Abstract
Heat sensitivity shows considerable functional variability in humans and laboratory animals, and is fundamental to inflammatory and possibly neuropathic pain. In the mouse, at least, much of this variability is genetic because inbred strains differ robustly in their behavioral sensitivity to noxious heat. These strain differences are shown here to reflect differential responsiveness of primary afferent thermal nociceptors to heat stimuli. We further present convergent behavioral and electrophysiological evidence that the variable responses to noxious heat are due to strain-dependence of CGRP expression and sensitivity. Strain differences in behavioral response to noxious heat could be abolished by peripheral injection of CGRP, blockade of cutaneous and spinal CGRP receptors, or long-term inactivation of CGRP with a CGRP-binding Spiegelmer. Linkage mapping supports the contention that the genetic variant determining variable heat pain sensitivity across mouse strains affects the expression of the Calca gene that codes for CGRPα.
Keywords: calcitonin gene-related peptide, genetic, Calca, nociceptors, pain
Humans display wide individual variability in sensitivity to pain. Although the relative importance of genes versus experience in human pain perception is unclear, recent studies have shown that mouse strains display large differences in behavioral pain sensitivity that are heritable (1). These same studies revealed genetic correlations between baseline thermal nociception and the hypersensitivity states associated with inflammatory and neuropathic pain (2). Of the strains examined, AKR and C57BL/6 mice displayed the largest and most consistent differences in several different assays of thermal nociception, with AKR being much less sensitive than C57BL/6. In contrast, AKR mice exhibit more robust heat hyperalgesia after inflammatory or nerve injury (1). Despite our considerable knowledge of the behavioral “phenomics” of baseline heat pain and hyperalgesia, there are almost no published data regarding the underlying cellular or molecular mechanisms. Here we show that the observed strain differences in response to thermal stimulation are caused by corresponding differences in the functioning of primary afferent nociceptors. The differences in nociceptor sensitivity, in turn, are caused by the presence of and sensitivity to the neuropeptide calcitonin gene-related polypeptide (CGRP). Finally, linkage mapping revealed a candidate gene likely responsible for the strain difference: Calca, the gene encoding CGRPα.
CGRPα is a secretory neuropeptide released from thin nerve fibers at their peripheral and central terminals, which is thought to contribute importantly to neurogenic inflammation in the skin and to central sensitization in the spinal cord (3-6). CGRP acts through a Gs protein-coupled receptor complex to activate cAMP-dependent protein kinase (PKA). PKA, via the transcription factor cAMP response element-binding protein, enhances expression of pronociceptive genes including the Calca gene itself (7-9). Moreover, many CGRP-containing sensory neurons in the rat dorsal root ganglion (DRG) are equipped with autoreceptors and respond to CGRP with calcium influx (10-12). Both activation of PKA and calcium influx are sufficient to induce sensitization to heat of nociceptive neurons and to enhance their stimulated release of CGRP (13-16). Nonetheless CGRP has not previously been shown to exert any acute sensitizing effects on cutaneous nociceptor endings or to induce heat hyperalgesia (17-20). We report here that CGRP gene expression, content, release, and function are all highly strain-dependent, that this factor accounts for the inherited strain differences in thermal response, and that, in appropriately chosen strains, CGRP does induce heat hyperalgesia.
Methods
More detailed descriptions can be found in supporting information, which is published on the PNAS web site.
Subjects. Naïve, adult (6-12 weeks of age) mice of both sexes were used in all experiments. Mouse strains included outbred CD-1 mice (ICR:Crl; Charles River Breeding Laboratories) and 12 inbred strains obtained from The Jackson Laboratory: 129P3, A, AKR, BALB/c, C3H/He, C57BL/6, C57BL/10, C58, CBA, DBA/2, RIIIS, and SM (all “J” substrains).
Paw-Withdrawal Test (21). The stimulus was a high-intensity beam (IITC, Woodland Hills, CA; setting = 20%, ≈45 W) aimed at the plantar surface of the mid-hindpaw of an inactive mouse. Paw-withdrawal latency (PWL) was measured to the nearest 0.1 s.
Single-Fiber Electrophysiology. The isolated skin-saphenous nerve preparation and the single-fiber recording technique were used in the present study (22). Receptive fields of identified single C-fibers were searched by mechanical probing and further characterized by using controlled radiant heat (32°C to 48°C in 20 s), controlled constant-force stimulation to the most sensitive spot, and von Frey filaments that were calibrated from 1 to 256 mN in a geometric series (see supporting information).
Immunoreactive CGRP (iCGRP) Release and Content. The hairy skin of both hindpaws was obtained from AKR and C57BL/6miceofboth sexes and passed through a series of incubation steps (5-min duration) to assess basal (at 32°C) and stimulated iCGRP release using enzyme immunoassay technology as described (15). Stimulation was performed by incubation at 44°C, 47°C, or 50°C, or in an isotonic solution containing 60 mM KCl or 1 μM capsaicin (Sigma).
To determine the iCGRP content, the skin flaps of both hind-paws were excised from male mice of both strains and homogenized after 15 min in 1 ml of 2 M acetic acid at 95°C by using an Ultra-Turrax T8 (IKA Works, Wilmington, NC). The suspension was centrifuged for 30 min at 10,000 × g, and 100 μl of the supernatant was diluted with water at a ratio of 1:100 to cope with the low pH of 3.0 and the high concentration of iCGRP. Two hundred microliters of the solution were finally mixed with the EIA buffer, and enzyme immunoassay was performed. The amounts of iCGRP released or contained are described with reference to 1-g wet weight of cutaneous tissue.
iCGRP Immunocytochemistry in DRG (3). Five mice of either strain were used; four to six lumbar DRGs from each animal were excised and conventionally processed (see supporting information) by using rabbit anti-CGRP as primary antibody (Amersham Pharmacia). Four DRG sections of each animal were randomly chosen for counting of the labeled neurons, and the numbers of immunopositive neurons are expressed as percentage of total number of neurons per section.
