Abstract
Bartonella endocarditis is a disease of emerging importance that causes serious complications and high rates of mortality. Due to the fastidious nature of Bartonella species and their high degrees of antibiotic susceptibility, cultures of clinical samples most often remain sterile and valvular biopsy specimens, the best specimens for PCR amplification, are seldom available. Therefore, serology appears to be the easiest diagnostic tool. In order to determine the best cutoff value for serology and its predictive values for the detection of Bartonella endocarditis, we studied 48 patients with culture- and/or PCR-confirmed Bartonella endocarditis. We also applied these serological criteria to 156 patients with blood culture-negative endocarditis. Furthermore, we compared the kinetics of the antibody responses to Bartonella spp. in order to estimate the value of serology for prediction of the occurrence of relapses. A titer of ≥1:800 for immunoglobulin G antibodies to either Bartonella henselae or B. quintana has a positive predictive value of 0.810 for the detection of chronic Bartonella infections in the general population and a value of 0.955 for the detection of Bartonella infections among patients with endocarditis. When this cutoff was applied to 156 patients with blood culture-negative endocarditis, we were able to diagnose Bartonella infections in an additional 45 patients with definite endocarditis for whom a positive Bartonella serology was the only evidence of infection. On follow-up, the kinetics of the decrease in antibody titers were significantly delayed in two patients with relapses. In conclusion, we recommend the determination of antibodies to both B. quintana and B. henselae and the use of a cutoff value of 1:800 for the diagnosis of Bartonella endocarditis. We propose that this criterion, which may also help with the detection of late relapses, be included as a major criterion in the Duke criteria for the diagnosis of infective endocarditis.
Four Bartonella species (primarily Bartonella quintana and B. henselae and, in one case each, B. elizabethae [5] and B. vinsonii subsp. berkhoffii [27]) have been implicated as causes of endocarditis in humans. Bartonella endocarditis most often occurs in middle-aged patients. Clinical diagnosis relies on a combination of epidemiological and clinical features such as male sex, homelessness, chronic alcoholism, contact with body lice and the absence of a previously known valvulopathy for B. quintana, and contact with cats or cat fleas and the presence of a previously known valvulopathy for B. henselae (10). B. henselae also causes other chronic infections such as chronic cat scratch disease, peliosis hepatis, and bacillary angiomatosis; and B. quintana causes chronic bacteremia and bacillary angiomatosis (20).
The most widely used method for the laboratory diagnosis of Bartonella infections is serology, with indirect immunofluorescence being the reference technique (26). In our laboratory, two cutoff values are used for the diagnosis of Bartonella infections. A titer of immunoglobulin G (IgG) antibodies to B. henselae of ≥1:50 is diagnostic of acute infections such as cat scratch disease with an evolution of less than 3 months (26). For the diagnosis of endocarditis, we use a titer of ≥1:1,600 for IgG antibodies to either B. quintana or B. henselae. Although a definite diagnosis of Bartonella endocarditis relies on culture, PCR, or immunohistochemical analysis of valvular biopsy specimens, we have previously demonstrated that such an antibody titer has a positive predictive value of 0.884 (25). However, this cutoff value had been determined with samples from a small number of patients with culture- and/or PCR-proven cases of Bartonella endocarditis (25). Moreover, the specificity of the serological method has been questioned due to cross-reactions among Bartonella species and between Bartonella species and Coxiella burnetii (16) and Chlamydia species (21). In a study described in a previous article (25), we tested sera from 11 patients with a previous diagnosis of Chlamydia endocarditis on the basis of serological data (9, 19). Adsorption procedures indicated that the reactive antibodies detected were most likely directed against Bartonella spp. and the existence of Chlamydia endocarditis should be questioned. To date, we have collected data for 38 patients with confirmed B. quintana endocarditis and 10 patients with B. henselae endocarditis.
The purpose of the study described in this report was (i) to determine the best serological cutoff value to be used for the diagnosis of Bartonella endocarditis, (ii) to detect additional cases of Bartonella endocarditis among patients with blood culture-negative endocarditis by use of this cutoff value, and (iii) to estimate the usefulness of serology for the prediction of relapses of Bartonella endocarditis by comparing patients who relapsed with those who recovered.
MATERIALS AND METHODS
Study design. (i) Case definition.
Patients were considered to have definite endocarditis on the basis of the criteria of Duke for the diagnosis of infective endocarditis (8). Patients were considered to be suffering from chronic cat scratch disease when the clinical evolution of a typical case of cat scratch disease was longer than 3 months or when visceral involvement was diagnosed. A diagnosis of chronic bacteremia was considered in patients who suffered from persistent bacteremia, as proven by positive blood cultures over a period of several weeks, without any echocardiographic indication of endocarditis.
