Abstract
A diagnostic method has been developed to detect anti-Leishmania donovani immunoglobulin G (IgG) in urine by enzyme-linked immunosorbent assay (ELISA). In measuring anti-L. donovani IgG, IgA, and IgM in urine, the method performed best in the detection of IgG. The sensitivity and specificity of the assay were determined with panels of urine samples from 62 visceral leishmaniasis (VL) patients, 59 healthy controls from areas of endemicity, 53 healthy controls from areas of nonendemicity, 59 malaria patients, 13 tuberculosis patients, 23 cutaneous leishmaniasis patients, and 7 patients with other diseases. Using L. donovani promastigote crude antigen, the test had 93.5% sensitivity (58 positives of 62 VL patient samples) and 89.3% specificity (191 negatives of 214 non-VL patient samples). The ELISA with acetone-treated L. donovani promastigote antigen raised the sensitivity and specificity to 95.0 and 95.3%, respectively. Western blot analysis revealed that most of the samples that cross-reacted with crude antigen in ELISA did not recognize any antigenic component of L. donovani crude antigen. We also checked 40 serum samples from the same group of VL patients for anti-L. donovani IgG and got 90.0% sensitivity with both crude and acetone-treated antigens. As collection of urine is much easier than collection of serum, the detection of anti-L. donovani IgG in urine with acetone-treated antigen will be useful in epidemiological studies. It could be an adjunct of laboratory diagnosis.
Visceral leishmaniasis (VL) is caused by protozoan parasites of the genus Leishmania and is transmitted by an insect vector, the phlebotomine sandfly. More than 47 countries are currently affected by leishmaniasis, with at least 200 million people at risk and approximately 100,000 new cases annually (2). Ninety percent of the cases occur in Bangladesh, India, Nepal, and Sudan. Bangladesh alone contributes about 15,000 new cases annually (10). Both the disease incidence and its severity—it is lethal if left untreated—are linked to poverty: malnutrition is associated with 8.7-times-higher risk for VL (7). Since the disease occurs mainly in areas where health services are poorly developed, the development of a simple, cheap, and reliable diagnostic method is necessary.
Demonstration of the parasites in bone marrow aspirates or needle biopsy specimens of the spleen and lymph node or by vitro cultivation are the definitive methods of diagnosis (27). However, these methods are insufficiently sensitive, and the techniques are invasive, painful, and even hazardous (24). A number of serological tests have been developed and evaluated for the diagnosis of VL, including immunofluorescent-antibody tests (40, 43), enzyme-linked immunosorbent assay (ELISA) (12, 14, 15, 20, 22, 25, 33, 42, 46), dot ELISA (23, 31, 36), immunoblot analysis (28, 34, 35), and the direct agglutination test (DAT) (6, 17, 18, 19, 29, 30, 32, 41). Due to its simplicity and high sensitivity and specificity, the DAT has already been introduced as a routine serological test for diagnosis of VL in India and Bangladesh, and the parameters of the test have been established under local conditions (1, 8, 9, 10, 13, 41).
In general, urine samples can be collected more easily than serum samples. To take advantage of this, several immunodiagnostic methods using urine have been established for some other diseases, like filariasis (21) and schistosomiasis (37). Kohanteb et al. (26) reported the detection of soluble antigen and antibody in the urine of VL patients by double-countercurrent immunoelectrophoresis, and de Colmenares et al. (11) detected antigenic compounds in urine by the Western blot technique. More recently, a latex agglutination test for the detection of Leishmania donovani antigen in urine was reported with good specificity but with sensitivity similar to that of microscopic diagnosis (3).
In this paper, we report a sensitive and specific ELISA to detect anti-L. donovani immunoglobulin G (IgG) in urine using acetone-treated promastigote antigen.
MATERIALS AND METHODS
Urine and serum samples.