Gene Expression Analysis. From each strain, four to eight mice of both sexes were killed. Lumbar DRGs (n = 6-12 per mouse) were harvested, and total RNA was isolated and reverse transcribed. Gene expression was quantified by using the 7700 Sequence Detector (TaqMan) and the SYBR Green PCR Core Reagent kit, as described in the manufacturer's manual (Applied Biosystems). The gene of interest was amplified by PCR, separated by gel electrophoresis, and isolated from the gel fragment (Qiaex, Qiagen, Valencia, CA). This product was used as a standard for the real-time PCR correlating threshold cycle to template copy number. In a first PCR, the cyclophilin or GAPDH content of each cDNA sample was quantified to control for variations in cDNA amounts and diluted accordingly. Amplification of the standard curve was performed at least in triplicate, with every cDNA sample in duplicates to control for tube internal failures. The values were averaged in each direction and normalized by referring to mean values of the housekeeping gene cyclophilin or GAPDH. The specificity of the PCR products was verified by gel electrophoresis. TaqMan-PCRs were performed in 25-μl volumes with a final concentration of 300 nmol/liter (for data in Fig. 1F) or 900 nmol/liter (for data in Fig. 3B) for each primer, with 95°C for 30 s and 60°C for 60 s, for 40 cycles. Sequences for Calca and Tac1 primers were determined by using public databases.
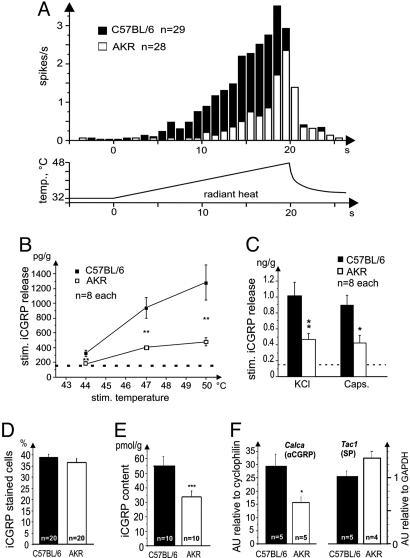
Fig. 1.
Differential sensory, neurosecretory, and transcriptional functions in primary afferent neurons of AKR and C57BL/6 mice. (A) Averaged heat responses of mechano-heat-sensitive C-fibers in the isolated skin-saphenous nerve preparation stimulated by radiant heat to the epidermis (lower trace shows stimulus profile). AKR: 8.1 spikes per stimulus ± 1.8 (SEM), threshold 44.3 ± 0.6°C; C57BL/6: 34.2 ± 0.8 spikes per stimulus, threshold 40.1 ± 0.54°C; both strain differences P < 0.01, Mann-Whitney U test. Detailed methods, further statistics, and data on mechanical sensitivity of the C-fibers are given in supporting information. (B) Stimulus-response curves of heat-induced (5 min) iCGRP release from isolated dorsal hindpaw skin. Baseline release (dotted line) was the same in both strains (152 ± 4 pg per g of skin in 5 min); all stimulated increases over baseline were significant (P < 0.01, Wilcoxon test). **, P < 0.01 compared to other strain, Mann-Whitney U test. (C) Responses induced by KCl (60 mM) and capsaicin (1 μM), baseline release is indicated by a dotted line; *, P < 0.05; **, P < 0.01. (D) Immunocytochemical strain comparison in lumbar DRGs. Percentage of iCGRP-staining neurons in four randomly chosen sections from each of five animals of either strain (n = 20 sections). Detailed methods and a representative photomicrograph can be found in supporting information. (E) Total iCGRP content of hairy skin; “n” refers to skin flaps from both hindpaws of 5/4 animals (***, P < 0.001; Mann-Whitney U test). For a table relating stimulated iCGRP release to total skin content and comparing between the mouse strains, see supporting information. (F) Quantitative gene expression of Calca, coding for αCGRP (Left), and Tac1, coding for preprotachykinin-A (substance P; SP) (Right). Amounts of mRNA in lumbar DRGs of four to five animals of either strain, determined by real-time PCR quantification (TaqMan) and expressed in arbitrary units (AU) relative to the housekeeping genes cyclophilin or GAPDH. *, P < 0.01, Mann-Whitney U test.
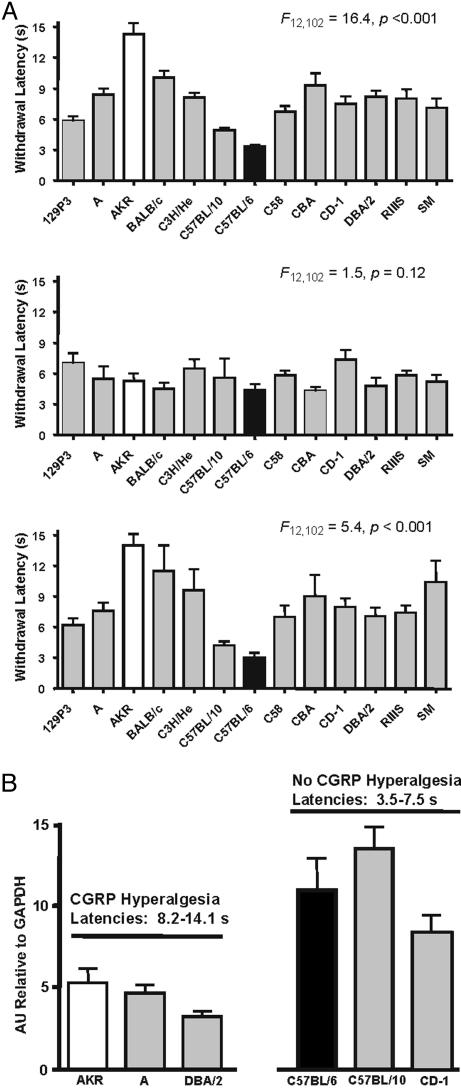
Fig. 3.