Patients were considered to be suffering from Bartonella infection following direct identification of B. henselae or B. quintana by culture, PCR, or immunohistochemical analysis of valvular tissue or lymph node biopsy specimens or by culture or PCR of blood specimens. In addition, patients with cat scratch disease who were positive for Bartonella by serology and granulomatous lymphadenitis by histological examination of a lymph node biopsy specimen were also considered to have Bartonella infection.
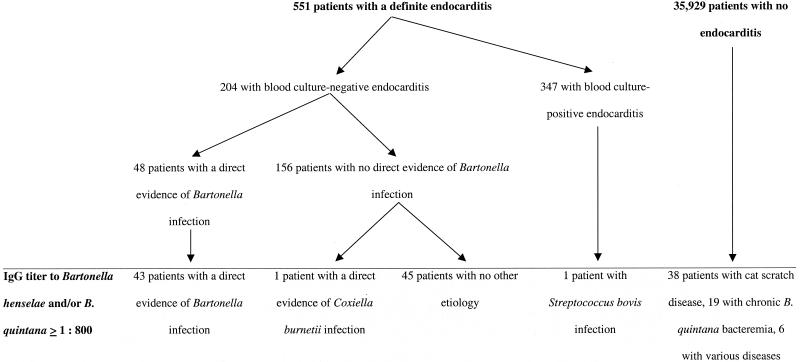
(ii) Patients.
The Unité des Rickettsies is a reference center for the diagnosis and study of rickettsioses and collected data for patients with blood culture-negative endocarditis between January 1995 and December 2000. During that period, we tested 45,000 serum specimens for anti-Bartonella antibodies. The serum specimens were obtained from 36,480 patients suspected of having various diseases including arthropod-transmitted infections, pneumonia, endocarditis, pericarditis, myocarditis, meningoencephalitis, or prolonged fever of unknown etiology. For patients for whom several specimens were available, we used the sample with the highest antibody titers. Of these 36,480 patients, 551 had endocarditis, including 204 patients with blood culture-negative endocarditis (Fig. 1). When available, blood or heart valve tissue from these patients was also sent to our laboratory. Systematic testing included Bartonella serology and PCR amplification and culture of valve and blood specimens. Among the 204 patients with blood culture-negative endocarditis, 48 patients had a diagnosis of Bartonella endocarditis on the basis of microbiological and/or PCR findings. Of these, 10 were infected with B. henselae and 38 were infected with B. quintana. On follow-up, the physicians in charge of these 48 patients were asked for serum specimens every 3 months for 1 year following the diagnosis. The treatment and outcome for each patient in the year following the diagnosis of culture-negative endocarditis were noted. Of the 48 patients, 46 were considered clinically and microbiologically cured following therapy, but 2 were considered to have relapses. Among the 156 patients with blood culture-negative endocarditis for whom no direct evidence of infection with a Bartonella sp. was obtained, data for 45 patients that we considered to have Bartonella endocarditis on the basis of elevated titers of antibodies to B. quintana and/or B. henselae were excluded from the determination of cutoff values.
FIG. 1.
Distribution of 36,480 patients whose serum samples were tested by the Unité des Rickettsies between January 1995 and December 2000 for antibodies to B. henselae or B. quintana by the MIF assay.
(iii) Serology.
B. quintana Oklahoma, B. quintana Marseille (isolated from a French patient from the present series), B. henselae Houston-1 (ATCC 49882T; American Type Culture Collection), and B. henselae Marseille (6) were used as antigens. The bacteria, grown in human endothelial cells (ECV 304 cells), were harvested, pelleted, and used as crude antigen in a microimmunofluorescence (MIF) assay (4, 26). The pellets were then resuspended in sterile distilled water so that each suspension had the same density of organisms, as determined optically following Gimenez staining. These antigens were applied with a drawing pen at opposing poles of each well on 30-well microscope slides (Dynatech Laboratories Ltd., Billingshurst, United Kingdom), air dried, and fixed in acetone for 10 min. Twofold serial dilutions of the sera (from 1:50 to 1:3,200) were made in phosphate-buffered saline with 3% nonfat powdered milk and applied to the antigens, and the mixtures were incubated in a moist chamber for 30 min at 37°C. After three washes in phosphate-buffered saline for 10 min for each wash, the slides were air dried and the reactive antibodies were detected with 1:300 dilutions of fluorescein isothiocyanate-conjugated goat anti-human IgG (γ chain; Fluoline G; BioMerieux, Marcy l'Etoile, France) by using the incubation times and the washing procedure described above. The slides were mounted in buffered glycerol (Fluoprep; BioMerieux) and read with a Zeiss fluorescence microscope at ×400 magnification.
