Abstract
GLUT1, the major glucose transporter in peripheral T lymphocytes, is induced upon T cell receptor activation. However, the role of GLUT1 during human thymocyte differentiation remains to be evaluated. Our identification of GLUT1 as the human T lymphotrophic virus (HTLV) receptor has enabled us to use tagged HTLV-receptor-binding domain fusion proteins to specifically monitor surface GLUT1 expression. Here, we identify a unique subset of CD4+CD8+ double-positive (DP) thymocytes, based on their GLUT1 surface expression. Whereas these cells express variable levels of CD8, they express uniformly high levels of CD4. Glucose uptake was 7-fold higher in CD4hiDP thymocytes than in CD4loDP thymocytes (P = 0.0002). Further analyses indicated that these GLUT1+ thymocytes are early post-β-selection, as demonstrated by low levels of T cell receptor (TCR)αβ and CD3. This population of immature GLUT1+DP cells is rapidly cycling and can be further distinguished by specific expression of the transferrin receptor. Importantly, the CXCR4 chemokine receptor is expressed at 15-fold higher levels on GLUT1+DP thymocytes, as compared with the DP GLUT1- subset, and the former cells show enhanced chemotaxis to the CXCR4 ligand CXCL12. Thus, during human thymopoiesis, GLUT1 is up-regulated after β-selection, and these immature DP cells constitute a population with distinct metabolic and chemotactic properties.
Keywords: GLUT-1, metabolism, thymus
Glucose provides a key supply of energy and carbon for all living organisms, and its transport is a universally conserved property. Vertebrate glucose transporters belong to the GLUT family, of which GLUT1, first identified in 1985 by Mueckler and colleagues (1), appears to be the main functional glucose-transporter isoform in T lymphocytes (2). Interestingly, however, GLUT1 does not appear to be expressed on either quiescent human T cells (2) or their murine counterparts (3, 4). Rather, GLUT1 expression is induced upon T cell receptor (TCR) activation, a process long recognized as being associated with increased glucose metabolism (5-8). We recently determined that GLUT1 is, indeed, an early marker of human T cell activation, with surface expression detected as early as 4 h after TCR stimulation (9).
In this context, glucose metabolism, in general, and GLUT1 expression, specifically, would be expected to be crucial to the survival, differentiation, and proliferation of developing T cells in the thymus. Indeed, >25 years ago, Whitesell and Regen (10) reported that the thymus contains two populations of cells: quiescent cells, where glucose transport equilibrates with a half-time of 30-50 min, and active cells, where the half-time is ≈1 min. However, studies attempting to correlate GLUT1 expression with glucose transport in the thymus were not undertaken, in large part, because specific GLUT1 detection has been hampered by a lack of reliable reagents recognizing exofacial GLUT1 determinants. Recently, however, Singer and colleagues (11) reported a large heterogeneity in GLUT1 expression in murine thymocyte subsets, with high expression restricted to immature double-negative (DN) and more mature CD8 single-positive (SP) cells. In contrast, the characteristics of human thymocyte populations expressing GLUT1 remain unexplored.
It is likely that those human thymocytes expressing GLUT1 are able to accrue increased energy and, as such, are capable of cell cycle entry and division. We hypothesized that identification of the precise population(s) of human thymocytes expressing GLUT1 would help to elucidate the relationships between thymic maturation steps and metabolic activity/demands. Indeed, we identify here a unique subset of metabolically active double-positive (DP) thymocytes, based on their surface expression of GLUT1 and concomitant expression of the CD71 transferrin receptor. These cells represent a subset of rapidly dividing immature DP cells that can be distinguished by high levels of CD4 and the CXCR4 chemokine receptor.
Methods
Thymocyte Preparation. Thymi were removed during corrective cardiac surgery of patients aged 4 mo to 7 y, in accordance with local ethics board regulations. Single-cell thymocyte suspensions were generated by physical disruption of tissue and filtration through 70-μm nylon screens.
Flow Cytometry. Directly labeled mAbs against the following antigens were used: CD4, CD8, CD3, CD71, TCRαβ, CD69, CD95, CD132, CD127, CD25, CD28, HLA-DR, CD49d, CD49e, CD29, glycophorin A, CXCR4, and isotype controls (Immunotech/Becton Dickinson). Surface expression of GLUT1 was monitored by binding to a recombinant human T lymphotrophic virus (HTLV)-2-envelope-receptor-binding domain (HRBD) fused to the EGFP coding sequence (HRBDEGFP), as described in ref. 12. For cell-cycle analysis, thymocytes were permeabilized and stained with an αKi-67 mAb (DAKO) or 7-amino-actinomycin-D (7-AAD; 400 μM, Sigma). Cell sorting was performed on a FACS ARIA flow cytometer (Becton Dickinson). Data analyses were performed by using the programs cellquest pro (Becton Dickinson) and flowjo (Tree Star, Ashland, OR).
Glucose Uptake. Thymocytes (6 × 105) were incubated in serum-free RPMI medium 1640 for 30 min then washed and incubated for 30 min in 500 μl of serum/glucose-free RPMI medium 1640. Uptake was initiated by adding labeled 2-deoxy-d[1-3H]glucose (Amersham Pharmacia Biosciences) to a final concentration of 0.1 mM (2 μCi/ml) (1 Ci = 37 GBq). Cells were incubated for 45 min at 37°C, washed in cold serum/glucose-free RPMI medium 1640, and solubilized in 500 μl of 0.1% SDS. Radioactivity was measured by liquid scintillation, and statistical analyses were performed by using Student's t test.
Chemotaxis Assay. Freshly isolated total thymocytes (5 × 105 in 100 μl of RPMI medium 1640) were loaded onto 5-μm pore polycarbonate transwell culture inserts (Costar). Six-hundred microliters of either RPMI medium 1640 or RPMI medium 1640 containing 300 ng/ml rCXCL12 (PeproTech, Rocky Hill, NJ) was added to the lower wells, and chemotaxis chambers were incubated at 37°C for 2.5 h. The contents of the lower wells were then recovered, and the number of migrating cells was counted on a hemocytometer. These cells were phenotyped by staining for CD4, CD8, TCRαβ, and CD71 expression with the corresponding mAbs. The chemotactic index was calculated as the ratio of the number of cells with a given phenotype migrating to CXCL12 to the number of cells migrating to medium alone.
