Abstract
Leukocytes are recruited from peripheral blood into milk as part of the inflammatory response to mastitis. However, excessive accumulation of inflammatory cells alters the quality of milk and the proteases produced by polymorphonuclear neutrophils (PMNs) and macrophages may lead to mammary tissue damage. To investigate PMN recruitment and the kinetics of their intracytoplasmic enzymes in inflammation, we generated mastitis in six cows by intramammary infusion of lipopolysaccharide (LPS). Clinical signs of acute mastitis were observed in all of the cows, and normal status was resumed by 316 h. Intracytoplasmic elastase, collagenase, and cathepsin activities were measured within live cells by flow cytometry in peripheral blood leukocytes and milk PMNs before and during the inflammatory process (at 10 time points between 4 and 316 h). The proportion of immature PMNs was appreciated by CD33 surface labeling measured in flow cytometry. Leukopenia was observed in the peripheral blood 4 h postinfusion, concomitant to an increase in somatic cell counts in milk. CD33+ PMNs were preferentially recruited from the peripheral blood to milk. Enzymatic activities were detected in PMNs, lymphocytes, and monocytes at levels depending on the cell type, sample nature, and time of collection. Milk PMNs had lower enzymatic activities than peripheral blood PMNs. This study showed that milk PMNs recruited during LPS-induced experimental mastitis have an immature phenotype and significantly lower enzymatic activities than peripheral blood PMNs. This suggests that CD33, an adhesion molecule, may be involved in the egress from blood to milk and that the enzymatic contents of PMNs are partly used during this process.
Bacterial infections of cattle are a major source of economic loss, and mastitis is the most prevalent disease of dairy cows (20). Polymorphonuclear neutrophils (PMNs) represent the major line of defense against bacterial infection of the mammary gland (26) because of their ability to phagocytose and kill opsonized or nonopsonized bacteria by using bactericidal enzymes and oxy radicals.
During mastitis, PMNs emigrate from the peripheral blood into milk through the mammary epithelium in response to chemotactic stimuli (cytokines such as interleukin-8, complement fractions such as C5a, some adhesion molecules, etc.) produced locally as a reaction to invading microorganisms or their components (4, 11, 21, 25). PMNs then phagocytose foreign particles or bacteria (22), discharge their granules' content (2, 6), and generate reactive oxygen metabolites (18). These processes are regulated, and the inflammatory response is proportional to the magnitude of the PMN influx (29).
Bovine PMNs have azurophil, secondary and tertiary granules containing a variety of proteins that differ between the types of granules (16). The enzymes stored within the granules are synthesized at different stages of myelopoietic differentiation (24). PMN granules contain both antibacterial peptides and enzymes (elastase, collagenase, cathepsins, phosphatases, lysozyme, etc.) that are involved in the elimination of bacteria but are also able to disrupt the extracellular matrix (ECM). During mastitis, PMN products are also able to modify milk proteins, thus altering the composition of milk (34; I. Michelutti, Y. Le Roux, and F. Laurent, 4th Meet. 3R, abstr. 1997, p. 357, vol. 4).
Little is known, however, of the enzymatic capacities of PMNs recruited in the mammary gland during mastitis. Here we examined the enzymatic activities of PMNs during experimental mastitis induced by lipopolysaccharide (LPS). Elastase, collagenase, and cathepsin activities were studied in peripheral blood leukocytes and milk PMNs. We analyzed the activity of enzymes involved in cell migration, PMN degranulation, phagocytosis, apoptosis, and cytotoxicity.
Collagenase, an enzyme produced by PMNs and monocytes/macrophages, facilitates the migration of blood leukocytes by degrading ECM collagen. Collagenase cleaves type I, II, III, and X collagens (32). Cathepsin C is a lysosomal enzyme contained in cytotoxic cells. Cathepsin G is stored in the primary (or azurophil) granules of PMNs (14) and in monocytes (10) and is released upon activation and phagocytosis (3). Elastase, another enzyme of PMN azurophil granules, present in macrophages and lymphocytes as well, is also involved in leukocyte migration, tissue destruction by digestion of ECM macromolecules, and phagocytosis. It has been reported to be able to cleave such leukocyte antigens as CD4 or CD8 and to modulate leukocyte adhesion by binding to or cleavage of ICAM-1 (8, 9, 12, 15).