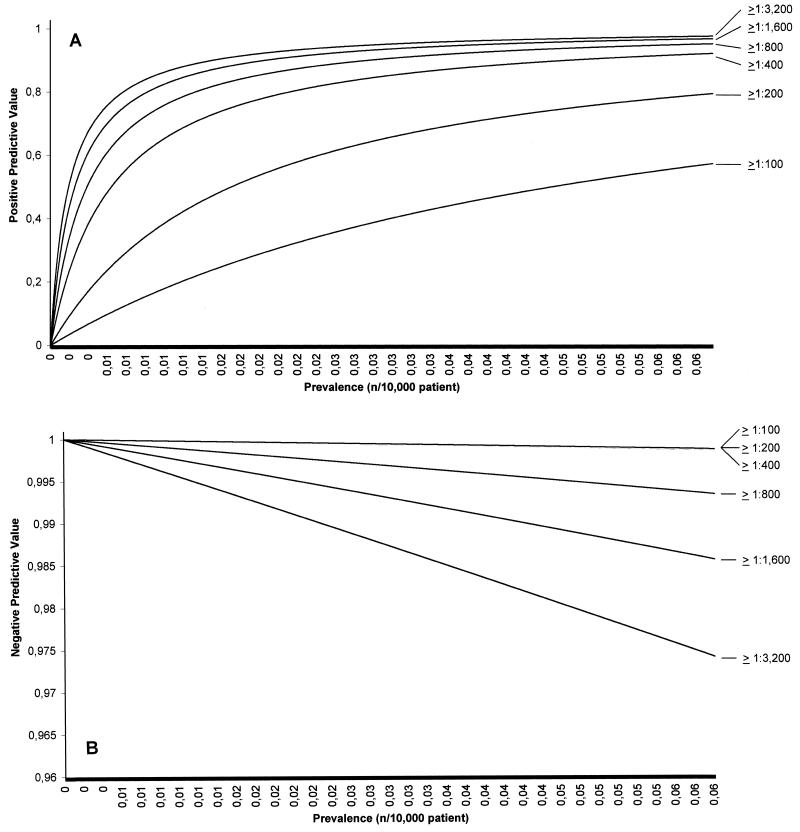
In order to determine the best cutoff value for the diagnosis of Bartonella endocarditis, the sensitivity, specificity, and positive and negative predictive values of titers of 1:100, 1:200, 1:400, 1:800, 1:1,600, and ≥ 1:3,200 for antibodies to B. henselae, B. quintana, and either species were compared for the whole population of 36,480 patients. The predictive values of the various cutoffs studied were evaluated in relationship to the expected prevalence of the disease among patients with endocarditis by use of Bayes's theorem, as follows: positive predictive value = (SE × PR)/[(SE × PR) + (1 − SPE) × (1 − PR)] and negative predictive value = [SPE × (1 − PR)]/[SPE × (1 − PR) + (1 − SE) × PR], where PR is the prevalence of the disease in the population concerned, SE is the sensitivity, and SPE is the specificity (Fig. 2). We excluded from this calculation the data for the 45 patients with a positive serology as sole evidence of Bartonella infection, as in this preliminary study correct interpretation of the results was not possible.
FIG. 2.
Evaluation of the positive (A) and negative (B) predictive values of the various cutoffs in titers of antibodies to Bartonella spp. for the detection of Bartonella endocarditis, tested by comparison of the predictive value of each titer in relationship to the expected prevalence of the disease by use of Bayes's theorem.
These serological criteria were then applied to 156 patients with blood culture-negative endocarditis with no etiological diagnosis.
Graphs and statistical analysis.
Bayesian curves and slopes of linear regression curves of the antibody titers were obtained by use of Microsoft Excel (version 7) software. The slopes of the linear regression curves of antibody titers were compared as variances by Student's t test with Epi info (version 6.0) software, which was also used to compare means. The observed differences were considered significant when the P value was <0.05 by two-tailed tests.
RESULTS
The data used for the diagnosis of Bartonella endocarditis in the 48 patients with Bartonella-proven endocarditis are presented in Table 1.
TABLE 1.
