Abstract
Ceruloplasmin (CP) is a multi-copper ferroxidase mainly synthesized by liver, secreted into the peripheral blood, playing a critical role in regulating the iron homeostasis. In the central nervous system (CNS), the CP expressed by astrocytes plays an important role in the transportation of iron from the blood across the blood-brain barrier (BBB) into the brain. Our previous study showed that conditional knockout of astrocytic CP with Cre-LoxP system (CpGfapcKO) not only improved the learning and memory abilities of elderly mice, but also impaired the learning and memory abilities of young mice. In order to further investigate the effects of CP on learning and memory with aging, we constructed mice model with tamoxifen-induced astrocyte specific knockout of CP, induced CP knockout at 12 months old, and observed the effects on mouse learning and memory at 18 months old. We were delighted to found that ablation of astrocytic CP by tamoxifen at 12 months old could similarly enhance the learning, memory and recognition abilities in 18-month-old mice. Iron deposition in the hippocampus associated with aging was mitigated, leading to a reduction in oxidative stress. The MAPK/JNK pathway exhibited attenuation, while the PI3K/Akt/GSK3 pathway showed enhancement. This combination is expected to result in the reduction of the phosphorylation level of MYC and the elevation of the nuclear translocation of MYC, which might then contribute to reduced cellular senescence. Additionally, the ROS/MAPK/Erk and ROS/MAPK/p38 pathways-dependent cell apoptosis in hippocampus was diminished. The hallmarks of Alzheimer's Disease (AD) were all significantly reduced. Ultimately, the alleviated cellular senescence along with the reduction in AD-related markers, coincided with an improvement in learning, memory, and recognition abilities. These findings further elucidated the role of CP in brain iron metabolism, offering a novel target and strategy for the prevention and treatment of neurodegenerative diseases, such as AD associated with aging.
Keywords: Ceruloplasmin, Astrocytes, Senescence, Recognition, Brain iron metabolism, Alzheimer's disease
1. Introduction
Ceruloplasmin (CP) is a multi-copper ferroxidase which is firstly isolated and discovered by Holmberg and Laurell in pig serum at 1940s [1,2]. CP is mainly synthesized by liver and secreted into the peripheral blood, while CP of the peripheral blood cannot cross the blood-brain barrier (BBB) and enter the central nervous system (CNS) [[3], [4], [5]]. The concentration of CP in peripheral blood serum is about 27.93 ± 1.79 mg/dL. However, the concentration of CP in cerebrospinal fluid (CSF) is only 0.628 ± 0.07 mg/dL, suggesting that CP is unable to cross BBB into the brain from peripheral blood [6]. Interestingly, there is also internal CP expression in the CNS [7,8]. As a multi-copper ferroxidase, CP plays a crucial role in facilitating the release of intracellular iron by assisting Ferroportin 1 (FPN1) [4]. Mutations in the CP gene that lead to aceruloplasminemia can disrupt brain iron metabolism, resulting in a range of neurodegenerative diseases. The severity of symptoms tends to worsen with aging [9,10].
There are tight junctions between endothelial cells at the BBB, preventing iron from freely entering the brain from peripheral blood. Astrocytes, which collaborate with brain microvascular endothelial cells (BMVECs) to form the BBB, are the main cell types in the brain responsible for synthesizing CP. Both glycosylphosphatidylinositol-anchored CP (GPI-CP) and soluble CP (sCP) catalyze the oxidation of ferrous iron (Fe2+) to ferric iron (Fe3+), thereby enhancing FPN1 activity for iron export on the basal surface of BMVECs [4]. Then the Fe3+ is captured by transferrin (Tf) in the interstitial fluid of brain and subsequently distributed to neurons and glial cells of the CNS [11]. In our previous study, we demonstrated that the conditional knockout of astrocytic CPusing the Cre-LoxP system (CpGfapcKO) significantly impacts the release of iron from FPN1 on BMVECs to the brain. This alteration resulted in iron accumulation within BMVECs and a reduction in iron levels in both the cerebral cortex and hippocampus. In aging mice, this intervention mitigates age-related brain iron accumulation, thereby decreasing oxidative stress and mitogen-activated protein kinase (MAPK)-dependent apoptosis in the cerebral cortex and hippocampus. Consequently, these effects lead to an enhancement of learning and memory capabilities [12].
However, for young mice, the iron deficiency induced by CpGfapcKO in the cerebral cortex and hippocampus leads to the damages in synaptic and myelin formation, as well as a reduction in adult neurogenesis within the hippocampus, leading to a dysfunction of learning and memory abilities [12]. CpGfapcKO mice express Cre enzyme as long as the Gfap promoter is activated during embryonic stage, resulting in the knockout of astrocytic CP during this period [[13], [14], [15]]. Therefore, if applied to clinical practice, it is unrealistic for an individual to ablate astrocytic CP during the embryonic stage and then wait for more than half a lifetime until old age, and it may also cause learning and memory impairment in youth.
In order to further explore the usability of regulating astrocytic CP in delaying aging in clinical practice, we bred mice have both hGFAP-creERT2 and Cpflox/flox by hybridizing hGFAP-creERT2 mice with Cpflox/flox mice, which were able to induce knockout of astrocytic CP at specific ages by injecting tamoxifen. This study used intraperitoneal injection of tamoxifen to induce CP knockout in mouse astrocytes at 12 months. At the age of 18 months old, Morris Water Maze (MWM) and New Object Recognition (NOR) behavioral experiments were performed. To our surprise, this method of tamoxifen-induced knockout of astrocytic CP at 12 months old can also significantly improve the learning, memory, and cognitive abilities of the aging mice. These studies will provide potential therapeutic target and novel strategy for inhibiting astrocytic CP to delay learning, memory, senescence, and Alzheimer's Disease (AD) onset with aging.
2. Materials and methods
2.1. Animals
All animal experiments were approved by ACUC (Animal Care and Use Committee) of Hebei Science and Technical Bureau, China, and conducted by the Animal Ethics Committee of Hebei Normal University, China. Cpflox/flox mice on C57BL/6 genetic background were purchased from the Beijing Vitalstar Biotechnology Company. The Loxp sites were inserted around exon 2 by CRISPR-Cas9-mediated homologous recombination. hGFAP-creERT2 mice on C57BL/6 genetic background were obtained from the Jia-wei Zhou's Lab (Institute of Neuroscience, Chinese Academy of Sciences). We generated mice with both hGFAP-creERT2 recombinase and Cpflox/flox (hGFAP-creERT2; Cpflox/flox mice) and the detail was shown in Fig. 2A. At the age of 12 months, all mice were injected with tamoxifen (100 mg/kg) intraperitoneally for 5 consecutive days to induce astrocytic CP knockout, and the experiment was conducted at the age of 18 months (Fig. 2A). APP/PS1 transgenic mice on C57BL/6 genetic background were purchased from Jackson Labs. We crossed the APP/PS1 transgenic mice with the hGFAP-creERT2; Cpflox/flox mice and we generated mice with APP/PS1, hGFAP-creERT2 and Cpflox/flox (APP/PS1; hGFAP-creERT2; Cpflox/flox mice). At the age of 6 months, all mice were injected with tamoxifen (100 mg/kg) intraperitoneally for 5 consecutive days to induce astrocytic CP knockout, and the experiment was conducted at the age of 8 months (Fig. 8A). Thy1-cre mice on C57BL/6 genetic background were purchased from Jackson Labs. Thy1-cre; Cpflox/flox mice (CpThy1cKO) were generated by crossing Thy1-cre mice with Cpflox/flox mice (Fig. S8A). All mice were housed with free access to water and food at the temperature of 21 ± 2 °C and a 12 h light/12 h dark cycle.
Fig. 2.
The hippocampal senescence was alleviated after tamoxifen-induced astrocytic CP knockout. (A) The reproduction and experiment of hGFAP-creERT2; Cpflox/flox mice. hGFAP-creERT2 transgenic mice were hybridized with Cpflox/flox mice, ultimately breeding mice possessing both hGFAP-creERT2 recombinase and Cpflox/flox (hGFAP-creERT2; Cpflox/flox mice) at least two generations later. At the age of 12 months, tamoxifen was injected intraperitoneally for 5 consecutive days (100 mg/kg) to induce astrocytic CP knockout, and the experiment was conducted at the age of 18 months (at this time, it is called 18 M CpGcKO + Tam mice). (B–C) Latency time (B) and distance (C) to the platform from the 1st day to the 5th day were recorded by MWM during the training trials (n = 10 for 3 M Cpflox/flox, 18 M Cpflox/flox and 18 M Cpflox/flox + Tam groups, n = 9 for 18 M CpGcKO + Tam group, and the data were analyzed by two-way ANOVA followed by the Bonferroni test. (D) After the training trails of 5 days, during the pole test at 6th day, the platform was removed and the frequency of the mice passed the area of hidden platform was recorded (n = 10 for 3 M Cpflox/flox, 18 M Cpflox/flox and 18 M Cpflox/flox + Tam groups, n = 9 for 18 M CpGcKO + Tam group). (E-F) The exploration time spent for the familiar and novel object (E) and the index of NOR (F) recorded by the NOR test (n = 8). (G) The hippocampal senescence detected by β-Galactosidase staining. Scale bar = 1 mm. (H) The levels of p16INK4a, p19ARF, p21Cip1, p15INK4b, p18INK4c, p19INK4d and p27Kip1 mRNAs in hippocampus measured by qPCR (n = 4). The data in charts (D), (F) and (H) were analyzed by one-way ANOVA followed by Fisher's LSD test (D, F) or Tukey's test (H), whereas the data in chart (E) were analyzed by paired two tailed t-test. All data are presented as the mean ± SEM, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ns (no significant difference).
Fig. 8.
The AD hallmarks were alleviated after tamoxifen-induced astrocytic CP knockout in APP/PS1 mice. (A) The strategy of APP/PS1; hGFAP-creERT2; Cpflox/flox mice breeding. hGFAP-creERT2 transgenic mice and Cpflox/flox mice were used for hybridization, and hGFAP-creERT2; Cpflox/WT mice were generated. At the same time, APP/PS1 transgenic mice and Cpflox/flox mice were also used for hybridization, and the APP/PS1; Cpflox/WT mice were generated. Then we used the hGFAP-creERT2; Cpflox/WT mice to hybridize with APP/PS1; Cpflox/WT mice, and the APP/PS1; hGFAP-creERT2; Cpflox/flox mice were obtained. At the age of 6 months, tamoxifen was injected intraperitoneally for 5 consecutive days (100 mg/kg) to induce astrocytic CP knockout, and the experiment was conducted at the age of 8 months (at this time, it is called APP/PS1; CpGcKO mice). (B–C) Latency time (B) and distance (C) to the platform from the 1st day to the 5th day were recorded by MWM (n = 7 per group). (D) After the training trails of 5 days, during the pole test at 6th day, the platform was removed and the frequency of the mice passed the area of hidden platform was recorded. (E-G) APP and Aβ oligomers levels in hippocampus measured by Western blot. The quantifications of (E) were presented in graph (F) and (G) (n = 3). (H, J) Immunofluorescence staining of Aβ in hippocampus, and the quantified data of the number of Aβ plaque in (H) were shown in (J) (n = 3), DAPI was used for nuclear staining. Scale bar = 1 mm. (I) Hippocampal iron contents and distribution measured by μ-XRF. The data were analyzed by two-way ANOVA followed by the Bonferroni test for (B, C), and one-way ANOVA followed by Fisher's LSD test for (D) or Tukey's test for (F, G, J). All data are presented as the mean ± SEM, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
The genotyping PCR primer sequences are shown as follows:
hGFAP-creERT2 :
Forward-Gcre2-F: 5′-GGTCGATGCAACGAGTGATGAGG-3’
Reverse-Gcre2-R: 5′-GCTAAGTGCCTTCTCTACACCTGCG-3’
Thy1-cre :
Forward-Tcre-F: 5′-ATTTGCCTGCATTACCGGTC-3’
Reverse-Tcre-R: 5′-ATCAACGTTTTCTTTTCGG-3’
APP/PS1 :
Forward-APP-F: 5′-GACTGACCACTCGACCAGGTTCTG-3’
Reverse-APP-R: 5′-CTTGTAAGTTGGATTCTCATATCCG-3’
Cpflox/flox :
Forward-Cp-seq-F: 5′-CAAAAAAGCCACTCAACAGCACC-3’
Reverse-Cp-seq-R: 5′- CAGCTTGCAGTCCATTTAGTC-3’
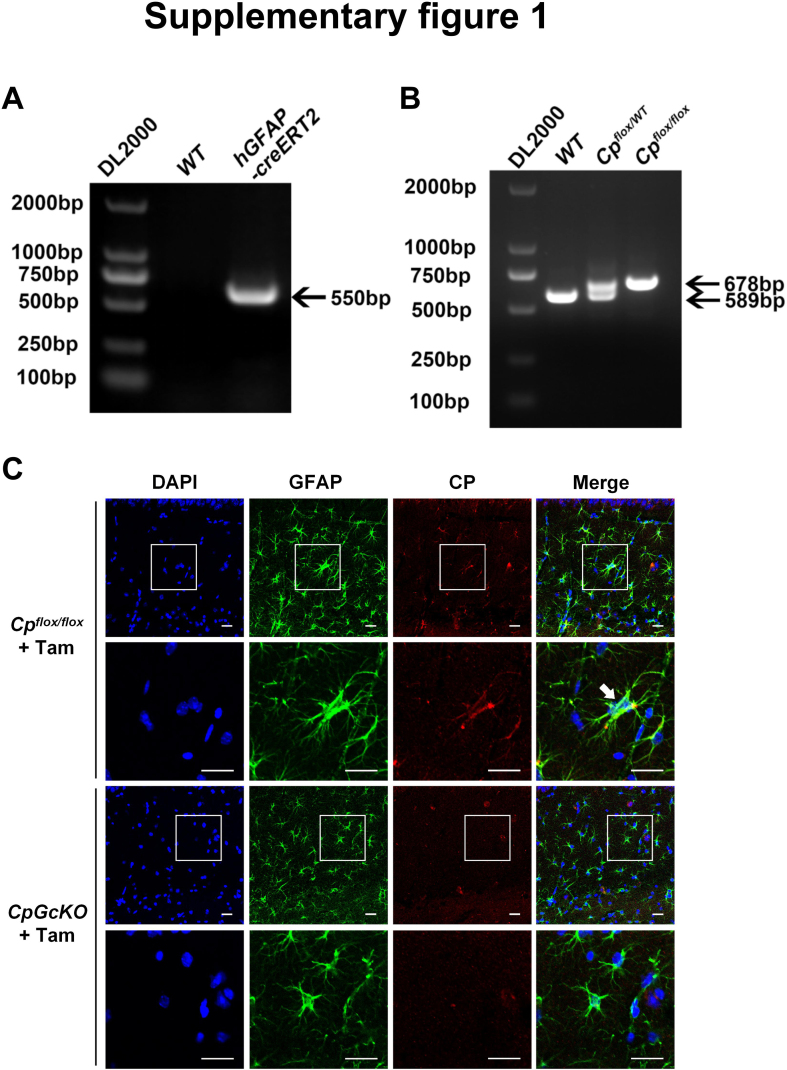
The genotyping results were shown in Fig. S1A and B.