These enzymes have a pathological role in inflammation in various diseases (5, 13). Elastase and cathepsin G play an important role in such inflammatory diseases as arthritis, as demonstrated by the fact that neutrophil elastase −/− or cathepsin G −/− mice are resistant to arthritis induction (1). In another study, we have also demonstrated alterations of enzymatic activities in human immunodeficiency virus-infected patients, who display lowered collagenase and cathepsin D activities in monocytes and increased elastase and cathepsin D activities in PMNs compared to controls (30).
Another aim of this study was to determine how peripheral and medullary stores of PMNs are used to support efflux to the mammary gland. We therefore examined the proportion of immature PMNs recruited during the inflammatory process. This was achieved by assessing the surface expression of CD33 on PMNs. CD33 is a molecule of the immunoglobulin superfamily expressed as one of the earliest markers of myeloid lineage commitment, which disappears from mature cells. Its structure suggests that it is likely to be involved in adhesion and cell emigration (27, 28).
Both CD33 expression and PMN enzymatic activities were monitored over time in sequentially collected peripheral blood and milk samples.
MATERIALS AND METHODS
Animals.
Six Prim' Holstein cows, free of intramammary infection, were selected from the experimental herd of the Laboratoire des Sciences Animales (ENSAIA, Nancy, France). All cows were in the early stages of lactation, i.e., periparturient with less than 6 weeks of lactation. Each cow was infused intramammarily in one rear quarter of the gland with 10 ml of pyrogen-free saline containing 10 μg of purified LPS from Escherichia coli (LPS B6; Sigma Chemical Co., St. Louis, Mo.).
Peripheral blood samples were collected before LPS inoculation and at 4, 8, 12, 16, 25, 36, 52, 64, 76, and 316 h postinfusion. Milk samples were collected from the experimental quarter at 4, 8, 12, 16, 25, 36, 52, 64, 76, and 316 h postinfusion. All of the peripheral blood and milk samples collected were examined within a few hours after sampling.
Blood samples.
Peripheral blood samples were collected into EDTA-containing Vacutainer tubes (Becton Dickinson, San Jose, Calif.). Peripheral white blood cell (WBC) counts in each sample were determined with an automated cell counter (Coulter Z1; Beckman Coulter, Fullerton, Calif.).
The proportions of lymphocytes, monocytes, and PMNs were determined by microscopic examination of May-Grünwald-Giemsa-stained smears (Dade Diff-Quick, Düdinger, Switzerland).
For each sample, 1 ml of whole blood was diluted 1:10 in phosphate-buffered saline (PBS), pH 7.4, and centrifuged at 200 × g for 10 min. The pellet was washed twice in PBS at 500 ×g for 5 min and then adjusted to 3 × 106 ± 0.5 × 106 cells/ml in PBS.
Milk samples.
Somatic cell counts in milk samples were assessed with a Fossomatic 5000 counter (Foss Electronic, Hillerd, Denmark).
Each milk sample was diluted 1:2 in PBS (pH 7.2) and centrifuged at 350 × g for 10 min. The cream-containing supernatant was centrifuged under the same conditions. The pellets of the two samples were pooled and centrifuged again in fresh PBS at 250 × g. The final cell pellet was suspended in Dulbecco modified Eagle's medium (Sigma) with 1 g of glucose per liter, supplemented with 4 mM l-glutamine (Sigma), 10% fetal calf serum (Sigma), 5 μg of insulin (Sigma) per ml, 5 μg of hydrocortisone (Sigma) per ml, 100 U of penicillin (Sigma) per ml, and 100 μg of streptomycin (Sigma) per ml. The final cell count was measured with an automated cell counter (Beckman Coulter), and the suspension was adjusted to 3 × 106 ± 0.5 × 106 cells/ml in PBS.
Leukocyte proteolytic activities.
Three CellProbe reagents, namely, GPLGP Collagenase, TP cathepsin, and AAPV elastase (Beckman Coulter) were used in this study. Each of these cytoenzymologic reagents comprises synthetic nonfluorescent enzyme substrates of two leaving groups conjugated to a dye molecule (Rhodamine 110). Conjugation of the leaving groups to the dye quenches the dye's fluorescence. The leaving groups conjugated to the dye diffuse through the cytoplasmic membrane of live cells and reach the intracellular compartment, where the relevant specific intracellular enzyme can cleave the reagent. Cleavage results in release of the dye within the cell, inducing fluorescence that can be detected by flow cytometry. The intensity of the fluorescence emitted is thus proportional to the enzymatic activity in each cell. The letters before each reagent's specificity indicate the amino acid sequence of the substrate that will be specifically cleaved by the respective enzyme.