Results
Surface GLUT1 Expression on a Subpopulation of DP Thymocytes Characterized by High CD4 Expression. Given the prime importance of the GLUT1 glucose transporter in cellular metabolism and T cell activation, it was crucial to determine the surface expression of GLUT1 during discrete stages of human thymocyte differentiation. Measurements of surface GLUT1 have been heretofore difficult to perform because of a lack of reliable antibodies directed against the extracellular portion of human GLUT1 (data not shown). Our studies, identifying GLUT1 as the HTLV receptor (12), have enabled us to use tagged HRBD fusion proteins (13) to specifically monitor surface GLUT1 expression.
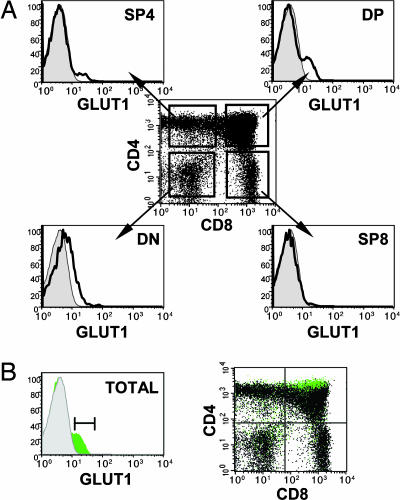
Surface GLUT1 expression was assessed on freshly isolated human thymocytes by using a soluble EGFP-tagged HRBD. The overall percentage of GLUT1+ thymocytes in individual thymi was remarkably constant, within a range of 11-14% (mean 12%, n = 5). Within the SP CD4 (SP4) and DP thymocyte populations, GLUT1+ thymocytes could be readily distinguished as discrete subsets (Fig. 1A). Additionally, a fraction of DN thymocytes expressing GLUT1 was detected; these cells likely correspond to those thymocytes undergoing a first burst of expansion before the rearrangement of TCR chains (14). Within SP4 thymocytes, GLUT1 expression was observed in the immature CD3- population [intermediate single-positive (ISP)4] but was not detected on the more mature CD3+ population (see Fig. 7, which is published as supporting information on the PNAS web site). Strikingly, within the DP cells, GLUT1+ thymocytes localized to a specific subpopulation, distinguishable from other DP cells by high surface CD4 levels (Fig. 1B). Indeed, the fluorescence of CD4 staining on the GLUT1+DP subset was significantly higher (P = 0.006) than that of the GLUT1- DP thymocytes (mean, 1.7-fold; range, 1.3-2; n = 5). Thus, DP thymocytes expressing GLUT1 can be distinguished by their high surface CD4 levels.
Fig. 1.
GLUT1 is expressed on a subpopulation of DP thymocytes characterized by high CD4 expression. (A) Surface expression of GLUT1 on freshly isolated human thymoctes was assessed by using an EGFP-tagged HRBD fusion protein that specifically binds GLUT1 (12). GLUT1 expression (solid line) is indicated relative to control staining. The relative expression of GLUT1 in DN, DP, SP4, and SP8 thymocyte subsets was determined by incubation of thymocytes with HRBDEGFP before staining with αCD4 and αCD8 mAbs. (B) The relative expression of CD4 and CD8 on GLUT1+ cells was monitored on CD4/CD8/GLUT1-stained total thymocytes. GLUT1+ cells were back-gated and superimposed (green) onto the total thymocyte population (black) on a CD4/CD8 dot plot. Results are representative of data obtained in five thymi.
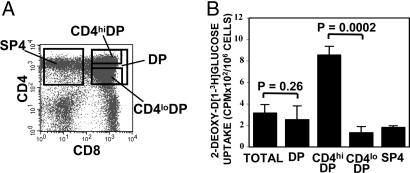
GLUT1-Expressing CD4hiDP Thymocytes Show Enhanced Glucose Uptake. To evaluate whether the cell surface expression of GLUT1 on CD4hiDP thymocytes has a physiological impact, thymocytes were assessed for their ability to uptake labeled glucose. DP and SP4 thymocyte populations were FACS-sorted, and DP subsets expressing high (CD4hiDP) and low (CD4loDP) levels of CD4 were further purified as indicated in Fig. 2A, representing an average 10% and 40%, respectively, of the total DP population. Notably, the CD4hiDP subset was defined by the localization of the GLUT1+ thymocyte population represented in Fig. 1B. Glucose uptake, as measured by the ability to uptake nonhydrolyzable 2-deoxy-d[1-3H]glucose, was approximately equivalent in unsorted thymocytes and sorted total DP thymocytes (P = 0.26). However, the level of glucose incorporation in the CD4hiDP population was 7-fold higher than in the CD4loDP population (P = 0.0002) (Fig. 2B). These results indicate that the presence of cell-surface GLUT1 on CD4hiDP thymocytes specifically promotes glucose transport in these cells, thus bearing important physiological significance.
Fig. 2.
GLUT1 expression on CD4hiDP thymocytes is of functional significance and promotes glucose uptake. (A) Total human thymocytes and DP and SP4 populations were FACS-sorted. DP cells were further sorted on the basis of CD4 expression, resulting in the isolation of CD4hiDP and CD4loDP subsets, as indicated in the dot plot. (B) Glucose uptake was assayed by incubating total and sorted thymocyte populations (6 × 105) with 2-deoxy-d[1-3H]glucose (0.1 mM) for 45 min at 37°C. Uptake is expressed as mean cpm for triplicate samples; error bars indicate SD. Statistical significance comparing glucose uptake in CD4hiDP, total DP, and CD4loDP cells is indicated.