Serology, culture, and DNA amplification results for 48 patients with Bartonella-induced endocarditis
| Patient (reference) | IgG titer by MIF assay
|
Results of a
|
Proven diagnosis | ||||
|---|---|---|---|---|---|---|---|
| B. henselae | B. quintana | Specific blood culture | PCR with fresh blood | Valvular culture | PCR with valve tissue | ||
| 1 (25) | 3,200 | 400 | ND | − | − | B. henselae | B. henselae |
| 2 (25) | 1,600 | 800 | − | ND | − | B. henselae | B. henselae |
| 3 (25) | 12,800 | 400 | − | − | B. henselae | B. henselae | B. henselae |
| 4 (25) | 0 | 0 | − | − | − | B. henselae | B. henselae |
| 5 (6) | 6,400 | 1,600 | B. henselae | B. henselae | ND | ND | B. henselae |
| 6 | 3,200 | 3,200 | − | − | B. henselae | B. henselae | B. henselae |
| 7 | 1,600 | 200 | ND | ND | − | B. henselae | B. henselae |
| 8 | 3,200 | 1,600 | − | ND | − | B. henselae | B. henselae |
| 9 | 1,600 | 800 | ND | ND | ND | B. henselae | B. henselae |
| 10 | 3,200 | 1,600 | B. henselae | B. henselae | ND | ND | B. henselae |
| 11 (25) | 12,800 | 51,200 | − | − | − | B. quintana | B. quintana |
| 12 (25) | 3,200 | 3,200 | − | − | − | B. quintana | B. quintana |
| 13 (25) | 51,200 | 51,200 | B. quintana | B. quintana | ND | ND | B. quintana |
| 14 (25) | 51,200 | 6,400 | − | − | B. quintana | B. quintana | B. quintana |
| 15 (7) | 3,200 | 3,200 | B. quintana | B. quintana | − | B. quintana | B. quintana |
| 16 (7) | 3,200 | 1,600 | B. quintana | B. quintana | − | B. quintana | B. quintana |
| 17 (7) | 3,200 | 3,200 | B. quintana | B. quintana | − | B. quintana | B. quintana |
| 18 (18) | 12,800 | 800 | − | B. quintana | B. quintana | B. quintana | B. quintana |
| 19 | 3,200 | 12,800 | B. quintana | B. quintana | B. quintana | B. quintana | B. quintana |
| 20 | 400 | 50 | ND | ND | B. quintana | B. quintana | B. quintana |
| 21 | 800 | 1,600 | ND | ND | B. quintana | B. quintana | B. quintana |
| 22 | 200 | 400 | B. quintana | ND | − | B. quintana | B. quintana |
| 23 | 200 | 12,800 | − | − | − | B. quintana | B. quintana |
| 24 | 1,600 | 800 | − | B. quintana | B. quintana | B. quintana | B. quintana |
| 25 | 6,400 | 3,200 | ND | ND | B. quintana | B. quintana | B. quintana |
| 26 | 3,200 | 1,600 | ND | ND | B. quintana | B. quintana | B. quintana |
| 27 | 6,400 | 800 | − | ND | − | B. quintana | B. quintana |
| 28 | 3,200 | 1,600 | − | ND | B. quintana | B. quintana | B. quintana |
| 29 | 1,600 | 1,600 | − | ND | B. quintana | B. quintana | B. quintana |
| 30 | 3,200 | 3,200 | − | ND | − | B. quintana | B. quintana |
| 31 | 3,200 | 1,600 | ND | ND | ND | B. quintana | B. quintana |
| 32 | 800 | 400 | − | − | − | B. quintana | B. quintana |
| 33 | 800 | 1,600 | − | − | B. quintana | − | B. quintana |
| 34 | 400 | 400 | − | − | B. quintana | B. quintana | B. quintana |
| 35 | 400 | 800 | ND | ND | B. quintana | B. quintana | B. quintana |
| 36 | 400 | 400 | − | ND | − | B. quintana | B. quintana |
| 37 | 1,600 | 3,200 | − | ND | − | B. quintana | B. quintana |
| 38 | 1,600 | 800 | − | ND | − | B. quintana | B. quintana |
| 39 | 1,600 | 1,600 | − | ND | B. quintana | B. quintana | B. quintana |
| 40 | 3,200 | 1,600 | B. quintana | B. quintana | B. quintana | B. quintana | B. quintana |
| 41 | 3,200 | 1,600 | − | ND | − | B. quintana | B. quintana |
| 42 | 1,600 | 3,200 | ND | ND | ND | B. quintana | B. quintana |
| 43 | 3,200 | 1,600 | ND | ND | ND | B. quintana | B. quintana |
| 44 | 3,200 | 3,200 | ND | ND | ND | B. quintana | B. quintana |
| 45 | 200 | 800 | − | ND | ND | B. quintana | B. quintana |
| 46 | 200 | 800 | − | ND | ND | B. quintana | B. quintana |
| 47 | 200 | 800 | − | ND | B. quintana | B. quintana | B. quintana |
| 48 | 200 | 800 | − | ND | ND | B. quintana | B. quintana |
+, positive; −, negative; ND, not determined.
Serology results.