Enzymatic activity assays were performed concomitantly, by using, for each sample, four 50-μl aliquots of washed cells prewarmed for 10 min in a 37°C water bath. Just prior to being used, CellProbe reagents were reconstituted with 250 μl of distilled water and 25 μl of the appropriate reagent was added to each warmed tube and gently mixed by hand. A blank control tube was performed by replacing the CellProbe reagent with 25 μl of PBS in the tube containing the prewarmed fourth aliquot for each sample. The reaction was allowed to proceed for 10 min precisely at 37°C in the water bath. The tubes were then placed on crushed ice for at least 10 min to stop the enzymatic reaction. Red blood cells were lysed at this stage with the Multi-Q-Prep system (Beckman Coulter). For milk somatic cells, 1 ml of cold PBS was added. Enzymatic activities were measured by flow cytometry (EPICS XL; Beckman Coulter) strictly within the next 15 min in order to avoid fluorescence release from the cells and nonspecific background labeling. Three gates were defined by using a forward scatter-side scatter biparametric histogram in order to discriminate among lymphocytes, monocytes, and PMNs. A control histogram displaying the same forward scatter ordinate but fluorescence on the abscissa allowed display of the fluorescence intensity of the three cell subsets. The percentage and mean fluorescence intensity of labeled cells were then recorded for each cell type by using monoparametric histograms.
PMNs subsets.
Several trials were performed to determine the cross-reactivity between an anti human CD33 monoclonal antibody and bovine peripheral blood cell samples. The percentage of labeled cells appeared to be consistent with the data obtained from humans (25), and we thus considered that the bovine homologue of CD33 was stained.
For each sample, 50 μl of fresh whole blood or milk somatic cells was mixed with 5 μl of anti-human CD33 monoclonal antibody (My9; Beckman Coulter). After 30 min of incubation at 4°C, 10 μl of fluorescein isothiocyanate-conjugated rabbit anti-mouse antibody (Dako, Glostrup, Denmark) diluted 1:40 in PBS was added and the cells were incubated for 30 min at 4°C. Peripheral red blood cells were lysed with the Multi-Q-Prep System (Beckman Coulter), and 300 μl of PBS was added to milk somatic cell samples. The proportion of labeled PMNs was then assessed by flow cytometry (EPICS XL; Beckman Coulter)
Statistical analyses.
The mean fluorescence intensity (MFI) ratio, defined as the MFI of the test tube divided by the MFI of the control, was calculated for each sample and for each enzymatic activity tested. Results of the six experiments were expressed as the mean level ± the standard deviation (SD). Gaussian distributions were tested by the Kolmogorov-Smirnov test, and then comparisons with baseline values (i.e., preinoculation samples) were performed by analysis of variance and unpaired or paired t tests adjusted for time (GraphPad Prism V2.1). The Mann-Whitney test was used when distributions were not Gaussian.
RESULTS
All of the cows displayed clinical signs of acute mastitis with fever (mean temperature before infusion, 37.8°C; mean maximal temperature, 39.8°C) and a decrease in milk production.
Cell counts.
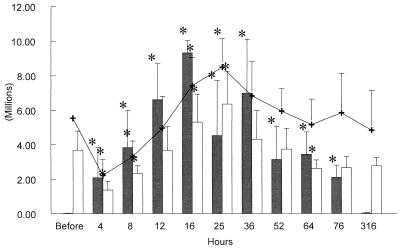
Mean peripheral WBC counts decreased significantly (P < 0.05) at 4 and 8 h postinfusion (Fig. 1) and then increased significantly between 16 and 25 h (P < 0.05) before returning to the baseline by 36 h postinfusion. Mean absolute numbers of PMNs showed the same kinetics but with a second wave of neutropenia by 64 h postinfusion (Fig. 1). Absolute monocyte numbers decreased between 4 and 12 h postinfusion and then increased by 316 h postinfusion. Absolute lymphocyte numbers decreased at 4 h postinfusion (P < 0.01) and then increased at 25, 36, 52, and 76 h postinfusion (P < 0.05).
FIG. 1.