Sixty-two urine samples from defined VL patients were collected from different medical college hospitals in Bangladesh. VL was diagnosed on the basis of clinical symptoms, including intermittent chronic fever for at least 1 month, hepatosplenomegaly, anemia, and wasting, along with hematological features of pancytopenia and reversed albumin globulin ratio and positive response to sodium antimony treatment. Among the 62 patients, 20 were confirmed parasitologically: Leishman-Donovan bodies were detected in splenic aspirates of seven patients and bone marrow aspirates of five patients, and promastigotes were demonstrated in eight patients after inoculations of aspirate materials in Novy, MacNeal, and Nicolle medium. Of the other patients, 39 were DAT positive, and the remaining 3 were aldehyde test positive (DAT was not done). In Bangladesh, the DAT (13) and the aldehyde test are used as routine serological tests for VL. During the collection of samples, all patients were in a course of treatment with sodium antimony gluconate at the World Health Organization recommended dose of 20 mg/kg of body weight (intramuscular or intravenous) or a maximum dose of 850 mg/day for 20 days (44). The average age of the patients was 26 years, ranging from 4 to 65 years. Fifty-nine control samples were taken from apparently healthy individuals in Bangladesh having no past history of kala-azar. In selecting healthy controls from areas of endemicity, we tried to match the age and sex with those of VL patients. Fifty-three samples from healthy Japanese individuals were used as healthy controls from areas of nonendemicity. Thirteen tuberculosis samples were collected from the Tuberculosis Hospital, Rajshahi, Bangladesh. Seven samples from patients with other diseases, including two patients with amebic liver abscess, two patients with aplastic anemia, one patient with aplastic anemia with nephrotic syndrome, one patient with aortic stenosis, and one patient with viral fever, were collected from Rajshahi Medical College Hospital, Rajshahi, Bangladesh. Fifty-nine malaria patient samples collected in the Solomon Islands and 23 cutaneous leishmaniasis (CL) patient samples from Ecuador were also used. Immediately after collection, NaN3 was added to each sample at a final concentration of 0.1% as a preservative to prevent bacterial growth. The samples were stored at 4°C except during the period of transportation to Japan. After the collection of urine, the samples were tested for antibodies within 12 months. In another study, it was shown that the filaria-specific IgG4 titer in urine samples preserved with 0.1% NaN3 and stored at 4°C did not change within 1 year (unpublished data). Also, filaria-specific IgG4 units in urine samples preserved with 0.1% NaN3 and stored at 37°C did not change within 4 weeks (21).
Forty serum samples from the same group of VL patients and 54 Japanese control sera, obtained from different individuals in the healthy control group from areas of nonendemicity for urine samples, were used in this study.
The participants were first informed that these samples would be carried to Japan for research purposes only and not for the diagnosis of their individual disease. Then, all the samples were collected with consent from the patients or from their parents when they were of minor age.
Parasite and antigen.
L. donovani strain DD8, isolated from a Bangladeshi patient, was used (38). Promastigotes were maintained in blood agar medium (USMARU; Difco) containing 20% defibrinated rabbit blood (Nippon Bio-Test Laboratories Inc., Tokyo, Japan) at 25°C (39). Mass cultivation of parasites was done in RPMI 1640 medium containing l-glutamine and NaHCO3 (Sigma, St. Louis, Mo.) supplemented with 100 U of penicillin G/ml, 50 μg of streptomycin/ml, and 1 mg of l-proline/ml. The parasites were harvested when a majority of the population of promastigotes attained elongated shape; they were washed three times with phosphate-buffered saline (PBS), pH 7.4, at 4°C by centrifugation at 1,600 × g for 15 min each time. The final pellets were kept at −40°C until they were used.
(i) Crude antigen.
The pellets were resuspended in 20 volumes of cold (4°C) PBS containing protease inhibitor cocktail (Sigma) followed by sonication in an ice bath. The lysate was centrifuged at 15,000 × g for 15 min at 4°C. The supernatant was collected, and the protein concentration was measured by protein assay (Bio-Rad, Richmond, Calif.) (1 μg of protein/1.3 × 105 promastigotes) and stored at −40°C.
(ii) Acetone-treated antigen.
The pellets were resuspended in 20 volumes of cold acetone, incubated at −20°C for 2 h, and then centrifuged at 15,000 × g for 10 min at −10°C. The supernatant was discarded, and the sediment was dried by gentle suctioning. The sediment was resuspended in 20 volumes of cold PBS (4°C) containing protease inhibitors, followed by sonication, centrifugation, and estimation of the protein concentration (1 μg of protein/4.2 × 105 promastigotes) as described for the crude antigen, and finally stored at −40°C.
ELISA.
ELISA was carried out in flat-bottom 96-well microtiter plates (MaxiSorp; Nunc, Roskilde, Denmark). The plates were coated with 5 μg of crude or acetone-treated antigen/ml (100 μl/well) in coating buffer (0.05 M carbonate-bicarbonate buffer, pH 9.6) and incubated overnight at 4°C. After being blocked with casein buffer (1% casein in 0.05 M Tris-HCl buffer with 0.15 M NaCl, pH 7.6) for 2 h at room temperature, the wells were loaded with 100 μl of urine (1:10 dilution in casein buffer) or serum (1:4,000 dilution in casein buffer) and incubated at 37°C for 1 h. The plates were washed four times with PBS containing 0.05% Tween 20 (pH 7.4) and incubated with peroxidase-conjugated goat anti-human IgG (Tago, Camarillo, Calif.), IgA (Zymed, South San Francisco, Calif.), or IgM (Tago) (1:4,000 dilution in casein buffer) at 37°C for 1 h. After being washed four times, the plates were incubated with substrate ABTS (KPL Inc., Gaithersburg, Md.) for 1 h at room temperature, and the optical density was measured at 415 nm. Each sample was assayed in duplicate.
