Abstract
The budding yeast Saccharomyces cerevisiae contains a family of Arf (ADP-ribosylation factor) GTPase activating protein (GAP) proteins with the Gcs1 + Age2 ArfGAP pair providing essential overlapping function for the movement of transport vesicles from the trans-Golgi network. We have generated a temperature-sensitive but stable version of the Gcs1 protein that is impaired only for trans-Golgi transport and find that deleterious effects of this enfeebled Gcs1-4 mutant protein are relieved by increased gene dosage of the gcs1-4 mutant gene itself or by the SFH2 gene (also called CSR1), encoding a phosphatidylinositol transfer protein (PITP). This effect was not seen for the SEC14 gene, encoding the founding member of the yeast PITP protein family, even though the Gcs1 and Age2 ArfGAPs are known to be downstream effectors of Sec14-mediated activity for trans-Golgi transport. Sfh2-mediated suppression of inadequate Gcs1-4 function depended on phospholipase D, whereas inadequate Gcs1-4 activity was relieved by increasing levels of diacylglycerol (DAG). Recombinant Gcs1 protein was found to bind certain phospholipids but not DAG. Our findings favor a model of Gcs1 localization through binding to specific phospholipids and activation of ArfGAP activity by DAG-mediated membrane curvature as the transport vesicle is formed. Thus, ArfGAPs are subject to both temporal and spatial regulation that is facilitated by Sfh2-mediated modulation of the lipid environment.
Keywords: vesicular transport, yeast GTPase activating protein, phospholipase D, phospholipid transfer protein
Eukaryotic cells possess membrane-bound compartments that perform distinct cellular functions and between which a large amount of material is trafficked. Indeed, the integrity of eukaryotic organelles depends on regulated transport of protein and membrane cargo between these compartments. Much of this transport takes place through the actions of membrane vesicles. As part of this vesicular transport, coat proteins are recruited for the formation of donor vesicles. Different subcellular sites of vesicular transport mediate the generation of distinct protein coat complexes. The organization of each coat complex is determined by the recruitment of specific regulatory factors (1).
The small GTPase ADP-ribosylation factor (Arf) regulates vesicle formation by coat recruitment (2). Arf proteins provide a molecular switch that works through a cycle of GTP binding and hydrolysis. The early stages of vesicle formation involve the recruitment of the inactive GDP-bound form of Arf that is a substrate for a guanine exchange factor (ArfGEF) (3) that exchanges GDP for GTP, thereby activating Arf. Upon nucleotide exchange, a myristate residue at the amino terminus of Arf becomes exposed and allows GTP-Arf to become stably anchored to the membrane (4). Coat proteins are recruited to this site, cargo is packaged, and a coated vesicle is formed. Such donor vesicles containing protein and lipid cargo are transported to the appropriate acceptor membrane compartment. Before vesicle fusion with an acceptor membrane, the protein coat is shed, with coat removal mediated by hydrolysis of Arf-bound GTP by the intrinsic GTPase activity of Arf through interaction with an Arf GTPase activating protein (GAP) (5, 6). ArfGAPs also play an important role in the production of transport vesicles (7, 8), perhaps by participating in a priming complex (9) that recycles Arf proteins for repeated rounds of coat recruitment.
The budding yeast Saccharomyces cerevisiae contains several ArfGAP proteins (10–12). Two of these, Gcs1 and Glo3, provide essential overlapping function for retrograde transport from the Golgi to the endoplasmic reticulum (11); another pair, Gcs1 and Age2, provide essential overlapping function for transport from the trans-Golgi network (13). In addition to the temporal regulation of ArfGAP function for both vesicle generation and vesicle fusion, ArfGAPs are also subject to spatial regulation to localize specific ArfGAPs to the appropriate vesicular-transport stage.
Local changes in lipid composition also play important regulatory roles in vesicular transport (14–16). Regulated lipid metabolism provides for temporal and spatial recruitment of specific coat proteins to membranes. In addition, altering the physical properties of lipids themselves is believed to accelerate vesicle formation and fusion by generating energetically favorable alterations in membrane structure. Recently, the binding of cytosolic proteins to both membrane-associated proteins and specific lipid components has revealed a “dual-key” mechanism for proper localization of proteins (15, 17). Phosphorylation of the inositol ring at the 3, 4, and/or 5 positions of phosphatidylinositol (PI) produces several distinct phospholipid species that have been demonstrated to recruit and/or alter the activity of proteins required for coat recruitment and shedding (16).