2.2. Morris water maze (MWM) test
The index of spatial learning and memory function were evaluated by using of MWM, as described previously [12,16,17]. The circular bucket was filled with water and divided into 4 quadrants (Diameter = 1.2 m, Height = 0.5 m) with the temperature of 22 ± 2 °C. The mice were transferred from experimental animal house to the behavioral test room to adapt for at least 0.5 h. The test included training trails for five consecutive days and pole testing for followed one day. The platform (Diameter = 9 cm) was visible at 1 cm over the water surface during the 1st day and 2nd day of training trails, while the platform was hidden at 0.5–1 cm under the water surface during the 3rd day to 5th day of training trails. Mice were allowed to seek the platform located in the first quadrant. The latency time and distance were recorded for up to 90 s. The test was conducted 4 times (4 trails) per day for 5 consecutive days. Between two adjacent tests, the mice were allowed to have a rest for at least 0.5 h. During the 6th pole test day, the platform was removed and the mice were allowed to freely swim for 90 s. The swimming locus was recorded by a camera connected to the computer. The number of the platform passing times of each mouse was recorded to assess the learning and memory abilities of mice.
2.3. Novel objects recognition (NOR) test
The NOR test was performed following a previous protocol with modifications [18]. The mice were first adapted to the 50 cm × 50 cm plastic chamber for 5 min. Subsequently, two identical objects were placed 5 cm away from the inner walls of two opposite positions. The mice were familiarized with these two identical objects for 10 min. After 24 h, one of the two objects was replaced with a new object of different shapes and colors. These mice were tested for 10 min using the Smart 3.0 video tracking analysis system to calculate the proportion of time the mice approached the new object and familiar object.
The calculation of new object preference index (Index of NOR) was shown as follows:
2.4. Cell culture
The Neuro-2a (N2a) cell line were cultured in the Dulbecco's modified Eagle's medium (DMEM) (Gibco, CA, USA) with 10 % fetal bovine serum (FBS) (Gibco) and 1 % penicillin-streptomycin (P1400, Solarbio, Beijing, China) in humidified incubator at 37 °C with 5 % CO2. Cells were treated with 100 μM ferroamine citrate (FAC) for 24 h. Then the FAC was removed, and the cells were washed twice with 0.01 M phosphate buffer solution (PBS). Next, we added FAC or deferoxamine (DFO) to the medium for 24 h again according to grouping in Fig. S6A.
2.5. Western blot
Protein expression was assessed by Western blot analysis as previously described [12,[19], [20], [21]]. Additionally, the nuclear protein was extracted according to the protocol of Nuclear Protein Extraction Kit (R0050, Solarbio). The primary antibodies used were used as follows: rabbit anti-mouse CP (1:10, 000; CP16-A, ADI, San Antonio, Texas, USA), rabbit anti-mouse Hephaestin (HP) (1:10, 000; HEPH11-S, ADI), rabbit anti-mouse L-ferritin (1:10, 000; ab109373, Abcam SF, CA, USA), rabbit anti-mouse H-ferritin (1:10, 000; ab183781, Abcam), rabbit anti-mouse FPN1 (1:10, 000; MTP11-S, ADI), mouse anti-mouse transferrin receptor 1 (TfR1) (1:10, 000; 13–6800, Invitrogen, Carlsbad, CA, USA), rabbit anti-mouse divalent metal transporter 1 with iron response element (DMT1(+IRE)) (1:10, 000; NRAMP21-S, ADI), rabbit anti-mouse divalent metal transporter 1 without iron response element (DMT1(-IRE)) (1:10, 000; NRAMP23-S, ADI), mouse anti-mouse β-actin (1:10, 000; CW0096, Cwbio Biotechnology limited company, Beijing, China), mouse anti-mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:10, 000; 60004-1-lg, Proteintech, USA), rabbit anti-mouse Phospho-c-MYC (p-MYC) (T58/S62) (1:10, 000; AF1258, Beyotime Biotechnology, Shanghai, China), rabbit anti-mouse c-MYC (MYC) (1:10, 000; AF6513, Beyotime Biotechnology), rabbit anti-mouse p16INK4a (1:10, 000; AF1672, Beyotime Biotechnology), mouse anti-mouse Histone H3 (1:10, 000; AF0009, Beyotime Biotechnology), rabbit anti-mouse Phospho-JNK (p-JNK) (T183/Y185) and JNK (1:10, 000; #4668S and #9252S, Cell Signaling Technology, St. Louis, MA, USA), rabbit anti-mouse Phospho-Akt (p-Akt) (S473) and Akt (1:10, 000; #4060S and #4691S, Cell Signaling Technology), rabbit anti-mouse Phospho-GSK3β (Y216)/GSK3α (Y279) (p-GSK3) (1:10, 000; AF5833, Beyotime Biotechnology), mouse anti-mouse GSK3α/β (1:10, 000; sc-7291, Santa Cruz Biotechnology, Dallas, TX, USA), rabbit anti-mouse APP/Aβ (1:10, 000; 36–6900, ThermoFisher, MA, USA), rabbit anti-mouse Phospho-Tau (p-Tau) (S396) (1:100, 000; ab109390, Abcam), rabbit anti-mouse p-Tau (T181) (1:5, 000; 28866-1-AP, Proteintech), rabbit anti-mouse p-Tau (S404) (1:5, 000; 81383-1-RR, Proteintech), rabbit anti-mouse Tau (1:5, 000; 10274-1-AP, Proteintech), rabbit anti-mouse Phospho-Erk (p-Erk) (T202/Y204) and Erk (1:5, 000; #4370S and #4695S, Cell Signaling Technology), rabbit anti-mouse Phospho-p38 (p-p38) (T180/Y182) and p38 (1:5, 000; #4511 and #8690S, Cell Signaling Technology), rabbit anti-mouse Bcl-2 (1:10, 000; 12789-1-AP-100, Proteintech), rabbit anti-mouse Bax (1:10, 000; 50599-2-lg, Proteintech), rabbit anti-mouse ACSL4 (1:10, 000; ab155282, Abcam), rabbit anti-mouse GPX4 (1:10, 000; ab125066, Abcam), rabbit anti-mouse Caspase 3 and Cleaved-caspase 3 (1:5, 000; #9622S, Cell Signaling Technology), rabbit anti-mouse IL-6 (1:10, 000; 21865-1-AP, Proteintech), rabbit anti-mouse TGF-β1 (1:10, 000; 21898-1-AP, Proteintech), rabbit anti-mouse IL-1β (1:10, 000; 16806-1-AP, Proteintech), rabbit anti-mouse TNF-α (1:10, 000; 17590-1-AP, Proteintech). After washing with 1 × TBST (pH = 7.6, 137.0 mM NaCl, 0.1 % Tween-20), the blots were incubated with an HRP anti-rabbit secondary antibody (1:10, 000; SA00001-2, Proteintech), or HRP anti-mouse secondary antibody (1:10, 000; SA00001-1, Proteintech) for 90 min at room temperature. After washing with 1 × TBST again, the proteins were treated by super ECL detection reagent kit (#36208ES60, Yeasen Biotechnology, Shanghai, China) and visually detected by chemiluminescence imager (Fujifilm Las-4000, Fujifilm, Japan).
2.6. Quantitative real-time PCR (qPCR)
Total RNA from the regions of cerebral cortex and hippocampus was extracted by TRIzol Reagent (15596018, ThermoFisher) and complementary DNA (cDNA) was synthesized with a PrimeScript™ RT reagent Kit (RR037A, Takara Biomedical Technology Co., Ltd., Beijing, China). Quantitative real-time PCR was performed by using UltraSYBR Mixture (CW0957L, Cwbio Biotechnology limited company, Beijing, China) and a Bio-Rad CFX 96 detection system (Bio-Rad, USA). The mRNA levels were normalized to β-actin mRNA. The PCR primers used are as follows:
p16INK4a :
Forward 5′- AACTCTTTCGGTCGTACCCC -3’
Reverse 5′- GCGTGCTTGAGCTGAAGCTA -3’
p19ARF :
Forward 5′- GCCGCACCGGAATCCT -3’
Reverse 5′- TTGAGCAGAAGAGCTGCTACGT -3’.
p15INK4b :
Forward 5′- CCTTTCAGGACGCGGTGTAA -3’
Reverse 5′- AAGGTACTGACTGCACCCAC -3’
p18INK4c :
Forward 5′- AGCCTCCTTAAAACTCTGCCG -3’
Reverse 5′- CTGTGCCGGTTTCTTATCCCT -3’
p19INK4d :
Forward 5′- GCAAGGAAAGGAGGGAGGTC -3’
Reverse 5′- CAGCTCCCGGTGAAGAAGG -3’
p21Kip :
Forward 5′- GTGGGTCTGACTCCAGCCC -3’
Reverse 5′- CCTTCTCGTGAGACGCTTAC -3’
p27Cip :
Forward 5′- AGATACGAGTGGCAGGAGGT -3’
Reverse 5′- ATGCCGGTCCTCAGAGTTTG -3’
β-actin :
Forward 5′- AGGCCCAGAGCAAGAGAGGTA -3’
Reverse 5′- TCTCCATGTCGTCCCAGTTG -3’
2.7. Immunofluorescence
The whole brains were separated and fixed in 4 % paraformaldehyde (PFA) for 24 h, and then cryoprotected in 30 % sucrose. The process of frozen section and immunofluorescence were the same as previously described [12,21]. Coronal sections of brains were cut at a 20 μm thickness in a freezing microtome (CM1950, Leica, German) and antigen retrieval was performed in 0.01 M citrate buffer (pH 6.0) in a microwave oven at 95 °C for 10 min. The samples were blocked with 2 % BSA, and incubated overnight at 4 °C with primary antibodies. The primary antibodies used were used as follows: mouse anti-mouse GFAP (1:1, 000; MAB360, Millipore, Temecula, CA, USA), rabbit-anti mouse CP (1:1, 000; CP16-A, ADI), rabbit-anti mouse HP (1:1, 000; HEPH11-S, ADI), mouse anti-mouse NeuN (1:1, 000; ab104224, Abcam), mouse anti-mouse Iba-1 (1:500; MABN92, Millipore), mouse anti-mouse CC1 (1:500; ab16794, Abcam), rabbit-anti mouse L-ferritin (1:1, 000; ab109373, Abcam), rabbit-anti mouse H-ferritin (1:1, 000; ab183781, Abcam), rabbit anti-mouse p-Tau (S396) (1:1, 000; ab109390, Abcam), rabbit anti-mouse Aβ (1:500; ab2539, Abcam), rabbit-anti mouse p16INK4a (1:500; GB111143-50, Servicebio, Wuhan, China). The following secondary antibodies and DAPI were used, and the incubations were at 37 °C for 50 min: Dylight 488 goat anti-mouse IgG (1:200; RS23210, Immunoway, TX, USA), Dylight 549 goat anti rabbit IgG (1:200; RS23320, Immunoway), Cy3–conjugated Affinipure Goat Anti-Mouse IgG (H + L) (1:200; SA00009-1, Proteintech), CoraLite488-conjugated Goat Anti-Rabbit IgG (H + L) (1:200; SA00013-2, Proteintech), DAPI (1:1, 000, 28718-90-3, Solarbio). Sections were washed with 0.01 M PBS (pH 7.2–7.4) after each staining step. For the precise area acquiring, the sections were photographed with the Olympus FV3000 (Olympus, Japan) co-focus microscope. For the whole hippocampus region acquiring, the sections were photographed with the TG Panoramic Tissue Cell Quantitative Analysis System (Tissue FAXS Plus S) (Tissue Gnostics, Austria).
