Abstract
In the innate immune system, the CD33 receptor modulates microglial activity. Its downregulation promises to slow Alzheimer disease, and it is already targeted in blood cancers. The mechanism underlying CD33 signaling is unresolved. Starting from the available crystal structure of its extracellular IgV-IgC1 domains, we have assembled a model of the human CD33 receptor by characterizing the oligomerization and structure of the IgC1, transmembrane, and cytosolic domains in solution. IgC1 homodimerizes via intermolecular β-strand pairing and packing. In contrast, the 21-residue transmembrane helix of CD33 appears monomeric and straight with a conserved thin neck, thick belly appearance followed by a positively charged, cytosolic patch. The cytosolic domain is dynamically unstructured. Sequence alignment and AlphaFold models indicate that IgC domains in the family of human Siglecs to which CD33 belongs are surprisingly variable. Only Siglec-6 is identified to analogously dimerize via IgC1. Our CD33 structural model suggests that the receptor is not signaling via a monomer-dimer shift. Rather, we propose that, aided but also constrained by dimerization, multivalent ligands may concentrate the receptor transmembrane and cytosolic domains sufficiently to trigger co-localization with an activating kinase.
Keywords: Alzheimer disease, CD33, membrane protein, NMR spectroscopy, receptor, Siglec, transmembrane signaling
Graphical Abstract

Introduction
Alzheimer’s disease (AD) is a fatal neurodegenerative disorder of the elderly that is characterized by the failure to clear extracellular amyloid-β (Aβ) peptides from the brain, the subsequent accumulation of intracellular neurofibrillary tangles of hyperphosphorylated tau protein, and neuroinflammation.1–3 While present therapies attempt to reduce Aβ peptide burden by employing antibody-based drugs,4 another promising avenue is to modulate the innate immune response to increase Aβ phagocytosis and reduce neuroinflammation. Both immunological responses are mediated by microglia, the resident phagocytes of the central nervous system. Studies suggest that the down- and upregulation of microglial cell surface receptors CD33 and TREM2, respectively, advance this goal.5 Here, we study the structure and oligomerization state of CD33 domains to gain further insight into its function and signaling mechanisms.
CD33 (Siglec-3) is a prominent member of the family of sialic acid-binding immunoglobulin-like lectins (Siglecs) that regulate the function of cells in the innate and adaptive immune systems through the recognition of sialoglycan ligands.6,7 It is an immunoinhibitory receptor expressed on myeloid progenitor cells, monocytes, macrophages, mast cells, dendritic cells, and brain microglia.8 All Siglecs are single-pass type 1 integral membrane proteins containing extracellular domains with generally one unique and homologous N-terminal V-set Ig domain (IgV), followed by variable numbers of C-set Ig domains (IgC). With only one IgC domain, CD33, together with Siglec-15, is the smallest Siglec receptor (Fig. 1A).6,7 IgV contains a sialoglycan-binding site centered on a conserved arginine residue that ligates the sialic acid carboxylate (Fig. 1B).7 CD33 binds the sialylated sulfated sequence Neu5Acα2-3[6SO3]Galβ1-4GlcNAc9 and its physiological ligands include keratan sulfate proteoglycans in the brain.10 By recognizing self-associated glycans, Siglecs are implicated in the discrimination of self and non-self.6,7 As such, they also play a role in autoimmune disease, and CD33 is additionally a drug target in acute myeloid leukemia.11
Figure 1. Structure of the extracellular CD33 domains and IgC1 domain characterization.

A, Domain organization of human CD33. The extracellular IgV-IgC1 domains are followed by transmembrane (TM) and cytosolic (CS) domains. B, Crystal structure of the IgV-IgC1 domains of human CD33 in complex with 3′-sialyllactose (PDB ID 5j06; chains A+D). Of the four molecules in the unit cell, two are shown in blue and red. C, Comparison of Siglec IgC topologies. IgC1 of CD33 dimerizes via βC pairing. For IgC2, two topologies are differentiated. In one βA pairs solely with the B-E sheet, whereas in the other an additional strand βA’ also pairs with the G-F-C sheet. This latter topology is designated IgC2* in contrast to the simpler IgC2 version. Occasionally, a β-strand (βC’) in the βC-βE linker is formed but helical conformation is also possible (Fig. 2D, F). D, Biophysical characterization of CD33(IgC1) in solution. 1H NMR and CD spectra of IgC1(D140-T232/C169S). The CD spectrum is characteristic of high β-sheet content (Table S1). It was recorded for 20 μM IgC1 in 10 mM KH2PO4/K2HPO4, pH 7.4 at 25 °C. The wide HN chemical shift dispersion is indicative of β-sheet hydrogen bonding. The NMR sample consisted of 0.4 mM IgC1 in 10 mM NaH2PO4/Na2HPO4, pH 7.4, 140 mM NaCl, 3mM KCl and was recorded at 35° C and 700 MHz.
Siglec activation often induces immunosuppression via the phosphorylation of immunoreceptor tyrosine-based inhibitory motifs (ITIM) in their cytosolic domain, and subsequent ITIM binding and activation of phosphatases.6,7 CD33 exhibits one ITIM and one ITIM-like motif. However, the signaling mechanism of Siglecs remains little understood. When cross-linked by antibodies it localizes to lipid raft domains in the membrane,12 suggesting that clustering of the receptors contributes to signaling. Subsequently, Src family kinases can phosphorylate the ITIM to convey the activated receptor state.13 For diverse receptors, the oligomerization state of the transmembrane (TM) domain determines signaling.14–18 For Siglecs, the role of the TM segment in signaling or clustering is unknown. Interestingly, IgC domains of several Siglecs have been reported to dimerize via disulfide bonds19–22 and the third and fourth IgC domains of mouse MAG (Siglec-4) have been reported to homodimerize non-covalently.23 For CD33, crystal structures are available for its IgV domain and IgV-IgC domain pairs in the absence and presence of ligands.24,25 While CD33 does not exhibit an unpaired Cys, the crystal structure of its IgV-IgC domains shows dimeric IgC contacts, and IgC-IgC contacts also mediate crystal packing (Fig. 1B and Fig. S1). Nonetheless, single crystals are invariably packed via repeating contacts and unit cells often contain oligomeric assemblies of unknown significance in solution. Here, we establish the oligomerization states of the IgC and TM domains, report the CD33 TM domain structure, and characterize the structural preferences of the cytosolic domain. Using this information, a model of the full-length CD33 structure is assembled and implications for Siglec signaling are discussed. Moreover, sequence alignment and AlphaFold models are used to relate our findings to the other human Siglecs.
Results
The IgC1 domain of CD33 dimerizes in solution
The IgV-IgC crystal structure has been deposited in the Protein Data Bank without an associated publication.24 Mainly for this reason, we discuss it here in relative detail. First, we note that IgC domains are categorized as either C1- or C2-type folds, for which the existence of the βD strand is a principal differentiator (Fig. 1C).26,27 The existence of βD for IgC of CD33 classifies this domain to adopt a C1-type fold (Fig. 2A). The structure suggests IgC dimerization to be mediated by the parallel pairing of βC-βC* strands where * denotes the second subunit (Fig. 1B–C and 2B). In addition, strand βD appears to be crucial for dimerization; side-chain packing between intermolecular βC-βD* and βC-βF* strands stabilize the interface (Fig. 2B–C) that buries a surface area of 808 Å2 (Fig. 1B). For example, Leu180 and Leu187 conspicuously contribute to hydrophobic packing (Fig. 2B–C) and elaborate electrostatic interactions surround an unusual Gly188-Pro189 sequence in strand βD (Fig. 2C). Gly188-Pro189 impose a change in βD-strand registry that points Arg190 towards βE and βC* to engage βC*(Ile176) in packing interactions and to position its guanidino group to donate hydrogen bonds to Thr191, Thr192, and Ser194 of the βD-βE hairpin (Fig. 2B–C). To enable this conformation, Val196 of strand βF hydrogen bonds with G188/NH and P189/CO (Fig. 2C). In turn, G188/CO hydrogen bonds with βF*(Gln213), a situation that is also observed for Arg190 (Fig. 2C). To examine the aggregation state of IgC1 and its putative dimerization interface in solution, we determined its molecular weight by gel filtration and analogously examined mutations of the dimerization interface.
Figure 2. Structural basis of CD33(IgC1) dimerization and relationship to other Siglecs.

A, Cartoon representation of the IgC1 domain of human CD33 (PDB ID 5ihb). With β strands A-B-E-D and C-F-G present, a C1-type Ig fold is adopted (Fig. 1C). B-C, Overview of the dimerization interface and structural integration of βD(Gly188-Pro189). D, ClustalΩ multiple sequence alignment of the βC-βD-βE region of the first IgC domains of human CD33rSiglecs. Select interdomain interactions are indicated. E, Analogous alignment of CD33 with the conserved human Siglecs. Conserved amino acids are colored by the Jalview multiple alignment editor28 using the ClustalX color scheme. F, Superposition of the first IgC domains of human CD33 and Siglec-5. The crystal structure of Siglec-5 (PDB ID 2zg2) was used. G, Superposition of the first IgC domains of human CD33 and CD22. The crystal structure of CD22 (PDB ID 5vkj) was used.
