the absence of any stimulation was undetectable except for IL-2 (5.43 ± 2.4 pg/mL).
The value induced by S. mutans, GtfC, or GtfD is significantly different from the values induced by TT (P < 0.01 [Student's t test]).
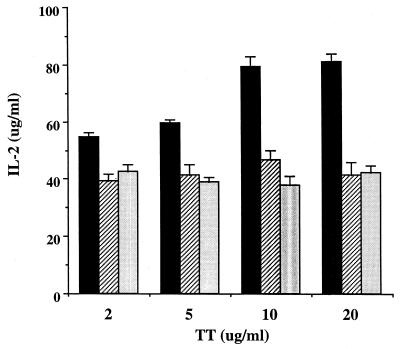
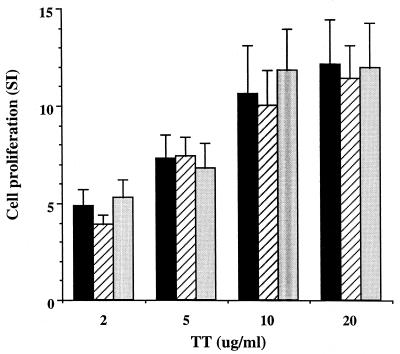
Because the significant down-regulation of IL-2 production by S. mutans was also observable in cell cultures with GtfC-GtfD preparations, we sought to determine whether GtfC or GtfD alone could modulate the cytokine production in T cells induced by other bacterial factors. The addition of S. mutans cell wall extracts (CWP) significantly reduced the level of detectable IL-2 induced by TT in all of the four subjects tested (Table 2). This result is analogous to the finding from another laboratory indicating that S. mutans released factors that selectively inhibited the production of IL-2 (16). To confirm that GTFs may act as the suppressing factors, the purified GtfC or GtfD was added along with TT in the same cultures. As shown in Table 2, both GtfC and GtfD exerted inhibitory effects on the TT-induced IL-2 level, down to 50% of the original response without the addition of GTFs. However, these results alone could not differentiate whether production or induction of IL-2 was affected. In separate cell cultures with only GtfC or GtfD added, a very low or undetectable level of IL-2 was found as shown earlier. The reduction of the level of IL-2 is persistent regardless of the dose of the tested antigen TT (Fig. 2), but reduction of IL-2 alone is not sufficient to inhibit lymphocyte proliferation (Fig. 3).
TABLE 2.
Reduction of TT-induced IL-2 in the presence of cell wall extracts and GtfC-GtfD
| Donor no. | Level of cytokine (% of inhibition) after stimulation with antigensa:
|
||||||
|---|---|---|---|---|---|---|---|
| TT (10) | TT-CWP (20) | TT-GtfC (10) | TT-GtfD (10) | CWP (20) | GtfC (10) | GtfD (10) | |
| 12 | 43.6 ± 3.5 | 18.9 ± 2.2 (57) | 20.1 ± 1.4 (54) | 24.7 ± 2.0 (43) | 8.1 ± 0.9 | 7.8 ± 1.2 | 12.2 ± 1.4 |
| 15 | 26.6 ± 2.5 | 5.1 ± 0.6 (81) | 6.2 ± 1.5 (77) | 7.5 ± 1.8 (72) | <5 | <5 | <5 |
| 21 | 79.2 ± 3.4 | 26.0 ± 2.5 (67) | 37.6 ± 2.1 (53) | 46.5 ± 2.4 (41) | 12.5 ± 0.7 | 7.1 ± 1.2 | 6.9 ± 1.3 |
| 22 | 15.8 ± 1.4 | 2.1 ± 0.6 (87) | 7.8 ± 1.6 (51) | 8.2 ± 2.2 (48) | <5 | 7.3 ± 0.5 | 9.4 ± 0.9 |
Values represent IL-2 levels (means ± standard deviations) in supernatants generated for 5 days by T cells (2 × 105/well) in the presence of irradiated PBMC as antigen-presenting cells and various antigens. Cytokines were detected with a quantitative ELISA. Percent inhibition was calculated as 100 − value for tested antigen/value with TT alone. Cytokine levels are shown in picograms per milliliter. Numbers in parentheses after antigens are amounts in micrograms per milliliter.