2.8. Synchrotron radiation micro-X-ray fluorescence (μ-XRF)
The whole brains of mice were separated and fixed in 4 % PFA for 24 h, and then cryoprotected in 30 % sucrose. Coronal sections of brains were cut at a 50 μm thickness in a freezing microtome (CM1950, Leica, German). The sections were mounted onto mylar film made of polycarbonate.
The μ-XRF microspectroscopy experiment was performed at 4W1B endstation, Beijing Synchrotron Radiation Facility (BSRF), which runs 2.5 GeV electron with current from 150 mA to 250 mA. The incident X-ray energy is monochromatized by W/B4C Double-Multilayer-Monochromator (DMM) at 15 keV and is focused down to a diameter of 50 μm by the polycapillary lens. The two-dimensional (2D) mapping is acquired by step-mode: the sample is held on a precision motor-driven stage, scanning 100 μm stepwise. The Si (Li) solid state detector is used to detect X-ray fluorescence emission lines with live time of 1 s. The data reduction and process were performed using PyMCA package [22]. The results were analyzed and mapped by using Origin 2019b software (Northampton, MA, USA).
2.9. Terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) staining
Hippocampal apoptosis was determined by TUNEL method following the manufacturer's protocol of the TUNEL BrightGreen Apoptosis Detection Kit (A112; Vazyme Biotech Co., Nanjing, China). DAPI was used for nuclear staining. The number of TUNEL-positive and DAPI-positive cells was quantified as previously described [12,21,23]. The counting area was situated in the same location for the four groups. Finally, the sections were photographed with the Olympus FV3000 (Olympus, Japan) co-focus microscope.
2.10. β-Galactosidase staining
Hippocampal cell senescence was determined by β-Galactosidase staining following the manufacturer's protocol of the Senescence β-Galactosidase Staining Kit (C0602; Beyotime Biotechnology). Finally, the sections were photographed with the TG Panoramic Tissue Cell Quantitative Analysis System (Tissue FAXS Plus S) (Tissue Gnostics).
2.11. CP gene expression data analysis
Gene expression analysis was conducted in silico from an online database. The CP gene expression data from various age groups (20–39 y, 40–49 y, 50–59 y, 60–69 y and 70–79 y) of humans were extracted from The Human Protein Atlas (https://www.proteinatlas.org/) [24]. Additionally, the CP gene expression data from 52 y to 102 y were found in the GEO database (https://www.ncbi.nlm.nih.gov/geo/): GSE28146 [25], GSE29378 [26], GSE36980 [27], GSE48350 [28] and GSE5281 [29,30]. Statistical analysis was then used for sorting the data.
2.12. Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.0 Software (GraphPad Software, CA, USA). All the data are presented as the mean ± SEM. For the line plots of multiple groups over time in behavioral tests, two-way ANOVA followed by multiple comparisons (Bonferroni test) were adopted. For the column charts of multiple groups, one-way ANOVA followed by multiple comparisons (Fisher's LSD test, Tukey's test or Dunnett's test) were adopted. For the violin charts presenting gene expression in amounts of individuals, the Kruskal-Wallis test followed by multiple comparisons (Dunn's test) were used for analysis. Linear regression was used for the tendency analysis. Unpaired two tailed t-tests were used for analysis of two independent groups, whereas paired two tailed t-tests were used for the same individual or group tested twice. Differences were considered statistically significant when the p-value was less than 0.05.
3. Results
3.1. CP gene expression signature exhibits a positive correlation with the aging process
To confirm whether the CP expression is up-regulated in the elderly population, we conducted data mining from The Human Protein Atlas and GEO database to investigate the variation tendency between CP gene expression levels in hippocampus and ages. The hippocampus is a brain region that is highly relevant to AD. We observed that the expression of hippocampal CP gene was significantly elevated in the middle-age to elderly-age population (40–49 y, 60–69 y, 70–79 y groups) compared to that in the adult population (20–39 y group) (Fig. 1A). We also observed a positive variation tendency via linear regression analysis between CP gene expression and age groups, ranging from the young old to the oldest old populations (52–102 y) (Fig. 1B). However, the positive correlation was less evident in females (Fig. 1C), whereas it was more pronounced in males (Fig. 1D). These findings suggest that the expression of CP gene increases with age, particularly from adulthood to old age, especially in males. Furthermore, the elevation of CP may play a significant role in the process of brain aging.
Fig. 1.
The CP gene expression was elevated with aging. (A) The expression data of the CP gene in the hippocampus among 20–39 y, 40–49 y, 50–59 y, 60–69 y and 70–79 y humans from GTEx data. The data were analyzed by Kruskal-Wallis test followed by Dunn's test (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. 20–39 y group). (B-D) Distribution and linear regression analysis of the hippocampal CP gene expression and the age of normal humans of total (B), female (C) and male (D) subjects (GEO: GSE28146, GSE29378, GSE36980, GSE48350 and GSE5281, ∗p < 0.05).
3.2. The cognitive abilities related to learning and memory were enhanced in aged mice following the knockout of astrocytic CP induced by tamoxifen
To reduce the CP level of hippocampus with aging, we generated tamoxifen-induced astrocytic CP knockout mice (Fig. 2A). The 12-month-old mice (equivalent to 40-year-old humans) were administered tamoxifen to induce astrocytic CP knockout, and the learning and memory abilities of these mice were assessed at the age of 18 months (equivalent to 60-year-old humans) (Fig. 2A). First of all, we identified the genotype of the mice. We confirmed the tamoxifen-induced knockout of astrocytic Cp gene with PCR (Fig. S1A and B). Using the immunofluorescence staining of GFAP (Glial fibrillary acidic protein, the marker of astrocytes) and CP in hippocampus, we furtherly verified that the astrocytic CP was declined in the 18-month-old hGFAP-creERT2;Cpflox/flox mice with tamoxifen injection (18 M CpGcKO + Tam) compared to that of 18-month-old Cpflox/flox mice with tamoxifen injection (18 M Cpflox/flox + Tam) (Fig. S1C).
Then we conducted behavioral experiments using the MWM and NOR. In the MWM tests, our findings indicated that the latency time and distance to the hidden platform of 18-month-old Cpflox/flox mice (18 M Cpflox/flox) and 18 M Cpflox/flox + Tam mice were significantly longer than those of 3-month-old Cpflox/flox mice (3 M Cpflox/flox). In contrast, the latency time and distance to the hidden platform of 18 M CpGcKO + Tam mice were significantly shorter than those of 18 M Cpflox/flox + Tam mice (Fig. 2B and C). During the 6th day of pole test, the platform was removed. The number of passing area of platform of 18 M Cpflox/flox mice and 18 M Cpflox/flox + Tam mice were decreased compared with 3 M Cpflox/flox mice, while of 18 M CpGcKO + Tam mice were increased compared with 18 M Cpflox/flox + Tam mice. (Fig. 2D).
In the NOR experiment, the results indicated that 3 M Cpflox/flox mice spent a significantly greater percentage of time exploring new objects compared to familiar ones. In contrast, both 18 M Cpflox/flox and 18 M Cpflox/flox + Tam mice exhibited nearly identical percentages of time spent on new versus familiar objects. Notably, 18 M CpGcKO + Tam mice dedicated a significantly longer duration to exploring new objects than to familiar ones (Fig. 2E). Compared to 3 M Cpflox/flox mice, the index of NOR was significantly decreased in 18 M Cpflox/flox mice and 18 M Cpflox/flox + Tam mice (Fig. 2F). In contrast, when compared to 18 M Cpflox/flox + Tam mice, the index of NOR was significantly increased in 18 M CpGcKO + Tam mice (Fig. 2F). These findings suggest that the learning and memory abilities of elderly tamoxifen-induced astrocytic CP knockout mice were enhanced.
3.3. Hippocampal senescence was mitigated following tamoxifen-induced astrocytic CP knockout
The learning and memory abilities were improved in old astrocytic CP deletion mice induced by tamoxifen, prompting us to investigate hippocampal senescence in these subjects. Utilizing β-galactosidase staining with the substrate X-Gal, we observed that especially in the DG region the blue product became more pronounced with advancing age in the mice, while the blue product became lighter in 18 M CpGcKO + Tam mice compared with that in 18 M Cpflox/flox + Tam mice (Fig. 2G). These findings suggest that tamoxifen-induced astrocytic CP knockout may mitigate hippocampal senescence.
To further explore the effect of tamoxifen-induced astrocytic CP knockout on hippocampal senescence, we used qPCR to detect the expression of cell cycle kinases inhibitor gene in hippocampus of 3 M Cpflox/flox, 18 M Cpflox/flox, 18 M Cpflox/flox + Tam and 18 M CpGcKO + Tam mice. The result indicated that the expression of p16INK4a and p19ARF which is the most representative markers of cellular senescence were significantly elevated by approximately 10-fold in 18 M Cpflox/flox mice and 18 M Cpflox/flox + Tam mice compared to those in 3 M Cpflox/flox mice, while the expression of p19INK4d and p27Kip1 were increased only about 2 folds in 18 M Cpflox/flox mice and 18 M Cpflox/flox + Tam mice compared to that in 3 M Cpflox/flox mice. The expression levels of p21Cip1, p15INK4b and p18INK4c did not exhibit significant changes with aging (Fig. 2H). However, the expression of p16INK4a, p19ARF, p15INK4b, p18INK4c, p19INK4d and p27Kip1 was significantly reduced in 18 M CpGcKO + Tam mice compared with 18 M Cpflox/flox + Tam mice, while the expression of p21Cip1 was not altered (Fig. 2H). These findings indicated that tamoxifen-induced astrocytic CP knockout could alleviate the substantial increased expression of p16INK4a and p19ARF mRNA in hippocampus with aging.
To further investigate the proportions of senescent cells of specific cell types, we conducted immunofluorescence experiments on the co-localization of p16INK4a with neurons (NeuN), astrocytes (GFAP) and microglia (Iba-1). Compared to the 18 M Cpflox/flox + Tam group, the proportion of NeuN+p16INK4a+ cells in the DG region of the hippocampus of the 18 M CpGcKO + Tam group was significantly reduced (Fig. S2A and B), whereas the proportions of GFAP+p16INK4a+ and Iba-1+p16INK4a+ cells were unchanged (Fig. S2C, D, E and F). These data suggested that the tamoxifen-induced astrocytic CP knockout mainly suppressed the senescence of neurons, rather than astrocytes nor microglia.
3.4. MYC phosphorylation was reduced and the MAPK/JNK and PI3K/Akt/GSK3 pathways were altered following tamoxifen-induced deletion of astrocytic CP
MYC is a regulatory protein involved in the cell cycle. The upregulation of MYC can promote the cell division and delay aging by inhibiting the expression of cell cycle kinase inhibitors, such as p15INK4b, p16INK4a, p21Cip1, and p27Kip1. MYC is regulated by the PI3K/Akt/GSK3 and MAPK/JNK signaling pathways, which facilitate its phosphorylation at T58 and S62 sites, ultimately leading to MYC degradation. Consequently, this process inhibits the nuclear translocation of MYC, increasing the expression of genes encoding cell cycle kinase inhibitors [31,32].