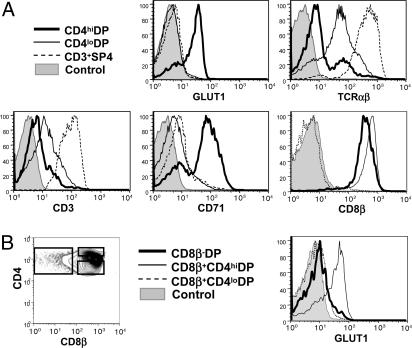
Characterization of the GLUT1+CD4hiDP Thymocyte Population. Given the specificity of GLUT1 expression and its distinctive metabolic impact within DP human thymocytes, further characterization of this unique population was warranted. Expression of thymocyte differentiation markers and cytokine and integrin receptors was, therefore, compared within CD4hiDP and CD4loDP subsets. In the representative thymus shown in Fig. 3, 68% of CD4hiDP thymocytes demonstrated positive GLUT1 expression, whereas GLUT1 was not detectable in the CD4loDP population (see Table 1, which is published as supporting information on the PNAS web site). Notably, the CD71 transferrin receptor, which is a marker of metabolic activity (15), was expressed at the surface of 73% of CD4hiDP thymocytes (mean = 62%; range, 43-73; n = 3), but was not detected on the CD4loDP population, further confirming the dynamic state of the former cells.
Fig. 3.
Characterization of CD4hiGLUT1+DP thymocytes.(A) The phenotype of CD4hiDP thymocytes was compared with that of CD4loDP and mature CD3+SP4 thymocytes. Cells were stained for CD4 and CD8 with GLUT1, TCRαβ, CD3, CD71, or CD8β mAbs. Representative histograms, showing relative expression levels in these three populations, are presented. (B) To precisely assess whether GLUT1 is expressed within β-selected cells, its expression in DP populations was analyzed within the CD8β- and CD8β+ subsets, as indicated in the histograms.
Phenotypic analyses of well defined thymic differentiation markers were used to investigate the maturation state of the metabolically active DP thymocyte subset. These CD4hiDP cells expressed detectable, albeit low, levels of the TCRαβ heterodimer, whereas expression on CD4loDP thymocytes was, on average, 4-fold higher (range, 2-6; n = 4; Fig. 3A). These data, indicating that the majority of CD4hiDP cells are at an earlier stage of thymic differentiation than CD4loDP cells, was confirmed by analysis of CD3 expression (Fig. 3A). Notably, however, GLUT1+CD4hiDP thymocytes represent a population that has undergone β-selection, because CD8β was detected at the surface of all cells (Fig. 3A). Conversely, when DP cells are divided into pre- and post-β-selection populations on the basis of surface CD8β expression, it can be observed that GLUT1 expression is restricted to those thymocytes that have undergone selection and are CD8β+ (Fig. 3B). These GLUT1+ thymocytes have clearly not yet negotiated the positive selection checkpoint, because CD69, an activation marker up-regulated during this process (16), was uniformly low within the CD4hiDP population, even though it was detected in a portion of CD4loDP thymocytes (see Fig. 8, which is published as supporting information on the PNAS web site). Other markers, including the γc cytokine subunit, CD25, and CD95 and the integrin receptors CD29, CD49d, and CD49e were expressed at comparable levels on the two DP populations (Fig. 8 and L.S., unpublished data).
The high proportion of CD4hiDP thymocytes expressing GLUT1 (≈70%) was comparable with the percentage of cells in this population expressing low levels of surface TCRαβ and high levels of the CD71 transferrin receptor. We therefore evaluated whether the GLUT1+ cells within the CD4hiDP population were also TCRαβlo and CD71+. Indeed, the vast majority of CD4hiDP thymocytes expressing GLUT1 expressed low levels of TCRαβlo and high levels of CD71 (Fig. 8), in marked contrast to the CD4loDP thymocyte subset, wherein <5% of the cells were characterized as GLUT1+, TCRαβlo, or CD71+. The ensemble of these data indicates that GLUT1+CD4hiDP thymocytes constitute a specific thymocyte phenotype, characterized as TCRαβloCD71+.
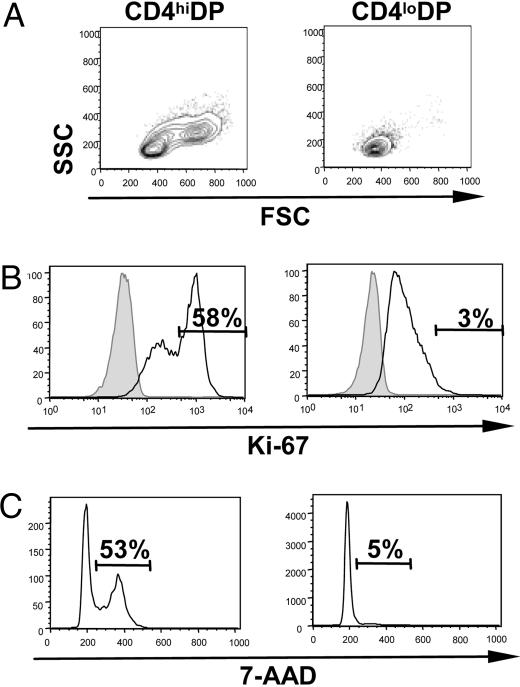
CD4hiDP Thymocytes Are Large Cycling Cells. GLUT1 levels have been shown to be closely linked to the metabolic and cell-cycle status of mature T cells (2, 3, 9). Given the differential GLUT1 expression between CD4hiDP and CD4loDP cells, we assessed whether these two subsets demonstrated differences in size and cell-cycle status. The forward scatter (FSC) and side angle scatter (SSC) of these subsets demonstrated that CD4loDP cells were uniformly small. In contrast, CD4hiDP thymocytes were heterogeneous, with a significant percentage of blast-like cells of increased size and granularity (mean, 64%; range, 51-88; n = 6; Fig. 4A). Moreover, high expression of Ki-67, induced during the mid-G1 phase of the cell cycle (17), was detected in 58% of CD4hiDP cells, as compared with only 3% of the CD4loDP subset (Fig. 4B). This striking difference in cell-cycle status between the two DP populations was further confirmed by DNA staining (using 7-amino-actinomycin D), with 53% of CD4hiDP thymocytes in the S/G2/M phases of the cell cycle, whereas only 5% of CD4loDP cells had exited from the quiescent G0/G1 stages (Fig. 4C).