FIG. 2.
Reduction of TT-induced IL-2 production from T cells by GtfC-GtfD. T cells from one donor were cultured with irradiated autologous PBMC in the presence of TT added at different concentrations as indicated. IL-2 production stimulated by TT alone (black bars) was inhibited by either GtfD (hatched bars) or GtfC (light gray bars) at a concentration of 10 μg/ml. Each bar represents the mean + standard deviation from triplicate assays. The percent inhibition by the addition of either GtfC or GtfD was statistically significant at each concentration of TT with the two-sample t test (P < 0.01).
FIG. 3.
No effects on TT-induced T-cell proliferation by GtfC-GtfD. The figure show the proliferation of T cells from the same donor as in Fig. 1 stimulated by TT at different concentrations as indicated without the addition of GtfC-GtfD (black bars) or in the presence of GtfD (hatched bars) or GtfC (light gray bars) at a concentration of 10 μg/ml. The proliferation of T cells was stimulated by TT in a dose-dependent manner, and the addition of either GtfC or GtfD had no effects on such proliferation. SI, stimulation index.
Because both IL-2 and IFN-γ are cytokines that contribute to Th1 polarization, the possible effects of GTFs on the TT-induced IFN-γ production were examined. In two subjects tested (subjects 12 and 15), both GtfC and GtfD exerted inhibitory effects on IFN-γ production down to 13 to 31%, respectively, when TT was added at a lower concentration (2 μg/ml). No significant changes were observed in the cultures induced by the addition of TT at a higher concentration (10 μg/ml), although TT alone stimulated higher levels of IFN-γ in cells from these two subjects. In the other two subjects (subjects 21 and 22), both GtfC and GtfD caused no or minor inhibition of the production of IFN-γ induced by either low or high doses of TT (Table 3). In addition, no significant change in TT-induced IL-10 production was detectable in the cultures from all tested subjects when GtfC or GtfD was added (data not shown). Taken together, these results suggested that GTFs of S. mutans might modulate the level of cytokine, particularly IL-2, induced by an irrelevant antigen.
TABLE 3.
Inhibition of TT-induced IFN-γ in the presence of cell wall extracts and GtfC-GtfD
| Donor no. | Level of cytokine (% of inhibition) after stimulation with antigena
|
|||||||
|---|---|---|---|---|---|---|---|---|
| TT (2)
|
TT (10)
|
|||||||
| Alone | + CWP (20) | + GtfC (10) | + GtfD (10) | Alone | + CWP (20) | + GtfC (10) | + GtfD (10) | |
| 12 | 526.1 | 329.9 (37) | 365.1 (31) | 362.6 (31) | 1,594.7 | 1,126.2 (29) | 824.7 (48) | 1,334.1 (16) |
| 15 | 399.3 | 379.1 (5) | 345.9 (13) | 346.5 (13) | 546.9 | 360.4 (34) | 382.7 (30) | 574.4 |
| 21 | 895.2 | 928.6 | 913.4 | 977.2 | 2,034.5 | 1,521.7 (25) | 1,741.7 (14) | 1,985.9 (2) |
| 22 | 330.9 | 310.7 (6) | 454.9 | 341.6 | 366.6 | 383.6 | 385.9 | 316.9 (14) |
Values represent average IFN-γ levels (picograms per milliliter) in supernatants generated for 5 days by T cells (2 × 105/well) in the presence of irradiated PBMC as antigen-presenting cells and various antigens. Cytokines were detected with a quantitative ELISA from quadruplicate assays with standard deviations within 5% of the mean. Percent inhibition was calculated as 100 − value for tested antigens/value for TT alone. Numbers in parentheses after antigens are amounts in micrograms per milliliter.