Kinetics of absolute numbers of milk somatic cells (black bars), peripheral blood PMNs (white bars), and peripheral blood WBC (+) over the 316 h of the LPS experiments. At each time point, the bar or line represents the mean ± SD of the six cows studied. ∗ = P < 0.05.
Mean milk somatic cell counts increased significantly (P < 0.05) by 4 h postinoculation and then plateaued at around 107/mm3 before returning to the baseline by 316 h postinoculation (Fig. 1).
CD33+ PMNs.
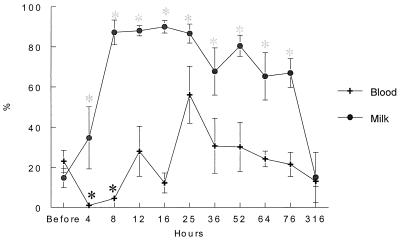
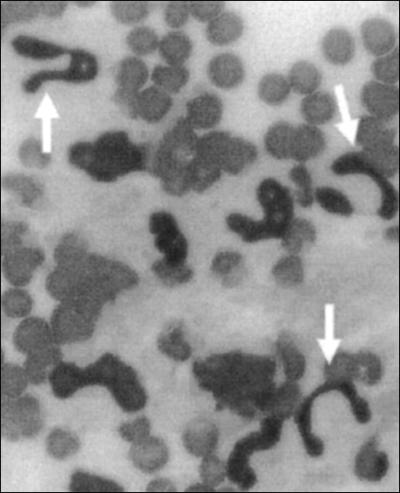
The baseline value of peripheral blood CD33+ PMNs was around 22% (Fig. 2). These cells became nearly undetectable in the peripheral blood by 4 h postinfusion (P < 0.01) and remained at significantly low levels at 8 h postinfusion (P < 0.05). A peripheral blood CD33+ PMN peak was then observed at 25 h postinfusion. In the meantime, CD33+ PMNs rose sharply in milk and plateaued by 8 h postinfusion, representing more than 70% of the milk PMNs (Fig. 2). By 316 h postinfusion, the percentage returned to the baseline, at around 15%. Examination of peripheral blood smears allowed us to see numerous images of PMN bands at the times when CD33+ cell counts were highest (Fig. 3)
FIG. 2.
Kinetics of the percentage (mean ± standard error) of CD33+ peripheral blood and milk PMNs over the 316 h of the LPS experiments. ∗ = P < 0.05.
FIG. 3.
Example of immature band PMNs (arrows) in peripheral blood at 25 h postinfusion. May-Grünwald-Giemsa staining was used. Initial magnification, ×200.
Enzymatic activities.
For the three types of cells tested, MFIs were significantly lower (P < 0.05) in controls (cells without substrate, background autofluorescence) than in experimental samples. This demonstrated that the three enzymatic activities investigated were detectable in the three cell types studied.
For all samples, collagenase activity was the highest in PMNs but the difference was statistically significant only with respect to that in lymphocytes (P < 0.01). Elastase and cathepsin activities were also statistically significantly higher (P < 0.05) in PMNs than in monocytes and lymphocytes. The three enzymatic activities studied were statistically significantly lower (P < 0.001) in milk PMNs than in peripheral blood PMNs.
Enzymatic activities over time. (i) Peripheral blood.
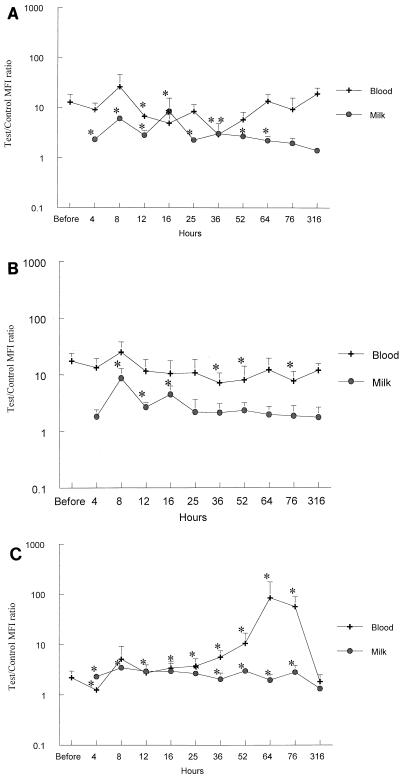
The collagenase activity of lymphocytes decreased significantly at 36 and 52 h and increased at 316 h (P < 0.05). Collagenase activity in monocytes was significantly decreased at 4, 16, 25, 36, and 52 h postinfusion (P < 0.03). Collagenase activity in PMNs (Fig. 4A) was decreased significantly at 12 to 16 h and at 36 h after infection (P < 0.05).