We have expressed IgC1 in P. pastoris with C169S to avoid unspecific disulfide formation (Fig. 2A). To render it suitable for molecular weight determination by gel filtration, we trimmed its glycosylation to single N-Acetylglucosamine (GlcNAc) moieties. Mass spectrometry confirmed a molecular weight of 12.7 kDa for our construct (Fig. S2). In this state, it remained folded with high β-sheet content as evidenced by CD and NMR spectroscopy (Fig. 1D, Fig. S2 and Table S1). Its apparent molecular weight in solution closely matched a dimer (Table 1 and Fig. S3). To unambiguously decide whether the dimerization interface of the crystal structure is engaged in solution, we introduced point mutations aimed to destabilize it. The L187D and L180D/L187D substitutions introduced negative charges in the hydrophobic dimer interface that would simply be solvent exposed for monomer IgC1 (Fig. 2B). L187D caused a shift to higher molecular weight (Table 1 and Fig. S3) indicative of an engaged but altered dimerization interface resulting in aggregation. L180D/L187D caused a further shift to higher molecular weight with a broad gel filtration profile indicative of more unspecific aggregation (Fig. S3). These behaviors negate solvent exposed charges expected for monomeric IgC1. To better understand the Gly188-Pro189 sequence motif, we studied the P189S mutation. Ser allows a wider conformational space than Pro (Fig. S4) and offers Ser194/Val196 a backbone HN atom for canonical βD-βE hydrogen bonding. However, utilizing this hydrogen would invariably abrogate the many contacts that sustain the unusual Gly188-Pro189 β-sheet conformation in the dimer (Fig. 2C). For example, βD-βF* hydrogen bonds would be lost. Gel filtration showed that C169S/P189S remained a dimer in solution (Table 1 and Fig. S3), suggesting that the contacts surrounding Gly188-Ser189 remained unchanged compared to Gly188-Pro189. This situation is reminiscent of Pro-mediated transmembrane helix kinks. In many such conformations, Pro is no longer required for kink formation as surrounding interactions have evolved to stabilize it.29 Thus, biochemical analysis supports IgC1 homodimerization in solution via deeply integrated βC-βC* strand pairing and βC-βD*/βF* packing.
Table 1.
Apparent human CD33(IgC1) molecular weights.
| IgC1 variant | Method Molecular weight determination | Apparent molecular weight [kDa] |
|---|---|---|
| C169S | Mass spectrometry | 12.7 |
| C169S | Gel filtrationa | 22.0 |
| C169S/P189S | Gel filtrationa | 23.5 |
| C169S/L187D | Gel filtrationa | 31.2 |
| C169S/L180D/L187D | Gel filtrationa | Unspecific aggregationb |
In 10 mM NaH2PO4/Na2HPO4, pH 7.4, 140 mM NaCl, 3mM KCl solution.
see Fig. S3.
Relevance of CD33 homodimerization mechanism to other Siglecs
CD33 dimerization depends on the presence of strand βD. We took advantage of this dependence to screen for the IgC1 dimerization motif of CD33 in the first IgC domain of all human Siglecs by aligning the sequences encompassing βC to βE (Fig. 2D–E). Human Siglecs are divided into four conserved receptors (SN, CD22, MAG and Siglec-15) and eleven CD33/Siglec-3-related Siglecs (CD33rSiglecs) that have evolved relatively rapidly.6,7 For Siglec-5, a crystal structure of the first IgC domain is available30 that lacks βD and, hence, represents an IgC2 fold (Fig. 2F and 1C). The sequence alignment suggests that all CD33rSiglecs except CD33 and Siglec-6 are homologous to Siglec-5 (Fig. 2D). AlphaFold2 models of these Siglec domains show βD to be replaced by either short helices or loops that would block any pairing at this location (Fig. S5). However, for many Siglecs, the region between βC and βE was modeled with low confidence and even Siglec-6 is modeled to lack βD despite its close identity with CD33 (Fig. S5). In sum, except for Siglec-6, the other CD33rSiglecs appear to adopt C2-type Ig-folds and are unlikely to homodimerize non-covalently.
For the conserved Siglecs, strand βD is present in the crystal structure of CD22 and MAG23,31 and predicted for SN (Fig. 2G and Fig. S5), classifying them as IgC1 domains. For Siglec-15, the structure between strands βC-βE is predicted with low confidence (Fig. S5). As part of the conserved Siglecs (Fig. 2E), it is tentatively assigned IgC1 topology. Sequence alignments illustrate longer βD sheets and longer βC-βD linkers than found in CD33 (Fig. 2E). The longer βC-βD linkers shield the dimerization interface and mismatch βC-βD heights (Fig. 2G). It therefore appears that, for the first IgC domains, homodimerization via parallel βC pairing is only possible for CD33 and Siglec-6.
CD33 forms a monomeric, 21-residue transmembrane helix
To characterize all CD33 domains, we next determined the structure of the CD33 TM domain in isotropic phospholipid bicelles by multidimensional heteronuclear NMR spectroscopy (Fig. 3A–B). Bicelles are established membrane mimics which form a central lipid bilayer disc that is stabilized by short-chain lipids.32 Given the absence of long-range interproton distance restraints for a linear α-helix, structural restraints relied on H-N, C-C and C′-N residual dipolar couplings (RDC). An ensemble of 20 structures was calculated by simulated annealing with a coordinate precision of 0.15 Å for backbone heavy atoms (Table S2 and Fig. S6). Residues Ala265-His285 adopted α-helical conformation (Fig. 3C), revealing a TM helix length of 21 residues. The first charged residue on the intracellular side, Lys283, establishes the cytosolic helix orientation (positive-inside rule)33 and, apart from this residue, no canonical membrane anchors such as Trp or Tyr are present (Fig. 3B–C). The side-chain distribution along the TM helix shows an interesting pattern. Within the extracellular leaflet, residues with small sidechains, Ala265, Gly266, Ala269 and Ala272, constitute a relatively thin helix (Fig. 3C–D). This contrasts the following relatively bulky residues; two Phe residues are particularly noteworthy. On the intracellular membrane-proximal side, a cluster of positive charges formed by R286-R287-K288 and R291 gives the membrane-proximal region a distinctively positive charge (Fig. 3B,D). Thus, the CD33 TM helix is composed of a thin neck and a thicker belly, which is followed by a positively charged, cytosolic patch.
Figure 3. Structure of the transmembrane domain of CD33.