None of the 48 patients had IgM antibodies reactive against Bartonella by MIF tests. IgG titers ranged from 0 to 1:51,200. Cross-reactions between the Bartonella species tested were observed for all patients with a positive result by serology. Of the 10 patients with B. henselae endocarditis, 8 (80%) had titers of IgG antibodies to B. henselae greater than those to B. quintana, 1 had equal titers of antibodies to both antigens, and 1 had negative results by serological tests with the three antigens tested. In all nine patients with positive serological test results, the titers of antibodies to both B. henselae strains tested were the same. Among the 38 patients with B. quintana endocarditis, 12 (31.6%) had titers of antibodies to B. quintana greater than those to B. henselae, 11 (28.9%) had equal titers of antibodies to B. quintana and B. henselae, and 15 (39.5%) had lower titers of IgG antibodies to B. quintana than to B. henselae. When the B. henselae and B. quintana antigens were studied separately, titers of ≥1:800 for antibodies to B. henselae were observed in 9 of 10 patients (90%) with B. henselae endocarditis but only 29 of 38 patients (76.3%) infected with B. quintana. Titers of ≥1:800 for antibodies to B. quintana were observed in 33 of 38 patients (86.8%) with B. quintana endocarditis and 6 of 10 patients (60%) infected with B. henselae. The specificity ranged from 0.955 to 0.999 for sera with titers of ≥1:100 and ≥1:3,200, respectively. The negative predictive value for the diagnosis of endocarditis was 0.999 for all titers tested. Titers less than 1:800 had a positive predictive value of <0.398 for the diagnosis of endocarditis, whereas titers ≥1:3,200 had a sensitivity of only 0.583. The positive and negative predictive values of all titers tested are presented in Fig. 2. The 1:800 dilution cutoff had a lower positive predictive value than the 1:1,600 dilution cutoff (0.398 versus 0.672) but a higher sensitivity (0.895 versus 0.771). Therefore, the 1:800 dilution was considered the best cutoff and was adopted in our study. The determination of titers of antibodies to B. henselae and B. quintana had similar sensitivities, specificities, and negative predictive values, but the positive predictive value was lower for antibodies to B. henselae (range, 0.005 and 0.206 for titers of ≥1:100 and ≥1;3,200, respectively) and higher for antibodies to B. quintana (range, 0.042 to 0.765).
The distributions of the 36,480 patients tested with regard to their Bartonella serologies are presented in Fig. 1. Among these 36,480 patients, 153 had titers of antibodies to a Bartonella species of 1:800 or more. Of these, 90 had endocarditis on the basis of the Duke endocarditis service criteria (8). Among these 90 patients, 43 had endocarditis proved to be caused by Bartonella, 45 had positive Bartonella serologies as the main evidence of infection and were classified as definitely having endocarditis, 1 had a documented C. burnetii infection and the positive serology was considered a cross-reaction (16), and 1 was infected with Streptococcus bovis. As none of the other patients from our series positive for endocarditis by blood culture exhibited a positive result for Bartonella by serological testing, the last patient described above may have had a dual infection. Sixty-three additional patients with titers of IgG antibodies to a Bartonella sp. ≥1:800 did not have endocarditis, 38 had cat scratch disease (including 17 with chronic infections), 19 were homeless and suffered from chronic B. quintana bacteremia, 1 presented with unexplained prolonged fever, 1 had unexplained pericarditis, 1 was diagnosed with a primary cytomegalovirus infection, 1 had legionellosis, 1 had a lymphoma, and 1 had leptospirosis. Altogether, a cutoff of ≥1:800 had a positive predictive value of 0.810 for the diagnosis of chronic B. henselae or B. quintana infections.
When the data for the 45 patients who we considered to be infected with a Bartonella sp. on the basis of a positive serological test result are discounted, among the 551 patients from our series with endocarditis, the cutoff value of 1:800 had a positive predictive value of 0.955 for the detection of Bartonella endocarditis, a negative predictive value of 0.990, a sensitivity of 0.895, and a specificity of 0.996.
When these diagnostic criteria were applied to 156 patients who had endocarditis but who were blood culture negative, 46 had a positive serological test result, including 1 patient with chronic Q fever and 45 patients with no other evidence of infection. Among these 45 patients, 19 (45.5%) had exposure factors consistent with B. quintana infection (13 were homeless, 6 were exposed to body lice, and 17 were alcoholics), 10 (22.2%) had exposure factors consistent with B. henselae infection (9 reported either cat scratches or bites and 5 reported cat flea bites), and 16 (35.5%) had no specific exposure factor. Follow-up serum specimens were not available from these 45 patients.
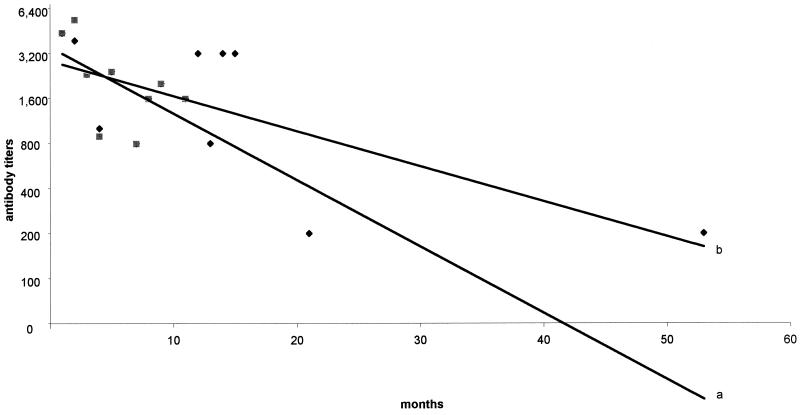
Follow-up serum specimens were obtained from 22 of the 48 patients with proven Bartonella infections (including the 2 patients who relapsed). The mean number of serum samples from these patients was 3.5 ± 1.5. The delay of sampling from the onset of therapy ranged from 1 to 53 months. Follow-up results are reported in Fig. 3. Compared to patients who recovered, the kinetics of the decrease in antibody titers in patients who relapsed was significantly delayed (P < 0.01) (Fig. 3). However, a single dilution difference was observed 1 year after the diagnosis, as patients who recovered had a median titer of 1:800 and those who relapsed had a titer of 1:1,600 (Fig. 3).