FIG. 4.
Enzymatic activity in peripheral blood and milk PMNs over time. Data are expressed as an MFI comparing the reaction tube and the control. Histograms were built with the mean MFI ratios ± SD of the six cows studied on a logarithmic scale. ∗ = P < 0.05. The enzymatic activities represented are, respectively, collagenase (A), cathepsin (B), and elastase (C).
Cathepsin activity in lymphocytes was stable over time. A significant decrease (P < 0.05) in cathepsin activity was noted at 16 and 36 h postinfusion in monocytes and at 36, 52, and 76 h postinfusion in PMNs (Fig. 4B).
Elastase activity in lymphocytes decreased significantly 4 h postinfusion (P = 0.024), was stable over time, and then increased at 64 h postinfusion (P = 0.026). Elastase activity in monocytes was relatively stable over time, with a significant decrease at 4 and 36 h postinfusion (P = 0.042 and 0.013, respectively). Elastase activity of PMNs (Fig. 4C) decreased significantly at 4 h postinfusion (P = 0.024) and then increased sharply between 25 and 76 h postinfusion (P < 0.03).
(ii) Milk samples.
Enzymatic activities were detected in milk PMNs between 4 and 316 h postinfusion. The results were compared to the 316-h postinfusion data because the somatic cell count was lower at 316 h postinfusion than at 4 h postinfusion and this lower level was more likely to represent the baseline.
Collagenase activity (Fig. 4A) was significantly increased (P < 0.05) for a long time (4 to 64 h postinfusion). Cathepsin activity (Fig. 4B) was significantly increased between 8 and 16 h postinfusion (P < 0.05). Elastase activity (Fig. 4C) was significantly increased over the whole period of study (P < 0.05).
DISCUSSION
This study allowed us to appreciate the kinetics of an inflammatory reaction induced in the mammary gland by infusion of LPS in terms of cellular recruitment and activation. One advantage of this model is that it allows us to examine significant cell numbers both in the peripheral blood and at the site of inflammation for a long time and after a precisely monitored single initiating event. The LPS infusion model thus allows an accurate evaluation of the initiation and regulation of the inflammatory reaction. Here we focused both on the recruitment of PMNs in the inflamed mammary gland and on the modifications of intracellular enzymatic activities induced by the ongoing inflammation.
Blood cell counts appeared to be rapidly and significantly affected by the inflammatory process. Four hours after LPS infusion, a sharp leukopenia was observed, with a reduction in both WBC and PMN counts to more than half of the baseline level. In parallel, milk somatic cell counts, which were lower than 22,000 cells/mm3 at the baseline, increased nearly 100-fold. Peripheral blood recovered from the sharp neutropenia by 12 h postinfusion, likely through the release of marginated PMNs (25). It may be hypothesized that the manipulation of the animals (stress), the inflammatory process itself, or both involved the secretion of corticosteroids liable to free marginated PMNs and to promote egress of PMNs from the bone marrow (7, 19, 31). This is also supported by the fact that the numbers of CD33+ PMNs, which initially decreased sharply, returned to baseline levels in the peripheral blood by 12 h. As peripheral blood counts continued to increase up to 25 h, other mechanisms were likely involved in the supply of these cells. Peripheral blood PMN counts also kept increasing, and at 25 h, these cells expressed higher levels of CD33, indicating that they might originate from the bone marrow and represent a medullary pool of incompletely mature PMNs. This was also suggested by the examination of peripheral blood smears showing immature forms of band PMNs. Interestingly, the majority of milk PMNs expressed CD33 during the whole duration of the experiment, suggesting that CD33+ cells were preferentially recruited.
Two peaks of milk cell counts were observed at 16 and 36 h. It may be hypothesized that many of these cells died between 16 and 25 h and that peripheral blood levels of PMNs were not high enough to maintain high milk PMN counts during that period. The immature CD33+ PMNs, likely released from the bone marrow, observed in the peripheral blood at 25 h were then able to reach the milk for the last wave of PMNs. At later times, as the inflammatory response was downregulated, a return to baseline values was observed in both compartments.