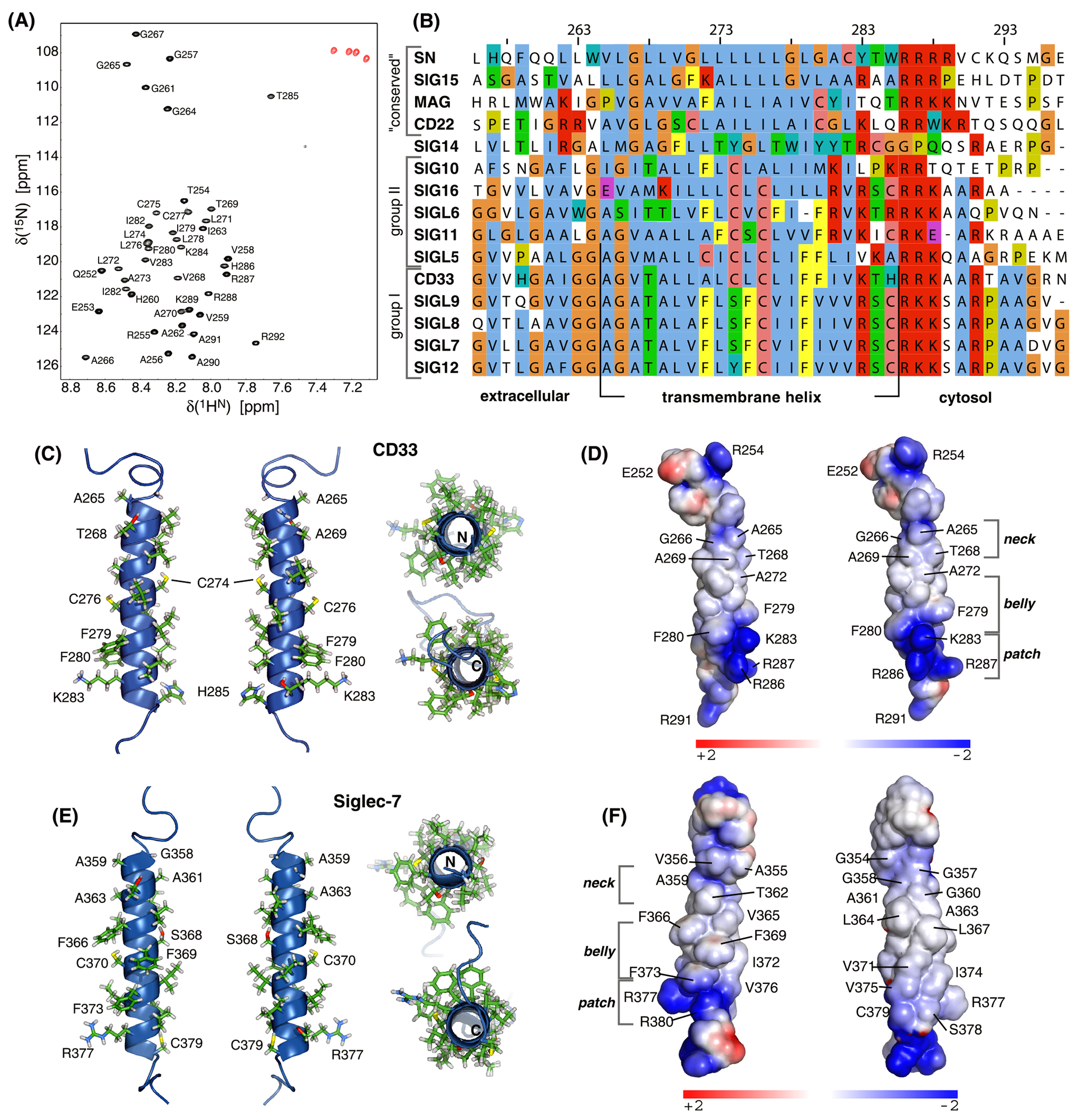
A, HN-N correlation NMR spectrum of 2H/13C/15N-labeled CD33(Q251-R291) in 350 mM DHPC, 105 mM DMPC, 6% D2O, 0.02% w/v NaN3, 25 mM HEPES•NaOH, pH 7.4 at 35° C and 700 MHz. B, ClustalΩ multiple sequence alignment of the human Siglec transmembrane domains. Siglecs are grouped into three classes and Siglec-14 as indicated. Conserved amino acids are colored by the Jalview multiple alignment editor28 using the ClustalX color scheme with manual modifications. C-D, Cartoon representation of the average CD33 TM domain structure and its surface charge distribution color-coded by electrostatic potential. E-F, Structural model including electrostatic potential of the TM domain of human Siglec-7. Electrostatic potentials were calculated using APBS.34
To convey a signal across the membrane, receptors may change the association state of their TM domain(s).14–16,18 In case of CD33, such a scenario would require TM helix homodimerization. To assess the default oligomerization state of the TM domain, we have determined the rotational correlation time, termed τc, of the bicelle-TM helix complex, which informs on particle size. With reference to the values of bicelle-immersed monomeric and dimeric integrin αIIbβ3 TM peptides of approximately 20 ns and 30 ns, respectively,35 the determined τc of 16.9 ± 0.2 ns bicelles identifies the CD33 TM helix as monomeric. At the studied lipid-to-peptide ratio, bicelles were in excess of peptides by a factor of 6.5 and the detection limit for a TM interaction is approximately 3 kcal/mol.36 At peptide-to-bicelle ratios down to the lowest examined value of 3.4, linewidth remained characteristic of monomeric peptide and concentration dependent spectral changes were minor (Fig. S7). Accordingly, there is no indication that the TM helix oligomerizes in standard lipids.
We also view the structural features of the TM helix not to be conducive for homodimeric helix-helix interactions.37,38 Packing in the “neck” region would lead to a relatively large crossing angle for a straight helix, which may require a longer TM helix length than 21 residues to remain membrane immersed.39 Likewise, the “belly”, centered on consecutive Phe residues (Phe279-Phe280), seems unsuited to establish defined contacts and its “back” is characterized by relatively featureless hydrophobic surface (Fig. 3C–D). Nonetheless, to explore packing possibilities AlphaFold3 modeling of a dimer and trimer were performed. First, we note that, for the monomer, the experimental structure differs from the AlphaFold2 model, which predicts a longer TM helix but exhibits low confidence for both the N- and C-terminal helix turns (Fig. S8). AlphaFold3 modeling of a dimer also predicts a longer helix that invokes curvature to pack belly-to-belly (Cys276 and Phe279) while placing the helix crossing generally in the neck region including the preceding Gly-Gly residues (Fig. S9A). In a trimer helix curvature increases even further with packing more complex but generally Phe279 in the center (Fig. S9B). The confidence metrics of the predictions suggest that the obtained models could represent plausible oligomeric architectures (medium to high Cα pLDDT (Fig. 9) with pTM scores of 0.57 and 0.51 for dimer and trimer, respectively). However, the confidence in the predicted interfacial interactions between subunits is relatively low (ipTM scores of 0.47 and 0.43 for dimer and trimer, respectively). This low confidence may relate to weak or non-specific binding interfaces, high structural flexibility, or a lack of co-evolutionary signals in the sequence. Thus, in the absence of external thermodynamic and structural features that favor oligomerization and TM helix extension, the CD33 receptor is unlikely to convey its ligand engagement via a monomer-dimer equilibrium of its TM domain.
A neck-belly-positive patch is universal to TM domains of CD33rSiglecs
To examine the significance of the CD33 TM domain structure for other Siglecs and to assess any recurring structural features, we have aligned all human Siglec TM domain sequences (Fig. 3B). The conserved receptors only contain the cytosolic positively charged patch of CD33 but otherwise appear relatively featureless. Siglec-14, 15 and 16 associate intramembranously with adaptor proteins such as DAP12.6,7 Siglec-14 appears most distinct from all other Siglecs; it lacks the positive patch and a high incidence of Tyr and Trp anchors in the intracellular membrane leaflet make this TM helix border ambiguous. In contrast, the Arg reported to make intramembrane contact with DAP1240 is relatively far N-terminal compared to Siglec-15 and 16. Siglec-16 exhibits the positive patch and shares a C/S/YxxC sequence motif with all other CD33rSiglecs except Siglec-10 and 14. Indeed, Siglec-10 is the greatest outlier among CD33rSiglecs, with thin neck-thick belly characteristics least pronounced. Besides C/S/YxxC, all remaining CD33rSiglecs also exhibit at least two Phe residues. Interestingly, for a number of receptors the Phe is shifted to center of the helix relative to CD33 and even increased to three Phe residues (Fig. 3B). To obtain structural insight into this subclass, we constructed a model of the Siglec-7 TM domain based on the CD33 structure (Fig. 3E–F). The Phe residues line one helix face, the belly, whereas its back is again relatively inconspicuous and the neck region also “thin”. The neck-belly characteristic together with the positively charged parch is therefore a hallmark of most CD33rSiglecs TM domains. We also note that a high incidence of backbone-sidechain hydrogen bonds through the C/S/YxxC motif and oftentimes additional such residues (Fig. 3C, E). Finally, the variation of the first charged residue on the intracellular side among CD33rSiglecs is noted. For Siglec-5, this suggests the TM helix to be longer by one residue compared to CD33. For Siglec-6, 11, and 14, an additional Arg appears two positions before the first Lys of CD33, which may also affect TM helix length. In addition to the discussed structural variations, differences in helix lengths and/or membrane immersion may separate the CD33rSiglecs into two groups at the level of their TM domains (Fig. 3B).
The CD33 cytosolic region is unstructured and rich in proline-induced extended conformations
The TM helix terminates at His285 and our TM construct ended at Arg291. Next, we examined the consequences of extending this construct to the C-terminus (Gln364) and characterized the structure of the cytosolic region. With an anticipated random-coil conformation, NMR spectroscopy is again well suited to characterize its average conformation (Fig. 4A). Although not a prerequisite to the binding of intracellular ligands, structural propensities away from random-coil conformation may indicate an anticipation of a bound conformation and a prominent binding region.41 As noted earlier, CD33 contains ITIM and ITIM-like motifs, predicted for residues Leu338-Leu343 and Thr356-Val361, respectively (Fig. 4C). The Src homology-2-containing tyrosine phosphatase 1 and 2 (Shp1 and 2) bind the ITIM motifs of CD33 via tandem SH2-SH2 domains.13,42 The structure of these domains in complex with unrelated peptides shows these ligands to be bound in extended conformation.43 In terms of NMR parameter, secondary 13Cα chemical shifts provide a readily interpretable parameter of secondary structure propensity.44,45 To determine these shifts, we have extended our TM domain backbone assignment to the cytosolic (CS) domain.
Figure 4. Structural properties of the cytosolic domain of CD33.

A, Superposition of the HN-N correlation NMR spectra of CD33(Q251-R291) and CD33(Q251-Q364), corresponding to the TM and TM-CS domains, respectively, at 35° C and 700 MHz. Proteins were 2H/13C/15N-labeled and reconstituted in 350 mM DHPC, 105 mM DMPC, 6% D2O, 0.02% w/v NaN3, 25 mM HEPES•NaOH, pH 7.4. B, Secondary 13Cα chemical shifts of the TM and TM-CS domains. For random coil conformations, shifts are close to zero, whereas positive and negative shifts denote helical and extended backbone propensities, respectively.44,45 C, ClustalΩ multiple sequence alignment of the depicted human CD33rSiglecs. Conserved amino acids are colored by the Jalview multiple alignment editor28 using the ClustalX color scheme. The immunoreceptor tyrosine-based inhibitory motifs (ITIM) are indicated.