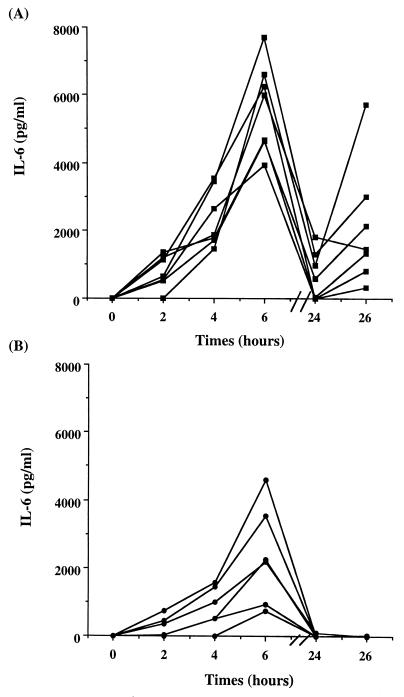
To test if the preferential induction of IL-6 through GTFs could also be demonstrated in vivo, the serum IL-6 level was subsequently investigated in a rat model. All seven rats injected with the wild-type GS-5 strain demonstrated in their serum an elevated IL-6 level after 2 h; the induction peaked at 6- to 10-fold at 6 h (4,000 to 8,000 pg/ml) after injection and persisted up to 26 h (Fig. 4A). On the other hand, all six rats injected intravenously with a similar dose of the NHS1DD isogenic mutant strain, which is defective in the expression of all three GTFs (GtfB-GtfC-GtfD), exhibited IL-6 levels (less than 4,500 pg/ml) significantly lower than those in rats challenged with the wild-type strain and dropping to the background level within 24 h (Fig. 4B). A similar pattern with a lower fold of induction was also observed for the NHS1DD-infected rats, suggesting that GTFs might not be the only components responsible for the induction of IL-6 in vivo. None of the rats in either of the tested groups had a detectable level of TNF-α in its serum. These results confirmed that GTFs of S. mutans could preferentially induce and potentiate the production of IL-6 from PBMC in vivo.
FIG. 4.
Serum IL-6 levels in rats intravenously infected with S. mutans. Wistar rats (average weights, 150 to 200 g) in the tested group (n = 13) were injected intravenously with 108 CFU of S. mutans GS-5 (A) (n = 7) or NHS1DD, a GS-5-derived isogenic mutant lacking expression of GTFs (B) (n = 6). Rats in the uninfected control group (n = 6) were injected with an equal volume of saline. Serum samples were taken periodically as indicated after injection. Cytokine levels were monitored by ELISA (DuoSet; rat IL-6; R & D Systems Inc.). The serum cytokine levels in the control group or in the tested group before infection were below the minimum detection level (<250 pg/ml). The difference of the means at 4 and 6 h for the GS-5 and NHS1DD groups is statistically significant by the two-sample t test (P < 0.01).
To correlate the significance of GTF-induced IL-6 production with the pathogenesis of infective endocarditis, IL-6 levels in serum samples from patients with endocarditis caused by different viridans streptococci that do or do not contain GTFs were examined. IL-6 could readily be detected in serum at various concentrations, ranging from 3.9 to 103.1 pg/ml, in patients infected with GTF-containing strains, including S. mutans, S. sanguis, and S. oralis (Table 4). Members of the Streptococcus anginosus group and Streptococcus mitis do not produce extracellular polysaccharides in the presence of sucrose and therefore are considered GTF-negative strains. With the exception of S. anginosus, the two other members of the S. anginosus group, Streptococcus constellatus and Streptococcus intermedius, induced levels of IL-6 that were lower or nondetectable during systemic infection. Interestingly, none of the patients exhibited detectable TNF-α in their serum except for the one infected with S. sanguis (Table 4). IL-6 was not detected in the serum of any volunteers in the healthy control group described above. These results suggested that GTFs might be important virulence factors for endocarditis in specifically inducing the proinflammatory cytokine IL-6 but might not be the only factors responsible.