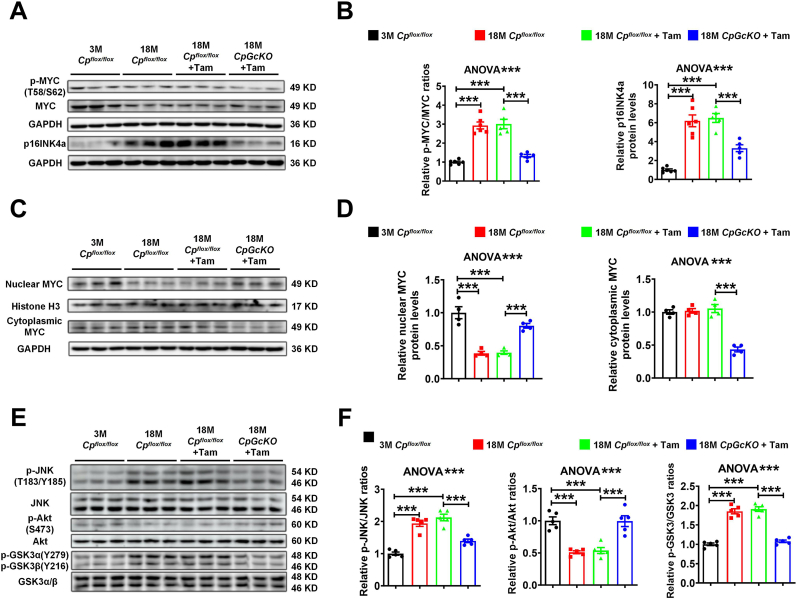
We detected hippocampal MYC phosphorylation of 3 M Cpflox/flox, 18 M Cpflox/flox, 18 M Cpflox/flox + Tam and 18 M CpGcKO + Tam mice. The experimental results indicated that with aging, the ratio of phosphorylated MYC (T58/S62) to total MYC increased (Fig. 3A and B). Compared to the 18 M Cpflox/flox + Tam mice, there was a significant decrease in the levels of MYC phosphorylation at T58/S62 in the hippocampus of 18 M CpGcKO + Tam mice (Fig. 3A and B). The expression level of p16INK4a, which is downstream of MYC, was significantly reduced in 18 M CpGcKO + Tam mice compared to 18 M Cpflox/flox + Tam mice (Fig. 3A and B). We extracted nuclear protein and cytoplasmic protein in the hippocampus of mice, subsequently assessing the changes in MYC protein levels both within the nucleus and cytoplasm. It was found that the nuclear MYC level of 18 M CpGcKO + Tam mice was significantly higher than that of 18 M Cpflox/flox + Tam mice. Conversely, the cytoplasmic MYC level was significantly lower in 18 M CpGcKO + Tam mice than that in 18 M Cpflox/flox + Tam mice (Fig. 3C and D).
Fig. 3.
The Akt/GSK3/MYC/p16INK4a and MAPK/JNK/MYC/p16INK4a pathways were altered in aged mice after tamoxifen-induced astrocytic CP knockout. (A-B) p-MYC (T58/S62), MYC expression level and p16INK4a expression level in hippocampus measured by Western blot. The quantification of (A) was presented in graph (B) (n = 6 for 3 M Cpflox/flox and 18 M Cpflox/flox groups, n = 5 for 18 M Cpflox/flox + Tam and 18 M CpGcKO + Tam groups). (C-D) Nuclear and cytoplasmic MYC levels in hippocampus measured by Western blot. The quantification of (C) was presented in graph (D) (n = 4). (E-F) p-JNK (T183/Y185), p-Akt (S473) and p-GSK3α (Y279) + p-GSK3β (Y216) levels in hippocampus measured by Western blot. The quantification of (E) was presented in graph (F) (n = 5). All data were analyzed by one-way ANOVA followed by Tukey's test, and presented as the mean ± SEM, ∗∗∗p < 0.001.
We also detected the MAPK/JNK, PI3K/Akt/GSK3 signaling pathways in hippocampus of mice. GSK3 is negatively regulated by the PI3K/Akt pathway, thereby constituting the PI3K/Akt/GSK3 signaling cascade [[33], [34], [35]]. With the aging of mice, there was a significant increase in the levels of JNK phosphorylation (T183/Y185) in the hippocampus, while Akt phosphorylation (S473) exhibited a significant decrease. Additionally, GSK3 phosphorylation (GSK3α (Y279) + GSK3β (Y216)), showed a notable increase (Fig. 3E and F). Compared to the 18 M Cpflox/flox + Tam mice, the levels of JNK phosphorylation (T183/Y185) in the hippocampus of 18 M CpGcKO + Tam mice were significantly reduced, as did GSK3 phosphorylation (GSK3α (Y279) + GSK3β (Y216)), which also showed significant reduction. In contrast, Akt phosphorylation (S473) levels significantly increased (Fig. 3E and F). These results suggested that tamoxifen-induced conditional knockout of CP in astrocytes may alleviate the age-related increases in JNK phosphorylation levels, the decreases in Akt phosphorylation levels, and the increases in GSK3 phosphorylation levels.
3.5. Hippocampal iron accumulation associated with aging was mitigated by the knockout of astrocytic CP induced by tamoxifen
To investigate the alterations in iron metabolism in hippocampus after tamoxifen-induced astrocytic CP deletion, we selected 18 M CpGcKO + Tam mice and utilized the same age of Cpflox/flox + Tam mice as control to assess changes in the expression of iron metabolism-related proteins in the hippocampus. The results showed that with aging, there was no significant change in the expression of DMT1(-IRE). The expression of H-ferritin, L-ferritin, FPN1 increased with aging, while the expression of TfR1, DMT1(+IRE) and HP decreased in hippocampus (Fig. 4A and B). Compared with 18 M Cpflox/flox + Tam mice, the 18 M CpGcKO + Tam mice performed decreases of H-ferritin, L-ferritin and FPN1 expression, but increases of TfR1, DMT1(+IRE) and HP expression in hippocampus (Fig. 4A and B).
Fig. 4.
The detection of iron metabolism in hippocampus of aged mice after tamoxifen-induced astrocytic CP knockout. (A-B) The levels of H-ferritin, L-ferritin, TfR1, FPN1, DMT1(+IRE), HP and DMT1(-IRE) in hippocampus detected by Western blot. Quantification of (A) are shown in chart (B) (n = 6 for 3 M Cpflox/flox and 18 M Cpflox/flox groups, n = 5 for 18 M Cpflox/flox + Tam and 18 M CpGcKO + Tam groups). (C) Iron contents and distribution in hippocampus measured by μ-XRF. All data were analyzed by one-way ANOVA followed by Tukey's test, and presented as the mean ± SEM, ∗∗∗p < 0.001, ns (no significant difference).
In order to further understand whether the iron levels were reduced in the hippocampus of elderly astrocytic CP knockout mice induced by tamoxifen at a specific time point, we conducted a study of iron measurement on the coronal plane with the hippocampus using μ-XRF. The results demonstrated that iron content in the hippocampus increased with aging, while compared with 18 M Cpflox/flox + Tam mice, there was a notable decrease in iron content within the hippocampus of 18 M CpGcKO + Tam mice (Fig. 4C). These findings suggested that inducing astrocytic CP knockout in mice at a specific time point could mitigate age-related iron deposition in the hippocampus.
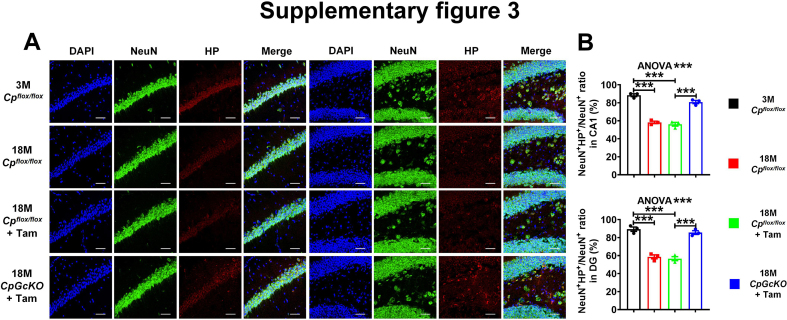
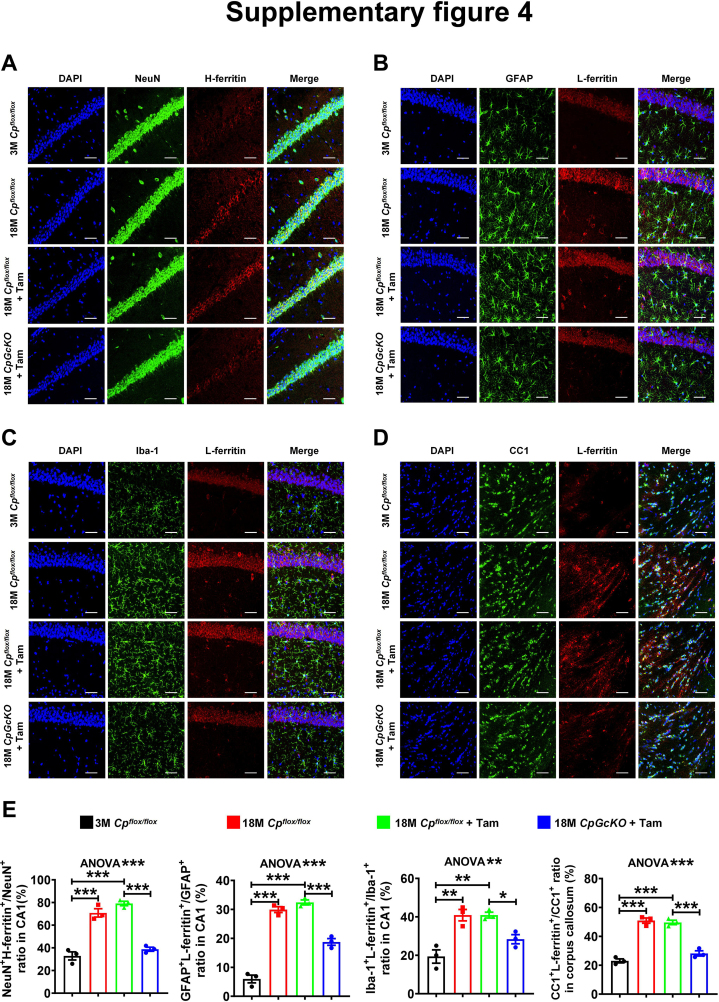
To further investigate the change of neuronal iron levels, we used immunofluorescence double labeling to label NeuN/H-ferritin and NeuN/HP in the hippocampus of mice. The results showed that with aging, the number of NeuN+H-ferritin+ cells in the DG (Fig. 5A and E) and CA1 (Fig. S4A and E) regions of hippocampus significantly increased, while the number of NeuN+HP+ cells significantly decreased (Fig. S3A and B). Compared with 18 M Cpflox/flox + Tam mice, the number of NeuN+H-ferritin+ cells in the DG (Fig. 5A and E) and CA1 (Fig. S4A and E) regions of hippocampus of 18 M CpGcKO + Tam mice was significantly reduced, but the number of NeuN+HP+ cells was significantly up-regulated (Fig. S3A and B). The results showed that inducing mice astrocytic CP knockout at a specific time point could alleviate the increase of the number of NeuN+H-ferritin+ cells but the decrease of the number of NeuN+HP+ cells caused by aging.
Fig. 5.
The levels of ferritin in neurons, astrocytes, microglia and oligodendrocytes in DG region of hippocampus of the aged mice after tamoxifen-induced astrocytic CP knockout. (A) The co-localization of NeuN and H-ferritin in DG of hippocampus. The ratio of NeuN+H-ferritin+ cells to the NeuN+ cells in DG of hippocampus (A) was shown in chart (E) (n = 4). (B) The co-localization of GFAP and L-ferritin in DG of hippocampus. The ratio of GFAP+L-ferritin+ cells to the GFAP+ cells in DG of hippocampus (B) was shown in chart (E) (n = 3). (C) The co-localization of Iba-1 and L-ferritin in DG of hippocampus. The ratio of Iba-1+L-ferritin+ cells to the Iba-1+ cells in DG of hippocampus (C) was shown in chart (E) (n = 3). (D) The co-localization of CC1 and L-ferritin in hippocampus. The ratio of CC1+L-ferritin+ cells to the CC1+ cells in hippocampus(D) was shown in chart (E) (n = 3). DAPI was used for nuclear staining. Scale bar = 50 μm. All data were analyzed by one-way ANOVA followed by Tukey's test, and presented as the mean ± SEM, ∗∗p < 0.01, ∗∗∗p < 0.001.