Fig. 4.
High CD4 expression characterizes a large cycling DP population. (A) The forward scatter (FSC) and side angle scatter (SSC) of CD4hiDP and CD4loDP thymocytes were analyzed by flow cytometry. (B) Cell-cycle entry of CD4hiDP and CD4loDP thymocytes was monitored by assessing intracellular expression of Ki-67. (C) DNA levels in CD4hiDP and CD4lloDP cells were monitored by 7-amino-actinomycin D (7-AAD) staining, and the relative percentages of thymocytes in S/G2/M are indicated. Results are representative of data obtained in two thymi.
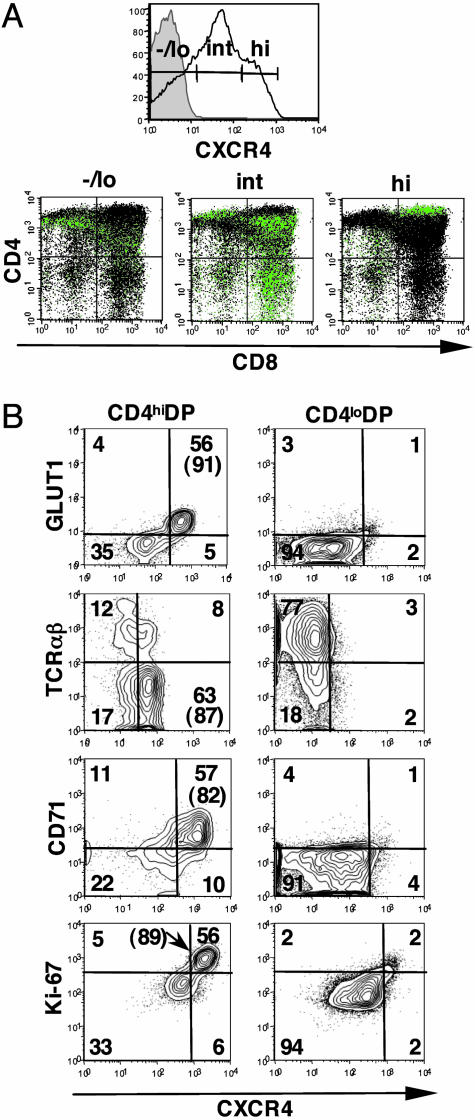
High Expression of CXCR4, the HIV-1 Coreceptor, Is Largely Restricted to GLUT1+CD71+CD4hiDP Thymocytes. CXCR4 is the most abundant and ubiquitously expressed chemokine receptor in the thymus (18), where it plays a critical role in thymocyte development (19). Coexpression of CXCR4 and CD4 is of major pathological importance, because these molecules are the major components of the receptor for T-tropic HIV-1 strains (20). Because metabolically active cells would provide an ideal environment for viral hijacking of cellular resources, we assessed CXCR4 levels on thymocyte populations in the context of GLUT1 expression. As previously reported, the vast majority of human thymocytes express CXCR4 (18, 21, 22), but, notably, the level of CXCR4 expression is highly variable, with fluorescence ranging over 3 logs (Fig. 5A). Thymocytes were divided on the basis of negative/low (-/lo), intermediate (int) and high (hi) levels of CXCR4, as shown in Fig. 5A, and the developmental stage of thymocytes in each group was determined. As reported, thymocytes with low or absent levels of CXCR4 were mainly SP4 and DN thymocytes, whereas those cells expressing intermediate levels of CXCR4 were of the SP8 and DP phenotype (22). A small percentage of CXCR4hi thymocytes were of the DN (2-4%, n = 5) and SP4 (3-7%, n = 5) phenotype, and, because these latter cells were CD3-, they represent the CD4+ISP thymic population (data not shown). Notably although, within the CD4hiDP subset, the vast majority of thymocytes could be classified as CXCR4hi (Fig. 5A and Table 1).
Fig. 5.
High expression of the HIV-1 coreceptor CXCR4 is largely restricted to GLUT1+CD4hiDP thymocytes. (A) CXCR4 expression was assessed on total thymocytes, and, based on the level of staining, thymocytes were subdivided into CXCR4-/lo, CXCR4int, and CXCR4hi populations, as indicated. The maturation state of these populations was determined by assessing the expression of CD4 and CD8 surface markers. The various CXCR4 subsets were back-gated and superimposed (green) onto the total thymocyte population (black) on a CD4/CD8 dot plot. (B) Expression of GLUT1, TCRαβ, CD71, and Ki-67 markers, with respect to CXCR4, were monitored on the CD4hiDP and CD4loDP cells and are presented as dot plots. The percentages of cells in each quadrant are indicated, and the quadrants were drawn with respect to high, rather than positive, CXCR4 expression (MFI of CXCR4 staining varies because of the use of different αCXCR4 fluorochoromes). The percentages of CXCR4hi cells expressing these various markers are indicated in parentheses.
We therefore investigated whether high CXCR4 expression was characteristic of cells within the CD4hiDP population that were phenotypically GLUT1+TCRαβloCD71+. Coexpression of GLUT1, TCRαβ, and CD71 with CXCR4 in the CD4hiDP and CD4loDP subsets was compared. As observed in the representative thymus in Fig. 5B, >80% of CXCR4hiCD4hiDP thymocytes coexpressed GLUT1 and CD71 and expressed low levels of TCRαβ. Moreover, these CXCR4hiDP cells demonstrated high levels of Ki-67. In marked contrast, <5% of CD4loDP thymocytes could be characterized as CXCR4hi. Indeed, CXCR4 staining on GLUT1+CD4hiDP cells was 15-fold higher than on the GLUT1-CD4loDP subset. High expression of CXCR4 is, thus, an important marker of the metabolically active immature DP thymocyte population characterized as GLUT1+TCRαβloCD71+CD4hi (see Fig. 9, which is published as supporting information on the PNAS web site).