First, we noted that extending the construct mitigated fraying of the TM helix; Lys283-Ala289 exhibited modestly higher, positive 13Cα shifts indicative of increased helical content (Fig. 4B). That is, two helix turns were somewhat stabilized. However, extending the TM construct invariably reduces the mobility of its terminal residues and the relatively small magnitude of 13Cα shift increases indicated that the influence of the cytosolic region on the TM domain structure was small. Starting with Ala290, secondary 13Cα shifts were close to zero, albeit with several distinct outliers (Fig. 4B). Shifts near zero represent random coil conformations,44,45 revealing the absence of a predefined ITIM binding conformation and stable secondary structure. Phosphorylation of Tyr, which takes place away far from the backbone, is not expected to change this disposition. The most conspicuous “outliers” in 13Cα shifts occurred in residues preceding proline residues, present at positions 301, 308, 319, 330 and 350 (Fig. 4B–C). Proline generally coerces preceding residues into extended conformations46 and the observed negative secondary 13Cα shifts reflect such conformations. For Pro319, this effect is mitigated by the conformational flexibility of preceding Gly318. When referencing the secondary 13Cα shifts to include nearest neighbor effects,47 these effects complied with the random coil behavior of this sequence (Fig. S10). For receptors, unfolded cytosolic domains are often observed in relation to their ability to serve as hubs for protein assembly and/or their ability to partake in phase separation.48,49 In sum, the intracellular portion of CD33 is dynamically unstructured but conspicuously influenced by its proline residues whereas ITIM-SH2 binding is not anticipated by deviating from random-coil conformation.
The prominent influence of proline residues in the CS domain prompted us to examine their conservation for the most similar CD33rSiglecs. Prolines are abundant for all examined Siglecs, but a clear conservation pattern was not evident (Fig. 4C). Interestingly, Siglecs-5, 8, 9, 11, and 12 are more Pro-rich than CD33 and Siglec-6 and 7. They also exhibit at least one stretch of consecutive prolines preceding ITIM1. The most conserved sequences in the cytosolic domains are the ITIM(-like) motifs with a consensus sequence (I/V/L/S)-X-Y-X-X-(L/V).6 A high amino acid conservation preceding and succeeding the ITIMs is also noted.
Human Siglec domain organization and oligomerization
CD33 is the first example of non-covalent IgC1 dimerization in Siglec structures. Dimerization via βC strand pairing is only shared with Siglec-6, which is evolutionary most closely related to CD33.50 Non-covalent dimerization was also reported for mouse MAG where the third and fourth IgC domains form hydrophobic contacts over an area of 2,037 Å2. 23 Nonetheless, MAG dimerization is too weak to be detected by gel filtration,23 highlighting the well-defined nature of the IgC1 dimer interface of CD33. As noted in Introduction, disulfide-linked dimerization was reported for Siglec-5 and 8.19,20 For Siglec-8, an unpaired Cys is present in the βC-βE linker of its second IgC domain (position 303; Fig. 5A–B). This position is equivalent to the dimerization interface of CD33. In contrast, for Siglec-5, the unpaired Cys residue localizes to strand βC of the third IgC domain (residue 364; Fig. 5A–B). This dimerization site would therefore be different from CD33.
Figure 5. Domain organization of human Siglecs.

A, Structural examples of IgC1 and IgC2* domain topologies (Fig. 1C) found in human Siglecs. Domains (d) are numbered consecutively starting with IgV as first domain. The indicated domains were superimposed with IgC1 of CD33 shown in red. For d4(IgC1) of Siglec-5 and d5(IgC1) of Siglec-11 dimerization via βD appears to be blocked by the βC-βE linkers. Interestingly, d4(IgC1) of Siglec-5 contains unpaired Cys364 in βC that may dimerize.19 The second IgC domain of Siglec-8 also exhibits unpaired Cys303 in the βC-βE linker that may dimerize.20 For CD33, the crystal structure is shown (PDB ID 5ihb), whereas for the other Siglecs AlphaFold2 models are displayed. These models are color-coded according to their per-residue model confidence score (pLDDT). B, Siglec domain organization according to AlphaFold2 models and available human crystal structures (CD22 5vkj, CD33 5ihb, Siglec-5 2zg2). In deviation from AlphaFold2 predictions, the first IgC domains of Siglec-6 and Siglec-15 were classified as C1-type based on sequence alignment (Fig. 2D–E).
We take the opportunity of analyzing IgC1 dimerization in CD33 to summarize the domain organization of human Siglecs. To complete this summary as much as possible, we have visually examined AlphaFold2 models of the remaining IgC domains. For these domains of CD33rSiglecs, C1-type folds were only found for the membrane-proximal Ig domains of Siglec-5, 10 and 11 (Fig. 5). In these cases, the βC-βD linkers appear to block the canonical dimerization interface (Fig. 5A), making dimerization unlikely. The other IgC domains lack βD, which classifies them as C2-type and makes their non-covalent dimerization via βC pairing improbable. They exhibit notable variability in the βC-βE intervening sequence but also in strand βA (Fig. 5A). While this strand pairs with βB, oftentimes it forms an extension that pairs with βG of the second β-sheet (Fig. 1C). It is common among IgC2 domains of Siglecs where we indicate it with an asterisk as IgC2* domain (Fig. 5A–B). In the conserved Siglecs, IgC1 domains appear to be more common (Fig. 5B). However, as judged from their AlphaFold2 models, none of the IgC1 domains appear capable of dimerizing in a manner similar to CD33. As such, Fig. 5B summarizes the current knowledge of human Siglec domain organization and oligomerization.
Structure of the CD33 receptor
CD33 is characterized by a sophisticated IgC1 dimerization interface that effects its non-covalent association into a dimer with separated TM helices and dynamically unstructured cytosolic domain. We have assembled this information into a model of the full-length receptor structure (Fig. 6A). The CS domain was represented by random conformations as was the IgC1-TM domain-domain linker. The relative compactness of the folded domains compared to the unfolded regions is conspicuous. This especially illustrates that TM domain dimerization could only make a difference to CS residues that are immediately membrane-proximal. Moreover, the relatively long IgC1-TM linker of 33 residues (Gly241-Gly263) suggests that IgV-IgC1 can adopt a wide range of orientations relative to the membrane.
Figure 6. Structural model of the human CD33 receptor and proposed signaling mechanism.

A, The IgV-IgC1 crystal structure (PDB ID 5ihb, chains A+D) is connected to the TM domain structure via a dynamically unstructured linker. The cytosolic domain is represented by unstructured conformations. B, Two dimeric CD33 molecules may be clustered by a multivalent ligand. C-F, First, a multivalent ligand binds CD33 to cluster it. The ensuing proximity of TM domains may attract them to membrane microdomains (lipid rafts; panel D). Alternatively, the proximity of clustered CS domains may trigger a liquid-liquid phase separation (LLPS) of the CS domains (panel E). Both processes may also combine (panel F). Each case would appose CD33 with a Src-family kinase in the raft area and/or the separated phase, allowing phosphorylation of the ITIMs to render the receptor active.
Discussion
Our experimental studies, sequence alignments, and analysis of AlphaFold2 structures reveal that human Siglecs exhibit a rich variety of IgC domain topologies with the non-covalent dimerization of the IgC1 domains of CD33 and Siglec-6 appearing to be the exception and not the rule (Fig. 5B). With the available IgV-IgC1 crystal structure,24 the achieved structural characterization of the TM and CS domains, and the determination of the oligomerization states of the TM and IgC1 domains, CD33 is the first Siglec for which a complete structural characterization was accomplished.
In contrast to disulfide-mediated homodimerization where only one point mutation is required to introduce Cys (Fig. 5B), βC pairing is stabilized by several interactions that rely on the presence of strand βD (Fig. 2A–C). Initially, a common ancestor to CD33 and Siglec-6 likely achieved partial dimer formation. As more stabilizing mutations accumulated, permanent dimerization was achieved attesting to an evolutionary advantage of dimeric over monomeric CD33. Principally, this advantage may lie at the level of ligand binding or receptor signaling. It also appears that CD33 drug targeting applications will achieve greatest efficiency when targeting the dimeric receptor structure (Fig. 1B).
