Abstract
Our subjective sensory experiences are thought to be heavily shaped by interactions between expectations and incoming sensory information. However, the neural mechanisms supporting these interactions remain poorly understood. By using combined psychophysical and functional MRI techniques, brain activation related to the intensity of expected pain and experienced pain was characterized. As the magnitude of expected pain increased, activation increased in the thalamus, insula, prefrontal cortex, anterior cingulate cortex (ACC) and other brain regions. Pain-intensity-related brain activation was identified in a widely distributed set of brain regions but overlapped partially with expectation-related activation in regions, including the anterior insula and ACC. When expected pain was manipulated, expectations of decreased pain powerfully reduced both the subjective experience of pain and activation of pain-related brain regions, such as the primary somatosensory cortex, insular cortex, and ACC. These results confirm that a mental representation of an impending sensory event can significantly shape neural processes that underlie the formulation of the actual sensory experience and provide insight as to how positive expectations diminish the severity of chronic disease states.
Keywords: functional MRI, mental imagery, placebo, psychophysical
The experience of a sensory event is highly subjective and can vary substantially from one individual to the next (1). Much of this individual variation may result from the manner in which past experience and future predictions about a stimulus are used to interpret afferent information. Consistent pairing of environmental cues with sensory events provides a learned historical context that is critically important for the prediction and processing of future sensations (2, 3). However, expectations that are inconsistent with sensory information can dramatically alter the sensory experience. In the case of pain, positive expectations can powerfully reduce the subjective experience of pain evoked by a consistently noxious stimulus, whereas negative expectations may result in the amplification of pain (4-7). Furthermore, expectations in which there is a high degree of certainty as to the outcome may activate descending control systems to diminish pain, whereas expectations associated with uncertain outcomes may amplify pain (8).
The prefrontal cortex (PFC), anterior insula, and anterior cingulate cortex (ACC) are activated during the anticipation of pain, but their exact role in pain expectation remains poorly delineated (9-12). Moreover, the neural mechanisms by which conscious predictions about the magnitude of pain influence the experience of pain remain poorly understood and largely unexploited in the treatment of pain. At the most fundamental level, expectation-induced modulation of pain must necessarily engage three neural processes. First, an active mental representation of an impending event must be formulated by incorporating past information with the present context and future implications of the stimulus. Second, brain regions supporting the mental representation of an impending event must engage a mechanism that can interact with brain regions processing pain. Finally, brain regions supporting the subjective experience of pain need to be modulated by expectations.
To delineate the mechanisms supporting expectation-modulation of pain, we recruited 10 normal, healthy volunteers to participate in a combined psychophysical/functional MRI (fMRI) investigation. Subjective reports of expected pain were used to identify brain mechanisms capable of supporting a mental representation of the expected experience. Expected pain was directly manipulated to determine which brain regions exhibiting pain-related brain activation were modulated by expectations. Finally, brain regions exhibiting overlapping activation during both actual pain and expected pain were identified to characterize the mechanisms that serve as an interface between expectation and experience.
Methods
Subjects. Ten healthy volunteers (eight males, two females, five whites, four Asians, one black) 24-46 years old (mean age, 30.3 y) participated in this study. All subjects gave written, informed consent, and all procedures were approved by the Institutional Review Board of Wake Forest University School of Medicine.
Stimulation. A TSA II thermal stimulator (Medoc Ramat Yishai, Israel) with a 16 × 16-mm contact surface was used to induce pain. The device was attached to the right lower leg, maintained at a baseline temperature of 35°C, and repositioned between trials. Stimulus temperatures consisted of 46, 48, and 50°C, with rise and fall rates of 6°C/s.
Experimental Paradigm. Each experimental trial lasted 120 s and consisted of a 30-s rest period, an expectation interval of variable duration, a 30-s period of painful stimulation, and a second rest period of variable length. The onsets and offsets of the expectation phases were signaled with 1-s duration tones. Increasingly intense stimulus temperatures (46, 48, and 50°C) were signaled by increasingly longer expectation intervals (7.5, 15, and 30 s). To reinforce the association between the duration of the expectation phase and the stimulus temperatures, subjects participated in a training session before the fMRI session.