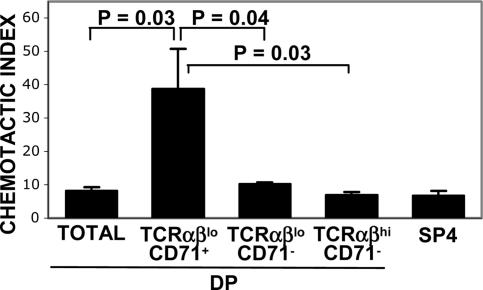
High Levels of CXCR4 on GLUT1+TCRαβloCD71+DP Thymocytes Promote CXCL12-Induced Migration. The natural ligand for CXCR4 is SDF-1/CXCL12 (23), a chemokine critical for thymic differentiation (19, 23). To determine whether the elevated cell-surface levels of CXCR4 on GLUT1+TCRαβloCD71+ DP thymocytes translated into an increased functional response to CXCL12, their relative chemotaxis was assessed within the context of total nonsorted thymocytes. Because of changes in CXCR4 and GLUT1 expression during incubation in the presence of chemokines and by serum alone (24), the GLUT1+TCRαβloCD71+CD4hiDP population with high CXCR4 was monitored a posteriori, by flow cytometry, on the basis of low TCRαβ and high CD71 expression (TCRαβloCD71+). Within the DP population, only 2-3% of TCRαβloCD71+ thymocytes migrated to medium alone. Whereas there was a low level of spontaneous migration of DP cells, these cells were phenotypically TCRαβhiCD71-. However, in the presence of CXCL12, there was a significant increase in the relative proportion of migrating TCRαβloCD71+ cells, representing 13% of DP thymocytes (see Fig. 10, which is published as supporting information on the PNAS web site). Indeed, whereas the addition of CXCL12 resulted in a 7- to 10-fold increase in the absolute numbers of total migrating DP thymocytes or TCRαβloCD71- and TCRαβhiCD71- DP cells (Fig. 6), the absolute numbers of migrating TCRαβloCD71+ DP cells increased by a mean of 40-fold (P < 0.05). Thus, high levels of CXCR4 on the GLUT1+TCRαβloCD71+ DP population are of functional significance and result in enhanced CXCL12-induced signaling and migration.
Fig. 6.
High levels of CXCR4 on CD4hiGLUT1+DP thymocytes are associated with enhanced CXCL12-induced migration. Migration of thymocyte populations in response to the CXCR4 ligand CXCL12 (300 ng/ml) was analyzed in an in vitro migration assay using total unsorted thymocytes. To assess the relative migration of the CXCR4hiGLUT1+DP population in comparison with other DP and SP subsets, the former DP cells were identified on the basis of low TCRαβ levels and high CD71 expression (TCRαβlo/CD71+), because CXCR4 and GLUT1 levels can be modulated by ex vivo culture and by the presence of chemokine. The chemotactic index, defined by the ratio of cells migrating to CXCL12 relative to medium alone, was determined a posteriori by CD4/CD8/TCRαβ/CD71 phenotype analyses of the cells that had migrated. The mean of duplicate samples (±SD) is presented. Statistical differences between the TCRαβlo/CD71+DP population and other thymocyte subsets are indicated.
Discussion
In the present study, we have identified a unique subset of DP thymocytes with high metabolic activity, as revealed by expression of the GLUT1 glucose transporter and increased glucose transport. Although these cells show variable levels of CD8, they express uniformly high levels of CD4. In accordance with their high levels of GLUT1, CD4hiDP cells are rapidly cycling, with ≈50% of cells in the S/G2/M phases of the cell cycle. We further evaluated these cells for expression of the transferrin receptor, because iron delivery, mediated by transferrin, is crucial for thymocyte differentiation and proliferation and, moreover, appears to be a specific marker of metabolically active murine thymocytes (15, 25). Indeed, transferrin receptor is expressed on the vast majority of GLUT1+CD4hiDP cells as compared with <5% of CD4loDP cells. Moreover, expression of CXCR4 is 15-fold higher on these GLUT1+DP thymocytes, as compared with the GLUT1-DP population, and this expression is associated with a higher relative migration in response to CXCL12. Thus, by studying the characteristics of T progenitor cells expressing the GLUT1 glucose transporter, we are able to classify a stage of human DP thymocyte differentiation by high expression of CD4 and CXCR4.
Our identification of this population may shed some light on the consequences of pre-TCR function (26) and proliferation during murine and human thymocyte differentiation. Although the maturation of human and murine thymocytes generally follows the same differentiation scheme, some differences exist. In humans, DN T cell progenitors differentiate into DP cells via a CD3-CD4+CD8- (ISP4) intermediate stage, whereas the equivalent murine cell is a CD3-CD4-CD8+ intermediate (16). In mice, the β-selection checkpoint occurs at a stage before the onset of expression of CD4 and CD8, more specifically, in CD25+CD44dim CD4-CD8- (DN4) cells. Those DN4 cells (CD25-CD44-/lo) that have undergone productive TCRβ gene rearrangements appear to be large and cycling, whereas the majority of DN4 cells with random TCRβ gene rearrangements are small and quiescent (27). Indeed, the majority of data suggests that the proliferation of DN4 cells is linked to a productive TCRβ rearrangement (28, 29).