Antibody levels were expressed as units on the basis of a standard curve. To construct the curve, pooled sera from four VL patients were serially diluted threefold (1:20,000 to 1:14,580,000) with casein buffer supplemented with sodium azide, and a set of the serially diluted sera was prepared for each microtiter plate. As antibody units, a value of 7,290 U was arbitrarily assigned to the 1:20,000 dilution and a value of 10 U to the 1:14,580,000 dilution. Antibody unit levels of >7,290 U were considered as 7,290 U. The cutoff point for anti-L. donovani IgG was set as the mean plus 3 standard deviations of log (unit + 1) values of the healthy controls from areas of nonendemicity and of the Japanese controls for urine and serum, respectively, to obtain maximum sensitivity and specificity. With crude antigen, the antilogarithmic cutoff values for urine and serum were 8.2 and 443.0 U, respectively. With acetone-treated antigen, the antilogarithmic values were 7.4 U for urine and 313.9 U for serum. The cutoff points for anti-L. donovani IgA and IgM were set as the means plus 3 standard deviations of log (optical density + 1) values of the healthy controls from areas of nonendemicity, and the antilogarithmic values were 0.045 for IgA and 0.008 for IgM.
Immunoblot analysis.
Crude antigen samples were electrophoresed on sodium dodecyl sulfate-8 to 16% polyacrylamide gels, 1.5 mm thick (Tefco, Tokyo, Japan), with a wide-range molecular mass marker (Tefco). The separated proteins were transferred to polyvinylidene difluoride membranes (0.45-μm pore size) under constant voltage at 100 V for 1.5 h in a transblotting chamber (Bio-Rad). The membrane was blocked for 20 min with casein buffer and incubated with two-times-diluted urine at 37°C for 1 h. After three washes with casein buffer, the membrane strips were incubated with peroxidase-conjugated goat anti-human IgG (1:1,000 dilution) for 1 h at 37°C. Bound antibodies were visualized with Konica immunostaining HRP-100 (Seikagaku Corp., Tokyo, Japan). The relative molecular weights of the detected bands were calculated with Quantity One software (version 2.7; PDI, Inc., New York, N.Y.).
RESULTS
Anti-L. donovani IgG.
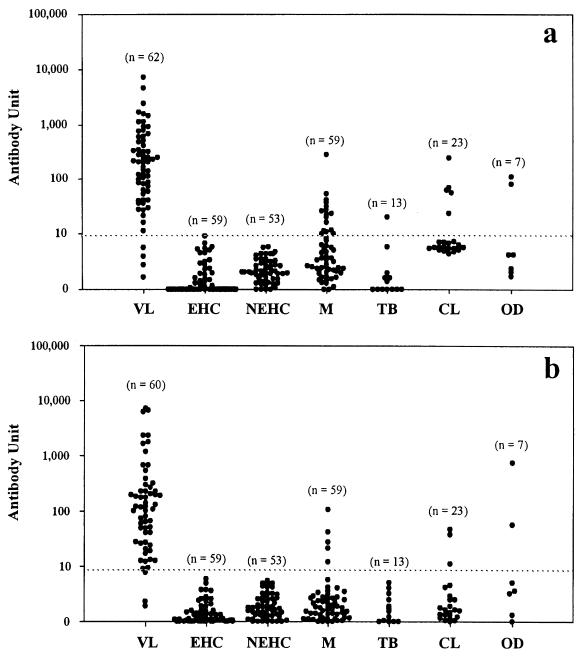
Urine samples from 62 VL and 214 non-VL patients were tested for IgG, using crude antigen (Fig. 1a). The test showed a sensitivity of 93.5% (58 positives of 62 VL patient samples) and a specificity of 89.3% (191 negatives of 214 non-VL patient samples). Urine samples from 60 VL and 214 non-VL patients were tested for IgG, using acetone-treated antigen (Fig. 1b). Two VL patient samples could not be tested because of inadequate quantity. The test showed increased sensitivity and specificity compared to the crude antigen, with a sensitivity of 95.0% (57 positives of 60 VL patient samples) and a specificity of 95.3% (204 negatives of 214 non-VL patient samples). In both assays, the healthy controls from areas of endemicity and nonendemicity showed 100% specificity. In the other-diseases category, two false positives—aplastic anemia and aplastic anemia with nephrotic syndrome—were detected in both assays. The specificities for malaria, tuberculosis, and CL were improved in the assay with acetone-treated antigen (Table 1).
FIG. 1.
Detection of anti-L. donovani IgG in urine of visceral leishmaniasis patients, healthy individuals, and controls with various diseases by ELISA with crude antigen (a) or acetone-treated antigen (b). Each symbol (•) stands for a single urine sample. The horizontal dotted lines represent the cutoff values: 8.2 (a) and 7.4 (b) U. EHC, healthy controls from areas of endemicity; NEHC, healthy controls from areas of nonendemicity; M, malaria; TB, tuberculosis; OD, other diseases; n, number of samples.
TABLE 1.