One or two days after the training session, subjects underwent fMRI scanning of 30 stimulus trials. To characterize modulation of pain by expectations, 33% of both the 48 and 50°C trials were falsely signaled during the fMRI session. In the case of 50°C trials, expectations of decreased pain were created by using an expectation interval of 15 s (normally signaling a 48°C stimulus). In the case of 48°C stimuli, expectations of increased pain were created by using an expectation interval of 30 s (normally signaling a 50°C stimulus). Throughout the training and fMRI acquisition series, each trial type was pseudorandomly presented to blinded subjects in a counterbalanced fashion in blocks of three stimulus conditions. To further minimize temporal effects over the course of the scanning session, data from blocks in which all three temperatures were correctly signaled (“true”) were analyzed separately from blocks in which one of the three temperatures was incorrectly signaled (“false”).
Psychophysics. In the training and fMRI sessions, overall ratings of both expected pain and experienced pain intensity were assessed by using mechanical visual analogue scales (VAS) (13, 14). During the training series, subjects also rated their expected pain and perceived pain intensity in a dynamic real-time fashion by using a computer-digitized VAS in separate trials. These real-time subjective ratings were normalized and averaged to characterize the time course of both expected pain and perceived pain intensity for subsequent fMRI analysis (15). During the fMRI session, subjects kept their eyes closed and provided ratings of expected pain and experienced pain intensity at the end of each series.
Image Acquisition and Analysis. For functional imaging, blood-oxygenation-level-dependent (BOLD) images were acquired continuously in each contiguous plane by using echo-planar imaging [echo time (TE) = 40 ms, repetition time (TR) = 3 s; 28 × 5-mm thick slices; 3.72 × 3.75-mm in-plane resolution; flip-angle, 90°; no slice gap; 1.5T General Electric Horizon LX scanner] with the single-epoch design (16). The interval between each acquisition series was 2 min. High-resolution structural scans also were acquired. The functional image analysis package fsl (Center for Functional Magnetic Resonance Imaging of the Brain, University of Oxford, Oxford) was used for image processing and statistical analysis (17-19). See Supporting Text, which is published as supporting information on the PNAS web site, for full details.
Brain activation that was significantly related to expected pain or to experienced pain was characterized by using a mixed-effects general linear-modeling procedure comprised of fixed-effects analysis (first level) within series and random effects between series (20). In all analyses, clusters of voxels that exceeded a Z score >2.3 and P < 0.01 (corrected for multiple comparisons) were considered statistically significant (21, 22).
To assess the brain regions with significant signal changes that were related to subjective psychophysical responses, we performed interseries group analyses across 10 subjects separately for expectation and pain. In these analyses, outputs of first-level analyses were proportionally weighted by VAS ratings for expectation and pain (16). For each subject, data from only the true blocks of fMRI series were included in the analyses. Analysis was restricted to gray matter voxels to minimize multiple comparisons.
To identify the brain regions that were modulated by increased or decreased expectations of pain, we examined data from false blocks. Group analyses were performed on each stimulus condition (expected 48°C, actual 48°C; expected 48°C, actual 50°C; and expected 50°C, actual 50°C combinations) to separately assess activation during expectation and during pain. As above, outputs from the first-level analyses were weighted by VAS ratings and restricted to voxels in the gray matter.
To further confirm that pain-induced brain activation was modulated by either decreased or increased expectations, we performed a direct statistical comparison between truly and falsely signaled trials (i.e., expected 50°C, actual 50°C versus expected 48°C, actual 50°C). In contrast to the above analyses, this analysis was not weighted according to subjective responses and was restricted to voxels with pain-related activation to minimize the number of multiple comparisons.
Analysis of Psychophysical and Heart Rate Data. Within-subjects ANOVAs were used to assess the effects of the expectation signal (duration of the expectation period) and stimulus temperature on psychophysical ratings of expected and perceived pain intensity. Regression analyses were used to determine the degree to which variability in perceived-pain-intensity ratings could be explained by ratings of expected pain intensity. To minimize potential confounds attributable to individual differences in pain sensitivity, these analyses were performed on percent changes (true - false/true × 100) in expected pain and percent changes (true - false/true × 100) in experienced pain.
To assess autonomic responses during the experimental task, heart rate was continuously monitored during the fMRI. Heart rate data were normalized by dividing the actual values by the initial value (rate derived from the first two beats) on a trial-by-trial basis. Within-subjects ANOVAs were used to identify heart rate differences between baseline (initial 30 s), expectation (6 s before the onset of the thermal stimulus), pain, and postpain periods.