Reversible abolition of multiple strain differences in heat nociception by exogenous administration of CGRP into the hindpaw, and related Calca gene expressions. (A) Bars represent mean ± SEM latency (s) to withdraw the paw from the noxious stimulus before (Top; average of four baseline measurements), 5 min (Middle), and 20 min (Bottom) after injection of 5 μg CGRP. F values provided are from one-way ANOVAs performed at each time point. (B) Basal Calca gene expression in CGRP “responders” (AKR, A, DBA/2) and “nonresponders” (C57BL/6, C57BL/10, CD-1). Bars represent mean ± SEM Calca expression in lumbar (L4-L6) DRGs relative to GAPDH; n = 3 per strain. Status regarding response to CGRP injection and range of basal latencies are provided above each group of strains. Calca expression in all low-latency “responder” strains significantly exceeded that of all high-latency “nonresponder” strains (ANOVA followed by LSD test).
Pharmacological Experiments. After habituation, naïve mice were tested four times (separated by 15 min) for basal PWLs on each paw. For peripheral injections, mice were briefly removed so that drugs could be administered s.c. into the right plantar hindpaw (in 20 μl of saline). For intrathecal (i.t.) injections, mice were removed from the test chambers, lightly anesthetized with isoflurane/oxygen, and given i.t. injections (in 2.5 μl of saline) (24) (see supporting information).
Injected compounds included rat CGRPα (0.5 per 20 μg per mouse), rat substance P (20 μg per mouse), rat CGRP8-37 (0.5 ng to 5 μg per mouse), and BIBN4096BS (10 ng to 1 μg per mouse). All compounds were obtained from Sigma, except for BIBN4096BS (25), which was generously provided by Boehringer Ingelheim. The anti-CGRP Spiegelmer (26) was synthesized and 5′-modified with 40-kDa polyethylene glycol (at Noxxon Pharma). The compound (or vehicle) was injected i.p. daily for 6 days after PWLs were assessed (on days 0 and 1) by an experimenter who was blind with respect to the pretreatment. On day 7, the behavioral tests were followed by CGRP or saline injections (s.c.) into the left and right plantar hindpaws, respectively, and further nociceptive testing at 5-min intervals. These experiments were performed with male C57BL/6 mice.
Quantitative Trait Locus (QTL) Mapping. Adult AKR and C57BL/6 mice were used to breed a (AKR × C57BL/6)F1 generation. Both reciprocals were used to breed (AKR × C57BL/6)F2 hybrids (n = 93; 50 males; 43 females). All mice were tested for paw-withdrawal test (21) sensitivity in the following manner: each hindpaw was tested 18 times, three times each (separated by at least 20 min) in two sessions per day (morning and afternoon) for 3 days. No striking repeated measures effects were noted, nor were there significant circadian or laterality effects, so all 36 measures were combined into a grand mean for each mouse. A full-genome search for linkage was conducted by using 110 polymorphic microsatellite markers separated by an average of 18.4 cM. QTLs were identified by interval mapping as implemented by map manager qtx (27).
In a follow-up mapping experiment, (BALB/c × C57BL/6)F2 hybrid mice (n = 108; 63 males, 45 females) were bred, phenotyped as described above, and tested for linkage to chromosome 7 only with the following markers (28-65 cM): D7Mit248, D7Mit350, D7Mit323, D7Mit98, and D7Mit71.
Finally, A.B6-Tyr+/J congenic mice were obtained from The Jackson Laboratory (stock no. 002565). In this fully congenic line, a region of chromosome 7 from the C57BL/6 strain (confirmed in our laboratory to include 41-65 cM) has been introgressed onto an A strain background. Mice of all three genotypes (n = 6-10 per genotype per sex per condition) were tested simultaneously for baseline PWLs and effect of intraplantar CGRP/vehicle injection as described above.
Results
Strain Difference in the Response of Nociceptors to Thermal Stimulation. The strain differences in heat-induced pain could be due to differences in responsiveness of primary afferent polymodal nociceptors or to complex differences in CNS signal processing; this was investigated by using the isolated skin-saphenous nerve preparation and single-fiber recording (22). The yield and proportion of mechano-heat sensitive C-fibers (C-MH) was about the same in AKR and C57BL/6 mice, as were conduction velocity and lack of spontaneous activity. However, nociceptors in C57BL/6 mice exhibited 4-fold greater response to radiant heat and began to fire at a 4°C lower threshold temperature compared to those from AKR (Fig. 1A and supporting information). In contrast, the same C-fibers did not show a major strain difference in response to controlled mechanical stimulations (see supporting information).
Strain Difference in Heat-Induced CGRP Release. We found that the strain difference in thermal nociceptors is associated with a corresponding difference in heat-induced release of iCGRP from isolated mouse skin (15). Another sensory neuropeptide, substance P, was also measured, but its release was close to the detection limit of the enzyme immunoassay and not significantly increased by noxious heat. The baseline iCGRP secretion at 32°C was about the same in both mouse strains, but C57BL/6 mice showed much greater stimulated release (up to 3.5-fold) at three levels of noxious heat compared to AKR mice (Fig. 1B).