TABLE 4.
Serum cytokines in patients with viridans streptococcus-induced endocarditis
| Case no.a | Bacterium | GTFs | IL-6 (pg/ml) | TNF-α (pg/ml) |
|---|---|---|---|---|
| 1 | S. mutans | + | 6.5 | —b |
| 2 | S. mutans | + | 103.1 | — |
| 3 | S. sanguis | + | 3.9 | 69.6 |
| 4 | S. oralis | + | 12.6 | — |
| 5 | S. oralis | + | — | — |
| 6 | S. mitis | — | 4.3 | — |
| 7 | S. mitis | — | — | — |
| 8 | S. constellatus | — | — | — |
| 9 | S. constellatus | — | — | — |
| 10 | S. intermedius | — | — | — |
| 11 | S. intermedius | — | — | — |
| 12 | S. anginosus | — | 23.9 | — |
| 13 | S. anginosus | — | 137.5 | — |
Blood samples of patients suffering from infective endocarditis at the acute stage were routinely collected from the Department of Infectious Diseases of National Taiwan University Hospital for the identification of bacterial origins.
—, not detected. The minimum detectable cytokine concentrations were estimated to be 1.6 and 7.8 pg/ml for IL-6 and TNF-α, respectively.
DISCUSSION
Based on the pathophysiological findings, endocarditis has been regarded as an immune complex-mediated syndrome characterized by depositions of circulating immune complexes identified in kidney, spleen, and cutaneous lesions (4). Necropsy studies have shown the presence of glomerulonephritis in a large proportion of human endocarditis patients, and immunofluorescence studies have characterized the lesion as mediated by immune complex deposition. Antibody specific for the infecting organism and bacterial cell wall constituents have been identified within immune complexes (3). IL-6 is a 21-kDa multifunctional protein that plays critical roles in the mediation of inflammatory and immune responses initiated after infection or injury (10). Therefore, the chronic inflammatory response observed in endocarditis might be related to the production of excess proinflammatory cytokines, such as TNF-α, IL-β, IL-6, and IL-8. Concomitantly, a clinical survey indicated that levels of cytokines, especially IL-6, in plasma were elevated on all occasions in patients with endocarditis (18). In this report, by examination with a purified system in vitro, we demonstrate that GTFs of S. mutans are primary bacterial products that preferentially induce IL-6 from T cells. Concomitantly, GTFs are the major constituents responsible for the elevated levels of IL-6 in serum when S. mutans enters the bloodstream, as demonstrated in a rat model. In this rat experiment, the induction of IL-6 occurred within an hour after intravenous injection of the wild-type S. mutans strain and peaked around 6 h afterwards. However, the induction of IL-6 in serum by the GTF-defective mutant was significantly reduced in terms of the levels of cytokines as well as the periods of persistence. The rat experiments provided direct evidence to indicate that GTFs of S. mutans play a significant role in the induction of the proinflammatory cytokine IL-6 in vivo, and this evidence correlated well with the results obtained in vitro.
Modulation of TT-induced IL-2 by S. mutans has been demonstrated previously (16), and lipoteichoic acid (LTA) from this and other gram-positive microorganisms was found to inhibit the biological functions of IL-2 through direct binding to IL-2 (17). Results of the present study suggested that GTFs of S. mutans might also play a significant role in the modulation of detectable levels of IL-2 induced by other antigens. Interestingly, the production of IFN-γ, another cytokine associated with a Th1-type response, was also inhibited in the T cells from some but not all individuals. However, LTA stimulated the production of IFN-γ in T cells in response to TT (17). Therefore, GTFs might play a role distinct from that of LTA in modulating induction or production of cytokines from T cells. The IL-10 that we detected in response to S. mutans and GTFs may not account for the decrease in IL-2 or IFN-γ, because the IL-10 level in the culture supernatant of TT-stimulated T cells was not changed when GTFs were added. In addition, the reduction in the level of IL-2 by GTF did not inhibit antigen-specific T-cell proliferation, suggesting that reduction of IL-2 production alone is not sufficient to inhibit lymphocyte proliferation. Similar phenomena have also been observed for the urease from Helicobacter pylori. The H. pylori urease inhibited mitogen-stimulated IL-2 production by PBMC and Jurkat T cells, but such inhibition had no effect on lymphocyte proliferation (15).