Results
Relationships Among Perceived Pain Intensity, Expected Pain Intensity, and Brain Activation. During the fMRI acquisition session, psychophysical ratings of expected pain intensity increased monotonically with the duration of the expectation phase (F = 41.990, P < 0.0001, data from four true blocks per subject). Subjects correctly expected that pain after a 15-s interval (2.5 ± 0.34) would be greater than that after a 7.5-s interval (1.1 ± 0.17 P < 0.05) and that pain after a 30-s interval (4.8 ±.66) would be greater than that after a 15-s interval (P < 0.05). Similarly, perceived pain intensity increased monotonically as stimulus temperature increased (F = 49.478, P < 0.0001), and subjects accurately distinguished among all temperatures (46°C, 0.9±.20; 48°C, 2.37 ± 0.38; 50°C, 4.7±.65; P < 0.05). A regression analysis revealed a strong relationship (β = 0.964, R2 = 0.880, P < 0.0001) between expected and experienced pain intensity, further confirming that subjects closely associated the three levels of expectation-phases durations with the magnitude of forthcoming painful experiences.
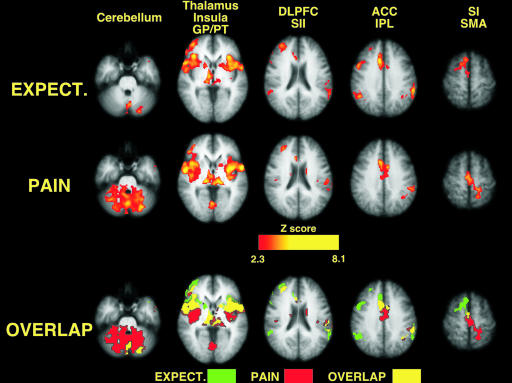
During the expectation period, numerous brain regions exhibited activation that was significantly related to the magnitude of expected pain (data from the four true blocks per subject, Fig. 1; and see Table 1, which is published as supporting information on the PNAS web site). Activation that was positively related to expected pain intensity was located in the cerebellum, thalamus, globus pallidus (GP)/putamen (PT), the anterior insula, the posterior insula/secondary somatosensory cortex (SII), the dorsolateral PFC (DLPFC), the ACC, the inferior parietal lobule, and the supplementary motor area (SMA). Regions exhibiting decreased activation in proportion to expected pain included the ventromedial PFC and the posterior cingulate cortex/precuneus. Both positively and negatively related activation foci were largely bilateral, with only the DLPFC exhibiting predominantly unilateral (right) activation.
Fig. 1.
Both expectation-related activation and pain-related activation were significantly related to the magnitude of expected and experienced pain intensity in correctly signaled trials (image left = right brain). Brain activation during expected pain overlaps extensively with activation during experienced pain.
Consistent with previous studies (23), a wide array of brain regions exhibited activation that was significantly related to the perceived intensity of pain (Fig. 1 and Table 1). Pain-intensity-related brain activation was distributed across the cerebellum, thalamus, GP/PT, anterior insula, posterior insula/SII, dorsolateral PFC, ACC, inferior parietal lobule, SMA, and the leg representation of the contralateral primary somatosensory cortex (SI). Similar to expectation-related activation, regions exhibiting decreased activation in proportion to perceived pain intensity included the ventromedial PFC and the posterior cingulate cortex/precuneus.
Pain-intensity-related activation overlapped extensively with expectation-related activation, despite the fact that these two cognitively distinct states were separated in time. Of the regions activated in either condition, SI was the only brain region displaying exclusively pain-intensity-related activation, whereas only the right portions of the inferior parietal lobule and SMA (premotor cortex) showed exclusive expectation-related activation. In areas with overlapping pain- and expectation-related activation, such as the insula, ACC, and SMA, rostral portions tended to have greater expectation-related activation, and caudal portions tended to have greater pain-intensity-related activation (Fig. 1, Table 1, and Fig. 5, which is published as supporting information on the PNAS web site).
The time course of activation within regions where expectation and pain-related activation overlapped is distinct from that of regions with exclusive expectation or pain-related activation. Moreover, the temporal profile of BOLD signal changes within regions of overlapping activation indicates that this overlap is physiological rather than hemodynamic in nature (see supporting information).