To differentiate neurosecretory-mediated from direct sensory mechanisms, two equally possible causes of the observed strain differences, we applied KCl (60 mM) as a nonspecific depolarizing stimulus. In response, the skin flaps of C57BL/6 mice released 2.7-fold more iCGRP (above baseline) relative to AKR mice, indicating an additional neurosecretory deficit in the latter strain (Fig. 1C). Capsaicin (10 μM) activation of TRPV1 also stimulated iCGRP release, which was again 2.7-fold greater in C57BL/6mice (Fig. 1C). These strain differences were not due to a difference in the number of CGRP-containing neurons as shown in an immunocytochemical study of lumbar DRGs (Fig. 1D and see supporting information). Rather, they were caused by the total skin content of iCGRP that was 1.6-fold larger in C57BL/6 than AKR mice (Fig. 1E and see supporting information). The reason for this difference in peptide content appears to be a difference in expression of the Calca gene encoding CGRPα, which was 1.8-fold higher in C57BL/6 versus AKR mice (Fig. 1F). No strain difference was observed in DRG expression of Tac1, encoding the other major pain-related peptide, substance P (Fig. 1F). Thus, the strain differences in heat responsiveness paralleled those in iCGRP content and chemically induced release. The functional link could be the autoreceptors present on many CGRP-containing DRG neurons and responding to CGRP with calcium influx (10-12). However, is there a causal relation between CGRP and heat sensitivity?
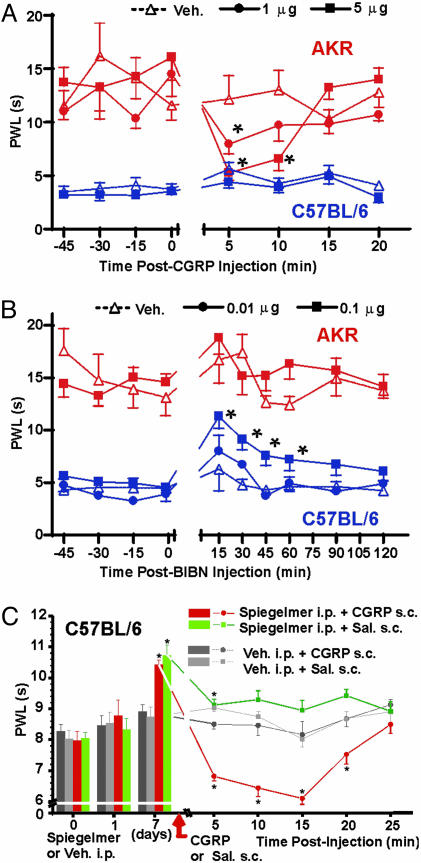
Abolition of the Behavioral Strain Difference by Pharmacological Manipulations of CGRP. Primary afferent neurons are known to release CGRP at both peripheral and spinal terminals upon noxious stimulation. In a series of behavioral experiments, we injected rat CGRPα and CGRP receptor antagonists into both compartments. Injected into the hindpaw of AKR mice, CGRP produced a dose-dependent lowering of PWLs to noxious heat (Fig. 2A). This hyperalgesia peaked 5 min after injection, and disappeared within 10-15 min, likely due to degradation of the injected peptide. The hyperalgesia was more robust in female versus male AKR mice (not shown). No dose of CGRP tested produced any measurable hyperalgesia in C57BL/6 mice. At 5 μg and at 5 min after injection, PWLs of CGRP-injected AKR and C57BL/6 mice were statistically equal, indicating a complete albeit temporary “rescue” of the strain difference (Fig. 2A). Strain-dependent CGRP-induced hyperalgesia was reproduced on another thermal assay, the hot-plate test, and appeared to be specific to heat because peripheral CGRP injection produced equivalent hypersensitivity to mechanical stimulation with von Frey filaments in both mouse strains (see supporting information).
Fig. 2.
Differential noxious heat sensitivity in AKR and C57BL/6 mice and its abolition by pharmacological manipulations at CGRP receptors and by CGRP inactivation. (A) Complete and reversible abolition of the AKR vs. C57BL/6 strain difference by injection of 5 μg of CGRPα into the right hindpaw; 1 μg of CGRPα produced a partial effect. Symbols represent mean ± SEM latency (s) to withdraw the paw from the noxious stimulus; n = 12-21 per strain per condition. *, P < 0.05 compared to vehicle-injected mice of same strain (Student's t test) and compared to PWL at time = 0; only the AKR-CGRP groups displayed a significant repeated measures effect. The left (noninjected) hindpaw was tested simultaneously; contralateral CGRP injection had no effect in either strain (data not shown). Injection of CGRPβ (5 and 10 μg) also produced no effect (data not shown). No CGRPα dose up to 20 μg produced any significant PWL changes in C57BL/6 mice (data not shown). (B) Reversible attenuation of the AKR vs. C57BL/6 strain difference in heat nociception by injection of 0.1 μg but not 0.01 μg of BIBN4096BS (BIBN) into the spinal cord. Symbols represent mean ± SEM withdrawal latency (s) from both hindpaws averaged; n = 5-8 per strain per condition. *, P < 0.05 compared to vehicle-treated mice of the same strain (Student's t test) and compared to PWL at time = 0; only the C57BL/6-BIBN (0.1 μg) group displayed a significant repeated measures effect. No BIBN dose up to 1.0 μg produced any significant PWL changes in AKR mice. (C) Reduced sensitivity to noxious heat in C57BL/6 mice after chronic inactivation of endogenous CGRP, and its complete reversal by exogenous CGRP administration. The radiant heat source was attenuated to adjust PWLs to be ≈8 s. The PEGylated anti-CGRP Spiegelmer NOX-504P was systemically injected (25 μg per mouse, i.p.) once daily over 6 days and caused a bilateral reduction on day 7 in PWLs as compared to vehicle injections (bar graph, means ± SEM). The subsequent CGRPα injection (5 μg, s.c.) into the left hindpaw caused a profound sensitization only in the CGRP-deprived but not vehicle-treated animals (line graph, means ± SEM). *, P < 0.01 (ANOVA followed by LSD test).