S. mutans expresses three GTFs (1, 11, 12) with distinct functions and localization. GtfB and GtfC are cell wall associated and synthesize primarily insoluble glucan, whereas GtfD is secreted and synthesizes water-soluble glucan. Comparison of amino acid sequences between the isozymes indicated that GtfB and GtfC share an overall 79% identity in amino acid residues and about 58% homology with GtfD. We found that S. mutans GTFs efficiently stimulated humoral and cellular immune responses in young adults; although the response to GtfD is higher, there is a robust response to GtfC as well (6, 8). In this report, we provide further evidence to indicate that GtfC and GtfD directed production of distinct cytokine profiles in T cells, specifically in regard to the induction of IL-6 in vitro and in vivo. No statistically significant difference was identified between GtfC and GtfD in terms of the levels of IL-6 induced in vitro, but the specificities of each of these closely related molecules in vivo as well as the question of distinct roles for GtfB, GtfC, and GtfD await further investigation.
Components of S. mutans other than GTFs have been shown to stimulate the production of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, from monocytes. One component is cell wall polysaccharide with a poly-l-rhamnose backbone with d-glucose side chains, and the other component is part of a family of immunologically and structurally related cell surface proteins termed antigen I/II (19, 20). Although both molecules were stimulators for production of cytokines in a variety of cells, including monocytes and endothelial and epithelial cells, the effects of these molecules in vivo have not yet been examined. Distinct from these studies, we demonstrated in the present study that GTFs show unique properties in stimulating the production of IL-6 in vitro and in vivo. Similar to protein I/II, GTFs are a family of structurally related enzymes found in several but not all viridans streptococcus species with different isozymes. Interestingly, patients with infective endocarditis caused by viridans streptococci that express GTFs had elevated IL-6 levels in their peripheral blood during the acute stage of infection. These results suggested that GTFs might play a similar role in humans during systemic infection in the activation of endogenous inflammatory mediators released by monocytes.
Acknowledgments
We thank H. K. Kuramitsu for providing the NHS1DD strain. We thank Tim J. Harrison, Reader in Molecular Virology, Department of Medicine, Royal Free and University College Medical School, UCL, for his kind review and help in the preparation of the manuscript.
This work was supported in part by the National Science Council (grant NSC-902320-B002-134) and the National Health Research Institute (grant NHRI-EX91-9139SI).