During human thymocyte differentiation, most TCRβ rearrangements appear to occur at a later stage of differentiation than those in mice, but the phenotype of thymocytes undergoing β-selection is somewhat controversial. The current model proposes that β-selection can begin in ISP4 cells before the expression of either the CD8α or CD8β chains (16). However, it has also been reported that β-selection occurs later, in DP cells characterized as CD4+CD8α+CD8β- (30) and in DP cells that subsequently up-regulated CD8β (31). Our data indicate that GLUT1 is expressed after β-selection, because all GLUT1+CD4hiDP thymocytes expressed cell-surface CD8β (Fig. 3A). These data are in agreement with a report showing that cycling cells are enriched within immature human DP thymocytes expressing CD8αβ heterodimers (31). Moreover, this population of DP cells is unique in that neither less mature CD8α+CD8β- nor more mature TCRαβhi thymocytes express markers of metabolic activity, such as GLUT1 or CD71.
Although the vast majority of DP cells have undergone productive TCRβ gene rearrangements, a small percentage of CD4ISP cells also express this receptor subunit (30, 32). It is, therefore, notable that we detected GLUT1 expression in a small percentage of these CD4ISP cells, likely corresponding to the subset of these β-selected cells reported to be actively cycling (see ref. 16 and Table 1). In the murine thymus, cell-surface GLUT1 expression has been observed on DN cells and SP8 cells (11). The high metabolic activity of murine SP8 cells is confirmed by their concomitant expression of CD71 (unpublished observations). The marked disparity between this murine thymic subset and the corresponding human CD8SP population, which is GLUT1-CD71-, suggests differences in the “biology” of thymic differentiation between mice and human, which warrant further investigation.
One intriguing finding is that GLUT1+DP human thymocytes express elevated levels of CD4, whereas both CD8α and CD8β levels are variable. Moreover, within the GLUT1+ISP population, surface CD4 levels are higher than those detected in the GLUT1- equivalents (L.S., unpublished data). Given the importance of the CD4-associated tyrosine kinase p56Lck in thymocyte differentiation (33), it is tempting to speculate that high CD4 levels provide a selective advantage for thymocytes at these stages of development. In support of this hypothesis, crosslinking of CD4, but not CD8, results in Lck phosphorylation (34) and in significantly higher Lck kinase activity (35). Additionally, in the presence of a dominant-negative Lck mutant, human thymocyte differentiation is blocked at the β-selection checkpoint, namely at the transition between ISP4 and DP stages (30). Thus, enhanced pre-TCR signaling, promoted by high levels of CD4-Lck association, may result in increased survival/proliferation of β-selected cells.
We found that surface CXCR4 is up-regulated between the CD4ISP and CD4hiDP stages, and is then markedly down-regulated on the more mature CD4loDP cells (Table 1). Thus, it appears that GLUT1 and CXCR4 promoters are subject to similar regulatory mechanisms. Indeed, CXCR4 and GLUT1 expression are both positively regulated by the phosphatidylinositol 3-kinase/AKT/mTOR signal transduction pathway (3, 36, 37). Moreover, it has long been known that the GLUT1 promoter is responsive to the hypoxia-inducible factor 1 α (HIF-1α) (38), and it has recently been shown that the CXCR4 promoter is also positively regulated by this factor (37, 39). Thus, a high coexpression of GLUT1 and CXCR4 is likely to be the consequence of similar transcription-dependent mechanisms. As discussed below, it will be important to determine whether the precise localization/migration of this DP population within the thymus translates into exposure to hypoxic conditions.
In mice, it is known that β-selection and subsequent proliferation correlate with a reversal in the polarity of thymocyte migration from the subcapsular zone back into the cortex (40). Attachment to the extracellular matrix (ECM) is a prerequisite for migration, and Schevach et al. (41) observed that human DP thymocytes can be divided into two subsets, based on their capacity to adhere to the ECM molecule fibronectin. Interestingly, those authors found that the “adherent” DP cells expressed higher levels of CD4 than did the “nonadherent” DP thymocytes. Indeed, we found that GLUT1+CD4hiDP cells demonstrated a 3-fold higher migration on fibronectin than the total DP population (L.S., unpublished data). The ensemble of these data suggests that the GLUT1+CXCR4hiTCRαβloCD71+DP population is likely to be associated with a critical phase of migration within the thymus.
Together with CD4, CXCR4 constitutes a major receptor for HIV-1 cell entry. Thymocyte destruction by CXCR4-tropic HIV-1 initially occurs in the DP population and subsequently manifests in CD4+SP cells (42). Moreover, the virus is initially detected in those DP cells expressing higher levels of CD4 and CXCR4 (43). Intriguingly, however, high levels of CD4 and CXCR4 on the GLUT1+DP thymocyte population may not be the sole elements responsible for their increased susceptibility to HIV infection. After viral entry, the internal environment of the target cell regulates the virus' capacity to undergo reverse transcription, nuclear import, integration, and the expression of viral proteins necessary to complete the viral life cycle. Numerous groups have reported that cell-cycle progression is an important determinant of a cell's susceptibility to HIV-1 infection (44-46). The active metabolism and rapidly cycling status of GLUT1+DP cells imply that they may be intrinsically more permissive to HIV-1 infection, notwithstanding their high CD4 and CXCR4 receptor levels. In support of this hypothesis, we found that HIV-1 core virions infect CXCR4hi thymocytes with significantly higher efficacy than other thymocyte populations (L.S., unpublished data). Moreover, HTLV-1, which uses GLUT1 as a receptor for viral entry (12), would also be expected to preferentially infect GLUT1+CD4hiDP cells. The infection of this population would potentially allow the transmission of HTLV to more mature thymocyte progeny, resulting in a reservoir of infected CD4+ T lymphocytes in vivo (47), thus having important implications for the maintenance of the virus in a quiescent T cell pool that would otherwise be resistant to infection.
Recent studies stemming from adult patients who have undergone hematopoietic stem cell transplantation and HIV-infected individuals who have benefited from highly active antiretroviral therapy have dispelled the notion that thymopoiesis does not occur in adults (48, 49). It will therefore be important to determine whether enhanced thymopoiesis in these patients is associated with increased percentages of metabolically active GLUT1+DP thymocytes.