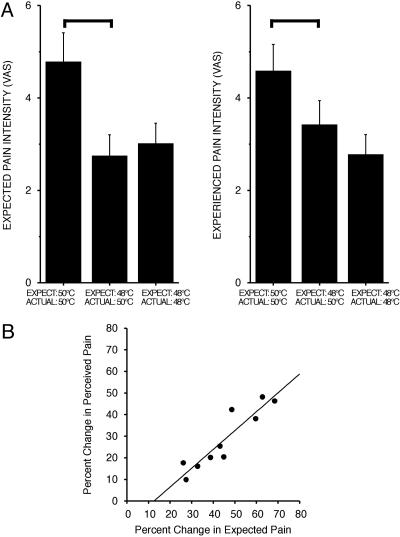
Pain Modulation by Expectations of Decreased Intensity. To assess pain modulation by expectations for decreased pain intensity, a 50°C stimulus was signaled by a 15-s interval normally used to signal a 48°C stimulus (total of three fMRI series per subject). Expectations of decreased intensity produced robust decreases in ratings of perceived pain intensity compared to those evoked by a 50°C stimulus correctly signaled by a 30-s interval (F = 49.32, P < 0.0001, Fig. 2A). The average decrease was 28.4% (range 9.9-48.1%) with 10 of 10 subjects exhibiting diminished pain intensity following diminished expectations of pain. Moreover, after expectations for decreased pain, ratings of experienced pain intensity, evoked by the incorrectly signaled 50°C stimulus, were diminished to the point that they were no longer reliably different from those evoked by a correctly signaled 48°C stimulus (F = 2.15, P < 0.1759). ANOVA confirmed that expectations were clearly manipulated. Expected pain during the falsely signaled 50°C stimulus (i.e., 15-s expectation period) was significantly less than that during a correctly signaled 50°C stimulus (30-s expectation period) (F = 60.69, P < 0.0001) and was statistically indistinguishable from that of a correctly signaled 48°C stimulus (F = 0.2944, P < 0.60, not significant).
Fig. 2.
Psychophysical ratings of experienced pain. (A) Expectations for decreased pain significantly reduce experienced pain (mean ± SD). Bars show significant differences (P < 0.01). (B) Differences in expected pain account for a significant percentage of the variability of the reduction in experienced pain (r2 = 0.85, P < 0.0002).
A regression analysis confirmed that decreased expectations powerfully modulated experienced pain intensity (Fig. 2B). Approximately 85% of the variability in the percent decrease in experienced-pain-intensity ratings could be explained by the percent decrease in expected pain produced by lowered expectations (β = 0.87, intercept = -11.03, R2 = 0.85, F = 44.522, P < 0.0002).
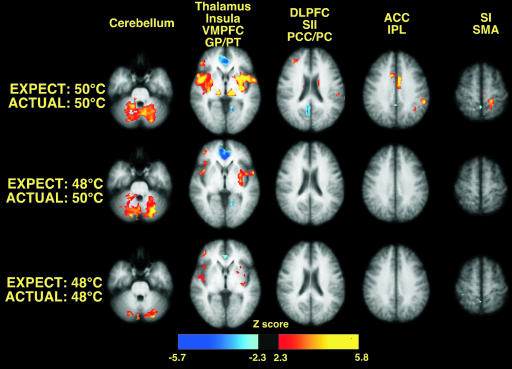
Expectations for decreased pain substantially reduced experienced-pain-intensity-related brain activation. On a qualitative level, pain-intensity-related activation after decreased expectations of pain closely resembled that evoked by correctly signaled 48°C stimuli. Detectable activation was no longer apparent in the ACC and the SI, and the degree of activation in the insula and other areas was qualitatively reduced (Fig. 3). In quantitative analyses, expectations for decreased pain produced statistically significant reductions of 50°C-stimulus-related activation in multiple brain regions (relative to activation evoked by correctly signaled 50°C stimuli, three series per condition per subject, Fig. 4; and see Table 2, which is published as supporting information on the PNAS web site). These functionally diverse regions included the cerebellum, thalamus, caudate, putamen, insula, dorsolateral PFC, SII, ACC, and SI. Of the regions exhibiting pain-intensity-related activation, only the ventromedial PFC, the posterior cingulate cortex, and the SMA were not significantly modulated by expectations for decreased pain. Importantly, this analysis was not weighted by perceived pain intensity, thus minimizing potential errors arising from the demand characteristics of the task. Thus, the reductions in 50°C-stimulus-related brain activation by expectations for reduced pain independently and objectively validate the subjective reports of decreased pain intensity.
Fig. 3.
Expectations for decreased pain significantly reduce pain-related brain activation during 50°C stimulation (image left = right brain).