As consecutive waves of PMNs could be seen first in the peripheral blood and then in milk, the three enzymatic activities we chose to study also appeared to be modified by the ongoing inflammatory process. The enzymes examined are involved in cell migration, PMN degranulation, phagocytosis, apoptosis, and cytotoxicity. Indeed, intracellular collagenase, cathepsin, and elastase activities could be specifically measured in bovine peripheral blood leukocytes and milk PMNs by flow cytometry. As observed previously (30) yet seldom reported, we were able to detect significant levels of enzymatic activity in lymphocytes, although at lower levels than in phagocytic cells. Peripheral blood lymphocytes, monocytes, PMNs, and milk cells contained enzymes with different levels of activity, depending on the cell type tested.
Mean enzymatic activities were lower in milk PMNs than in peripheral blood PMNs. PMNs that reach the mammary gland thus appear to have used up part of their enzymatic content during their migration in order to cross the endothelium, ECM, and epithelium. Activation may then lead them to go on releasing enzymes locally in milk. Consistent with our observations, Gruner et al. (17) have shown that the myeloperoxidase content decreases by 39% after diapedesis across the blood-milk barrier in vivo. Owen and Campbell also reported that PMN enzymes can be released or expressed on the cell surface to degrade ECM components (24). A recent study further demonstrated that diapedesis across the mammary epithelium reduces the phagocytic activity and oxidative burst of PMNs (33). Naidu and Newbould (23) have postulated that the poor phagocytic and oxidative burst activity of milk PMNs, compared to that of blood PMNs, was due partially to low energy stores within these cells. Those authors observed a 38% loss of the initial glycogen content of PMNs after diapedesis into the mammary gland.
PMN collagenase activity was almost always lower in milk than in peripheral blood, but between 4 and 64 h postinfusion, milk PMN collagenase activity was significantly higher than the baseline. The highest level was noted at 16 h postinfusion, concomitant with a significant decrease in peripheral blood collagenase activity. This suggests that although PMNs use up some of their collagenase content during diapedesis, those with the best activity seem to be preferentially recruited from the peripheral blood.
The cathepsin activity measured in this study represents the activities of cathepsins C and G. Cathepsin G is localized in the same granules as elastase in PMNs. In the peripheral blood, minor modifications were observed, merely suggesting slightly less activity in the more immature cells of late samples. The kinetics of cathepsin activities in milk cells showed that significantly higher cathepsin activity could be detected between 8 and 16 h postinfusion. This suggests that PMNs with the highest enzymatic activity are recruited in the early stages of inflammation.
Elastase activity was high and stable in milk PMNs over the whole inflammatory period and returned to the baseline level by 316 h postinfusion. However, this stability in milk PMNs was not reflected in peripheral blood. In the latter, PMN elastase activity decreased significantly 4 h after infusion and then increased by 8 h postinfusion. This might reflect either the fact that PMNs with the richest elastase content were first recruited or the fact that marginated PMNs are in a resting state and need some time to increase their enzymatic activities (i.e., by 8 h postinfusion). More interestingly, from 25 to 76 h postinfusion, there was a sharp 1-log rise in peripheral blood PMN elastase activity. This did not appear to correlate with the immaturity of the cells, i.e., with CD33 expression. However, it did coincide with the significant decrease in milk cell counts. Assuming that elastase-rich PMNs are preferentially recruited (i.e., 4 h postinfusion), this could reflect the retention of such cells in the peripheral blood when chemotactic signals decrease as the inflammatory reaction subsides.
In conclusion, this study, performed with a well-monitored model of experimental mastitis, allowed us to obtain precise information on the kinetics of PMN recruitment at the inflammation site, together with data regarding their maturation stage and enzymatic activities. The concomitant study of peripheral blood and milk samples allowed a thorough analysis of the peripheral consequences of local inflammation, indicating that all pools of available PMNs (peripheral, marginated, and medullary) can participate in recruitment at the inflammatory site.