Sensitivity and specificity of ELISA for the detection of different immunoglobulin classes in urine and serum using L. donovani crude antigen or acetone-treated antigen
| Sample | Antigen | Ig class | Sensitivity for VLa | Specificitya,b
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| EHC | NEHC | M | TB | CL | OD | ||||
| Urine | Acetone treated | IgG | 57/60 (95.0) | 59/59 (100) | 53/53 (100) | 54/59 (91.5) | 13/13 (100) | 20/23 (87.0) | 5/7 (71.4) |
| Crude | IgG | 58/62 (93.5) | 59/59 (100) | 53/53 (100) | 44/59 (74.6) | 12/13 (92.3) | 18/23 (78.3) | 5/7 (71.4) | |
| IgM | 30/45 (66.7) | 12/13 (92.3) | 14/14 (100) | − | − | − | 4/7 (57.1) | ||
| IgA | 17/45 (37.8) | 13/13 (100) | 14/14 (100) | − | − | − | 3/7 (42.9) | ||
| Serum | Acetone treated | IgG | 36/40 (90.0) | − | 54/54 (100) | − | − | − | − |
| Crude | IgG | 36/40 (90.0) | − | 54/54 (100) | − | − | − | − | |
Number positive/total (percent).
EHC, healthy controls from areas of endemicity; NEHC, healthy controls from areas of nonendemicity; M, malaria; TB, tuberculosis; OD, other diseases; −, not tested.
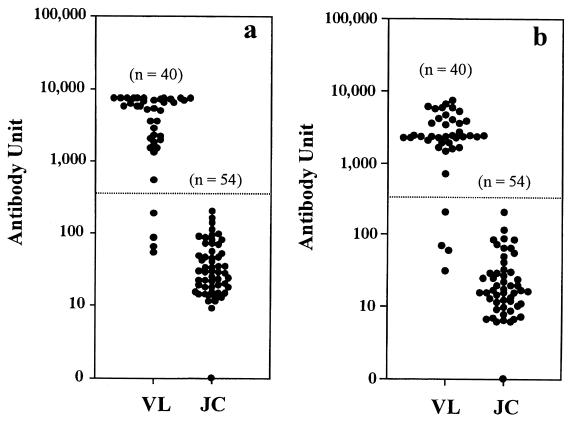
Serum samples from 40 VL patients and 54 Japanese controls were tested for IgG using both crude and acetone-treated antigens (Fig. 2). Both assays showed the same sensitivity and specificity, which were 90.0 and 100%, respectively (Table 1).
FIG. 2.
Detection of anti-L. donovani IgG in sera of VL patients and healthy Japanese controls by ELISA with crude antigen (a) or acetone-treated antigen (b). Each symbol (•) stands for a single serum sample. The horizontal dotted lines represent the cutoff values: 443.0 (a) and 313.9 (b). JC, Japanese controls; n, number of samples.
Anti-L. donovani IgA and IgM.
Urine samples from 45 VL and 34 non-VL patients were tested for IgA and IgM with crude antigen (Table 1). Both assays showed poor sensitivities, 37.8% for IgA and 66.7% for IgM. Samples from four patients with other diseases (one patient with amebic liver abscess, two patients with aplastic anemia, and one patient with aplastic anemia with nephrotic syndrome) gave false-positive results with IgA. One healthy control from an area of endemicity and three patients with other diseases (two patients with aplastic anemia and one patient with aplastic anemia with nephrotic syndrome) gave false-positive results with IgM.
Immunoblot analysis of the urine samples.
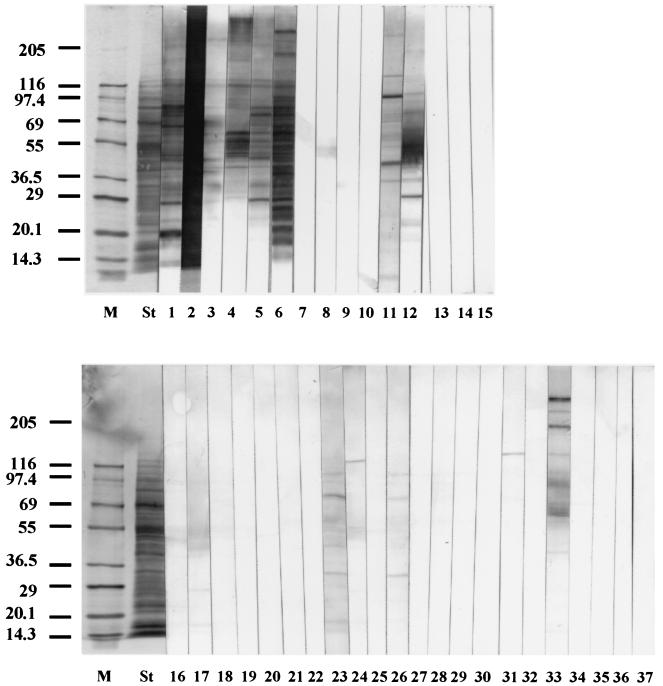
Urine samples for immunoblot analysis were selected according to the ELISA result of an anti-L. donovani IgG assay using crude antigen. All 23 false-positive and 4 false-negative VL patient samples, along with randomly selected control samples (six positive VL patient samples and two negative healthy samples from areas of endemicity) were tested for IgG reactivity (Fig. 3). All positive VL patient samples recognized numerous antigenic components ranging from 10 to 283 kDa. Antigenic components of 126, 116, 94, 84, 65, 60, 55, 45, 41, and 29 kDa were frequently recognized by VL patient samples, of which 126 and 116 kDa were common to all. Only one false-negative VL patient sample faintly recognized a single band at the 51-kDa position. This sample proved to be positive with the acetone-treated antigen in ELISA. The other three false negatives did not react, but two of them were parasite positive. Two negative (healthy controls from areas of endemicity) samples and 8 out of 15 malaria patient samples did not recognize any component. Two aplastic anemia patient samples recognized several antigenic components, of which 60, 45, 41, and 29 kDa were shared among some of the VL patient samples. The tuberculosis patient sample recognized the 132-kDa component. Of five CL patient samples, one recognized several components, of which the 65-kDa component was common in some of the VL patient samples. Another CL patient sample recognized two components very faintly, and the other three did not recognize any.