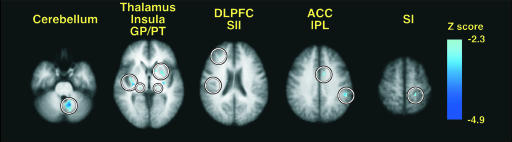
Fig. 4.
Brain regions where expectations for decreased pain significantly reduce pain-related (50°C) brain activation. Direct statistical comparisons between correctly and incorrectly signaled trials revealed that numerous sites (circled) exhibited pain-related activation when subjects expected a 48°C stimulus instead of a 50°C stimulus (image left = right brain).
Autonomic Responses During Expectation and Pain. Normalized heart rate served as an index for autonomic responses. During the expectation phase, heart rate changes (percentage of base-line) were not significantly affected by the expected stimulus (expect 46°C, 98.8%; expect 48°C, 98.7%; expect 50°C, 100.1%; F = 1.311, P < 0.2941), suggesting minimal autonomic arousal. In contrast, during the pain phase, heart rate changes monotonically increased in a manner significantly related to stimulus temperature (actual 46°C, 98.9%; actual 48°C, 100.3%; actual 50°C, 104.5%; F = 8.22, P < 0.0029).
Expectations of Increased Pain. To determine whether perceived pain intensity could be increased by increased expectations for pain, subjects were examined during trials in which a 48°C stimulus was signaled by a 30-s expectation period that was normally used to signal a 50°C stimulus (three series per condition per subject). ANOVA confirmed that subjects expected greater pain after a 30-s expectation period (VAS, 4.5) than after a 15-s expectation period (VAS, 2.6; F = 34.564, P < 0.0002). In sharp contrast to the pain modulation evoked by expectations for decreased pain, expectations for increased pain did not significantly alter psychophysical ratings of experienced pain (F = 2.499, P < 0.148; correct expectation, VAS 2.1; increased expectation, VAS 2.4). Accordingly, comparisons of fMRI data during increased expectations of pain are not reported.
Discussion
Expectations of decreased pain profoundly reduce both the subjective experience of pain and pain-related brain activation. These reductions are widespread and encompass a functionally diverse set of brain regions, including the thalamus, SI, SII, insula, ACC, PFC, and cerebellum. Despite their diversity, all these brain regions are known to exhibit activation that is significantly related to the subjective experience of pain (24-26). The modulation of pain-related activation by expectations is positively related with both the subjective expectation of pain magnitude and the magnitude of brain activation supporting a mental representation of impending pain.
Mental Representation of Impending Pain. A number of brain regions, including the PFC, insula, ACC, the globus pallidus/putamen, thalamus, and cerebellum, exhibited activation that was significantly related to subjective reports of expected-pain magnitude. This expectation-related activation was not accompanied by any reliable increases in heart rate, suggesting that expectations of pain were not accompanied by significant fear, anxiety, and/or alteration of affect, because these psychological responses often coincide with changes in autonomic responses (27-29). Subjects had been selected on their ability to tolerate the range of noxious thermal stimuli and had, before functional imaging, experienced the full range of noxious stimuli used in the experiment. Both procedures serve to minimize fear. Taken together with the observation that expectation-related brain activation was clearly graded in proportion to the magnitude of the expected pain, this finding suggests that the PFC, insula, and ACC work together with their associated subcortical regions to support the mental representation of an impending stimulus.
A mental representation of an impending event relies heavily on information from past experience and, therefore, must incorporate information from brain regions associated with memory recall. The hippocampus is involved in neural processes supporting the retrieval of past memories and is reciprocally connected with the amygdala in a fashion that may support affective modulation of memory recall (30-32). Both structures have long been known to be important for somatic memories, and both project to brain regions that exhibit graded activation during expectations of pain (33). Of expectation-related regions, the PFC receives input from the amygdala and the hippocampus (via the parahippocampal gyrus and adjacent regions) and is known to be important for the monitoring of retrieved mnemonic information (34-37). The ACC and the insula receive input from the amygdala, hippocampus, and parahippocampal regions (38-40) and are activated during memory tasks (32). Furthermore, the ACC, insula, and PFC are highly interconnected (39-42) and, with their associated subcortical regions, likely work together to support the construction of a mental representation of an impending event.