In order to further explore the changes of iron levels of astrocytes and microglia, we used immunofluorescence double labeling method to label GFAP/L-ferritin and Iba-1/L-ferritin in the hippocampus of mice. The results showed that the number of GFAP+L-ferritin+ cells in the DG (Fig. 5B and E) and CA1 (Fig. S4B and E) regions of hippocampus significantly increased with aging, and the number of Iba-1+L-ferritin+ cells also significantly increased in the DG (Fig. 5C and E) and CA1 (Fig. S4C and E) regions of hippocampus with aging. Compared with 18 M Cpflox/flox + Tam mice, the number of GFAP+L-ferritin+ cells in the DG (Fig. 5B and E) and CA1 (Fig. S4B and E) regions of hippocampus was significantly reduced, and the number of Iba-1+L-ferritin+ cells was also significantly reduced in the DG (Fig. 5C and E) and CA1 (Fig. S4C and E) regions of hippocampus of 18 M CpGcKO + Tam mice. In addition, we also found that the density of GFAP positive cells and Iba-1 positive cells in the CA1 and DG regions of hippocampus significantly increased with the age of mice (Fig. S5A). Compared with 18 M Cpflox/flox + Tam mice, the GFAP positive cell density and Iba-1 positive cell density in the CA1 and DG regions of the hippocampus decreased in the 18 M CpGcKO + Tam mice (Fig. S5A). We also measured the hippocampal cytokines and found that IL-6 and IL-1β were decreased in 18 M CpGcKO + Tam mice compared with 18 M Cpflox/flox + Tam mice (Fig. S5B and C), suggested the inflammation increased with aging was mitigated after tamoxifen-induced astrocytic CP deletion.
In order to further explore the changes of iron levels in oligodendrocytes, we used immunofluorescence double labeling to label the CC1/L-ferritin in the corpus callosum and hippocampus of these mice. The results showed that the number of CC1+L-ferritin+ cells in the hippocampus (Fig. 5D and E) and corpus callosum (Fig. S4D and E) increased significantly with aging. Compared with 18 M Cpflox/flox + Tam mice, the number of CC1+L-ferritin+ cells in the hippocampus (Fig. 5D and E) and corpus callosum (Fig. S4D and E) significantly decreased.
These results indicated that the injection of tamoxifen-induced astrocytic CP knockout could alleviate L-ferritin accumulation of astrocytes, microglia and oligodendrocytes with aging. Additionally, the increase in the number of astrocytes and microglia with age was also mitigated.
3.6. Phosphorylation of MYC (T58/S62) was down-regulated after the iron chelation of FAC-treated N2a
To confirm the effect of iron level on the phosphorylation of MYC (T58/S62), we established the iron overload model and iron overload-restored model of N2a cell line in vitro. The result showed that the H-ferritin and L-ferritin increased obviously after FAC treatment, while decreased after DFO treatment at a dose-dependent manner and gradually recovered (Fig. S6A and B). The level of phosphorylation of MYC (T58/S62) was not altered at all under the condition of FAC-induced iron overload, but decreased after DFO treatment-induced mitigation of iron overload (Fig. S6B). These results suggested that down-regulating the iron level might lead to the attenuation of phosphorylation of MYC (T58/S62).
3.7. Hippocampal cell apoptosis was significantly reduced in aged mice following the knockout of astrocytic CP induced by tamoxifen
Excessive iron deposition can generate a large amount of oxidative stress via Fenton reaction, which may ultimately result in cell apoptosis through MAPK/Erk and MAPK/p38 signaling pathways [19,36,37]. In our previous study, 18 M CpGfapcKO mice exhibited an alleviated cell apoptosis via decreased iron-ROS-MAPK pathway signal [12]. So, can the alleviation of iron deposition caused by tamoxifen-induced astrocytic CP knockout also reduce the oxidative stress-induced cell apoptosis as the CpGfapcKO mice mentioned above? Using Western blot analysis, we found that compared with 3 M Cpflox/flox mice, the expression of 4-HNE, Erk1/2 phosphorylation (T202/Y204), p38 phosphorylation (T180/Y182), and Cleaved-caspase3/Caspase3 ratio in the hippocampus of 18 M Cpflox/flox mice and 18 M Cpflox/flox + Tam mice were significantly increased. Compared with 18 M Cpflox/flox + Tam mice, the expression of 4-HNE, the Erk1/2 phosphorylation (T202/Y204), the p38 phosphorylation (T180/Y182) and Cleaved-caspase3/Caspase3 ratio were significantly reduced in 18 M CpGcKO + Tam mice (Fig. 6A and B). TUNEL staining found that the number of TUNEL labeled positive cells in the hippocampus increased significantly with aging, and the increase would be alleviated after tamoxifen-induced astrocytic CP knockout (Fig. 6C, D and E). However, the expression levels of ferroptosis related proteins ACSL4 and GPX4 did not change significantly among all these four groups (Fig. 6A and B). These suggests that tamoxifen-induced knockout of astrocytic CP can also alleviate the increase in oxidative stress levels associated with aging by reducing iron deposition. Consequently, this intervention weakens the MAPK/Erk and MAPK/p38 signaling pathways, and was accompanied by a reduction in cell apoptosis.
Fig. 6.
MAPK/Erk, MAPK/p38 pathways, cell apoptosis and ferroptosis-related protein measurement in hippocampus. (A-B) p-Erk1/2 (T202/Y204) level, p-p38 (T180/Y182) level, 4-HNE expression, ACSL4 expression, GPX4 expression and Cleaved-caspase3 level in hippocampus measured by Western blot. The quantification of (A) was presented in graph (B) (For Cleaved-caspase3, n = 5; for others, n = 3). (C-E) TUNEL staining in the CA1 (C) and DG (D) regions of hippocampus. The number of apoptotic cells was quantified and presented in graph (E) (n = 3). DAPI was used for nuclear staining. Scale bar = 50 μm. All data were analyzed by one-way ANOVA followed by Tukey's test, and presented as the mean ± SEM, ∗∗p < 0.01, ∗∗∗p < 0.001, ns (no significant difference).
3.8. The levels of hippocampal APP, Aβ oligomers and phosphorylation of Tau were reduced in aged tamoxifen-induced astrocytic CP knockout mice
AD is a common neurodegenerative disease, especially the risk of disease increases with age [38,39]. We performed detection of the levels of APP, Aβ oligomers and phosphorylation of Tau. Western blot showed up-regulated levels of APP, Aβ oligomers with aging, while the levels of APP, Aβ oligomers of 18 M CpGcKO + Tam mice was down-regulated compared with 18 M Cpflox/flox + Tam mice (Fig. 7A, D and E). The phosphorylation of Tau was detected by Western blot and immunofluorescence staining. We found that the phosphorylation levels of Tau (S396, T181 and S404) were all obviously increased with aging (Fig. 7B, F, G and H). Conversely, these elevated phosphorylation levels of Tau were reduced in the 18 M CpGcKO + Tam group compared to 18 M Cpflox/flox + Tam group (Fig. 7B, F, G and H). Additionally, the covered areas of p-Tau (S396) in hippocampus were increased with aging, but decreased in 18 M CpGcKO + Tam mice compared to 18 M Cpflox/flox + Tam mice (Fig. 7C and I). These suggested that inducing mice astrocytic CP knockout at 12 months old could delay the progression of AD pathology.
Fig. 7.
The levels of APP, Aβ oligomers and Tau phosphorylation in hippocampus. (A) APP and Aβ oligomer levels in hippocampus, as measured by Western blot analysis. The quantification of the data shown in (A) was presented in the graphs in (D) (APP) and (E) (Aβ oligomers) (n = 4). (B) Tau phosphorylation (S396, T181 and S404) levels in hippocampus, as determined by Western blot analysis. The quantification of the data shown in (B) was presented in the graphs in (F–H) (n = 3). (C) Immunofluorescence staining of p-Tau (S396) in hippocampus. The quantification of the covered areas of p-Tau (S396) was shown in chart (I) (n = 3). DAPI was used for nuclear staining. Scale bar = 1 mm. All data were analyzed by one-way ANOVA followed by Tukey's test, and presented as the mean ± SEM, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.9. Hippocampal AD hallmarks of APP/PS1 mice were mitigated by tamoxifen-induced astrocytic CP knockout
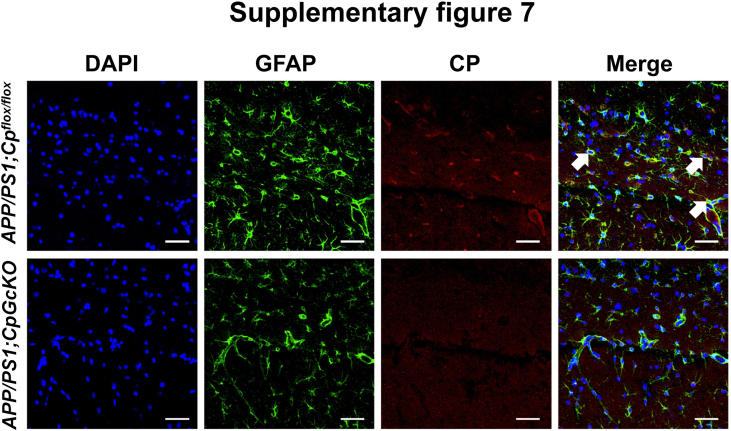
To identify the impact of astrocytic CP knockout on the AD hallmarks in APP/PS1 mice, we hybridized the APP/PS1 mice with hGFAP-creERT2;Cpflox/flox mice, and finally generated APP/PS1; hGFAP-creERT2;Cpflox/flox mice (APP/PS1;CpGcKO) (Fig. 8A). The astrocytic CP was knockout by tamoxifen intraperitoneal injection at 6 months old. The WT, Cpflox/flox, APP/PS1;Cpflox/flox mice at the same age of 6 months old were also intraperitoneally injected with tamoxifen. At the age of 8 months old, we have these mice behavioral experiment. First of all, we utilized double-immunofluorescence staining of GFAP and CP in hippocampus both of the APP/PS1;Cpflox/flox and APP/PS1;CpGcKO group. The result showed that the CP was absent of GFAP positive cells (Astrocytes) in APP/PS1;CpGcKO mouse (Fig. S7), suggesting the astrocytic CP in APP/PS1 mouse was knockout successfully.
Next, we carried out MWM behavioral experiments. Results showed that the latency time and distance to the hidden platform of APP/PS1;Cpflox/flox mice were significantly longer than those in WT and Cpflox/flox mice during the training test (Fig. 8B and C). Conversely, the latency time and distance to the hidden platform of APP/PS1;CpGcKO mice were significantly shorter than those in APP/PS1;Cpflox/flox mice (Fig. 8B and C). The platform was removed at the 6th day of pole test. We found that the frequency of passing times of platform area was decreased in APP/PS1;Cpflox/flox mice compared with WT and Cpflox/flox mice, while increased in APP/PS1;CpGcKO mice compared with APP/PS1;Cpflox/flox mice (Fig. 8D).
We also detected the hippocampal APP and Aβ oligomers levels in WT, Cpflox/flox, APP/PS1;Cpflox/flox and APP/PS1;CpGcKO mice by using Western blot. Results showed that the hippocampal APP and Aβ oligomers levels increased significantly in APP/PS1;Cpflox/flox mice compared to WT and Cpflox/flox mice, while decreased in APP/PS1;CpGcKO mice compared to APP/PS1;Cpflox/flox mice (Fig. 8E, F and G). We also observed hippocampal Aβ plaques via immunofluorescence staining. We discovered that the Aβ plaques were absent in the hippocampus of WT and Cpflox/flox mice, but obvious in the group of APP/PS1;Cpflox/flox mice. The number of Aβ plaques were down-regulated in APP/PS1;CpGcKO mice compared to APP/PS1;Cpflox/flox mice (Fig. 8H and J). These suggested that inducing APP/PS1 mice astrocytic CP knockout can mitigate the pathology of AD.
To verify the changed of hippocampal iron metabolism in APP/PS1 mice after tamoxifen-induced astrocytic CP deletion, we conducted the iron level and distribution measurement by using of μ-XRF. The result showed that the hippocampal iron content increased in APP/PS1;Cpflox/flox mice compared to WT and Cpflox/flox mice, while the iron content decreased in APP/PS1;CpGcKO mice compared to APP/PS1;Cpflox/flox mice (Fig. 8I).
We also detected the expression of hippocampal iron metabolism related proteins. The expression of FPN1, L-ferritin and H-ferritin decreased, and the expression of TfR1 increased, while the expression of DMT1(+IRE) was unchanged in APP/PS1;CpGcKO mice compared to APP/PS1;Cpflox/flox mice (Fig. 9A and B), suggesting the iron content could be reduced in APP/PS1 mice via tamoxifen-induced astrocytic CP knockout.
Fig. 9.
The detection of iron metabolism-related protein, Erk/apoptosis, p38/apoptosis, Akt/GSK3/MYC/p16INK4a and JNK/MYC/p16INK4a pathways in tamoxifen-induced astrocytic CP knockout in APP/PS1 mice. (A) The levels of hippocampal TfR1, FPN1, L-ferritin, DMT1(+IRE) and H-ferritin detected by Western blot. (B) Quantification of graph (A) (n = 3). (C) p-Erk1/2 (T202/Y204) level, p-p38 (T180/Y182) level, Bcl-2 expression, Bax expression, Cleaved-caspase3 level and 4-HNE expression in hippocampus measured by Western blot. (D) Quantification of graph (C) (n = 3). (E) p-JNK (T183/Y185) level, p-Akt (S473) level, p-GSK3α (Y279) + p-GSK3β (Y216) level, p-MYC (T58/S62) level and p16INK4a expression level in hippocampus measured by Western blot. (F) Quantification of graph (E) (n = 3). All data were analyzed by one-way ANOVA followed by Tukey's test, and presented as the mean ± SEM, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ns (no significant difference).