Based on the data reported here, we propose a redefined model of human DP thymocyte differentiation, whereby surface CD4 levels are highest immediately after β-selection (see Fig. 11, which is published as supporting information on the PNAS web site). The ensuing DP cells are phenotypically large and cycling, expressing the glucose transporter GLUT1, the transferrin receptor CD71, and high levels of the CXCR4 chemokine receptor. These metabolically active cells then differentiate into mature DP cells, with an up-regulation of TCRαβ and CD3 and a down-regulation of CD4. At this point of differentiation, the cells exit from the cell cycle, becoming small and quiescent, with a concomitant decrease in cell-surface GLUT1, CD71, and CXCR4. Thus, during human thymocyte development, the up-regulation of GLUT1, CD71, CXCR4, and CD4 after β-selection allows the characterization of a stage of immature DP cells with distinct metabolic and chemotactic properties.
Supplementary Material
Acknowledgments
We thank operating room personnel of Pr. Metras at the Hôpital la Timone (Marseille, France), without whose assistance this study would not have been possible; H. Spits and collaborators, for generously sharing their unpublished data and giving us the opportunity for scientific exchange; A. Singer, for insightful discussions on the “particularities” of murine thymic differentiation; H. Rodewald, for helpful input; C. Dupperay and R. Galendo for expert assistance with FACS sorting; M. Toribio (Universidad Autónoma de Madrid, Madrid), for kindly providing material; and C. Mongellaz and other members of our laboratories for continuous input. L.S. was supported by the Agence Nationale de Recherches sur le SIDA, N.M. was supported by the Ministère de l'Éducation Nationale, de l'Enseignement Supérieur et de la Recherche, S.K. was supported by the Centre National de la Recherche Scientifique, and M.S. and N.T. were supported by the Institut National de la Santé et de la Recherche Médicale. This work was funded by a grant from the Agence Nationale de Recherches sur le SIDA (to M.S. and N.T.).
Author contributions: L.S., S.K., and N.T. designed research; L.S. and S.K. performed research; N.M., J.-L.B., and M.S. contributed new reagents/analytic tools; L.S., S.K., N.M., J.-L.B., M.S., and N.T. analyzed data; and L.S. and N.T. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DN, double-negative; DP, double-positive; HTLV, human T lymphotrophic virus; HRBD, HTLV-2-envelope-receptor-binding domain; ISP, intermediate single-positive; SP, single-positive; TCR, T cell receptor.
References
- 1.Mueckler, M., Caruso, C., Baldwin, S. A., Panico, M., Blench, I., Morris, H. R., Allard, W. J., Lienhard, G. E. & Lodish, H. F. (1985) Science 229, 941-945. [DOI] [PubMed] [Google Scholar]
- 2.Chakrabarti, R., Jung, C. Y., Lee, T. P., Liu, H. & Mookerjee, B. K. (1994) J. Immunol. 152, 2660-2668. [PubMed] [Google Scholar]
- 3.Rathmell, J. C., Elstrom, R. L., Cinalli, R. M. & Thompson, C. B. (2003) Eur. J. Immunol. 33, 2223-2232. [DOI] [PubMed] [Google Scholar]
- 4.Frauwirth, K. A., Riley, J. L., Harris, M. H., Parry, R. V., Rathmell, J. C., Plas, D. R., Elstrom, R. L., June, C. H. & Thompson, C. B. (2002) Immunity 16, 769-777. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, E. H., Barkhan, P. & Hale, A. J. (1963) Br. J. Haematol. 9, 101-111. [DOI] [PubMed] [Google Scholar]
- 6.Hedeskov, C. J. (1968) Biochem. J. 110, 373-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roos, D. & Loos, J. A. (1970) Biochim. Biophys. Acta 222, 565-582. [DOI] [PubMed] [Google Scholar]
- 8.Peters, J. H. & Hausen, P. (1971) Eur. J. Biochem. 19, 509-513. [DOI] [PubMed] [Google Scholar]
- 9.Manel, N., Kinet, S., Battini, J. L., Kim, F. J., Taylor, N. & Sitbon, M. (2003) Blood 101, 1913-1918. [DOI] [PubMed] [Google Scholar]
- 10.Whitesell, R. R. & Regen, D. M. (1978) J. Biol. Chem. 253, 7289-7294. [PubMed] [Google Scholar]
- 11.Yu, Q., Erman, B., Bhandoola, A., Sharrow, S. O. & Singer, A. (2003) J. Exp. Med. 197, 475-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manel, N., Kim, F. J., Kinet, S., Taylor, N., Sitbon, M. & Battini, J. L. (2003) Cell 115, 449-459. [DOI] [PubMed] [Google Scholar]
- 13.Kim, F. J., Seiliez, I., Denesvre, C., Lavillette, D., Cosset, F. L. & Sitbon, M. (2000) J. Biol. Chem. 275, 23417-23420. [DOI] [PubMed] [Google Scholar]
- 14.Haks, M. C., Oosterwegel, M. A., Blom, B., Spits, H. M. & Kruisbeek, A. M. (1999) Semin. Immunol. 11, 23-37. [DOI] [PubMed] [Google Scholar]
- 15.Brekelmans, P., van Soest, P., Voerman, J., Platenburg, P. P., Leenen, P. J. & van Ewijk, W. (1994) Cell. Immunol. 159, 331-339. [DOI] [PubMed] [Google Scholar]
- 16.Spits, H. (2002) Nat. Rev. Immunol. 2, 760-772. [DOI] [PubMed] [Google Scholar]
- 17.Gerdes, J., Lemke, H., Baisch, H., Wacker, H. H., Schwab, U. & Stein, H. (1984) J. Immunol. 133, 1710-1715. [PubMed] [Google Scholar]