FIG. 3.
Immunoblot profiles of L. donovani crude antigen polypeptides reacted with urine of VL patients and various controls. Lanes: M, molecular mass marker; St, separated polypeptides stained with Coomassie brilliant blue; 1 to 6, ELISA-positive VL; 7 to 10, ELISA-negative VL; 11 and 12, aplastic anemia; 13 and 14, healthy controls from areas of endemicity; 16 to 30, malaria; 31, tuberculosis; 32 to 36, CL; 15 and 37, casein buffer. The positions of molecular-mass markers in kilodaltons are shown on the left.
DISCUSSION
Detection of parasites is most definitive in the diagnosis of VL. However parasitological diagnosis is invasive and often not sensitive. Zijlstra et al. (45) found sensitivities of 93.1, 67.1, and 56.3% for the demonstration of causative parasites in spleen, bone marrow, and lymph node aspirates, respectively, in a study of 87 hospitalized patients. A community-based survey in Bangladesh showed that only 29 of 125 (23%) clinically suspected persons were confirmed parasitologically, but all responded favorably to treatment (8). In another study conducted in Bangladesh with 715 parasitologically confirmed and 558 parasitologically negative but clinically confirmed kala-azar patients, 97.5% responded successfully to sodium antimony treatment; among them, >90% showed response within 3 to 5 days of treatment (10). In these situations and because of the serious therapeutic implications related to an incorrect or late diagnosis of VL, there is a strong need for an accurate laboratory test to confirm the clinical diagnosis. Additionally, this disease occurs mostly in young populations. A study in Brazil reported that 78% of cases occurred in children <5 years of age (4). Another study in Bangladesh reported that a majority (98.2%) of VL patients were below 25 years old (10). Therefore, the diagnostic method should be easy and readily acceptable to such populations. Our ELISA with urine would meet this requirement in terms of relative ease in collecting samples. In this assay system, serum was not a better sample than urine. The ELISA will be particularly useful in epidemiological studies.
One of the problems of serological assays for VL is the existence of cross-reactions with other diseases, including African trypanosomiasis (sleeping sickness), mucocutaneous leishmaniasis, cutaneous leishmaniasis, malaria, tuberculosis, leprosy, and amebiasis, which are coendemic in some parts of the world (5, 17, 23, 31). In our study, a considerable number of cross-reactions with malaria, tuberculosis, CL, and aplastic anemia were noted with the crude antigen. With the use of acetone-treated antigen, one out of four false-negative VL patient samples turned to positive and 13 out of 23 false-positive samples (10 malaria, 1 tuberculosis, and 2 CL patient samples) turned to negative and improved the total sensitivity to 95.0% and the specificity to 95.3%. The immunoblot analysis revealed that most of the samples which proved to be negative with the acetone-treated antigen did not recognize any antigenic component, whereas those samples remaining positive detected several antigenic components. As acetone treatment caused the removal of lipid from parasite antigens, cross-reactions due to lipid portions must have been reduced.
Use of the acetone-treated antigen could not reduce cross-reactions in aplastic anemia. Two of the three aplastic anemia patient samples showed very high antibody titers with all classes of immunoglobulins. The patients did not have any history of VL. As aplastic anemia patients need frequent blood transfusions, the possibility of receiving blood with a high antibody titer against L. donovani cannot be ruled out. Further investigations are necessary to distinguish these two, as both diseases cause pancytopenia.
In a posttreatment follow-up, Hailu (16) reported that the rate of negative change was 50% up to 1 year and 89% from 2 to 8 years with serum-based ELISA. In contrast, with the DAT it was 0% by 1 year and only 33% by 2 to 8 years. This indicates that the performance of ELISA is better than that of the DAT in the posttreatment follow-up. It will be worthwhile to test our urine-based ELISA after treatment. In addition, the potential use of ELISA in early detection of asymptomatic and subclinical infection and the prognosis after early treatment also need evaluation.