Functional aspects of the CD33 structure
As opposed to disulfide-linked receptors, the many contacts achieving non-covalent IgC1 dimerization lock the relative IgC1-IgC1 domain-domain orientation more decisively. This will aid the initial assembly of the dimeric receptor and confer a preferred domain-domain orientation for the IgV pair (Fig. 6A). IgV connects to IgC1 via a short linker, a buried surface area of 402 Å, and is restrained by the IgV-IgC1 disulfide bond (Fig. 1B). Moreover, Siglec-6 dimerizes via its first IgC domain in contrast to the probable disulfide linkages of Siglec-5 and 8 in subsequent domains (Fig. 5B). For some Siglecs, instances of unexpectedly selective ligand targeting are documented,51–53 suggesting that not only are ligand affinities important but also the arrangement of binding sites on multivalent ligands. In general, the number, coupling and properties of the IgC domains may encode such spatial preference. Non-covalent dimerization may be another element to encode a spatial binding preference even more precisely. As such, non-covalent dimerization of CD33 and Siglec-6 may encode a spatial binding configuration that is important to their specific functional contexts.
CD33 signaling hypotheses
Receptors signal ligand binding into the cytosol. For receptors consisting of more than one subunit, a signal is often transduced by shifting the monomer-dimer equilibrium of their TM helices following ligand binding.15,16 If this scenario were to apply to CD33, a TM domain monomer-dimer equilibrium of CD33 would have to be coupled to ligand binding by IgV. The IgV-IgC1 crystal structure is available free and bound with 3′-sialyllactose or 6′-sialyllactose.24,54,55 However, ligand binding does not change the IgV backbone structure and IgV-IgC1 orientation significantly, or impact IgC1 dimerization in the crystal (Fig. S11). It is therefore unlikely that ligand binding to IgV leads to a structural rearrangement that is transmitted to or beyond IgC1. In addition, the TM domain of CD33 was found to be monomeric and appears unsuited to dimerize specifically (Fig. 3C–D) unless helix curvature and extension could be induced (Fig. S9). Furthermore, a relatively long linker of 33 residues connects IgC1 to the TM domain (Fig. 6A). This length would uncouple any TM helix monomer-dimer equilibrium from the corresponding IgC1 equilibrium or a change in IgV-IgC1 domain-domain orientation.56 The long linker may facilitate ligand binding by extending IgV beyond the glycocalyx coat of the membrane. We therefore view a shift in the monomer-dimer equilibrium of CD33, away from the IgC1 dimer or away from the TM monomer, as unlikely to contribute to its activation.
If a multivalent ligand engages IgV, it can nonetheless oligomerize the receptor (Fig. 6B). For CD33, P22 monomers had no effect on basal cellular phagocytosis whereas P22 conjugated to microparticles increased phagocytosis.25 If ligand-binding sites are clustered, receptors will, in turn, also cluster in the membrane. In other words, clustering concentrates the receptor TM and CS domains in the membrane and cytosol, respectively. It has been reported that ligand-bound CD33 and other activated Siglecs localize to membrane microdomains (lipid rafts).12,57 Accordingly, clustered TM domains might display an altered lipid preference, promoting their transfer to lipid rafts (Fig. 6C–D) where a Src family kinase could ultimately phosphorylate the ITIMs.13 In this altered lipid environment, the TM structure and/or oligomerization could change (Fig. S9) but, at present, this only seems to be a theoretical possibility.
The CS domain is not anticipating a bound conformation and, outside of the ITIMs, prolines distinctly modulate its dynamically unstructured ensemble (Fig 4B). Its dynamically unstructured conformations further imply that any information originating in the TM domains is quickly lost to the CS residues farther from the membrane (Fig. 6A). Receptor clustering therefore restricts the lateral diffusion of CD33 in the membrane and concentrates the CS domain without dramatically changing their structural properties. Nonetheless, merely concentrating the CS domain may, in analogy to other immune receptors,48 allow them to partake in a liquid-liquid phase separation (LLPS). LLPS could occur alongside CD33 localization into lipid rafts (Fig. 6F), or independent of it once a critical concentration is locally achieved (Fig. 6E), to also allow a productive co-localization with a Src family kinase.13 In addition to impacting ligand binding, dimerization of CD33 and Siglec-6, would reduce the entropic cost of clustering the receptor and, thus, lower the threshold of receptor activation.
Conclusions
Receptors may transmit a signal across the membrane by changing their conformation and/or by switching the oligomeric state of their subunits. While a full understanding of CD33 signaling requires additional studies, the presented structural model of inactive CD33 represents an important milestone towards this goal. Given the putative absence of changes in subunit structure and oligomerization upon ligand binding, our study suggests CD33 signaling to operate via a clustering mechanism where the concentration of the TM and/or CS domains represents the activating signal.
Materials and Methods
Expression and purification of CD33 IgC1 domain
An insert encompassing Asp140-Thr232 of the human CD33 gene was generated by PCR and subcloned into the pPIC9K yeast expression vector (Invitrogen) with the α-mating signal sequence, and a N-terminal Flag-tag for detection. Additionally, the C169S substitution was implemented to avoid unspecific disulfide bond formation (Fig. 1B). Electrocompetent histidine auxotrophic Pichia (Komagataella phaffii) cells (his4-Δ1; BioGrammatics, Inc. BG12) were transformed. Single colonies were picked and cultured in 4 ml of BMGY medium (1% yeast extract, 2% peptone, 4·10−5% biotin, 100 mM KH2PO4/K2HPO4, pH 7.5, 1.34% yeast nitrogen base, and 1% glycerol) for 24 hours (30 °C and 200 rpm). The culture was spun down and the cell pellet was resuspended in 4 ml of BMMY medium (1% yeast extract, 2% peptone, 4·10−5% biotin, 100 mM KH2PO4/K2HPO4, pH 7.5, 1.34% yeast nitrogen base, and 0.5% methanol) to a OD600 of 1 and grew for 24 hours (30 °C and 200 rpm). After 24 hours, additional methanol was added (0.5%), and cells grown for another 24 hours. Subsequently, cells were pelleted by centrifugation and the supernatant analyzed by Western blot using the monoclonal anti-Flag antibody conjugated with M2-alkaline phosphatase (Sigma Aldrich, cat. no. A9469). The best expressing clone was grown in BMGY medium for 16 hours at 30°C and 250 rpm. This culture was spun down and the cell pellet was resuspended in 1 liter of BMMY medium to a OD600 of 2. Cells were grown for 72 hours at 25 °C and 250 rpm, and methanol was replenished every 12 hours to a concentration of 0.5%. The supernatant was collected by pelleting the cells by centrifugation (4 °C and 4000g for 20 min.), the pH was adjusted to 7.5 using 8 M NaOH, stirred on ice for 2 hours, and filtered (0.2 μm). The supernatant was applied on a HiTrap IMAC HP column (GE Amersham, Inc.) charged with Ni2+. The column was washed with 50 mM KH2PO4/K2HPO4, pH 7.5, 300 mM NaCl, 20 mM imidazole. Then, the bound protein was eluted in 50 mM KH2PO4/K2HPO4, pH 7.5, 300 mM NaCl, 300 mM imidazole. For deglycosylation, Endo H (UniProt entry P04067)58 was added to a final concentration of 0.04 μM and incubated overnight at 37 °C. The eluate was concentrated to 0.2 ml and applied on a Superdex 75 Increase 10 300 GL column equilibrated with 10 mM NaH2PO4/Na2HPO4, pH 7.4, 140 mM NaCl, 3mM KCl. For molecular weight determination (Table 1), the column was calibrated in the same buffer at a flow rate of 0.5 ml/min on a cytiva ÄKTA go system using albumin (67.0 kDa), ovalbumin (43.0 kDa), chymotrypsinogen A (25.0 kDa), RNAse A (13.7 kDa), and Blue Dextran 2000 as part of the cytiva gel filtration calibration kit.
Circular dichroism (CD) spectroscopy
IgC1 variants were exchanged into 10 mM K2HPO4/KH2PO4, pH 7.4, 25mM KCl solution by four ultrafiltration-dilution cycles (1:10 dilution) and adjusted to a concentration of 20 μM employing ε280nm= 12,490 M−1cm−1. 59 CD measurements were carried out at 25 °C on a JASCO J-810 spectropolarimeter by acquiring spectra from 190 to 260 nm in a quartz cell of 0.1 cm path length. Sixteen scans, recorded in 0.5 nm steps at a rate of 50 nm/min with a 1 nm bandwidth and a 4 s integration time, were accumulated. Spectra were corrected for solvent contributions. The observed ellipticity in millidegrees, Θ, was converted to the mean residue ellipticity, [Θ]MRW, using [Θ]MRW= (MRW × Θ) / (10 × d × c), where d is the path length in cm, c is the protein concentration in mg/ml, and MRW (mean residue weight) equals MW/(n-1) with MW denoting the molecular weight of the polypeptide chain in Daltons and n representing the number of amino acids in the chain. Peptide secondary structure content was estimated using the CONTIN-LL program60 via the DichroWeb interface.61,62
AlphaFold models
AlphaFold2 structural models associated with the Uniprot entries of human Siglecs were used. Dimeric and trimeric models of the CD33 TM domain were generated using the AlphaFold3 webserver (https://alphafold.com). For each oligomeric state, the sequence Ala265-Arg291 was submitted as multiple identical chains. Default parameters were used during the predictions and models were generated in the absence of lipids. The highest-ranking models as scored by AlphaFold3 were selected.