The behavioral CGRP hyperalgesia in AKR mice could only be partially reversed by peripherally administered CGRP antagonists, because both BIBN4096BS and CGRP8-37 showed (functional) partial agonist activity, themselves producing dose-dependent hyperalgesia in AKR but not C57BL/6 mice upon hindpaw injection (see supporting information). However, BIBN4096BS injected i.t. into the spinal cord produced robust, dose-dependent (0.01-0.1 μg) antinociception in C57BL/6 but not AKR mice, largely abolishing the behavioral strain difference (Fig. 2B). Conversely, CGRP itself injected i.t. produced hyperalgesia in AKR but not C57BL/6 mice (see supporting information). In summary, the pharmacological experiments verified a further robust strain difference: differential response to exogenously administered CGRP and blockade of CGRP receptors. The findings are all consistent with the hypothesis that AKR mice are relatively insensitive to heat because they are deficient in CGRP release at both ends of primary afferent neurons in response to noxious thermal stimulation.
Abolition of the Behavioral Strain Difference by a CGRP-Binding Spiegelmer. We overcame the limited efficacy of the peripherally injected CGRP antagonists by using a recently developed CGRP-binding Spiegelmer (STAR-F12-Δ43-48). This biostable l-RNA aptamer inactivates CGRPα with an IC50 of 3 nM, whereas nonmatching l-RNA is biologically inert (26). NOX-504P, a PEGylated variant of the Spiegelmer (28) injected daily, produced a marked prolongation of PWLs in C57BL/6 mice in both hindpaws by day 7 (Fig. 2C). Subsequent CGRP injection into one hindpaw caused a profound hyperalgesia in Spiegelmer-treated mice, very similar in time course and magnitude to what had been observed in untreated AKR mice. Even the (saline-injected) contralateral paw showed a slight sensitization, presumably because of some systemic distribution of the CGRP simultaneously injected into the other paw (Fig. 2C). Thus, depriving C57BL/6 mice of their ample CGRP reversed the strain difference, giving them a heat-insensitive and CGRP-sensitive AKR-like phenotype within 1 week.
Variable CGRP Expression Is Sufficient to Explain Differences in Thermal Sensitivity Across Numerous Mouse Strains. In an attempt to evaluate the generality of the C57BL/6 and AKR phenotypes, we tested 10 other inbred strains and outbred CD-1 mice for peripheral CGRP-induced hyperalgesia. Overall, six strains displayed significant hyperalgesia in response to intraplantar injection of 5 μg of CGRP, and seven strains did not (see supporting information). Furthermore, the magnitude of CGRP hyperalgesia was significantly correlated with that strain's baseline nociceptive sensitivity (r = 0.80, P < 0.001), such that at 5 min after injection, all strain differences were eliminated (Fig. 3A). The original strain differences returned at 20 min after injection after the injected CGRP had been metabolized. Six of these strains were assessed for Calca expression in lumbar DRGs relative to GAPDH; in every case, the CGRP-sensitive strains exhibited lower levels of Calca mRNA (Fig. 3B), and mean strain baseline latency was significantly correlated with Calca gene expression (r = -0.68, P < 0.01). We also tested six strains for their sensitivity to noxious heat after peripheral substance P injection, and found that every strain displayed heat hyperalgesia (see supporting information). This finding indicates a likely role of substance P in thermal nociception, but precludes its involvement in mediating the strain differences in heat sensitivity.
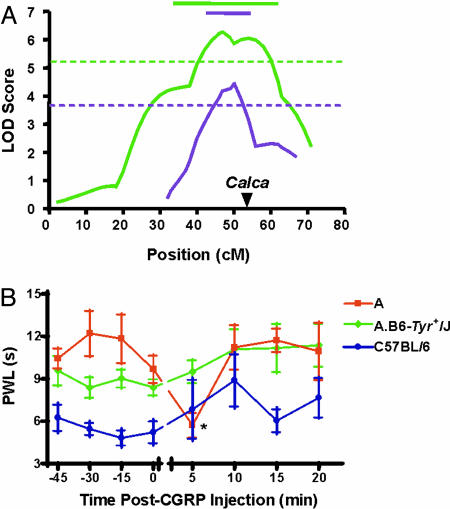
Candidacy of the Calca Gene as the Proximal Cause of the Strain Differences. What gene(s) and sequence variants are responsible for these strain differences? Using a QTL mapping strategy employing (C57BL/6 × AKR)F2 hybrid mice and a full-genome search for linkage (performed independently from the studies described above), we identified only one major gene locus affecting thermal nociception on the paw-withdrawal test. This QTL is on chromosome 7 (maximum LOD score of 6.3) (Fig. 4A). The gene(s) at this locus [peak, 50 cM; confidence interval (CI), 34-62 cM) accounted for >27% of the overall trait variance, and almost 40% of the genetic variance. The linkage varied by sex, being larger in female mice (LOD = 8.1); this is of interest because the hyperalgesic effect of peripheral CGRP administration in AKR mice was also larger in females. In a separate QTL mapping study focused on chromosome 7, we replicated this strong linkage in a (C57BL/6 × BALB/c)F2 intercross (LOD score, 4.5; peak, 50 cM; CI, 42-54; Fig. 4A). Finally, we tested A.B6-Tyr+/J congenic mice, in which a region of chromosome 7 (41-65 cM) from C57BL/6 mice has been introgressed onto the background of A strain mice. We found that the congenic mice displayed significantly lower baseline PWLs compared to A strain mice (an effect larger in females than in males), and furthermore that the congenic mice displayed no evidence whatsoever of CGRP-induced hyperalgesia (Fig. 4B). Calca, the gene coding for CGRP, is located near the peak of linkage at 54 cM. Therefore, these data suggest that polymorphisms in the Calca gene are proximally responsible for the contrasting thermal sensitivity of C57BL/6 versus AKR, BALB/c and A mice. We have sequenced the entire coding region of the mouse Calca gene in AKR mice and found no nucleotide variants compared to the C57BL/6 sequence in publicly available databases. Two single nucleotide polymorphisms between the strains were identified in the promoter region (-1496 and -1499), but the potential significance of these for the observed strain differences in heat sensitivity and CGRP expression remains to be documented.