FIG. 3.
Comparison of kinetics of titers of IgG antibodies to either B. quintana or B. henselae in 20 patients with Bartonella endocarditis who recovered (curve a) and 2 patients who relapsed (curve b), shown as linear trend curves. Grey squares, titers in serum of patients who recovered; black diamonds, titers in serum of patients who relapsed.
DISCUSSION
Eighty-one cases of Bartonella endocarditis have been reported in the international literature, mostly by our group (1-3, 5-7, 10-15, 18, 23, 24, 28, 29). In a previous study, we estimated that bartonellae are responsible for 3% of all cases of endocarditis (30). To date, we have identified 48 patients with Bartonella endocarditis on the basis of positive culture and/or PCR results.
The serological tests available at present may not reliably distinguish between antibody responses to Bartonella species and C. burnetii (16) or Chlamydia species (21). Moreover, the demonstrated antigenic cross-reactivity between Bartonella and Chlamydia species has raised questions about the reliability of the diagnoses of cases of Chlamydia endocarditis described in the literature.
Using the MIF assay, we have previously demonstrated that a titer of 1:1,600 had a positive predictive value of 0.884 for the detection of Bartonella endocarditis in the general population (25). In the present study, such a cutoff value had a positive predictive value of 0.672 for the detection of Bartonella endocarditis in the general population but a sensitivity of only 0.771, whereas a titer of 1:800 had a positive predictive value of 0.398 and a sensitivity of 0.895. The lower positive predictive value observed in the present study compared to that observed in our previous study (25) may be explained by the recent inclusion in our series of homeless patients who suffer from chronic B. quintana bacteremia and who thus exhibit elevated antibody titers.
Given the potential severity of Bartonella endocarditis and the good outcomes achieved when patients with Bartonella endocarditis are treated with aminoglycosides (D. Raoult, unpublished data), we considered it more useful to adopt the titer which had the greatest sensitivity, i.e., a titer of 1:800 for antibodies against B. quintana and/or B. henselae. We also determined that this cutoff had a positive predictive value of 0.955 for the detection of Bartonella infections among patients with endocarditis (Fig. 2).
The specificities of serological tests for the identification of the species causing Bartonella-induced endocarditis have been questioned due to the cross-reactivities among Bartonella species. In our study, we observed cross-reactions among Bartonella species for almost all patients. Moreover, 68% of patients with proven B. quintana infections even exhibited higher titers of antibodies to B. henselae than to B. quintana. The combination of results of tests for both antigens allowed the diagnosis of Bartonella endocarditis in 43 of 48 patients with proven Bartonella endocarditis, whereas testing for antibodies only to B. henselae or B. quintana led to the diagnosis of Bartonella infection in only 38 and 39 patients, respectively. Therefore, we recommend the determination of titers of antibodies to both B. quintana and B. henselae for the diagnosis of Bartonella endocarditis and propose that a cutoff of 1:800 for the titer of the antibody to either B. quintana and/or B. henselae be included as a major criterion in the diagnostic score of the Duke endocarditis service (17).
The 1:800 cutoff titer had a positive predictive value of 0.810 for the detection of chronic Bartonella infections, including endocarditis, in our population of 36,480 patients tested. This high predictive value of elevated antibody titers for the detection of chronic Bartonella infections appears to be specific to our laboratory, as many other series reported in the literature, in which other antigens were used, have reported elevated antibody titers even for patients with acute infections (31).
Using MRL-BA slides (MRL Diagnostics, Cypress, Calif.), we commonly obtained titers of >1:400 for patients with acute cat scratch disease, whereas using our own antigens, we most commonly determined titers of 1:50 to 1:200 (unpublished data). The difference in the antibody responses between patients with acute and chronic Bartonella infections observed in our laboratory is not unique. Such a phenomenon is also observed in patients with Q fever, with a cutoff for the detection of acute infections of ≥1:200 for phase II IgG antibody and a cutoff for chronic infections of ≥1:800 for phase I IgG antibody (22). In patients with chronic Bartonella or C. burnetii infections, the recurrent presence of bacteria in the bloodstream may result in a prolonged antigenic stimulation and thus antibody titers higher than those during acute infections, during which the antigenic stimulation is of short duration.