Integration of Expectations and Afferent Information. Modulation of pain by expectation is intimately linked with both subjective reports of expected pain and with expectation-induced brain activation. During expectations of decreased pain, nearly 85% of the variability in changes in the experience of pain could be accounted for by changes in the expected magnitude of pain. Consistent with findings from other studies of mental representation, brain regions involved in the processing of expectations overlapped considerably with those involved in the processing of afferent sensory information (43). These areas likely represent critical pathways for the integration of expectation-related information with afferent sensory information. Expectation-related information from both the ACC and anterior insula can be transmitted to several somatosensory regions. Information from the anterior insula can be transmitted sequentially to the posterior insula, SII, and inferior parietal cortex (40-42). Information from the insula and ACC may also be transmitted through direct connections to the SI (44, 45). Thus, all of these cerebral cortical areas receiving afferent nociceptive information can be readily modulated by expectation-induced information. Because expectations are future predictions derived from both past experience and present context, this flow of expectation-related information may be crucial for the development of a perceptual set. Such a perceptual set could prime brain regions for processing afferent information of a predetermined nature, thereby minimizing computational complexity while increasing speed and accuracy of afferent processing (3, 46). Given the highly distributed and parallel nature of pain processing (26, 47), it is unlikely that a perceptual set in a single brain region could effectively modulate the afferent processing of nociceptive information. Instead, a perceptual set needs to be a highly distributed process as well. The multiple sites at which expectation-related and afferent-related information interact appear likely to comprise a widely distributed mechanism supporting a perceptual set.
The integration of expectation-related information with afferent information appears to be critical for a complete cognitive experience of pain. Patients with insular cortical lesions have been reported to identify a noxious stimulus as painful but are unable to properly appreciate the meaning of their pain (48). Similarly, terminal cancer patients who have had prefrontal lobotomies appear to fully appreciate novel pain but exhibit diminished appreciation of the implications of their cancer-related pain (49-51). Furthermore, classic studies on dogs raised in sensory-deprived environments indicate that animals with minimal prior experience with pain exhibit aberrant responses to novel painful stimuli (52). In addition to contributing to expectations, active mental representations of past or impending sensory events likely play critical roles in discriminative processes where afferent information is compared with information from memory.¶
The present findings confirm that brain mechanisms supporting expectations of pain powerfully interact with brain mechanisms processing afferent nociceptive information to dramatically alter the subjective experience of pain. Positive expectations (i.e., expectations for decreased pain) produce a reduction in perceived pain (28.4%) that rivals the effects of a clearly analgesic dose of morphine (0.08 mg/kg of body weight, an ≈25% reduction in pain) (53). These data provide a neural mechanism that can, in part, explain the positive impact of optimism in chronic disease states (54, 55). Moreover, the potent pain modulation evoked by positive expectations underscores the potential of cognitive therapy for the treatment of pain.
Supplementary Material
Acknowledgments
We thank the Functional MRI of the Brain Image Analysis Group (FMRIB), Oxford University for the fsl analysis software. This study was supported by National Institutes of Health Grant R01 NS39426.
Author contributions: T.K., J.G.M., P.J.L., and R.C.C. designed research; T.K., J.G.M., P.J.L., and R.C.C. performed research; T.K. and R.C.C. analyzed data; and T.K., J.G.M., P.J.L., and R.C.C. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACC, anterior cingulate cortex; fMRI, functional MRI; PFC, prefrontal cortex; SI, primary somatosensory cortex; SII, secondary somatosensory cortex; SMA, supplementary motor area; VAS, visual analogue scale.
Footnotes
Oshiro, Y., Quevedo, A. S., Mc Haffie, J. G., Kraft, R. A. & Coghill, R. C. (2004) Soc. Neurosci. Abstr. 34, 746.12 (abstr.).
References
- 1.Coghill, R. C., McHaffie, J. G. & Yen, Y. F. (2003) Proc. Natl. Acad. Sci. USA 100, 8538-8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James, W. (1890) The Principles of Psychology (Holt, New York).