Fig. 4.
Location of a major gene affecting thermal nociception on the paw-withdrawal test on mid-chromosome 7. (A) The green curve shows logarithm of the odds (LOD) scores (on a Kosambi-corrected map) for linkage at successive locations (in cM) along chromosome 7 in (AKR × C57BL/6)F2 mice, from a full-genome search for linkage. No other strongly suggestive QTLs or strong epistatic interactions were observed. The purple curve shows LOD scores in a subsequent (BALB/c × C57BL/6)F2 intercross, genotyped only at five markers on chromosome 7. In both cases, a recessive model, yielding the best fit, is shown. Horizontal dotted lines represent “highly significant” (99.9th percentile) genome-wide LOD score thresholds for each experiment as determined by permutation analysis. Horizontal solid lines represent the 1-LOD drop-off confidence interval for each experiment. Calca (arrowhead) falls within confidence intervals in both experiments. (B) Congenic mice (A.B6-Tyr+/J) featuring a C57BL/6-derived genomic segment on mid-chromosome 7 placed on an A strain genetic background display reduced basal PWLs and no CGRP-induced hyperalgesia. Symbols represent mean ± SEM. (s) PWLs before and after CGRP injection at time = 0, n = 6-10 per genotype. Vehicle injection produced no changes in any genotype (data not shown). Shown are data from female mice; male congenic mice displayed a smaller (but still statistically significant) reduction in PWLs.
Discussion
Like interindividual differences in pain sensitivity in humans, the cause of differences in heat pain sensitivity across mouse strains could have lain anywhere from the outer cornified layer of the skin to the highest levels of central sensory-motor processing. In fact, we located the difference in the responsiveness of C-MH primary afferent nociceptors, which are much more sensitive to heat in C57BL/6 mice than in AKR mice. The strain difference is associated with a major difference in the sensitivity to and the expression, content, and release of CGRP, one of the two major modulatory peptides in C-MH neurons. Although perhaps not the only factor determining differential heat response in these strains, C-MH sensitivity is probably the major factor, because appropriate pharmacological manipulations of CGRP levels, and the availability of CGRP receptors, completely eliminated the response differential between AKR and C57BL/6 mice, and of other strains as well. In spinal nociceptive transmission, CGRP played an additional role, consistent with its sensitizing action in the rat dorsal horn (5, 6). CGRP-rich C57BL/6 mice showed an hypoalgesic effect of blocking the CGRP receptor, whereas the CGRP-poor AKR mice showed a sensitizing effect of supplementing the neuropeptide.
The present data provide direct evidence for a potential auto- and/or paracrine role of CGRP in modulating acute noxious heat transduction. CGRP has long been predicted to play such a role, because it is released from primary afferent nociceptors by a variety of noxious stimuli. In fact, CGRP release is commonly used as an index of nociceptor activation. However, injection of CGRP into rodent and human skin has generally been without a direct hyperalgesic effect, although spinal administration sensitized nociceptive transmission and enhanced effects of other mediators, such as substance P (5, 18-20). The reason may be that the literature on CGRP as a modulator of heat nociception, at least in the mouse, has been complicated by the previously unknown strain-dependence of CGRP sensitivity and gene expression. That is, the failure of prior studies to demonstrate peripheral CGRP effects might be due to the use of CGRP-rich and, thus, CGRP-insensitive mouse or rat strains (18, 19).
Sensitivity to the peripheral sensitizing action of CGRP seems to be a consequence of CGRP availability rather than an independent phenomenon, as the CGRP-deficient AKR mice were sensitive to CGRP, and the CGRP-insensitive C57BL/6 mice became sensitive when their CGRP was scavenged by the anti-CGRP Spiegelmer. CGRP sensitivity may also increase under pathophysiological conditions such as after spinal nerve L5 lesion; the resulting persistent hyperalgesia in the rat was reported to be reversed by intraplantar injection of a CGRP receptor blocker (29). In another rat model of partial nerve injury, enlarged subsets of residual sensory neurons with enhanced CGRP immunostaining were observed in close vicinity to injured ones with reduced CGRP expression (30). Under these circumstances, the self-adjusting balance between the availability of CGRP and sensitivity to CGRP may be disturbed. Normally, this balance may allow the C57BL/6-like strains to express large amounts of CGRP, down-regulating CGRP sensitivity, and the CGRP-poor AKR-like strains to develop a high sensitivity to CGRP. The balance could set strain-typical basal heat sensitivities. However, perturbation in the event of neuropathy would presumably more affect the CGRP-sensitive strains such as AKR. In the worst case, a positive feedback could build up, leading to a “vicious cycle” of nociceptor autosensitization, whereby the nociceptors that release the CGRP are not necessarily the same ones that express autoreceptors and become sensitized. Indeed, AKR mice are by far the most susceptible of all strains tested to develop heat hyperalgesia after partial nerve injury (2).