REFERENCES
- 1.Adkison, A. M., S. Z. Raptis, D. G. Kelley, and C. T. Pham. 2002. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J. Clin. Investig. 109:363-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggiolini, M., and B. Dewald. 1984. Exocytosis by neutrophils. Contemp. Top. Immunobiol. 14:221-246. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini, M., J. Schnyder, U. Bretz, B. Delwald, and W. Ruch. 1980. Cellular mechanisms of proteinase release from inflammatory cells and the degradation of extracellular proteins. Ciba Found. Symp. 75:105-121. [DOI] [PubMed] [Google Scholar]
- 4.Barber, M. R., and T. J. Yang. 1998. Chemotactic activities in nonmastitic and mastitic mammary secretions: presence of interleukin-8 in mastitic but not nonmastitic secretions. Clin. Diagn. Lab. Immunol. 5:82-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieth, J. G. 2001. The elastases. J. Soc. Biol. 195:173-179. [PubMed] [Google Scholar]
- 6.Borregaard, N. 1997. Development of neutrophil granule diversity. Ann. N. Y. Acad. Sci. 832:62-68. [DOI] [PubMed] [Google Scholar]
- 7.Brenner, I., P. N. Shek, J. Zamecnik, and R. J. Shephard. 1998. Stress hormones and the immunological responses to heat and exercise. Int. J. Sports Med. 19:130-143. [DOI] [PubMed] [Google Scholar]
- 8.Bristow, C. L., L. K. Lyford, D. P. Stevens, and P. M. Flood. 1991. Elastase is a constituent product of T cells. Biochem. Biophys. Res. Commun. 181:232-239. [DOI] [PubMed] [Google Scholar]
- 9.Cai, T. Q., and S. D. Wright. 1996. Human leukocyte elastase is an endogenous ligand for the integrin CR3 (CD11b/CD18, Mac-1, alpha M beta 2) and modulates polymorphonuclear leukocyte adhesion. J. Exp. Med. 184:1213-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell, E. J., E. K. Silverman, and M. A. Campbell. 1989. Elastase and cathepsin G of human monocytes. Quantification of cellular content, release in response to stimuli, and heterogeneity in elastase-mediated proteolytic activity. J. Immunol. 143:2961-2968. [PubMed] [Google Scholar]
- 11.Caswell, J. L., D. M. Middleton, and J. R. Gordon. 1999. Production and functional characterization of recombinant bovine interleukin-8 as a specific neutrophil activator and chemoattractant. Vet. Immunol. Immunopathol. 67:327-340. [DOI] [PubMed] [Google Scholar]
- 12.Champagne, B., P. Tremblay, A. Cantin, and Y. St Pierre. 1998. Proteolytic cleavage of ICAM-1 by human neutrophil elastase. J. Immunol. 161:6398-6405. [PubMed] [Google Scholar]
- 13.Corteling, R., D. Wyss, and A. Trifilieff. 2002. In vivo models of lung neutrophil activation. Comparison of mice and hamsters. BMC Pharmacol. 2:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delwald, B., R. Rindler Ludwig, U. Bretz, and M. Baggiolini. 1975. Subcellular localization and heterogeneity of neutral proteases in neutrophilic polymorphonuclear leukocytes. J. Exp. Med. 141:709-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doring, G., F. Frank, C. Boudier, S. Herbert, B. Fleischer, and G. Bellon. 1995. Cleavage of lymphocyte surface antigens CD2, CD4, and CD8 by polymorphonuclear leukocyte elastase and cathepsin G in patients with cystic fibrosis. J. Immunol. 154:4842-4850. [PubMed] [Google Scholar]
- 16.Gennaro, R., B. Dewald, U. Horisberger, H. U. Gubler, and M. Baggiolini. 1983. A novel type of cytoplasmic granule in bovine neutrophils. J. Cell Biol. 96:1651-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruner, E. R., R. J. Harmon, and R. W. Hemken. 1982. A comparison of antibacterial granule components in neutrophil leukocytes from bovine blood and milk after endotoxin infusion. J. Dairy Sci. 65:194-198. [Google Scholar]