When studying the evolution of antibodies to Bartonella following the diagnosis of endocarditis, we observed significantly delayed responses in two patients who relapsed in comparison with the response times in those who recovered (Fig. 3). The follow-up of patients with positive results for Bartonella by serological tests may be suitable for the prediction of late relapses, but further studies are needed to confirm these data.
In conclusion, in our study, the MIF assay had a high predictive value for the detection of chronic Bartonella infections, especially endocarditis, when it was applied to the sera of patients with blood culture-negative endocarditis and was also helpful in detecting late relapses in patients with delayed decreases in antibody titers.
Acknowledgments
We thank M. Archambaud, X. Belenfant, C. Benoit-Lemercier, F. Bruneel, D. Clave, P. Collet, L. Deforges, S. J. Eykyn, E. James, P. Jourdain, O. Launay, H. Lelièvre, J.-L. Mainardi, T. J. Marrie, J. Nash, and C. Roure for providing clinical material.
REFERENCES
- 1.Baorto, E., R. M. Payne, L. N. Slater, F. Lopez, D. A. Relman, K. W. Min, and J. W. St. Geme. 1998. Culture-negative endocarditis caused by Bartonella henselae. J. Pediatr. 132:1051-1054. [DOI] [PubMed] [Google Scholar]
- 2.Breathnach, A. S., J. M. Hoare, and S. J. Eykyn. 1997. Culture-negative endocarditis: contribution of bartonella infections. Heart 77:474-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruneel, F., J. D'Estanque, P. E. Fournier, G. Arlet, M. Thuong, M. Wolff, J. P. Bedos, S. Lariven, and B. Regnier. 1998. Isolated right-sided Bartonella quintana endocarditis in an immunocompetent adult. Scand. J. Infect. Dis. 30:424-425. [DOI] [PubMed] [Google Scholar]
- 4.Dalton, M. J., L. E. Robinson, J. Cooper, R. L. Regnery, J. G. Olson, and J. E. Childs. 1995. Use of Bartonella antigens for serologic diagnosis of cat-scratch disease at a National Referral Center. Arch. Intern. Med. 155:1670-1676. [PubMed] [Google Scholar]
- 5.Daly, J. S., M. G. Worthington, D. J. Brenner, W. C. Moss, D. G. Hollis, R. S. Weyant, A. G. Steigerwalt, R. E. Weaver, M. I. Daneshvar, and S. P. O'Connor. 1993. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J. Clin. Microbiol. 31:872-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drancourt, M., R. J. Birtles, G. Chaumentin, F. Vandenesch, J. Etienne, and D. Raoult. 1996. New serotype of Bartonella henselae in endocarditis and cat-scratch disease. Lancet 347:441-443. [DOI] [PubMed] [Google Scholar]
- 7.Drancourt, M., J. L. Mainardi, P. Brouqui, F. Vandenesch, A. Carta, F. Lehnert, J. Etienne, E. Vigier, F. Goldstein, J. Acar, and D. Raoult. 1995. Bartonella (Rochalimaea) quintana endocarditis in homeless patients: report of three cases. N. Engl. J. Med. 332:419-423. [DOI] [PubMed] [Google Scholar]
- 8.Durack, D. T., A. S. Lukes, and D. K. Bright. 1994. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am. J. Med. 96:200-222. [DOI] [PubMed] [Google Scholar]
- 9.Etienne, J., D. Ory, D. Thouvenot, D. Raoult, R. Loire, J. P. Delahaye, and J. Beaune. 1992. Chlamydial endocarditis: a report of 10 cases. Eur. Heart J. 13:1422-1426. [DOI] [PubMed] [Google Scholar]
- 10.Fournier, P. E., H. Lelievre, S. J. Eykyn, J. L. Mainardi, T. J. Marrie, F. Bruneel, C. Roure, J. Nash, D. Clave, E. James, C. Benoit-Lemercier, L. Deforges, H. Tissot-Dupont, and D. Raoult. 2001. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine 80:245-251. [DOI] [PubMed] [Google Scholar]
- 11.Guyot, A., A. Bakhai, N. Fry, J. Merritt, H. Malnick, and T. Harrison. 1999. Culture-positive Bartonella quintana endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 18:145-147. [DOI] [PubMed] [Google Scholar]
- 12.Hadfield, T. L., R. Warren, M. Kass, E. Brun, and C. Levy. 1993. Endocarditis caused by Rochalimaea henselae. Hum. Pathol. 24:1140-1141. [DOI] [PubMed] [Google Scholar]
- 13.Holmes, A. H., T. C. Greenough, G. J. Balady, R. L. Regnery, B. E. Anderson, J. C. Oikeane, J. D. Fonger, and E. L. McCrone. 1995. Bartonella henselae endocarditis in an immunocompetent adult. Clin. Infect. Dis. 21:1004-1007. [DOI] [PubMed] [Google Scholar]