Expression and purification of transmembrane CD33 peptides
Using the cDNA of human CD33 (Mammalian Gene Collection Clone ID 5217182, UniProt entry P20138) as template, an insert encompassing Gln251-Gln364 was generated by PCR and subcloned into the pET-44 expression vector (Novagen, Inc.) with maltose binding protein as N-terminal fusion protein and an intervening TEV protease cleavage site. A shorter variant, encompassing Gln251-Arg291, was subsequently constructed using standard techniques. Expression was induced in E. coli BL21(DE3)pLysS,T1R cells (Sigma-Aldrich, Inc.) cultured at 37 °C in M9 minimal medium, containing combinations of 99% 13C-D glucose, 99% 15NH4Cl, and 99% D2O, at an OD600 of 1.0 by adding isopropyl β-d-1-thiogalactopyranoside to 1.0 mM. Cells were lysed by sonication in 50 mM NaH2PO4/Na2HPO4, pH 7.4, 300 mM NaCl, 100 mM SDS, 20 mM imidazole, 2 mM β-mercaptoethanol. The clarified lysate was applied on a HiTrap IMAC HP column (GE Amersham, Inc.) charged with Ni2+ for immobilized metal affinity chromatography. The column was washed with 50 mM NaH2PO4/Na2HPO4, pH 7.4, 300 mM NaCl, 25 mM SDS solution, followed by 50 mM NaH2PO4/Na2HPO4, pH 7.4, 300 mM NaCl, 8 M urea, 20 mM imidazole, and 50 mM NaH2PO4/Na2HPO4, pH 7.4, 300 mM NaCl, 8 M urea, 50 mM imidazole. Then, bound protein was eluted in 50 mM NaH2PO4/Na2HPO4, pH 7.4, 300 mM NaCl, 8 M urea, 300 mM imidazole solution. The peptide was cleaved from the fusion protein using TEV protease at a molar ratio of 1:50 overnight at 30 °C in 50 mM Tris·HCl, pH 8.0, 1 mM DTT solution, leaving a Gly as the N-terminal residue preceding Gln251. Uncleaved protein and fusion protein were removed by IMAC and the column flow-through was applied on a Hamilton PRP-3 reverse-phase HPLC column. The peptides were eluted using a linear gradient from 70%/30% buffer A (H2O, 0.1% TFA) / buffer B (70% acetonitrile, 30% 1-propanol, 0.1% TFA) to 10%/90% in 30 min. Subsequent to freeze-drying, peptide purity was verified by SDS-PAGE and NMR.
NMR sample preparation
TM peptide concentrations were measured in acetronitrile-water solution by UV spectroscopy (ε205nm= 149,580 M−1cm−1 and ε280nm= 3,105 M−1cm−1 for Gln251-Arg291 and Gln251-Gln364, respectively) and defined amounts of peptide were freeze-dried. Peptides were taken up in 320 μL of 350 mM 1,2-dihexanoyl-sn-glycero-3-phosphocholine (DHPC), 105 mM 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 6% D2O, 0.02% w/v NaN3 buffered by 25 mM HEPES·NaOH, pH 7.4 to yield concentrations of 1.1 mM and 0.8 mM for Gln251-Arg291 and Gln251-Gln364, respectively. The Gln251-Arg291 peptide-bicelle complex was aligned relative to the magnetic field by a stretched, negatively charged polyacrylamide gel of 320 μl volume, which was polymerized from a 4.2% w/v solution of acrylamide (AA), 2-acrylamido-2-methyl-1-propanesulfonate (AMPS) and bisacrylamide (BIS) with a monomer-to-crosslinker ratio of 49:1 (w/w) and a molar ratio of 95:5 of AA to AMPS.63 For IgC1(D140-T232 with C169S) a 0.4 mM sample (ε280nm= 12,615 M−1cm−1) in 10 mM NaH2PO4/Na2HPO4, pH 7.4, 140 mM NaCl, 3mM KCl was prepared.
NMR spectroscopy
NMR experiments were carried out on a cryoprobe-equipped Bruker Avance 700 spectrometer at 35 °C. Data were processed and analyzed with the nmrPipe package and CARA. 2H/13C/15N-labeled N peptide and TROSY-type H-N detection64 was used for HNCA, HNCACB, HN(CA)CO and HNCO-based backbone assignments, the measurement of 3JC′Cγ and 3JNCγ couplings,65 and the detection of 1JNH, 1JCαC′, 1JC′N as well as 1JNH+1DNH, 1JCαC′+1DCαC′, 1JC′N+1DC′N couplings66–69 using isotropic and aligned samples, respectively.
Structure calculation of the CD33 TM domain
The bicelle-embedded CD33 TM peptide structure of the well-folded Ala265-His285 residues was calculated by simulated annealing, starting at 3000 K using the program XPLOR-NIH.70 The peptide termini were represented by random-coil conformations. Backbone dihedral angle constraints were derived from N, Hα Cα, Cβ and C′ chemical shifts using the program TALOS-N.71 χ1 side-chain angle restraints were derived from the 3JC′Cγ and 3JNCγ coupling constants.65 In addition to standard force field terms for covalent geometry (bonds, angles, and improper dihedrals) and nonbonded contacts (Van der Waals repulsion), dihedral angle and interproton distance restraints were implemented using quadratic square-well potentials, and a backbone-backbone hydrogen-bonding potential and torsion angle potential of mean force were employed.72,73 The difference between predicted and experimental residual dipolar couplings (RDC; Δ1D) was described by a quadratic harmonic potential. The final values for the force constants of the different terms in the simulated annealing target function are as follows: 1,000 kcal·mol−1·Å−2 for bond lengths; 500 kcal·mol−1·rad−2 for angles and improper dihedrals, which serve to maintain planarity and chirality; 4 kcal·mol−1·Å−4 for the quartic Van der Waals repulsion term; 30 kcal·mol−1·Å−2 for interproton distance restraints; 500 kcal·mol−1·rad−2 for dihedral angle restraints; 0.3 kcal·mol−1·Hz−2 for 1DNH RDC restraints and 1DC′N and 1DCαC′ scaled relative to 1DNH according to their dipolar interaction constants; 1.0 for the torsion angle potential; and a directional force of 0.20 and a linearity force of 0.05 for the hydrogen-bonding potential. A total of 20 structures were calculated (Fig. S6 and Table S2).
Supplementary Material
Acknowledgements
This work was supported by a grant from the National Institutes of Health to T.S.U. (R01AG072442).
Abbreviations
- AD
Alzheimer’s disease
- Aβ
amyloid-β
- CS
cytosolic
- ITIM
immunoreceptor tyrosine-based inhibitory motifs
- LLPS
liquid–liquid phase separation
- RDC
residual dipolar coupling
- Siglec
sialic acid-binding immunoglobulin-like lectin
- TM
transmembrane
Footnotes
Accession numbers
Structure coordinates, chemical shifts and structural constraints been deposited to PDB and BMRB databases with accession codes 9bet and 31167, respectively.
Supporting Information
Figures depicting dimerization of IgC1 in the crystallographic unit cell, characterization of the CD33 IgC1 domain expressed in P. pastoris, gel filtration of wild-type CD33(IgC1) and mutants, Ramachandran plots of CD33(IgV-IgC1), superposition of the first human Siglec IgC domains, structural ensemble of the CD33 TM domain, concentration dependence of CD33 TM spectral parameters, AlphaFold2 model of the human CD33 TM domain, AlphaFold3 models of homodimeric and -trimeric CD33 TM domains, POTENCI secondary 13Cα chemical shifts of the TM-CS domains, and superposition of free and ligand-bound CD33 IgV-IgC1 domains. Tables of secondary structure content of CD33(IgC1) and structural statistics for the human CD33 TM domain structure.
The authors declare no competing financial interest.