- 18.Hampton, M. B., A. J. Kettle, and C. C. Winterbourn. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007-3017. [PubMed] [Google Scholar]
- 19.Hetherington, S. V., and P. G. Quie. 1985. Human polymorphonuclear leukocytes of the bone marrow, circulation, and marginated pool: function and granule protein content. Am. J. Hematol. 20:235-246. [DOI] [PubMed] [Google Scholar]
- 20.Hortet, P., and H. Seegers. 1998. Loss in milk yield and related composition changes resulting from clinical mastitis in dairy cows. Prev. Vet. Med. 37:1-20. [DOI] [PubMed] [Google Scholar]
- 21.Imhof, B. A., and D. Dunon. 1997. Basic mechanism of leukocyte migration. Horm. Metab. Res. 29:614-621. [DOI] [PubMed] [Google Scholar]
- 22.Ingham, H. R., Sisson, P. R., Middleton, R. L., Narang, H. K., Codd, A. A., and J. B. Selkon. 1981. Phagocytosis and killing of bacteria in aerobic and anaerobic conditions. J. Med. Microbiol. 14:391-399. [DOI] [PubMed] [Google Scholar]
- 23.Naidu, T. G., and F. H. Newbould. 1973. Glycogen in leukocytes from bovine blood and milk. Can. J. Comp. Med. Vet. Sci. 37:47-55. [PMC free article] [PubMed] [Google Scholar]
- 24.Owen, C. A., and E. J. Campbell. 1999. The cell biology of leukocyte-mediated proteolysis. J. Leukoc. Biol. 65:137-150. [DOI] [PubMed] [Google Scholar]
- 25.Paape, M. J., A. J. Guidry, N. C. Jain, and R. H. Miller. 1991. Leukocyte defense mechanisms in the udder. Flemish Vet. J. 62(Suppl.):95-109. [Google Scholar]
- 26.Paape, M. J., K. Shafer-Weaver, A. V. Capuco, K. Van Oostveldt, and C. Burvenich. 2000. Immune surveillance of mammary tissue by phagocytic cells. Adv. Exp. Med. Biol. 480:259-277. [DOI] [PubMed] [Google Scholar]
- 27.Peiper, S. C., and R. G. Andrews. 1995. M9 CD33 cluster workshop report, p. 837-840. In S. F. Schlossman, L. Boumsell, W. Gilks, J. M. Harlan, T. Kishimoto, C. Morimoto, J. Ritz, S. Shaw, R. Silverstein, T. Springer, T. F. Tedder, and R. F. Todd (ed.), Leukocyte typing. V. White cell differentiation antigens. Oxford University Press, Oxford, United Kingdom.
- 28.Peiper, S. C., and H. H. Guo. 1997. CD33 Workshop Panel report, p. 972-974. In T. Kishimoto, H. Kikutani, A. E. G. K. von dem Borne, S.M. Goyert, D.Y. Mason, M. Miyasaka, L. Moretta, K. Okumura, S. Shaw, T. Springer, K. Sugamura, and H. Zola (ed.), Leucocyte typing. VI. White cell differentiation antigens. Garland Publishing, Inc., New York, N.Y.
- 29.Phalipon, A., and P. J. Sansonetti. 1999. Microbial-host interactions at mucosal sites. Host response to pathogenic bacteria at mucosal sites. Curr. Top. Microbiol. Immunol. 236:163-189. [DOI] [PubMed] [Google Scholar]
- 30.Prin-Mathieu, C., V. Baty, G. Faure, H. Schumacher, M. N. Kolopp-Sarda, T. May, P. Canton, and M. C. Bene. 2001. Assessment by flow cytometry of peripheral blood leukocyte enzymatic activities in HIV patients. J. Immunol. Methods 252:139-146. [DOI] [PubMed] [Google Scholar]
- 31.Schalm, O. W., J. Lasmanis, and N. C. Jain. 1976. Conversion of chronic staphylococcal mastitis to acute gangrenous mastitis after neutropenia in blood and bone marrow produced by an equine anti-bovine leukocyte serum. Am. J. Vet. Res. 37:885-890. [PubMed] [Google Scholar]
- 32.Shapiro, S. D., E. J. Campbell, R. M. Senior, and H. J. Welgus. 1991. Proteinases secreted by human mononuclear phagocytes. J. Rheumatol. 18:95-98. [PubMed] [Google Scholar]
- 33.Smits, E., C. Burvenich, A. J. Guidry, R. Heyneman, and A. Massart-Leen. 1999. Diapedesis across mammary epithelium reduces phagocytic and oxidative burst of bovine neutrophils. Vet. Immunol. Immunopathol. 56:259-269. [DOI] [PubMed] [Google Scholar]
- 34.Sordillo, L. M., K. Shafer-Weaver, and D. DeRosa. 1997. Symposium: bovine immunology. Immunology of the mammary gland. J. Dairy Sci. 80:1851-1865. [DOI] [PubMed] [Google Scholar]