- 14.Jacoby, G. A., C. M. Hay, R. B. Colvin, and B. D. Walker. 1997. A 38-year-old man with digital clubbing, low-grade fever, and a murmur—Bartonella endocarditis, probably due to Bartonella quintana. N. Engl. J. Med. 336:205-210. [DOI] [PubMed] [Google Scholar]
- 15.Jalava, J., P. Kotilainen, S. Nikkari, M. Skurnik, E. Vanttinen, O. P. Lehtonen, E. Eerola, and P. Toivanen. 1995. Use of the polymerase chain reaction and DNA sequencing for detection of Bartonella quintana in the aortic valve of a patient with culture-negative infective endocarditis. Clin. Infect. Dis. 21:891-896. [DOI] [PubMed] [Google Scholar]
- 16.La Scola, B., and D. Raoult. 1996. Serological cross reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J. Clin. Microbiol. 34:2270-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, J. S., D. J. Sexton, N. Mick, R. Nettles, V. G. J. Fowler, T. Ryan, T. Bashore, and G. R. Corey. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 30:633-638. [DOI] [PubMed] [Google Scholar]
- 18.Mainardi, J. L., M. Drancourt, J. M. Roland, J. L. Gestin, D. Raoult, J. F. Acar, and F. W. Goldstein. 1996. Bartonella (Rochalimaea) quintana endocarditis in an Algerian farmer. Clin. Microbiol. Infect. 1:275-276. [DOI] [PubMed] [Google Scholar]
- 19.Marrie, T. J., M. Harczy, O. E. Mann, R. W. Landymore, A. Raza, S. P. Wang, and J. T. Grayston. 1990. Culture-negative endocarditis probably due to Chlamydia pneumoniae. J. Infect. Dis. 161:127-129. [DOI] [PubMed] [Google Scholar]
- 20.Maurin, M., R. J. Birtles, and D. Raoult. 1997. Current knowledge of Bartonella species. Eur. J. Clin. Microbiol. Infect. Dis. 16:487-506. [DOI] [PubMed] [Google Scholar]
- 21.Maurin, M., F. Eb, J. Etienne, and D. Raoult. 1997. Serological cross-reactions between Bartonella and Chlamydia species: implications for diagnosis. J. Clin. Microbiol. 35:2283-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 12:518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel, R., J. O. Newell, G. W. Procop, and D. H. Persing. 1999. Use of polymerase chain reaction for citrate synthase gene to diagnose Bartonella quintana endocarditis. Am. J. Clin. Pathol. 112:36-40. [DOI] [PubMed] [Google Scholar]
- 24.Piroth, L., P. Menecier, and J. P. Kisterman. 1996. Negative blood culture endocarditis: search for the intracellular germ. Presse Med. 25:1348. [PubMed] [Google Scholar]
- 25.Raoult, D., P. E. Fournier, M. Drancourt, T. J. Marrie, J. Etienne, J. Cosserat, P. Cacoub, Y. Poinsignon, P. Leclercq, and A. M. Sefton. 1996. Diagnosis of 22 new cases of Bartonella endocarditis. Ann. Intern. Med. 125:646-652. [DOI] [PubMed] [Google Scholar]
- 26.Raoult, D., H. Tissot-Dupont, and M. Enea Mutillod. 1994. Positive predictive value of Rochalimaea henselae antibodies in the diagnostic of cat scratch disease (CSD). Clin. Infect. Dis. 19:335. [DOI] [PubMed] [Google Scholar]
- 27.Roux, V., S. J. Eykyn, S. Wyllie, and D. Raoult. 1999. First report of Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture negative endocarditis in man. J. Clin. Microbiol. 38:1698-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spach, D. H., K. P. Callis, D. S. Paauw, Y. B. Houze, F. D. Schoenknecht, D. F. Welch, H. Rosen, and D. J. Brenner. 1993. Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J. Clin. Microbiol. 31:692-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spach, D. H., A. S. Kanter, N. A. Daniels, D. J. Nowowiejski, A. M. Larson, R. A. Schmidt, B. Swaminathan, and D. J. Brenner. 1995. Bartonella (Rochalimaea) species as a cause of apparent “culture-negative” endocarditis. Clin. Infect. Dis. 20:1044-1047. [DOI] [PubMed] [Google Scholar]
- 30.Watanakunakorn, C., and T. Burkert. 1993. Infective endocarditis at a large community teaching hospital, 1980-1990—a review of 210 episodes. Medicine 72:90-102. [DOI] [PubMed] [Google Scholar]
- 31.Zbinden, R., N. Michael, M. Sekulovski, A. von Graevenitz, and D. Nadal. 1997. Evaluation of commercial slides for detection of immunoglobulin G against Bartonella henselae by indirect immunofluorescence. Eur. J. Clin. Microbiol. Infect. Dis. 16:648-652. [DOI] [PubMed] [Google Scholar]