- 18.Taylor, J. R., Jr., Kimbrell, K. C., Scoggins, R., Delaney, M., Wu, L. & Camerini, D. (2001) J. Virol. 75, 8752-8760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plotkin, J., Prockop, S. E., Lepique, A. & Petrie, H. T. (2003) J. Immunol. 171, 4521-4527. [DOI] [PubMed] [Google Scholar]
- 20.Feng, Y., Broder, C. C., Kennedy, P. E. & Berger, E. A. (1996) Science 272, 872-877. [DOI] [PubMed] [Google Scholar]
- 21.Zaitseva, M. B., Lee, S., Rabin, R. L., Tiffany, H. L., Farber, J. M., Peden, K. W., Murphy, P. M. & Golding, H. (1998) J. Immunol. 161, 3103-3113. [PubMed] [Google Scholar]
- 22.Berkowitz, R. D., Beckerman, K. P., Schall, T. J. & McCune, J. M. (1998) J. Immunol. 161, 3702-3710. [PubMed] [Google Scholar]
- 23.Bleul, C. C., Farzan, M., Choe, H., Parolin, C., Clark-Lewis, I., Sodroski, J. & Springer, T. A. (1996) Nature 382, 829-833. [DOI] [PubMed] [Google Scholar]
- 24.Jourdan, P., Abbal, C., Noraz, N., Hori, T., Uchiyama, T., Vendrell, J. P., Bousquet, J., Taylor, N., Pene, J., Yssel, H. (1998) J. Immunol. 160, 4153-4157. [PubMed] [Google Scholar]
- 25.Macedo, M. F., de Sousa, M., Ned, R. M., Mascarenhas, C., Andrews, N. C. & Correia-Neves, M. (2004) Immunology 112, 543-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodewald, H. R. & Fehling, H. J. (1998) Adv. Immunol. 69, 1-112. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman, E. S., Passoni, L., Crompton, T., Leu, T. M., Schatz, D. G., Koff, A., Owen, M. J. & Hayday, A. C. (1996) Genes Dev. 10, 948-962. [DOI] [PubMed] [Google Scholar]
- 28.Falk, I., Biro, J., Kohler, H. & Eichmann, K. (1996) J. Exp. Med. 184, 2327-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tourigny, M. R., Mazel, S., Burtrum, D. B. & Petrie, H. T. (1997) J. Exp. Med. 185, 1549-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blom, B., Verschuren, M. C., Heemskerk, M. H., Bakker, A. Q., van Gastel-Mol, E. J., Wolvers-Tettero, I. L., van Dongen, J. J. & Spits, H. (1999) Blood 93, 3033-3043. [PubMed] [Google Scholar]
- 31.Carrasco, Y. R., Trigueros, C., Ramiro, A. R., de Yebenes, V. G. & Toribio, M. L. (1999) Blood 94, 3491-3498. [PubMed] [Google Scholar]
- 32.Ramiro, A. R., Trigueros, C., Marquez, C., San Millan, J. L. & Toribio, M. L. (1996) J. Exp. Med. 184, 519-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molina, T. J., Kishihara, K., Siderovski, D. P., van Ewijk, W., Narendran, A., Timms, E., Wakeham, A., Paige, C. J., Hartmann, K. U., Veillette, A., et al. (1992) Nature 357, 161-164. [DOI] [PubMed] [Google Scholar]
- 34.Luo, K. X. & Sefton, B. M. (1990) Mol. Cell. Biol. 10, 5305-5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravichandran, K. S. & Burakoff, S. J. (1994) J. Exp. Med. 179, 727-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohn, A. D., Summers, S. A., Birnbaum, M. J. & Roth, R. A. (1996) J. Biol. Chem. 271, 31372-31378. [DOI] [PubMed] [Google Scholar]
- 37.Phillips, R. J., Mestas, J., Gharaee-Kermani, M., Burdick, M. D., Sica, A., Belperio, J. A., Keane, M. P. & Strieter, R. M. (2005) J. Biol. Chem. 280, 22473-22481. [DOI] [PubMed] [Google Scholar]
- 38.Shih, S. C. & Claffey, K. P. (1998) Int. J. Exp. Pathol. 79, 347-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schioppa, T., Uranchimeg, B., Saccani, A., Biswas, S. K., Doni, A., Rapisarda, A., Bernasconi, S., Saccani, S., Nebuloni, M., Vago, L., et al. (2003) J. Exp. Med. 198, 1391-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrie, H. T. (2003) Nat. Rev. Immunol. 3, 859-866. [DOI] [PubMed] [Google Scholar]
- 41.Salomon, D. R., Mojcik, C. F., Chang, A. C., Wadsworth, S., Adams, D. H., Coligan, J. E. & Shevach, E. M. (1994) J. Exp. Med. 179, 1573-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenzweig, M., Clark, D. P. & Gaulton, G. N. (1993) AIDS 7, 1601-1605. [DOI] [PubMed] [Google Scholar]
- 43.Kitchen, S. G. & Zack, J. A. (1997) J. Virol. 71, 6928-6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korin, Y. D. & Zack, J. A. (1998) J. Virol. 72, 3161-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unutmaz, D., KewalRamani, V. N., Marmon, S. & Littman, D. R. (1999) J. Exp. Med. 189, 1735-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dardalhon, V., Jaleco, S., Kinet, S., Herpers, B., Steinberg, M., Ferrand, C., Froger, D., Leveau, C., Tiberghien, P., Charneau, P., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 9277-9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richardson, J. H., Edwards, A. J., Cruickshank, J. K., Rudge, P. & Dalgleish, A. G. (1990) J. Virol. 64, 5682-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCune, J. M., Loftus, R., Schmidt, D. K., Carroll, P., Webster, D., Swor-Yim, L. B., Francis, I. R., Gross, B. H. & Grant, R. M. (1998) J. Clin. Invest. 101, 2301-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Douek, D. C., Vescio, R. A., Betts, M. R., Brenchley, J. M., Hill, B. J., Zhang, L., Berenson, J. R., Collins, R. H. & Koup, R. A. (2000) Lancet 355, 1875-1881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.