References
- (1).Masters CL; Bateman R; Blennow K; Rowe CC; Sperling RA; Cummings JL Alzheimer’s disease, Nat. Rev. Dis. Primers 2015, 1, 15056. [DOI] [PubMed] [Google Scholar]
- (2).Mucke L Alzheimer’s disease, Nature 2009, 461, 895–897. [DOI] [PubMed] [Google Scholar]
- (3).Frigerio CS; Wolfs L; Fattorelli N; Thrupp N; Voytyukt I; Schmidt I; Mancuso R; Chen WT; Woodbury ME; Srivastava G; Moller T; Hudry E; Das S; Saido T; Karran E; Hyman B; Perry VH; Fiers M; De Strooper B The Major Risk Factors for Alzheimer’s Disease: Age, Sex, and Genes Modulate the Microglia Response to A beta Plaques, Cell Reports 2019, 27, 1293-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).van Dyck CH; Swanson CJ; Aisen P; Bateman RJ; Chen C; Gee M; Kanekiyo M; Li D; Reyderman L; Cohen S; Froelich L; Katayama S; Sabbagh M; Vellas B; Watson D; Dhadda S; Irizarry M; Kramer LD; Iwatsubo T Lecanemab in Early Alzheimer’s Disease, N. Engl. J. Med 2022, 388, 9–21. [DOI] [PubMed] [Google Scholar]
- (5).Griciuc A; Tanzi RE The role of innate immune genes in Alzheimer’s disease, Curr. Opin. Neurol 2021, 34, 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Crocker PR; Paulson JC; Varki A Siglecs and their roles in the immune system, Nat. Rev. Immunol 2007, 7, 255–266. [DOI] [PubMed] [Google Scholar]
- (7).Angata T; Varki A Discovery, classification, evolution and diversity of Siglecs, Mol. Asp. Med 2023, 90, 101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Murugesan G; Weigle B; Crocker PR Siglec and anti-Siglec therapies, Curr. Opin. Chem. Biol 2021, 62, 34–42. [DOI] [PubMed] [Google Scholar]
- (9).Bull C; Nason R; Sun L; Van Coillie J; Sorensen DM; Moons SJ; Yang Z; Arbitman S; Fernandes SM; Furukawa S; McBride R; Nycholat CM; Adema GJ; Paulson JC; Schnaar RL; Boltje TJ; Clausen H; Narimatsu Y Probing the binding specificities of human Siglecs by cell-based glycan arrays, Proc. Natl. Acad. Sci. U. S. A 2021, 118, e2026102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Gonzalez-Gil A; Porell RN; Fernandes SM; Maenpaa E; Li TA; Li T; Wong PC; Aoki K; Tiemeyer M; Yu ZJ; Orsburn BC; Bumpus NN; Matthews RT; Schnaar RL Human brain sialoglycan ligand for CD33, a microglial inhibitory Siglec implicated in Alzheimer’s disease, J. Biol. Chem 2022, 298, 101960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Jen EY; Ko CW; Lee JE; Del Valle PL; Aydanian A; Jewell C; Norsworthy KJ; Przepiorka D; Nie L; Liu J; Sheth CM; Shapiro M; Farrell AT; Pazdur R FDA Approval: Gemtuzumab Ozogamicin for the Treatment of Adults with Newly Diagnosed CD33-Positive Acute Myeloid Leukemia, Clin. Cancer Res 2018, 24, 3242–3246. [DOI] [PubMed] [Google Scholar]
- (12).Perez-Oliva AB; Martinez-Esparza M; Vicente-Fernandez JJ; Miguel RCS; Garcia-Penarrubia P; Hernandez-Caselles T Epitope mapping, expression and post-translational modifications of two isoforms of CD33 (CD33M and CD33m) on lymphoid and myeloid human cells, Glycobiology 2011, 21, 757–770. [DOI] [PubMed] [Google Scholar]
- (13).Paul SP; Taylor LS; Stansbury EK; McVicar DW Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2, Blood 2000, 96, 483–490. [PubMed] [Google Scholar]
- (14).Bocharov EV; Mayzel ML; Volynsky PE; Goncharuk MV; Ermolyuk YS; Schulga AA; Artemenko EO; Efremov RG; Arseniev AS Spatial Structure and pH-dependent Conformational Diversity of Dimeric Transmembrane Domain of the Receptor Tyrosine Kinase EphA1, J. Biol. Chem 2008, 283, 29385–29395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lau T-L; Kim C; Ginsberg MH; Ulmer TS The structure of the integrin alphaIIbbeta3 transmembrane complex explains integrin transmembrane signalling, EMBO J. 2009, 28, 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Arkhipov A; Shan YB; Das R; Endres NF; Eastwood MP; Wemmer DE; Kuriyan J; Shaw DE Architecture and Membrane Interactions of the EGF Receptor, Cell 2013, 152, 557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Call ME; Wucherpfennig KW; Chou JJ The structural basis for intramembrane assembly of an activating immunoreceptor complex, Nat. Immunol 2010, 11, 1023–U1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Sahoo AR; Buck M Structural and Functional Insights into the Transmembrane Domain Association of Eph Receptors, Int. J. Mol. Sci 2021, 22, 8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Cornish AL; Freeman S; Forbes G; Ni J; Zhang M; Cepeda M; Gentz R; Augustus M; Carter KC; Crocker PR Characterization of siglec-5, a novel glycoprotein expressed on myeloid cells related to CD33, Blood 1998, 92, 2123–2132. [PubMed] [Google Scholar]
- (20).Floyd H; Ni J; Cornish AL; Zeng ZZ; Liu D; Carter KC; Steel J; Crocker PR Siglec-8 - A novel eosinophil-specific member of the immunoglobulin superfamily, J. Biol. Chem 2000, 275, 861–866. [DOI] [PubMed] [Google Scholar]
- (21).Siddiqui S; Schwarz F; Springer S; Khedri Z; Yu H; Deng LQ; Verhagen A; Naito-Matsui Y; Jiang WP; Kim D; Zhou J; Ding BB; Chen X; Varki N; Varki A Studies on the Detection, Expression, Glycosylation, Dimerization, and Ligand Binding Properties of Mouse Siglec-E, J. Biol. Chem 2017, 292, 1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Yu ZB; Maoui M; Wu LT; Banville D; Shen SH mSiglec-E, a novel mouse CD33-related siglec (sialic acid-binding immunoglobulin-like lectin) that recruits Src homology 2 (SH2)-domain-containing protein tyrosine phosphatases SHP-1 and SHP-2, Biochem. J 2001, 353, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Pronker MF; Lemstra S; Snijder J; Heck AJR; Thies-Weesie DME; Pasterkamp RJ; Janssen BJC Structural basis of myelin-associated glycoprotein adhesion and signalling, Nat. Commun 2016, 7, 13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Dodd RB; Meadows W; Qamar S; Johnson CM; Kronenberg-Versteeg D; St George-Hyslop P Structure of the immune receptor CD33, 2017, 10.2210/pdb2215IHB/pdb. [DOI] [Google Scholar]
- (25).Miles LA; Hermans SJ; Crespi GAN; Gooi JH; Doughty L; Nero TL; Markulic J; Ebneth A; Wroblowski B; Oehlrich D; Trabanco AA; Rives ML; Royaux I; Hancock NC; Parker MW Small Molecule Binding to Alzheimer Risk Factor CD33 Promotes A beta Phagocytosis, iScience 2019, 19, 110-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Williams AF; Barclay AN The Immunoglobulin Superfamily - Domains For Cell-Surface Recognition, Annu. Rev. Immunol 1988, 6, 381–405. [DOI] [PubMed] [Google Scholar]
- (27).Williams AF A Year In The Life Of The Immunoglobulin Superfamily, Immunol. Today 1987, 8, 298-&. [DOI] [PubMed] [Google Scholar]
- (28).Clamp M; Cuff J; Searle SM; Barton GJ The Jalview Java alignment editor, Bioinformatics 2004, 20, 426–427. [DOI] [PubMed] [Google Scholar]
- (29).Yohannan S; Faham S; Yang D; Whitelegge JP; Bowie JU The evolution of transmembrane helix kinks and the structural diversity of G protein-coupled receptors, Proc. Natl. Acad. Sci. U. S. A 2004, 101, 959–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Zhuravleva MA; Trandem K; Sun PD Structural implications of Siglec-5-mediated sialoglycan recognition, J. Mol. Biol 2008, 375, 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Ereno-Orbea J; Sicard T; Cui H; Mazhab-Jafari MT; Benlekbir S; Guarne A; Rubinstein JL; Julien JP Molecular basis of human CD22 function and therapeutic targeting, Nat. Commun 2017, 8, 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Sanders CR; Schwonek JP Characterization Of Magnetically Orientable Bilayers In Mixtures Of Dihexanoylphosphatidylcholine And Dimyristoylphosphatidylcholine By Solid-State Nmr, Biochemistry 1992, 31, 8898–8905. [DOI] [PubMed] [Google Scholar]
- (33).Vonheijne G Membrane-Protein Structure Prediction - Hydrophobicity Analysis And The Positive-Inside Rule, J. Mol. Biol 1992, 225, 487–494. [DOI] [PubMed] [Google Scholar]
- (34).Baker NA; Sept D; Joseph S; Holst MJ; McCammon JA Electrostatics of nanosystems: Application to microtubules and the ribosome, Proc. Natl. Acad. Sci. U. S. A 2001, 98, 10037–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Suk JE; Situ AJ; Ulmer TS Construction of Covalent Membrane Protein Complexes and High-throughput Selection of Membrane Mimics, J. Am. Chem. Soc 2012, 134, 9030–9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Situ AJ; Schmidt T; Mazumder P; Ulmer TS Characterization of Membrane Protein Interactions by Isothermal Titration Calorimetry, J. Mol. Biol 2014, 426, 3670–3680. [DOI] [PubMed] [Google Scholar]