Extrapolating our present findings, one would predict that complete deletion of the Calca (CGRPα) gene should lead to a profound loss of noxious heat sensitivity. Indeed, such knockout mice (on a C57BL/6 background) are unable to develop any heat hyperalgesia in different models of inflammation, and they show reduced behavioral responses also to capsaicin, i.p. acetic acid, and to formalin, indicative of a reduced function of the proton-activated capsaicin receptor channel, TRPV1, and of impaired spinal sensitization, respectively (31, 32). However, basic heat responsiveness of the CGRP null mutants is unchanged from wild types (31, 33), suggesting that other genes may be compensating for the lack of Calca expression in the knockouts. TRPV1, although activated also by noxious heat, is not essential for basic primary afferent heat sensitivity (34), but again the null mutants of this ionotropic channel are lacking heat hyperalgesia in different models of inflammation (35, 36). Thus, it is conceivable that TRPV1 is a target of CGRP′s sensitizing action, at least under inflammatory conditions. The signal transduction cascade downstream of CGRP receptors is in accord with nociceptor sensitization to heat (see Introduction). However, the direct correlation of basic heat sensitivity with CGRP gene expression level and the desensitization after prolonged anti-CGRP Spiegelmer treatment may require further explanations, perhaps considering the other heat-activated ion channels, TRPV2 and TRPV3.
Polymorphisms near the Calca gene, and resulting differences in CGRP actions in peripheral tissues and spinal cord, might account for the striking variability among humans in their sensitivity to thermal and inflammatory pain; that is, pain related to nociceptor sensitization to heat and ameliorated by cooling. Our findings might also be related to individual susceptibilities to recurrent pain attacks such as in cluster headaches or migraine. Migraine is thought to be due to abnormal discharge of hypersensitive nociceptive afferents innervating the meninges. The hypersensitivity, in turn, is thought to be related to neuropeptides, especially CGRP released from nociceptor endings and causing neurogenic inflammation. Patients who suffer from migraine, but not normal subjects, experience typical delayed headaches after CGRP infusion (37). Correspondingly, CGRP receptor antagonism by BIBN4096 BS was shown to be effective for the acute treatment of migraine in a recent proof-of-concept clinical trial (23). The present data suggest that a key difference between migraineurs and normal subjects may be their CGRP sensitivity.
In our inbred mouse strains, sensitivity to CGRP was shown to result from a genetically low (or experimentally lowered) availability of CGRP that also leads to a relative insensitivity to noxious heat. Low versus high expression of the Calca (CGRPα) gene was found to be a proximal determinant of the peripheral CGRP peptide level. A region on mid-chromosome 7 near Calca was the only locus found in the mouse genome that was coinherited with noxious heat sensitivity. Thus, it is likely that a specific mid-chromosome 7 polymorphism identified in the promoter region upstream of Calca is the cause of the heritable difference in CGRP expression level and heat sensitivity. At the very least, CGRP appears to have been underestimated in its role of adjusting the gain in heat nociception.
Supplementary Material
Acknowledgments
This work was supported by U.S. Public Health Service Grant DA15191 and the Canada Research Chairs and Canada Foundation for Innovation Programs (to J.S.M.), the Canadian Institutes for Health Research (R.Q.), German-Israeli Foundation Grant I-771-250.4/2002, and European Commission fp6 no. 502 800PAINGENES (to M.M., M.D., and P.W.R.).
Author contributions: J.S.M., M.D., and P.W.R. designed research; J.S.M., F.M., F.S., K.S., K.Z., H.R., J.-S.A., N.B., H.A.H., C.H., K.V.S.N., A.L.R., J.R., A.S., S.B.S., S.S., S.G.L., M.M., and P.W.R. performed research; K.M., A.V., S.K., and R.Q. contributed new reagents/analytic tools; J.S.M., E.J.C., and P.W.R. analyzed data; and J.S.M., R.Q., M.D., and P.W.R. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CGRP, calcitonin gene-related peptide; iCGRP, immunoreactive CGRP; DRG, dorsal root ganglion; PWL, paw-withdrawal latency.
References
- 1.Mogil, J. S., Wilson, S. G., Bon, K., Lee, S. E., Chung, K., Raber, P., Pieper, J. O., Hain, H. S., Belknap, J. K., Hubert, L., et al. (1999) Pain 80, 67-82. [DOI] [PubMed] [Google Scholar]
- 2.Mogil, J. S., Wilson, S. G., Bon, K., Lee, S. E., Chung, K., Raber, P., Pieper, J. O., Hain, H. S., Belknap, J. K., Hubert, L., et al. (1999) Pain 80, 83-93. [DOI] [PubMed] [Google Scholar]
- 3.Bernardini, N., Neuhuber, W., Reeh, P. W. & Sauer, S. K. (2004) Neuroscience 126, 585-590. [DOI] [PubMed] [Google Scholar]