REFERENCES
- 1.Aoki, H., T. Shiroza, M. Hayakawa, S. Sato, and H. K. Kuramitsu. 1986. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect. Immun. 53:587-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baddour, L. M. 1988. Twelve-year review of recurrent native valve infectious endocarditis: a disease of the modern antibiotic era. Rev. Infect. Dis. 10:1163-1170. [DOI] [PubMed] [Google Scholar]
- 3.Bayer, A. S., and A. N. Theofilopoulos. 1990. Immunopathogenetic aspects of infective endocarditis. Chest 97:204-212. [DOI] [PubMed] [Google Scholar]
- 4.Brown, M., and G. E. Griffin. 1998. Immune responses in endocarditis. Br. Heart J. 79:1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, S. C., K. T. Luh, L. J. Deng, and W. C. Hsieh. 1987. Bacteriology of viridans streptococcal bacteremia. Chin. J. Microbiol. Immunol. 20:311-318. [PubMed] [Google Scholar]
- 6.Chia, J. S., S. W. Lin, C. S. Yang, and J. Y. Chen. 1997. Antigenicity of a synthetic peptide from glucosyltransferase of Streptococcus mutans in humans. Infect. Immun. 65:1126-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chia, J. S., C. S. Yang, and J. Y. Chen. 1993. Analysis of a DNA polymorphic region in the gtfB and gtfC genes of Streptococcus mutans. Infect. Immun. 65:1563-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chia, J. S., C. M. You, C. Y. Hu, B. L. Chang, and J. Y. Chen. 2001. Human T-cell responses to the glucosyltransferases of Streptococcus mutans. Clin. Diagn. Lab. Immunol. 8:441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fekete, T. 1990. Controversies in the prevention of infective endocarditis related to dental procedures. Dent. Clin. N. Am. 34:79-90. [PubMed] [Google Scholar]
- 10.Gadient, R. A., and U. H. Otten. 1997. Interleukin-6 (IL-6)—a molecule with both beneficial and destructive potentials. Prog. Neurobiol. 52:379-390. [DOI] [PubMed] [Google Scholar]
- 11.Hanada, N., and H. K. Kuramitsu. 1988. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect. Immun. 56:1999-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanada, N., and H. K. Kuramitsu. 1989. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect. Immun. 57:2079-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horaud, T., and F. Delbos. 1984. Viridans streptococci in infective endocarditis: species distribution and susceptibility to antibiotics. Eur. Heart J. 5(Suppl. C):39-44. [DOI] [PubMed] [Google Scholar]
- 14.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer, F., K. T. Wilson, and S. P. James. 2000. Modulation of innate cytokine responses by products of Helicobacter pylori. Infect. Immun. 68:6265-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plitnick, L. M., J. A. Banas, D. M. Jelley-Gibbs, J. O'Neil, T. Christian, and S. P. Mudzinski. 1998. Inhibition of interleukin-2 by a Gram-positive bacterium, Streptococcus mutans. Immunology 95:522-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plitnick, L. M., R. A. Jordan, J. A. Banas, D. M. Jelley-Gibbs, M. C. Walsh, M. T. Preissler, and E. J. Gosselin. 2001. Lipoteichoic acid inhibits interleukin-2 (IL-2) function by direct binding to IL-2. Clin. Diagn. Lab. Immunol. 8:972-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawczynska-Englert, I., T. Hryniewiecki, and D. Dzieranowska. 2000. Evaluation of serum cytokine concentrations in patients with infective endocarditis. J. Heart Valve Dis. 9:705-709. [PubMed] [Google Scholar]
- 19.Soell, M., F. Holveck, M. Scholler, D. Wachsmann, and J. P. Klein. 1994. Binding of Streptococcus mutans SR protein to human monocytes: production of tumor necrosis factor, interleukin 1, and interleukin 6. Infect. Immun. 62:1805-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soell, M., E. Lett, F. Holveck, M. Scholler, D. Wachsmann, and J. P. Klein. 1994. Activation of human monocytes by streptococcal rhamnose glucose polymers is mediated by CD14 antigen, and mannan binding protein inhibits TNF-α release. J. Immunol. 154:851-860. [PubMed] [Google Scholar]
- 21.Tsumori, H., and H. Kuramitsu. 1997. The role of the Streptococcus mutans glucosyltransferases in the sucrose-dependent attachment to smooth surfaces: essential role of the GtfC enzyme. Oral Microbiol. Immunol. 12:274-280. [DOI] [PubMed] [Google Scholar]
- 22.Vernier, A., M. Diab, M. Soell, G. Haan-Archipoff, A. Beretz, D. Wachsmann, and J. P. Klein. 1996. Cytokine production by human epithelial and endothelial cells following exposure to oral viridans streptococci involves lectin interactions between bacteria and cell surface receptors. Infect. Immun. 64:3016-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]