Moreover, the hippocampal iron/ROS/MAPK/apoptosis, MAPK/JNK/MYC, PI3K/Akt/GSK3/MYC pathways were detected by using Western blot. The p38 phosphorylation (T180/Y182), Cleaved-caspase3/Caspase3 ratio and the expression of 4-HNE in the hippocampus of APP/PS1;Cpflox/flox mice were significantly up-regulated compared with those in WT and Cpflox/flox mice, while the Bcl-2/Bax expression ratio down-regulated and the Erk1/2 phosphorylation (T202/Y204) level was unchanged. Compared with APP/PS1;Cpflox/flox mice, The Erk1/2 phosphorylation (T202/Y204), p38 phosphorylation (T180/Y182), Cleaved-caspase3/Caspase3 ratio and the expression of 4-HNE were significantly reduced in APP/PS1;CpGcKO mice, while the Bcl-2/Bax expression ratio significantly increased (Fig. 9C and D). In APP/PS1;Cpflox/flox mice, the levels of JNK phosphorylation (T183/Y185) in the hippocampus significantly increased, Akt phosphorylation (S473) significantly decreased, GSK3 phosphorylation (GSK3α (Y279) + GSK3β (Y216)) significantly increased, compared with WT and Cpflox/flox mice (Fig. 9E and F). Compared with APP/PS1;Cpflox/flox mice, the levels of JNK phosphorylation (T183/Y185) in the hippocampus of APP/PS1;CpGcKO mice significantly decreased, while Akt phosphorylation (S473) levels significantly increased, and GSK3 phosphorylation (GSK3α (Y279) + GSK3β (Y216)) significantly decreased (Fig. 9E and F). The levels of MYC phosphorylation (T58/S62) and p16INK4a expression showed a significant decrease in APP/PS1;CpGcKO mice compared to APP/PS1;Cpflox/flox mice (Fig. 9E and F).
The above findings suggest that tamoxifen-induced astrocytic CP knockout in APP/PS1 mice may alleviate the iron/ROS/MAPK/p38 and iron/ROS/MAPK/Erk pathways to reduce apoptosis. Meanwhile, the CP deficiency may also weaken MAPK/JNK/MYC and PI3K/Akt/GSK3/MYC pathways to diminish the cell senescence. The alleviated apoptosis and senescence may then ultimately result in improved learning and memory abilities and mitigated AD hallmarks.
4. Discussion
Our previously study has explained the effect of astrocytic CP knockout on the brain iron metabolism [12]. Astrocytic CP plays a crucial role in the transport of iron from the peripheral blood across the BBB into the brain [11,40]. Astrocytic CP knockout reduces iron content in the cerebral cortex and hippocampus of mice, while iron is deposited and retained in BMVECs. Astrocytic CP deficiency affects the iron output of FPN1 from the basal surface of BMVECs to the cerebral cortex and hippocampus. The CpGfapcKO-induced iron reduction resulted in alleviated iron accumulation in the old mice, and the iron-induced oxidative stress was also declined. MAPK-dependent cell apoptosis was mitigated, leading to improvement of learning and memory abilities. However, when the mice were young, CpGfapcKO-induced decreased in iron levels affected synaptic and myelination formation, leading to impairment of learning and memory abilities. Studies have shown that as AD progression, CP protein of CSF was elevated obviously [41]. The data mining of Human Protein Atlas and GEO databases about the relationship between CP expressions and ages was conducted. We found that the CP expressions of hippocampus were sharply increased when the human entering middle age and young old age, and the hippocampal CP expression continuously elevated till oldest old age even over 100 years old (Fig. 1). Therefore, the knockout of astrocytic CP at middle age may become a better strategy for alleviating brain senescence.
Considering of the negative contribution of CpGfapcKO-induced iron reduction to the young mice, we designed a new model that we can induce the astrocytic CP knockout at the middle age which can avoid the harmful effect on the young mice. We hybridized hGFAP-creERT2 mice with Cpflox/flox mice to generate mice carrying both hGFAP-creERT2 and Cpflox/flox. At the age of 12 months, tamoxifen was intraperitoneally injected into the hGFAP-creERT2; Cpflox/flox mice. At the age of 18 months, we observed various indicators of cognitive behavior in mice. (Fig. 2A and Fig. S1).
Firstly, we found that the learning and memory abilities of MWM detection and the hippocampal-dependent cognitive abilities of NOR detection were significantly improved in 18 M CpGcKO + Tam mice, compared with those in 18 M Cpflox/flox + Tam mice, and tamoxifen-induced astrocytic CP knockout alleviated the damage of learning and memory caused by the aging of mice (Fig. 2B–F). These were similar to the results of old CpGfapcKO mice [12].
The expression level of p16INK4a protein, which is the most representative of aging, also significantly increases during aging in the body [42,43]. In our previous study, the MDA and ROS levels were significantly increased with mice aging [12]. Excessive ROS not only enhances the MAPK/p38 and MAPK/Erk pathways, but also affects the PI3K/Akt and MAPK/JNK signaling pathways [[33], [34], [35]]. MYC is a cell cycle regulatory protein that is regulated by the PI3K/Akt/GSK3 and MAPK/JNK signaling pathways, causing phosphorylation at the sites of S62 and T58, making it prone to degradation, which is not conducive to the nuclear translocation of MYC and the cell cycle kinase inhibitor genes are disinhibited, mainly including p16INK4a [31,32,44].
In order to investigate whether the alleviation of hippocampal iron accumulation and the reduction of oxidative stress in aging mice could be attributed to the tamoxifen-induced knockout of astrocytic CP, we examined how this alteration might affect the PI3K/Akt/GSK3/MYC and MAPK/JNK/MYC signaling pathways, as well as the expression level of the senescence marker p16INK4a protein. It was observed that the knockout of astrocytic CP induced by tamoxifen resulted in an increase in Akt phosphorylation levels, a decrease in GSK3 phosphorylation levels, a reduction in MYC phosphorylation levels, an enhancement of MYC nuclear translocation levels, and a decrease in the expression level of p16INK4a protein (Fig. 3). β-Galactosidase staining revealed a mitigation in the degree of aging in 18 M CpGcKO + Tam mice, especially in the DG region of the hippocampus (Fig. 2G). We also additionally detected the proportion of p16INK4a positive neurons, astrocytes and microglia in the DG region of the hippocampus. We found that the obvious reduction was in the proportion of p16INK4a positive neurons, while the proportions of p16INK4a positive astrocytes and p16INK4a positive microglia were not altered at all. These results indicate the astrocytic CP knockout-induced alleviation of hippocampal cell senescence mainly occurred in neurons (Fig. S2). To sum up, the above findings suggest that the alleviation of iron accumulation resulting from tamoxifen-induced astrocytic CP knockout leads to a reduction in oxidative stress (Fig. 6A). This alteration may affect the PI3K/Akt/GSK3 and MAPK/JNK signaling pathways, subsequently decreasing MYC phosphorylation, enhancing MYC nuclear translocation, and down-regulating the expression of p16INK4a protein. The expression levels of other cell cycle kinase inhibitors, such as p19ARF mRNA was also reduced, which may result in a mitigation of senescence in the hippocampus of the mice. Furthermore, this may reserve the learning and memory impairment caused by aging (Fig. 2, Fig. 3).
The iron content in the brain increases significantly with aging, and excessive iron content can promote the progression of AD. As is well known, with increasing age, the large amounts of free radicals generated by iron deposition induced Fenton reaction are closely related to the progression of neurodegenerative diseases [45]. Previous studies have also reported that excessive ROS caused by iron deposition can activate the MAPK signaling pathway, leading to cell apoptosis [19,36,37]. The serious deposition of brain iron is one of the most important factors in the occurrence and development of neurodegenerative diseases [11,46]. In this study, we discovered that tamoxifen-induced astrocytic CP knockout led to a decrease in hippocampal iron content and alleviated iron deposition caused by aging (Fig. 4, Fig. 5, Fig. S4 and Fig. S5). Besides, we also confirmed that the attenuation of iron accumulation in cells (N2a) could lessen down the phosphorylation of MYC (T58/S62) (Fig. S6), which might promote MYC nuclear translocation to inhibit p16INK4a expression. As another type of ferroxidase, HP is mainly expressed in neurons and also participates in the cellular iron exportation process [47,48]. Our research results showed that the level of HP of neurons in 18 M CpGcKO + Tam mice increased, enhancing the release of iron from neurons through HP, further reducing neuronal iron levels (Fig. S3). In addition, consistent with our previous research [12], the iron/ROS/MAPK/p38 and iron/ROS/MAPK/Erk pathways with apoptosis were alleviated in 18 M CpGcKO + Tam mice (Fig. 6).
The degree of AD hallmarks increases with aging of mice [49,50]. Iron has also been confirmed to be a promoting factor of Aβ sedimentation and Tau hyperphosphorylation, which are both landmark factors in the pathogenesis of AD [45]. Nasal administration of DFO can alleviate AD symptoms in APP/PS1 mice [51]. Overexpression of Mitochondrial ferritin in SH-SY5Y cells attenuated the Aβ-induced neurotoxicity via sequestrating free iron into the mitochondrial storage protein [52]. To investigate whether the improvement of learning, memory and cognitive abilities is also related to the hallmark molecules of AD in 18 M CpGcKO + Tam mice, we measured the levels of Aβ sedimentation and Tau phosphorylation. We found a significant decrease in Tau phosphorylation, APP levels and Aβ oligomers in the hippocampus of 18 M CpGcKO + Tam mice, which could be associated with in an improvement of the learning, memory, and cognitive abilities (Fig. 7).
Aβ oligomers can be classified into two major groups: On-pathway Aβ oligomers and off-pathway Aβ oligomers. The on-pathway Aβ oligomers are capable of forming Aβ fibrils, while the off-pathway Aβ oligomers fail to form fibrils [53,54]. On-pathway Aβ oligomers especially Aβ dimers are significantly up-regulated in the brain of AD patients and AD mice with aging, increasing the Aβ plaque load of brain [[55], [56], [57]]. However, the off-pathway Aβ oligomers such as Aβ trimers are present as early as at 1 year old in the human brain [58]. Moreover, the Aβ trimers can exist for many years or even several decades before amyloid fibrils deposition in brain [57,59]. These findings indicate that off-pathway Aβ oligomers can exist in the brain of normal and young mice. In our previous study, we used anti-off-pathway oligomer protein A11 antibody for detecting Aβ oligomers in young age and old age mice, where we found that the levels of Aβ oligomers in old age mice were significantly higher than those in young age mice [12]. Some notable recent studies also have demonstrated a detectable presence of Aβ in the brains of WT (non-APP/PS1 transgenic) mice. The concentrations of soluble Aβ in the brain tissue of 4-month-old WT mice, as detected by ELISA was about 0.3 pmol/g, which was 5–10 times lower than that of 4-month-old APP/PS1 mice [60]. The signal strength of Aβ in PET images in WT mice increased with aging (2–12 months old), and the 4G8 antibody staining also revealed small Aβ plaques in 12-month-old WT mice [61]. Therefore, these studies suggest that WT mice exhibit low-levels of Aβ in brain which can be elevated with normal aging, and primarily support our findings that the Aβ oligomers detected in non-APP/PS1 transgenic mice (Fig. 7).
In order to simulate the therapeutic effect of tamoxifen-induced astrocytic CP knockout on AD treatment, we bred APP/PS1; hGFAP-creERT2;Cpflox/flox mice. At the age of 6 months old, all mice were injected with tamoxifen intraperitoneally for five consecutive days to induce astrocytic CP knockout, and the experiment was conducted at the age of 8 months (Fig. S7). We found that inducing astrocytic CP knockout also can improve the learning and memory abilities of APP/PS1 mice, with the number of Aβ plaques and the levels of APP and Aβ oligomers in the hippocampus reduced. The hippocampal iron levels in APP/PS1;CpGcKO mice were also decreased, with 4-HNE expression down-regulated, MAPK/p38 and MAPK/Erk signaling pathways altered and apoptosis in the hippocampus reduced. The hippocampal MAPK/JNK/MYC and PI3K/Akt/GSK3/MYC pathways were also altered, with senescence mitigated and the learning and memory abilities improved in the AD mice (Fig. 8, Fig. 9). Previously research has shown that global CP knockout could exacerbate iron deposition in the hippocampus of AD mice, significantly increasing oxidative stress levels, and up-regulating the number of apoptotic cells, leading to a significant deterioration of AD symptoms in mice [36]. The possible reasons for the different results are as follows: Firstly, knockout of astrocytic CP cannot affect peripheral blood iron levels, which is different from global knockout of CP [12]. Secondly, patients with aceruloplasminemia (global CP absent) have very high levels of blood ferritin [[62], [63], [64]], and a large amount of ferritin can bind to TfR1 on BMVECs to promote iron uptake by the brain [65,66]. Thirdly, in addition to the astrocytic CP, there is CP expression of neurons [67]. We also bred neuronal CP knockout (CpThy1cKO) mice, and research showed that there were significant increases of iron levels in the cerebral cortex and hippocampus of CpThy1cKO mice (Fig. S8). This means that neuronal CP may play an important role in neuronal iron release, while CP on astrocytes promotes BMVECs iron release from peripheral blood into brain and regulates brain iron intake. The lack of neuronal CP in global CP deficient mice may block neuronal iron output and lead to brain iron deposition.