- (37).Moore DT; Berger BW; DeGrado WF Protein-protein interactions in the membrane: Sequence, structural, and biological motifs, Structure 2008, 16, 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Fleming KG; Engelman DM Specificity in transmembrane helix-helix interactions can define a hierarchy of stability for sequence variants, Proc. Natl. Acad. Sci. U. S. A 2001, 98, 14340–14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Lau T-L; Dua V; Ulmer TS Structure of the integrin alphaIIb transmembrane segment, J. Biol. Chem 2008, 283, 16162–16168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Angata T; Hayakawa T; Yamanaka M; Varki A; Nakamura M Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates, Faseb J. 2006, 20, 1964–1973. [DOI] [PubMed] [Google Scholar]
- (41).Garcia-Alvarez B; de Pereda JM; Calderwood DA; Ulmer TS; Critchley D; Campbell ID; Ginsberg MH; Liddington RC Structural determinants of integrin recognition by Talin, Mol. Cell 2003. 11 49–58. [DOI] [PubMed] [Google Scholar]
- (42).Walter RB; Raden BW; Zeng R; Hausermann P; Bernstein ID; Cooper JA ITIM-dependent endocytosis of CD33-related Siglecs: role of intracellular domain, tyrosine phosphorylation, and the tyrosine phosphatases, Shp1 and Shp2, J. Leukoc. Biol 2008, 83, 200–211. [DOI] [PubMed] [Google Scholar]
- (43).Liu YL; Lau J; Li WG; Tempel W; Li L; Dong AP; Narula A; Qin S; Min JR Structural basis for the regulatory role of the PPxY motifs in the thioredoxin-interacting protein TXNIP, Biochem. J 2016, 473, 179–187. [DOI] [PubMed] [Google Scholar]
- (44).Spera S; Bax A Empirical Correlation Between Protein Backbone Conformation And C-Alpha And C-Beta C-13 Nuclear-Magnetic-Resonance Chemical-Shifts, J. Am. Chem. Soc 1991, 113, 5490–5492. [Google Scholar]
- (45).Wishart DS; Case DA (2001) Use of chemical shifts in macromolecular structure determination, In Nuclear Magnetic Resonance Of Biological Macromolecules, Pt A (Thomas L. James VD., Uli Schmitz, Ed.), pp 3–34, Academic Press, Waltham, Massachusetts. [DOI] [PubMed] [Google Scholar]
- (46).Schimmel PR; Flory PJ Conformational Energies And Configurational Statistics Of Copolypeptides Containing L-Proline, J. Mol. Biol 1968, 34, 105–120. [DOI] [PubMed] [Google Scholar]
- (47).Nielsen JT; Mulder FAA POTENCI: prediction of temperature, neighbor and pH-corrected chemical shifts for intrinsically disordered proteins, J. Biomol. NMR 2018, 70, 141–165. [DOI] [PubMed] [Google Scholar]
- (48).Xiao Q; McAtee CK; Su XL Phase separation in immune signalling, Nat. Rev. Immunol 2022, 22, 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Wen L; Moser M; Ley K Molecular mechanisms of leukocyte beta2 integrin activation, Blood 2022, 139, 3480–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Bornhofft KF; Goldammer T; Rebl A; Galuska SP Siglecs: A journey through the evolution of sialic acid-binding immunoglobulin-type lectins, Dev. Comp. Immunol 2018, 86, 219–231. [DOI] [PubMed] [Google Scholar]
- (51).Wisnovsky S; Mackl L; Malaker SA; Pedram K; Hess GT; Riley NM; Gray MA; Smith BAH; Bassik MC; Moerner WE; Bertozzi CR Genome-wide CRISPR screens reveal a specific ligand for the glycan-binding immune checkpoint receptor Siglec-7, Proc. Natl. Acad. Sci. U. S. A 2021, 118, e2015024118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Ramya TNC; Weerapana E; Liao LJ; Zeng Y; Tateno H; Liao LA; Yates JR; Cravatt BF; Paulson JC In Situ trans Ligands of CD22 Identified by Glycan-Protein Photocross-linking-enabled Proteomics, Mol. Cell. Proteomics 2010, 9, 1339–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).O’Reilly MK; Paulson JC (2010) Multivalent Ligands For Siglecs, In Methods In Enzymology, Vol 478: Glycomics, pp 343–363, Elsevier Academic Press Inc, San Diego. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Dodd RB; Meadows W; Qamar S; Johnson CM; Kronenberg-Versteeg D; St George-Hyslop P Structure of the immune receptor CD33 in complex with 3’-sialyllactose, 2017, 10.2210/pdb2215J2206/pdb. [DOI] [Google Scholar]
- (55).Dodd RB; Meadows W; Qamar S; Johnson CM; Kronenberg-Versteeg D; St George-Hyslop P Structure of the immune receptor CD33 in complex with 6’-sialyllactose, 2017, 10.2210/pdb2215J2210B/pdb. [DOI] [Google Scholar]
- (56).Schmidt T; Ye F; Situ AJ; An W; Ginsberg MH; Ulmer TS A Conserved Ectodomain-Transmembrane Domain Linker Motif Tunes the Allosteric Regulation of Cell Surface Receptors, J. Biol. Chem 2016, 291, 17536–17546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Ando M; Shoji T; Tu WJ; Higuchi H; Nishijima K; Iijima S Lectin-dependent localization of cell surface sialic acid-binding lectin Siglec-9, Cytotechnology 2015, 67, 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Robbins PW; Trimble RB; Wirth DF; Hering C; Maley F; Maley GF; Das R; Gibson BW; Royal N; Biemann K Primary Structure Of The Streptomyces Enzyme “Endo-Beta-N-Acetylglucosaminidase-H, J. Biol. Chem 1984, 259, 7577–7583. [PubMed] [Google Scholar]
- (59).Gill SC; Vonhippel PH Calculation Of Protein Extinction Coefficients From Amino-Acid Sequence Data, Anal. Biochem 1989, 182, 319–326. [DOI] [PubMed] [Google Scholar]
- (60).Provencher SW; Glockner J Estimation Of Globular Protein Secondary Structure From Circular-Dichroism, Biochemistry 1981, 20, 33–37. [DOI] [PubMed] [Google Scholar]
- (61).Whitmore L; Wallace BA DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data, Nucleic Acids Res. 2004, 32, W668–W673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Lobley A; Whitmore L; Wallace BA DICHROWEB: an interactive website for the analysis of protein secondary structure from circular dichroism spectra, Bioinformatics 2002, 18, 211–212. [DOI] [PubMed] [Google Scholar]
- (63).Ulmer TS; Ramirez BE; Delaglio F; Bax A Evaluation of backbone proton positions and dynamics in a small protein by liquid crystal NMR spectroscopy, J. Am. Chem. Soc 2003, 125, 9179–9191. [DOI] [PubMed] [Google Scholar]
- (64).Pervushin K; Riek R; Wider G; Wuthrich K Attenuated T-2 relaxation by mutual cancellation of dipole- dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution, Proc. Natl. Acad. Sci. U. S. A 1997, 94, 12366–12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Hu JS; Grzesiek S; Bax A Two-dimensional NMR methods for determining (chi 1) angles of aromatic residues in proteins from three-bond J(C’C gamma) and J(NC gamma) couplings, J. Am. Chem. Soc 1997, 119, 1803–1804. [Google Scholar]
- (66).Kontaxis G; Clore GM; Bax A Evaluation of cross-correlation effects and measurement of one-bond couplings in proteins with short transverse relaxation times, J. Magn. Reson 2000, 143, 184–196. [DOI] [PubMed] [Google Scholar]
- (67).Jaroniec CP; Ulmer TS; Bax A Quantitative J correlation methods for the accurate measurement of 13C[prime]-13C[agr] dipolar couplings in proteins, J. Biomol. NMR 2004, 30, 181–194. [DOI] [PubMed] [Google Scholar]
- (68).Chou JJ; Delaglio F; Bax A Measurement of one-bond N-15-C-13 ‘ dipolar couplings in medium sized proteins, J. Biomol. NMR 2000, 18, 101–105. [DOI] [PubMed] [Google Scholar]
- (69).Fitzkee NC; Bax A Facile measurement of H-1-N-15 residual dipolar couplings in larger perdeuterated proteins, J. Biomol. NMR 2010, 48, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Schwieters CD; Kuszewski JJ; Tjandra N; Clore GM The Xplor-NIH NMR molecular structure determination package, J. Magn. Reson 2003, 160, 65–73. [DOI] [PubMed] [Google Scholar]
- (71).Shen Y; Bax A (2015) Protein Structural Information Derived from NMR Chemical Shift with the Neural Network Program TALOS-N, In Artificial Neural Networks, 2nd Edition, pp 17–32, Humana Press Inc, Totowa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Grishaev A; Bax A An empirical backbone-backbone hydrogen-bonding potential in proteins and its applications to NMR structure refinement and validation, J. Am. Chem. Soc 2004, 126, 7281–7292. [DOI] [PubMed] [Google Scholar]
- (73).Kuszewski J; Gronenborn AM; Clore GM Improvements and extensions in the conformational database potential for the refinement of NMR and X-ray structures of proteins and nucleic acids, J. Magn. Reson 1997, 125, 171–177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