- 4.Richardson, J. D. & Vasko, M. R. (2002) J. Pharmacol. Exp. Ther. 302, 839-845. [DOI] [PubMed] [Google Scholar]
- 5.Sun, R.-Q., Tu, Y.-J., Lawand, N. B., Yan, J.-Y., Lin, Q. & Willis, W. D. (2004) J. Neurophysiol. 92, 2859-2866. [DOI] [PubMed] [Google Scholar]
- 6.Ebersberger, A., Issa, P. B., Vanegas, H. & Schaible, H.-G. (2000) Neuroscience 99, 171-178. [DOI] [PubMed] [Google Scholar]
- 7.Juaneda, C., Dumont, Y. & Quirion, R. (2000) Trends Pharmacol. Sci. 21, 432-438. [DOI] [PubMed] [Google Scholar]
- 8.Hokfelt, T., Bartfai, T. & Bloom, F. E. (2003) Lancet Neurol. 2, 463-472. [DOI] [PubMed] [Google Scholar]
- 9.Anderson, L. E. & Seybold, V. S. (2004) J. Neurochem. 91, 1417-1429. [DOI] [PubMed] [Google Scholar]
- 10.Ye, Z., Wimalawansa, S. J. & Westlund, K. N. (1999) Neuroscience 92, 1389-1397. [DOI] [PubMed] [Google Scholar]
- 11.Ma, W., Chabot, J.-G., Powell, K. J., Jhamandas, K., Dickerson, I. M. & Quirion, R. (2003) Neuroscience 120, 677-694. [DOI] [PubMed] [Google Scholar]
- 12.Segond von Banchet, G., Pastor, A., Biskup, C., Schlegel, C., Benndorf, K. & Schaible, H.-G. (2002) Neuroscience 110, 131-145. [DOI] [PubMed] [Google Scholar]
- 13.Rathee, P. K., Distler, C., Obreja, O., Neuhuber, W., Wang, G. K., Wang, S. Y., Nau, C. & Kress, M. (2002) J. Neurosci. 22, 4740-4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guenther, S., Reeh, P. W. & Kress, M. (1999) Eur. J. Neurosci. 11, 3143-3150. [DOI] [PubMed] [Google Scholar]
- 15.Kessler, F., Habelt, C., Averbeck, B., Reeh, P. W. & Kress, M. (1999) Pain 83, 289-295. [DOI] [PubMed] [Google Scholar]
- 16.Bolyard, L. A., Van Looy, J. W. & Vasko, M. R. (2000) Pain 88, 277-285. [DOI] [PubMed] [Google Scholar]
- 17.Reeh, P. W., Kocher, L. & Jung, S. (1986) Brain Res. 384, 42-50. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura-Craig, M. & Gill, B. K. (1991) Neurosci. Lett. 124, 49-51. [DOI] [PubMed] [Google Scholar]
- 19.Saxen, M. A., Welch, S. P. & Dewey, W. L. (1993) Life Sci. 53, 397-405. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen-Bjergaard, U., Nielsen, L. B., Jensen, K., Edvinsson, L., Jansen, I. & Olesen, J. (1991) Peptides 12, 333-337. [DOI] [PubMed] [Google Scholar]
- 21.Hargreaves, K., Dubner, R., Brown, F., Flores, C. & Joris, J. (1988) Pain 32, 77-88. [DOI] [PubMed] [Google Scholar]
- 22.Reeh, P. W. (1986) Neurosci. Lett. 15, 141-146. [DOI] [PubMed] [Google Scholar]
- 23.Olesen, J., Diener, H.-C., Husstedt, I. W., Goadsby, P. J., Hall, D., Meier, U., Pollentier, S., Lesko, L. M. & BIBN 4096 Clinical Proof of Concept Study Group (2004) N. Engl. J. Med. 350, 1104-1110. [DOI] [PubMed] [Google Scholar]
- 24.Hylden, J. L. K. & Wilcox, G. L. (1980) Eur. J. Pharmacol. 67, 313-316. [DOI] [PubMed] [Google Scholar]
- 25.Doods, H., Hallermayer, G., Wu, D., Entzeroth, M., Rudolf, K., Engel, W. & Eberlein, W. (2000) Br. J. Pharmacol. 129, 420-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vater, A., Jarosch, F., Buchner, K. & Klussmann, S. (2003) Nucleic Acids Res. 31, e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manly, K. F. & Olson, J. M. (1999) Mamm. Genome 10, 327-334. [DOI] [PubMed] [Google Scholar]
- 28.Vater, A. & Klussmann, S. (2003) Curr. Opin. Drug Discov. Dev. 6, 253-261. [PubMed] [Google Scholar]
- 29.Jang J. H., Nam T. S., Paik K. S. & Leem, J. W. (2004) Neurosci. Lett. 360, 129-132. [DOI] [PubMed] [Google Scholar]
- 30.Li, X.-Q., Verge, V. M. K., Johnston, J. M. & Zochodne, D. W. (2004) J. Neuropath. Exp. Neurol. 63, 1092-1103. [DOI] [PubMed] [Google Scholar]
- 31.Zhang, L., Hoff, A. O., Wimalawansa, S. J., Cote, G. J., Gagel, R. F. & Westlund, K. N. (2001) Pain 89, 265-273. [DOI] [PubMed] [Google Scholar]
- 32.Salmon, A.-M., Damaj, M. I., Marubio, L. M., Epping-Jordan, M. P., Merlo-Pich, E. & Changeux, J.-P. (2001) Nat. Neurosci. 4, 357-358. [DOI] [PubMed] [Google Scholar]
- 33.Salmon, A.-M., Damaj, I., Sekine, S., Picciotto, M. R., Marubio, L. & Changeux, J.-P. (1999) NeuroReport 10, 849-854. [DOI] [PubMed] [Google Scholar]
- 34.Woodbury, C. J., Zwick, M., Wang, S., Lawson, J. J., Caterina, M. J., Koltzenburg, M., Albers, K. M., Koerber, H. R. & Davis, B. M. (2004) J. Neurosci. 24, 6410-6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis, J. B., Gray, J., Gunthorpe, M. J., Hatcher, J. P., Davey, P. T., Overend, P., Harries, M. H., Latcham, J., Clapham, C., Atkinson, K., et al. (2000) Nature 405, 183-187. [DOI] [PubMed] [Google Scholar]
- 36.Caterina, M. J., Leffler, A., Malmberg, A. B., Martin, W. J., Trafton, J., Petersen-Zeitz, K. R., Koltzenburg, M., Basbaum, A. I. & Julius, D. (2000) Science 288, 306-313. [DOI] [PubMed] [Google Scholar]
- 37.Lassen, L. H., Haderlev, P. A., Jacobsen, V. B., Iversen, H. K., Sperling, B. & Olesen, J. (2002) Cephalalgia 22, 54-61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.