In conclusion, tamoxifen-induced astrocytic CP knockout at the age of 12 months old can alleviate the iron deposition with aging, mitigating the excessive oxidative stress. The PI3K/Akt/GSK3 and MAPK/JNK pathways were changed, with MYC phosphorylation reduced, which may result in increased nuclear translocation of MYC, and decreased p16INK4a expression. The cell senescence was inhibited and the learning and memory abilities were improved (Fig. 10). This model of conditional tamoxifen-induced gene knockout of astrocytic CP avoided the harmful influence of the Cre-LoxP system-induced gene knockout of astrocytic CP at the young age of mice. Our results indicate again that astrocytic CP plays an important role in brain iron input, and the knockout of astrocytic CP can alleviate cell apoptosis and senescence by reducing iron deposition-mediated mitigated oxidative stress at the old age. DFO iron-chelation treatment has toxic side-effect on the body, and the DFO-induced iron loss may affect iron-related signaling pathways, resulting in ophthalmic toxicity, neurotoxicity and anemia [[68], [69], [70], [71]]. In addition, the half-life of DFO in the body was very short [72]. Our study found a new potential treatment target of the brain senescence and AD, and provide a better potential prevention and therapeutic strategy than that about DFO on the disease related to AD.
Fig. 10.
Schematic diagram of the mechanism of tamoxifen-induced astrocytic CP knockout on learning and memory improvement. Tamoxifen-induced astrocytic CP knockout inhibits the FPN1/CP iron influx pathway into the brain, alleviating iron accumulation in the brain. Therefore, the levels of oxidative stress decreased. The MAPK/JNK and PI3K/Akt/GSK3 pathways are altered, which may lead to a down-regulation of phosphorylation of MYC (T58/S62), with the nuclear translocation of MYC up-regulated, p16INK4a expression inhibited and senescence mitigated. AD-like hallmarks, such as Aβ aggregation and Tau phosphorylation, are also reduced, which may be the result of attenuated iron deposition. Finally, the learning and memory abilities are improved during old age.
CRediT authorship contribution statement
Zhong-Da Li: Writing – review & editing, Writing – original draft, Conceptualization. Shaomeng Kang: Investigation, Formal analysis, Data curation. Haiyan Li: Visualization, Formal analysis, Data curation. Peng Yu: Visualization, Funding acquisition, Formal analysis, Data curation. Ruikun Xie: Visualization, Validation. Chenchen Li: Methodology, Investigation. Qi Jing: Software, Resources. Zhengzheng Gong: Methodology. Li Li: Data curation. Zhengning Li: Resources. Mengyu Geng: Resources. Zihan Zhang: Resources. Yang Li: Supervision, Conceptualization. Yan-Zhong Chang: Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (32070962 and U23A20169) and the Science and Technology Project of Hebei Education Department (ZD2022004).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2025.103611.
Contributor Information
Yang Li, Email: yang.li@siat.ac.cn.
Yan-Zhong Chang, Email: y.z.chang@hebtu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
figs6.
figs7.
figs8.
Data availability
Data will be made available on request.
References
- 1.Holmberg C.G., Laurell C.B. Investigations in serum copper; nature of serum copper and its relation to the iron-binding protein in human serum. Acta Chem. Scand. 1947;1:944–950. doi: 10.3891/acta.chem.scand.01-0944. [DOI] [PubMed] [Google Scholar]
- 2.Holmberg C.G., Laurell C.B. Histaminolytic activity of a copper protein in serum. Nature. 1948;161:236. doi: 10.1038/161236a0. [DOI] [PubMed] [Google Scholar]
- 3.Patel B.N., David S. A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. J. Biol. Chem. 1997;272:20185–20190. doi: 10.1074/jbc.272.32.20185. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy R.C., Kosman D.J. Iron transport across the blood-brain barrier: development, neurovascular regulation and cerebral amyloid angiopathy. Cell. Mol. Life Sci. 2015;72:709–727. doi: 10.1007/s00018-014-1771-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel B.N., Dunn R.J., Jeong S.Y., Zhu Q., Julien J.P., David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J. Neurosci. 2002;22:6578–6586. doi: 10.1523/JNEUROSCI.22-15-06578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Riccardis L., Buccolieri A., Muci M., Pitotti E., De Robertis F., Trianni G., Manno D., Maffia M. Copper and ceruloplasmin dyshomeostasis in serum and cerebrospinal fluid of multiple sclerosis subjects. Biochim. Biophys. Acta, Mol. Basis Dis. 2018;1864:1828–1838. doi: 10.1016/j.bbadis.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Patel B.N., Dunn R.J., David S. Alternative RNA splicing generates a glycosylphosphatidylinositol-anchored form of ceruloplasmin in mammalian brain. J. Biol. Chem. 2000;275:4305–4310. doi: 10.1074/jbc.275.6.4305. [DOI] [PubMed] [Google Scholar]
- 8.Gaasch J.A., Lockman P.R., Geldenhuys W.J., Allen D.D., Van der Schyf C.J. Brain iron toxicity: differential responses of astrocytes, neurons, and endothelial cells. Neurochem. Res. 2007;32:1196–1208. doi: 10.1007/s11064-007-9290-4. [DOI] [PubMed] [Google Scholar]
- 9.Zanardi A., Nardini I., Raia S., Conti A., Ferrini B., D'Adamo P., Gilberti E., DePalma G., Belloli S., Monterisi C., Coliva A., Rainone P., Moresco R.M., Mori F., Zurlo G., Scali C., Natali L., Pancanti A., Giovacchini P., Magherini G., Tovani G., Salvini L., Cicaloni V., Tinti C., Tinti L., Lana D., Magni G., Giovannini M.G., Gringeri A., Caricasole A., Alessio M. New orphan disease therapies from the proteome of industrial plasma processing waste- a treatment for aceruloplasminemia. Commun. Biol. 2024;7:140. doi: 10.1038/s42003-024-05820-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kharel Z., Kharel H., Phatak P.D. Diagnosing aceruloplasminemia: navigating through red herrings. Ann. Hematol. 2024;103:2173–2176. doi: 10.1007/s00277-024-05743-7. [DOI] [PubMed] [Google Scholar]
- 11.Wu Q., Ren Q., Meng J., Gao W.J., Chang Y.Z. Brain iron homeostasis and mental disorders. Antioxidants (Basel) 2023;12:1997. doi: 10.3390/antiox12111997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z.D., Li H., Kang S., Cui Y.G., Zheng H., Wang P., Han K., Yu P., Chang Y.Z. The divergent effects of astrocyte ceruloplasmin on learning and memory function in young and old mice. Cell Death Dis. 2022;13:1006. doi: 10.1038/s41419-022-05459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia A.D., Doan N.B., Imura T., Bush T.G., Sofroniew M.V. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 14.Ganat Y.M., Silbereis J., Cave C., Ngu H., Anderson G.M., Ohkubo Y., Ment L.R., Vaccarino F.M. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J. Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong W., Gong P., Pan X., Ren Z., Liu Y., Qi G., Li J.L., Sun W., Ge W.P., Zhang C.L., Duan S., Qin S. Temporal-spatial generation of astrocytes in the developing diencephalon. Neurosci. Bull. 2024;40:1–16. doi: 10.1007/s12264-023-01131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda S., Sato N., Niisato K., Takeuchi D., Kurinami H., Shinohara M., Rakugi H., Kano M., Morishita R. Validation of Aβ1-40 administration into mouse cerebroventricles as an animal model for Alzheimer disease. Brain Res. 2009;1280:137–147. doi: 10.1016/j.brainres.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 17.Chen C., Li X., Gao P., Tu Y., Zhao M., Li J., Zhang S., Liang H. Baicalin attenuates alzheimer-like pathological changes and memory deficits induced by amyloid β1-42 protein. Metab. Brain Dis. 2015;30:537–544. doi: 10.1007/s11011-014-9601-9. [DOI] [PubMed] [Google Scholar]
- 18.Da Mesquita S., Louveau A., Vaccari A., Smirnov I., Cornelison R.C., Kingsmore K.M., Contarino C., Onengut-Gumuscu S., Farber E., Raper D., Viar K.E., Powell R.D., Baker W., Dabhi N., Bai R., Cao R., Hu S., Rich S.S., Munson J.M., Lopes M.B., Overall C.C., Acton S.T., Kipnis J. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560:185–191. doi: 10.1038/s41586-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P., Wu Q., Wu W., Li H., Guo Y., Yu P., Gao G., Shi Z., Zhao B., Chang Y.Z. Mitochondrial ferritin deletion exacerbates β-amyloid-induced neurotoxicity in mice. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/1020357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z.D., Geng M.Y., Dou S.R., Wang X., Zhang Z.H., Chang Y.Z. Caffeine decreases hepcidin expression to alleviate aberrant iron metabolism under inflammation by regulating the IL-6/STAT3 pathway. Life (Basel) 2022;12:1025. doi: 10.3390/life12071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z.D., Liu F., Zeng Y., Liu Y., Luo W., Yuan F., Li S., Li Q., Chen J., Fujita M., Zhang G., Li Y. EGCG suppresses PD-1 expression of T cells via inhibiting NF-κB phosphorylation and nuclear translocation. Int. Immunopharmacol. 2024;133 doi: 10.1016/j.intimp.2024.112069. [DOI] [PubMed] [Google Scholar]
- 22.Solé V.A., Papillon E., Cotte M., Walter P., Susini J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta B Atomic Spectrosc. 2007;62:63–68. [Google Scholar]
- 23.Li J., Ding Y., Zhang J., Zhang Y., Cui Y., Zhang Y., Chang S., Chang Y.Z., Gao G. Iron overload suppresses hippocampal neurogenesis in adult mice: implication for iron dysregulation-linked neurological diseases. CNS Neurosci. Ther. 2024;30 doi: 10.1111/cns.14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pontén F., Gry M., Fagerberg L., Lundberg E., Asplund A., Berglund L., Oksvold P., Björling E., Hober S., Kampf C., Navani S., Nilsson P., Ottosson J., Persson A., Wernérus H., Wester K., Uhlén M. A global view of protein expression in human cells, tissues, and organs. Mol. Syst. Biol. 2009;5:337. doi: 10.1038/msb.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blalock E.M., Buechel H.M., Popovic J., Geddes J.W., Landfield P.W. Microarray analyses of laser-captured hippocampus reveal distinct gray and white matter signatures associated with incipient Alzheimer's disease. J. Chem. Neuroanat. 2011;42:118–126. doi: 10.1016/j.jchemneu.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller J.A., Woltjer R.L., Goodenbour J.M., Horvath S., Geschwind D.H. Genes and pathways underlying regional and cell type changes in Alzheimer's disease. Genome Med. 2013;5:48. doi: 10.1186/gm452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hokama M., Oka S., Leon J., Ninomiya T., Honda H., Sasaki K., Iwaki T., Ohara T., Sasaki T., LaFerla F.M., Kiyohara Y., Nakabeppu Y. Altered expression of diabetes-related genes in Alzheimer’s disease brains: the Hisayama study. Cerebr. Cortex. 2014;24:2476–2488. doi: 10.1093/cercor/bht101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berchtold N.C., Cribbs D.H., Coleman P.D., Rogers J., Head E., Kim R., Beach T., Miller C., Troncoso J., Trojanowski J.Q., Zielke H.R., Cotman C.W. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15605–15610. doi: 10.1073/pnas.0806883105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang W.S., Dunckley T., Beach T.G., Grover A., Mastroeni D., Walker D.G., Caselli R.J., Kukull W.A., McKeel D., Morris J.C., Hulette C., Schmechel D., Alexander G.E., Reiman E.M., Rogers J., Stephan D.A. Gene expression profiles in anatomically and functionally distinct regions of the normal aged human brain. Physiol. Genom. 2007;28:311–322. doi: 10.1152/physiolgenomics.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang W.S., Reiman E.M., Valla J., Dunckley T., Beach T.G., Grover A., Niedzielko T.L., Schneider L.E., Mastroeni D., Caselli R., Kukull W., Morris J.C., Hulette C.M., Schmechel D., Rogers J., Stephan D.A. Alzheimer's disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4441–4446. doi: 10.1073/pnas.0709259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almeida J., Mota I., Skoda J., Sousa E., Cidade H., Saraiva L. Deciphering the role of p53 and TAp73 in neuroblastoma: from pathogenesis to treatment. Cancers (Basel) 2022;14:6212. doi: 10.3390/cancers14246212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vervoorts J., Lüscher-Firzlaff J., Lüscher B. The ins and outs of MYC regulation by posttranslational mechanisms. J. Biol. Chem. 2006;281:34725–34729. doi: 10.1074/jbc.R600017200. [DOI] [PubMed] [Google Scholar]