- 3.Posner, M. I., Snyder, C. R. & Davidson, B. J. (1980) J. Exp. Psychol. 109, 160-174. [PubMed] [Google Scholar]
- 4.Price, D. D. (2000) Science 288, 1769-1772. [DOI] [PubMed] [Google Scholar]
- 5.Dannecker, E. A., Price, D. D. & Robinson, M. E. (2003) J. Pain 4, 74-81. [DOI] [PubMed] [Google Scholar]
- 6.Robinson, M. E., Gagnon, C. M., Riley, J. L., III, & Price, D. D. (2003) J. Pain 4, 284-288. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti, F., Arduino, C. & Amanzio, M. (1999) J. Neurosci. 19, 3639-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ploghaus, A., Becerra, L., Borras, C. & Borsook, D. (2003) Trends Cogn. Sci. 7, 197-200. [DOI] [PubMed] [Google Scholar]
- 9.Koyama, T., Tanaka, Y. Z. & Mikami, A. (1998) NeuroReport 9, 2663-2667. [DOI] [PubMed] [Google Scholar]
- 10.Ploghaus, A., Tracey, I., Gati, J. S., Clare, S., Menon, R. S., Matthews, P. M. & Rawlings, J. N. P. (1999) Science 284, 1979-1984. [DOI] [PubMed] [Google Scholar]
- 11.Porro, C. A., Baraldi, P., Pagnoni, G., Serafini, M., Facchin, P., Maieron, M. & Nichelli, P. (2002) J. Neurosci. 22, 3206-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porro, C. A., Cettolo, V., Francescato, M. P. & Baraldi, P. (2003) NeuroImage 19, 1738-1747. [DOI] [PubMed] [Google Scholar]
- 13.Price, D. D., McGrath, P. A., Rafii, A. & Buckingham, B. (1983) Pain 17, 45-56. [DOI] [PubMed] [Google Scholar]
- 14.Price, D. D., Bush, F. M., Long, S. & Harkins, S. W. (1994) Pain 56, 217-226. [DOI] [PubMed] [Google Scholar]
- 15.Koyama, Y., Koyama, T., Kroncke, A. P. & Coghill, R. C. (2004) Pain 107, 256-266. [DOI] [PubMed] [Google Scholar]
- 16.Koyama, T., McHaffie, J. G., Laurienti, P. J. & Coghill, R. C. (2003) NeuroImage 19, 976-987. [DOI] [PubMed] [Google Scholar]
- 17.Talairach, J. & Tournoux, P. (1988) Co-planar Stereotaxic Atlas of the Human Brain (Thieme, New York).
- 18.Jenkinson, M., Bannister, P., Brady, M. & Smith, S. (2002) NeuroImage 17, 825-841. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson, M. & Smith, S. (2001) Med. Image Anal. 5, 143-156. [DOI] [PubMed] [Google Scholar]
- 20.Woolrich, M. W., Ripley, B. D., Brady, M. & Smith, S. M. (2001) NeuroImage 14, 1370-1386. [DOI] [PubMed] [Google Scholar]
- 21.Friston, K. J., Worsley, K. J., Frackowiak, R. S. J., Mazziotta, J. C. & Evans, A. C. (1994) Hum. Brain Mapp. 1, 210-220. [DOI] [PubMed] [Google Scholar]
- 22.Worsley, K. J., Evans, A. C., Marrett, S. & Neelin, P. (1992) J. Cereb. Blood Flow Metab. 12, 900-918. [DOI] [PubMed] [Google Scholar]
- 23.Coghill, R. C. (2002) in Surgical Management of Pain, ed. Burchiel, K. J. (Thieme, New York), pp. 919-932.
- 24.Porro, C. A., Cettolo, V., Francescato, M. P. & Baraldi, P. (1998) J. Neurophysiol. 80, 3312-3320. [DOI] [PubMed] [Google Scholar]
- 25.Derbyshire, S. W. G., Jones, A. K. P., Gyulai, F., Clark, S., Townsend, D. & Firestone, L. L. (1997) Pain 73, 431-445. [DOI] [PubMed] [Google Scholar]
- 26.Coghill, R. C., Sang, C. N., Maisog, J. M. & Iadarola, M. J. (1999) J. Neurophysiol. 82, 1934-1943. [DOI] [PubMed] [Google Scholar]
- 27.Rhudy, J. L. & Meagher, M. W. (2000) Pain 84, 65-75. [DOI] [PubMed] [Google Scholar]
- 28.Ploghaus, A., Narain, C., Beckmann, C. F., Clare, S., Bantick, S., Wise, R., Matthews, P. M., Rawlins, J. N. & Tracey, I. (2001) J. Neurosci. 21, 9896-9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson, J. R., Jr., Drevets, W. C., Snyder, A. Z., Gusnard, D. A. & Raichle, M. E. (2001) Proc. Natl. Acad. Sci. USA 98, 688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saunders, R. C., Rosene, D. L. & Van Hoesen, G. W. (1988) J. Comp. Neurol. 271, 185-207. [DOI] [PubMed] [Google Scholar]
- 31.Anderson, A. K. & Phelps, E. A. (2001) Nature 411, 305-309. [DOI] [PubMed] [Google Scholar]
- 32.Smith, A. P., Henson, R. N., Dolan, R. J. & Rugg, M. D. (2004) NeuroImage 22, 868-878. [DOI] [PubMed] [Google Scholar]
- 33.Murray, E. A. & Mishkin, M. (1983) Brain Res. 270, 340-344. [DOI] [PubMed] [Google Scholar]
- 34.Porrino, L. J., Crane, A. M. & Goldman-Rakic, P. S. (1981) J. Comp. Neurol. 198, 121-136. [DOI] [PubMed] [Google Scholar]
- 35.Goldman-Rakic, P. S., Selemon, L. D. & Schwartz, M. L. (1984) Neuroscience 12, 719-743. [DOI] [PubMed] [Google Scholar]
- 36.Fuster, J. M. (1997) The Prefrontal Cortex (Lippincott, Philadelphia).