Acknowledgments
We are grateful to A. R. M. Saifuddin Ekram (Department of Medicine, Rajshahi Medical College) for guidance in sample collection from Rajshahi Medical College Hospital.
REFERENCES
- 1.Addy, M., D. K. Som, C. Das, S. Bhattacharya, T. Bowmik, P. Rakshit, P. Patra, A. Nandy, and A. B. Choudhury. 1989. Evaluation of direct agglutination test (DAT) in the diagnosis and screening of kala-azar. Indian Med. Gaz. 123:184-187. [Google Scholar]
- 2.Ashford, R. W., P. Desjeux, and P. de Raadt. 1992. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitol. Today 8:104-105. [DOI] [PubMed] [Google Scholar]
- 3.Attar, Z. J., M. L. Chance, S. el-Safi, J. Carney, A. Azazy, M. El-Hadi, C. Dourado, and M. Hommel. 2001. Latex agglutination test for the detection of urinary antigens in visceral leishmaniasis. Acta Trop. 78:11-16. [DOI] [PubMed] [Google Scholar]
- 4.Badaro, R., T. C. Jones, R. Lorenco, B. J. Cerf, D. Sampio, E. M. Carvalho, H. Rocha, R. Teixeira, and W. D. Johnson, Jr. 1986. A prospective study of visceral leishmaniasis in an endemic area of Brazil. J. Infect. Dis. 154:639-649. [DOI] [PubMed] [Google Scholar]
- 5.Badaro, R., S. G. Reed, A. Barral, G. Orge, and T. C. Jones. 1986. Evaluation of the micro enzyme-linked assay (ELISA) for antibodies in American visceral leishmaniasis: antigen selection for detection of infection-specific response. Am. J. Trop. Med. Hyg. 35:72-78. [DOI] [PubMed] [Google Scholar]
- 6.Boelaert, M., S. El Safi, D. Jacquet, A. De Muynck, P. V. D. Stuyft, and D. Le Ray. 1999. Operational validation of the direct agglutination test for diagnosis of visceral leishmaniasis. Am. J. Trop. Med. Hyg. 60:129-134. [DOI] [PubMed] [Google Scholar]
- 7.Cerf, B. J., T. C. Jones, R. Badaro, D. Sampaio, R. Teixeira, and W. D. Johnson, Jr. 1987. Malnutrition as a risk factor for severe visceral leishmaniasis. J. Infect. Dis. 156:1030-1033. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury, M. S., A. el Harith, A. al Massum, E. al Karim, and A. al Rahman. 1993. Prevalence of agglutinating anti-Leishmania antibodies in two multi-thousand Bengali communities. Parasitol. Res. 79:444-450. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury, M. S., A. al Masum, E. al Karim, S. Semia'o-Santos, K. M. Rahman, H. ar-Rashid, and A. el Harith. 1993. Applicability of direct agglutination test (DAT) at a rural health setting in Bangladesh and feasibility of local antigen production. Arch. Inst. Pasteur Tunis 70:333-344. [PubMed] [Google Scholar]
- 10.Chowdhury, S., F. Haque, A. al-Masum, A. el Harith, and E. Karim. 1991. Positive response to sodium antimony gluconate administration in visceral leishmaniasis seropositive patients. Am. J. Trop. Med. Hyg. 44:390-393. [DOI] [PubMed] [Google Scholar]
- 11.De Colmenares, M., M. Portus, C. Riera, M. Gallego, M. J. Aisa, S. Torras, and C. Munoz. 1995. Detection of 72-75-kD and 123-kD fractions of Leishmania antigen in urine of patients with visceral leishmaniasis. Am. J. Trop. Med. Hyg. 52:427-428. [DOI] [PubMed] [Google Scholar]
- 12.El Amin, E. R. M., P. A. Wright, P. A. Kager, J. J. Laarman, and K. W. Pondman. 1985. ELISA using intact promastigotes for immunodiagnosis of kala-azar. Trans. R. Soc. Trop. Med. Hyg. 79:344-350. [DOI] [PubMed] [Google Scholar]
- 13.El-Masum, M. A., D. A. Evans, D. M. Minter, and A. El Harith. 1995. Visceral leishmaniasis in Bangladesh: the value of DAT as a diagnostic tool. Trans. R. Soc. Trop. Med. Hyg. 89:185-186. [DOI] [PubMed] [Google Scholar]
- 14.Fargeas, C., M. Hommel, R. Maingon, C. Dourado, M. Monsigny, and R. Mayer. 1996. Synthetic peptide-based enzyme-linked immunosorbent assay for serodiagnosis of visceral leishmaniasis. J. Clin. Microbiol. 34:241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guimares, M. C., B. J. Celeste, E. A. DeCastilho, J. R. Mineo, and J. M. Diniz. 1981. Immunoenzymic assay (ELISA) in mucocutaneous leishmaniasis, kala-azar and chagas' disease: an epimastigote Trypanosoma cruzi antigen able to distinguish between anti-Trypanosoma and anti-Leishmania antibodies. Am. J. Trop. Med. Hyg. 30:942-947. [DOI] [PubMed] [Google Scholar]