- 33.Zhu C., Shen S., Zhang S., Huang M., Zhang L., Chen X. Autophagy in bone remodeling: a regulator of oxidative stress. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.898634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell T.M., Richardson D.R. The good Samaritan glutathione-S-transferase P1: an evolving relationship in nitric oxide metabolism mediated by the direct interactions between multiple effector molecules. Redox Biol. 2023;59 doi: 10.1016/j.redox.2022.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prasad M., Gatasheh M.K., Alshuniaber M.A., Krishnamoorthy R., Rajagopal P., Krishnamoorthy K., Periyasamy V., Veeraraghavan V.P., Jayaraman S. Impact of glyphosate on the development of insulin resistance in experimental diabetic rats: role of NFκB signalling pathways. Antioxidants (Basel) 2022;11:2436. doi: 10.3390/antiox11122436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y.S., Zhang L.H., Yu P.P., Gou Y.J., Zhao J., You L.H., Wang Z.Y., Zheng X., Yan L.J., Yu P., Chang Y.Z. Ceruloplasmin, a potential therapeutic agent for Alzheimer's disease. Antioxidants Redox Signal. 2018;28:1323–1337. doi: 10.1089/ars.2016.6883. [DOI] [PubMed] [Google Scholar]
- 37.Wu W.S., Zhao Y.S., Shi Z.H., Chang S.Y., Nie G.J., Duan X.L., Zhao S.M., Wu Q., Yang Z.L., Zhao B.L., Chang Y.Z. Mitochondrial ferritin attenuates β-amyloid-induced neurotoxicity: reduction in oxidative damage through the Erk/P38 mitogen-activated protein kinase pathways. Antioxidants Redox Signal. 2013;18:158–169. doi: 10.1089/ars.2011.4285. [DOI] [PubMed] [Google Scholar]
- 38.Hou Y., Dan X., Babbar M., Wei Y., Hasselbalch S.G., Croteau D.L., Bohr V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 39.Zhao R. Can exercise benefits be harnessed with drugs? A new way to combat neurodegenerative diseases by boosting neurogenesis. Transl. Neurodegener. 2024;13:36. doi: 10.1186/s40035-024-00428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian Z.M., Ke Y. Rethinking the role of ceruloplasmin in brain iron metabolism. Brain Res. Rev. 2001;35:287–294. doi: 10.1016/s0165-0173(01)00056-x. [DOI] [PubMed] [Google Scholar]
- 41.Ayton S., Janelidze S., Roberts B., Palmqvist S., Kalinowski P., Diouf I., Belaidi A.A., Stomrud E., Bush A.I., Hansson O. Acute phase markers in CSF reveal inflammatory changes in Alzheimer’s disease that intersect with pathology, APOE ε4, sex and age. Prog. Neurobiol. 2021;198 doi: 10.1016/j.pneurobio.2020.101904. [DOI] [PubMed] [Google Scholar]
- 42.Nardella C., Clohessy J.G., Alimonti A., Pandolfi P.P. Pro-senescence therapy for cancer treatment. Nat. Rev. Cancer. 2011;11:503–511. doi: 10.1038/nrc3057. [DOI] [PubMed] [Google Scholar]
- 43.Colucci M., Zumerle S., Bressan S., Gianfanti F., Troiani M., Valdata A., D’Ambrosio M., Pasquini E., Varesi A., Cogo F., Mosole S., Dongilli C., Desbats M.A., Contu L., Revankdar A., Chen J., Kalathur M., Perciato M.L., Basilotta R., Endre L., Schauer S., Othman A., Guccini I., Saponaro M., Maraccani L., Bancaro N., Lai P., Liu L., Pernigoni N., Mele F., Merler S., Trotman L.C., Guarda G., Calì B., Montopoli M., Alimonti A. Retinoic acid receptor activation reprograms senescence response and enhances anti-tumor activity of natural killer cells. Cancer Cell. 2024;42:646–661.e9. doi: 10.1016/j.ccell.2024.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.García-Gutiérrez L., Delgado M.D., León J. MYC oncogene contributions to release of cell cycle brakes. Genes (Basel) 2019;10:244. doi: 10.3390/genes10030244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward R.J., Zucca F.A., Duyn J.H., Crichton R.R., Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13:1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Long H., Zhu W., Wei L., Zhao J. Iron homeostasis imbalance and ferroptosis in brain diseases. MedComm. 2023;4 doi: 10.1002/mco2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji C., Steimle B.L., Bailey D.K., Kosman D.J. The ferroxidase hephaestin but not amyloid precursor protein is required for ferroportin-supported iron efflux in primary hippocampal neurons. Cell. Mol. Neurobiol. 2018;38:941–954. doi: 10.1007/s10571-017-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song N., Wang J., Jiang H., Xie J. Ferroportin1 and hephaestin overexpression attenuate iron-induced oxidative stress in MES23.5 dopaminergic cells. J. Cell. Biochem. 2010;110:1063–1072. doi: 10.1002/jcb.22617. [DOI] [PubMed] [Google Scholar]
- 49.De Strooper B., Karran E. The cellular phase of Alzheimer's disease. Cell. 2016;164:603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 50.Cosacak M.I., Bhattarai P., Kizil C. Alzheimer’s disease, neural stem cells and neurogenesis: cellular phase at single-cell level. Neural Regen. Res. 2020;15:824–827. doi: 10.4103/1673-5374.268896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo C., Wang T., Zheng W., Shan Z.Y., Teng W.P., Wang Z.Y. Intranasal deferoxamine reverses iron-induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer's disease. Neurobiol. Aging. 2013;34:562–575. doi: 10.1016/j.neurobiolaging.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Kupershmidt L., Youdim M.B.H. The neuroprotective activities of the novel multi-target iron-chelators in models of Alzheimer's disease, amyotrophic lateral sclerosis and aging. Cells. 2023;12:763. doi: 10.3390/cells12050763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glabe C.G. Structural classification of toxic amyloid oligomers. J. Biol. Chem. 2008;283:29639–29643. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mrdenovic D., Pieta I.S., Nowakowski R., Kutner W., Lipkowski J., Pieta P. Amyloid β interaction with model cell membranes - what are the toxicity-defining properties of amyloid β? Int. J. Biol. Macromol. 2022;200:520–531. doi: 10.1016/j.ijbiomac.2022.01.117. [DOI] [PubMed] [Google Scholar]
- 55.Shankar G.M., Li S., Mehta T.H., Garcia-Munoz A., Shepardson N.E., Smith I., Brett F.M., Farrell M.A., Rowan M.J., Lemere C.A., Regan C.M., Walsh D.M., Sabatini B.L., Selkoe D.J. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azargoonjahromi A. The duality of amyloid-β: its role in normal and Alzheimer's disease states. Mol. Brain. 2024;17:44. doi: 10.1186/s13041-024-01118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Almeida Z.L., Vaz D.C., Brito R.M.M. Morphological and molecular profiling of amyloid-β species in Alzheimer's pathogenesis. Mol. Neurobiol. 2025;62:4391–4419. doi: 10.1007/s12035-024-04543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lesné S.E., Sherman M.A., Grant M., Kuskowski M., Schneider J.A., Bennett D.A., Ashe K.H. Brain amyloid-β oligomers in ageing and Alzheimer's disease. Brain. 2013;136:1383–1398. doi: 10.1093/brain/awt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larson M.E., Lesné S.E. Soluble Aβ oligomer production and toxicity. J. Neurochem. 2012;120(Suppl 1):125–139. doi: 10.1111/j.1471-4159.2011.07478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cankar N., Beschorner N., Tsopanidou A., Qvist F.L., Colaço A.R., Andersen M., Kjaerby C., Delle C., Lambert M., Mundt F., Weikop P., Jucker M., Mann M., Skotte N.H., Nedergaard M. Sleep deprivation leads to non-adaptive alterations in sleep microarchitecture and amyloid-β accumulation in a murine Alzheimer model. Cell Rep. 2024;43 doi: 10.1016/j.celrep.2024.114977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian Z., Wang Z., Li B., Meng X., Kuang Z., Li Y., Yang Y., Ye K. Thy1-ApoE4/C/EBPβ double transgenic mice act as a sporadic model with Alzheimer's disease. Mol. Psychiatr. 2024;29:3040–3055. doi: 10.1038/s41380-024-02565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pérez-Aguilar F., Burguera J.A., Benlloch S., Berenguer M., Rayón J.M. Aceruloplasminemia in an asymptomatic patient with a new mutation. Diagnosis and family genetic analysis. J. Hepatol. 2005;42:947–949. doi: 10.1016/j.jhep.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 63.Fasano A., Colosimo C., Miyajima H., Tonali P.A., Re T.J., Bentivoglio A.R. Aceruloplasminemia: a novel mutation in a family with marked phenotypic variability. Mov. Disord. 2008;23:751–755. doi: 10.1002/mds.21938. [DOI] [PubMed] [Google Scholar]
- 64.Xu W.Q., Ni W., Wang R.M., Dong Y., Wu Z.Y. A novel ceruloplasmin mutation identified in a Chinese patient and clinical spectrum of aceruloplasminemia patients. Metab. Brain Dis. 2021;36:2273–2281. doi: 10.1007/s11011-021-00799-0. [DOI] [PubMed] [Google Scholar]
- 65.Fan K., Jia X., Zhou M., Wang K., Conde J., He J., Tian J., Yan X. Ferritin nanocarrier traverses the blood brain barrier and kills glioma. ACS Nano. 2018;12:4105–4115. doi: 10.1021/acsnano.7b06969. [DOI] [PubMed] [Google Scholar]
- 66.Huang C.W., Chuang C.P., Chen Y.J., Wang H.Y., Lin J.J., Huang C.Y., Wei K.C., Huang F.T. Integrin α(2)β(1)-targeting ferritin nanocarrier traverses the blood-brain barrier for effective glioma chemotherapy. J. Nanobiotechnol. 2021;19:180. doi: 10.1186/s12951-021-00925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoo K.Y., Hwang I.K., Eum W.S., Kim D.W., Kwon Y.G., Kang T.C., Choi S.Y., Kim Y.S., Won M.H. Differential effects and changes of ceruloplasmin in the hippocampal CA1 region between adult and aged gerbils after transient cerebral ischemia. Neurosci. Res. 2006;55:134–141. doi: 10.1016/j.neures.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 68.Kong Y., Liu P.K., Li Y., Nolan N.D., Quinn P.M.J., Hsu C.W., Jenny L.A., Zhao J., Cui X., Chang Y.J., Wert K.J., Sparrow J.R., Wang N.K., Tsang S.H. HIF2α activation and mitochondrial deficit due to iron chelation cause retinal atrophy. EMBO Mol. Med. 2023;15 doi: 10.15252/emmm.202216525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosin B., Sahel J.A. Lifting the iron curtain of vision. EMBO Mol. Med. 2023;15 doi: 10.15252/emmm.202217259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu D., Wen X., Liu W., Hu H., Ye B., Zhou Y. Comparison of the effects of deferasirox, deferoxamine, and combination of deferasirox and deferoxamine on an aplastic anemia mouse model complicated with iron overload. Drug Des. Dev. Ther. 2018;12:1081–1091. doi: 10.2147/DDDT.S161086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Porter J.B., Faherty A., Stallibrass L., Brookman L., Hassan I., Howes C. A trial to investigate the relationship between DFO pharmacokinetics and metabolism and DFO-related toxicity. Ann. N. Y. Acad. Sci. 1998;850:483–487. doi: 10.1111/j.1749-6632.1998.tb10528.x. [DOI] [PubMed] [Google Scholar]
- 72.Hamilton J.L., Imran Ul-Haq M., Abbina S., Kalathottukaren M.T., Lai B.F., Hatef A., Unniappan S., Kizhakkedathu J.N. In vivo efficacy, toxicity and biodistribution of ultra-long circulating desferrioxamine based polymeric iron chelator. Biomaterials. 2016;102:58–71. doi: 10.1016/j.biomaterials.2016.06.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.