- 37.Henson, R. N., Shallice, T. & Dolan, R. J. (1999) Brain 122, 1367-1381. [DOI] [PubMed] [Google Scholar]
- 38.Mufson, E. J., Mesulam, M. M. & Pandya, D. N. (1981) Neuroscience 6, 1231-1248. [DOI] [PubMed] [Google Scholar]
- 39.Vogt, B. A. & Pandya, D. N. (1987) J. Comp. Neurol. 262, 271-289. [DOI] [PubMed] [Google Scholar]
- 40.Friedman, D. P., Murray, E. A., O'Neil, J. B. & Mishkin, M. (1986) J. Comp. Neurol. 252, 323-347. [DOI] [PubMed] [Google Scholar]
- 41.Mufson, E. J. & Mesulam, M. (1982) J. Comp. Neurol. 212, 23-37. [DOI] [PubMed] [Google Scholar]
- 42.Mesulam, M.-M. & Mufson, E. J. (1982) J. Comp. Neurol. 212, 38-52. [DOI] [PubMed] [Google Scholar]
- 43.Kosslyn, S. M., Ganis, G. & Thompson, W. L. (2001) Nat. Rev. Neurosci. 2, 635-642. [DOI] [PubMed] [Google Scholar]
- 44.Darian-Smith, C., Darian-Smith, I., Burman, K. & Ratcliffe, N. (1993) J. Comp. Neurol. 335, 200-213. [DOI] [PubMed] [Google Scholar]
- 45.Huffman, K. J. & Krubitzer, L. (2001) Cereb. Cortex 11, 849-867. [DOI] [PubMed] [Google Scholar]
- 46.Bushnell, M. C., Duncan, G. H., Dubner, R., Jones, R. L. & Maixner, W. (1985) J. Neurosci. 5, 1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coghill, R. C., Talbot, J. D., Meyer, E., Gjedde, A., Evans, A. C., Bushnell, M. C. & Duncan, G. H. (1994) J. Neurosci. 14, 4095-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berthier, M., Starkstein, S. & Leiguarda, R. (1988) Ann. Neurol. 24, 41-49. [DOI] [PubMed] [Google Scholar]
- 49.Freeman, W. & Watts, J. W. (1948) Ann. Intern. Med. 28, 747-754. [DOI] [PubMed] [Google Scholar]
- 50.King, H. E., Clausen, J. & Scarff, J. E. (1950) J. Nerv. Ment. Dis. 112, 93-96. [PubMed] [Google Scholar]
- 51.White, J. C. & Sweet, W. H. (1968) Pain and the Neurosurgeon: A Forty Year Experience (Thomas, Springfield, IL).
- 52.Melzack, R. & Scott, T. H. (1957) J. Comp. Physiol. Psychol. 50, 155-161. [DOI] [PubMed] [Google Scholar]
- 53.Price, D. D., Gruen, A. V., Miller, J., Rafii, A. & Price, C. (1985) Pain 22, 261-269. [DOI] [PubMed] [Google Scholar]
- 54.Drossman, D. A., McKee, D. C., Sandler, R. S., Mitchell, C. M., Cramer, E. M., Lowman, B. C. & Burger, A. L. (1988) Gastroenterology 95, 701-708. [DOI] [PubMed] [Google Scholar]
- 55.Costello, N. L., Bragdon, E. E., Light, K. C., Sigurdsson, A., Bunting, S., Grewen, K. & Maixner, W. (2002) Pain 100, 99-110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.