- 16.Hailu, A. 1990. Pre- and post-treatment antibody levels in visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 84:673-675. [DOI] [PubMed] [Google Scholar]
- 17.Harith, A. E., A. H. J. Kolk, J. Leeuwenburg, R. Muigai, S. Kiugu, S. Kiugu, and J. J. Laarman. 1986. A simple and economical direct agglutination test for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 80:583-587. [DOI] [PubMed] [Google Scholar]
- 18.Harith, A. E., A. H. J. Kolk, P. A. Kager, J. Leeuwenburg, F. J. Faber, R. Muigai, S. Kiugu, and J. J. Laaarman. 1987. Evaluation of a newly developed direct agglutination test (DAT) for serodiagnosis and seroepidemiological studies of visceral leishmaniasis: comparison with IFAT and ELISA. Trans. R. Soc. Trop. Med. Hyg. 81:603-606. [DOI] [PubMed] [Google Scholar]
- 19.Harith, A. E., A. H. J. Kolk, J. Leeuwenburg, R. Muigai, E. Huigen, T. Jelsma, and P. A. Kager. 1988. Improvement of a direct agglutination test for field studies of visceral leishmaniasis. J. Clin. Microbiol. 26:1321-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hommel, M., W. Peters, J. Ranque, M. Quilici, and G. Lanotte. 1978. The micro-ELISA technique in the serodiagnosis of visceral leishmaniasis. Ann. Trop. Med. Parasitol. 72:213-218. [DOI] [PubMed] [Google Scholar]
- 21.Itoh, M., M. V. Weerasooriya, X.-G. Qui, N. K. Gunawardena, M. T. Anantaphruti, S. Tesana, P. Rattanaxay, Y. Fujimaki, and E. Kimura. 2001. Sensitive and specific enzyme-linked immunosorbent assay for the diagnosis of Wuchereria bancrofti infection in urine samples. Am. J. Trop. Med. Hyg. 65:362-365. [DOI] [PubMed] [Google Scholar]
- 22.Jaffe, C. L., and D. McMahon-Pratt. 1987. Serodiagnostic assay for visceral leishmaniasis employing monoclonal antibodies. Trans. R. Soc. Trop. Med. Hyg. 81:587-594. [DOI] [PubMed] [Google Scholar]
- 23.Jaffe, C. L., and M. Zalis. 1988. Use of purified parasite proteins from Leishmania donovani for the rapid serodiagnosis of visceral leishmaniasis. J. Infect. Dis. 157:1212-1220. [DOI] [PubMed] [Google Scholar]
- 24.Kar, K. 1995. Serodiagnosis of leishmaniasis. Crit. Rev. Microbiol. 21:123-152. [DOI] [PubMed] [Google Scholar]
- 25.Kaul, P., N. Malla, S. Kaur, R. C. Mahajan, and N. K. Ganguly. 2000. Evaluation of a 200-kDa amastigote-specific antigen of L. donovani by enzyme-linked immunosorbent assay (ELISA) for the diagnosis of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 94:173-175. [DOI] [PubMed] [Google Scholar]
- 26.Kohanteb, J., S. M. Ardehali, and H. R. Rezai. 1987. Detection of Leishmania donovani soluble antigen and antibody in the urine of visceral leishmaniasis patients. Trans. R. Soc. Trop. Med. Hyg. 81:578-580. [DOI] [PubMed] [Google Scholar]
- 27.Marsden, P. D. 1979. Current concepts in parasitology—leishmaniasis. N. Engl. J. Med. 300:350-352. [DOI] [PubMed] [Google Scholar]
- 28.Mary, C., D. Lamouroux, S. Dunan, and M. Quilici. 1992. Western blot analysis of antibodies to Leishmania infantum antigens: potential of the 14-kD and 16-kD antigens for diagnosis and epidemiologic purposes. Am. J. Trop. Med. Hyg. 47:764-771. [DOI] [PubMed] [Google Scholar]
- 29.Mbati, P. A., J. I. Githure, J. M. Kagai, G. Kirigi, F. Kibati, K. Wasunna, and D. K. Koech. 1999. Evaluation of a standardized direct agglutination test (DAT) for the diagnosis of visceral leishmaniasis in Kenya. Ann. Trop. Med. Parasitol. 93:703-710. [PubMed] [Google Scholar]
- 30.Meredith, S. E. O., N. C. M. Kroon, E. Sondorp, J. Seaman, M. G. A. Goris, C. W. van Ingen, H. Oosting, G. J. Schoone, W. J. Terpstra, and L. Oskam. 1995. Leish-KIT, a stable direct agglutination test based on freeze-dried antigen for serodiagnosis of visceral leishmaniasis. J. Clin. Microbiol. 33:1742-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pappas, M. G., J. W. Hajkowski, and W. T. Hockmeyer. 1983. Dot enzyme linked immunosorbent assay (dot-ELISA): a microtechnique for the rapid diagnosis of visceral leishmaniasis. J. Immunol. Methods 64:205-214. [DOI] [PubMed] [Google Scholar]
- 32.Rab, M. A., and D. A. Evans. 1997. Detection of anti-Leishmania antibodies in blood collected on filter paper by the direct agglutination test. Trans. R. Soc. Trop. Med. Hyg. 91:713-715. [DOI] [PubMed] [Google Scholar]
- 33.Raj, V. S., A. Ghosh, V. S. Dole, R. Madhubala, P. J. Myler, and K. D. Stuart. 1999. Serodiagnosis of leishmaniasis with recombinant ORFF antigen. Am. J. Trop. Med. Hyg. 61:482-487. [DOI] [PubMed] [Google Scholar]
- 34.Rolland-Burger, L., X. Rolland, C. W. Grieve, and L. Monjour. 1991. Immunoblot analysis of the humoral immune response to Leishmania donovani infantum polypeptides in human visceral leishmaniasis. J. Clin. Microbiol. 29:1429-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salotra, P., A. Raina, and N. S. Negi. 1999. Immunoblot analysis of the antibody response to antigens of Leishmania donovani in Indian kala-azar. Br. J. Biomed. Sci. 56:263-267. [PubMed] [Google Scholar]
- 36.Senaldi, G., H. Xiao-Su, D. C. Hoessli, and C. Bordier. 1996. Serological diagnosis of visceral leishmaniasis by a dot-enzyme immunoassay for the detection of a Leishmania donovani-related circulating antigen. J. Immunol. Methods 193:9-15. [DOI] [PubMed] [Google Scholar]
- 37.Shaker, Z. A., M. A. Kaddah, S. B. Hanallah, and M. I. El-Khodary. 1998. Production of monoclonal antibodies against target schistosomal antigen secreted in the urine of Schistosoma mansoni-infected patients. Int. J. Parasitol. 28:1893-1901. [DOI] [PubMed] [Google Scholar]
- 38.Shamsuzzaman, S. M., M. Furuya, A. K. M. S. Choudhury, M. Korenaga, and Y. Hashiguchi. 2000. Characterisation of Bangladeshi Leishmania isolated from kala-azar patients by isoenzyme electrophoresis. Parasitol. Int. 49:139-145. [DOI] [PubMed] [Google Scholar]
- 39.Shamsuzzaman, S. M., M. Furuya, M. Korenaga, K. Imamura, and Y. Hashiguchi. 1999. Use of urine samples from healthy humans, nephritis patients or other animals as an alternative to foetal calf serum in the culture of Leishmania (L.) donovani in vitro. Ann. Trop. Med. Parasitol. 93:613-620. [DOI] [PubMed] [Google Scholar]
- 40.Shaw, J. J., and A. Voller. 1964. The detection of circulating antibody to kala-azar by means of immunofluorescence techniques. Trans. R. Soc. Trop. Med. Hyg. 58:349-352. [DOI] [PubMed] [Google Scholar]
- 41.Singla, N., G. S. Singh, S. Sundar, and V. K. Vinayak. 1993. Evaluation of direct agglutination test as an immunodiagnostic tool for kala-azar in India. Trans. R. Soc. Trop. Med. Hyg. 87:276-278. [DOI] [PubMed] [Google Scholar]
- 42.Srivastava, L., J. C. Suri, and R. K. Sanyal. 1979. ELISA test in diagnosis of kala-azar in current epidemic in Bihar. J. Commun. Dis. 11:183-186. [Google Scholar]
- 43.Walton, B. C., V. M. Brooks, and I. Arojone. 1972. Serodiagnosis of American leishmaniasis by indirect fluorescent antibody test. Am. J. Trop. Med. Hyg. 21:296-299. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. 1984. Leishmaniasis. World Health Organization Technical Report Series 701. World Health Organization, Geneva, Switzerland.
- 45.Zijlstra, E. E., M. S. Ali, A. M. el-Hassan, I. A. el-Toum, M. Satti, H. W. Ghalib, and P. A. Kager. 1992. Kala-azar: a comparative study of parasitological methods and the direct agglutination test in diagnosis. Trans. R. Soc. Trop. Med. Hyg. 86:505-507. [DOI] [PubMed] [Google Scholar]
- 46.Zijlstra, E. E., N. S. Daifalla, P. A. Kager, E. A. G. Khalil, A. M. El-Hassan, S. G. Reed, and H. W. Ghalib. 1998. rK39 enzyme-linked immunosorbent assay for diagnosis of Leishmania donovani infection. Clin. Diagn. Lab. Immunol. 5:717-720. [DOI] [PMC free article] [PubMed] [Google Scholar]