One protein that modifies lipid metabolism and affects vesicular transport is Sec14. This protein has PI/phosphatidylcholine (PC) transfer activity in vitro, and genetic analysis indicates that Sec14 controls vesicular transport from the trans-Golgi network by altering the local lipid environment (18–20). Sec14 is necessary for vesicle formation from the trans-Golgi network, presumably by ensuring a lipid composition that is permissive for proteins that mediate Golgi-derived transport. Interestingly, PI binding by Sec14 is dispensable for Golgi-derived vesicular transport, for an engineered version of Sec14 that can bind only PC restores vesicular transport and cell growth to cells lacking endogenous Sec14 function (20).
Five other yeast proteins with similarity to Sec14 define the Sfh (Sec14 homology) family (21, 22). Unlike Sec14, these Sfh proteins are not essential and can transfer only PI. Despite this inability to bind PC, the overexpression of Sfh2 or Sfh4 (and, to a lesser extent, Sfh5) but not other members of the Sfh family allows yeast cells to grow despite the otherwise lethal absence of Sec14. Signaling through PC turnover mediated by phospholipase D (PLD) (Spo14) acts as a positive regulator of Sec14-dependent vesicular transport; all known second-site mutations that bypass the essential function of Sec14 absolutely require Spo14. Suppression of defective Sec14 function by increased abundance of Sfh4 also depends completely on Spo14, whereas the efficiency of Sfh2 effects in cells lacking Sec14 is only moderately improved by Spo14. The combined evidence points to a model whereby members of the Sec14/Sfh family modify lipid metabolism to stimulate poorly characterized activities required for effective vesicular transport (23).
We have shown that two lipid-responsive proteins that function in the Sec14 pathway are the Gcs1 and Age2 ArfGAPs (24). Here, we show that Sfh2, but not Sec14, improves an impairment in trans-Golgi transport otherwise impaired because of inadequate Gcs1 activity by modulating lipid composition, demonstrating distinct roles for Sfh lipid-binding proteins and revealing an important role for lipid metabolism in regulating ArfGAP function for vesicular transport.
Materials and Methods
Yeast Strains, Plasmids, and Growth Conditions. All yeast strains were derived from strain W303 (11). The haploid strain YTW8A (MATα age2Δ::HIS3 gcs1Δ::LEU2 ade2–1 his3–11 leu2–3,112 trp1–1 ura3–1 [pMG4–4]) is a meiotic segregant from diploid strain AAY20 (MATα/MATa age2Δ::HIS3/+ gcs1Δ::LEU2/+ ade2–1/ade2–1 his3–11/his3–11 leu2–3/lue2–112 trp1–1/trp1–1 ura3–1/ura3–1 [pMG4–4]) heterozygous for GCS1 and AGE2 null mutations and harboring the gcs1-4 plasmid pMG4–4 (derived from the TRP1 CEN plasmid pRS314). The gcs1-4 mutation was generated by PCR-mediated mutagenesis (11) of the GCS1 gene, followed by cotransformation of gcs1::HIS3 age2::LEU2 haploid cells (kept alive with a plasmid-borne AGE2 gene) with the mutated population of GCS1 genes and a gapped pRS314 plasmid missing the SpeI/ClaI fragment. After transformation and in vivo gap repair of the pRS314 plasmid with mutagenized GCS1 molecules, viable cells lacking the AGE2 plasmid were screened for temperature-sensitive gcs1 mutations. Dosage suppressor genes that allowed for growth of YTW8A cells at 37°C were selected by using a 2-μm-based URA3 yeast DNA library; genomic inserts were identified by DNA sequencing.
Growth of yeast and standard genetic manipulations were performed essentially as described in ref. 25. Cell concentrations were determined by using a Coulter electronic particle counter (Model ZM).
Assays of Vesicular Transport. To assess endocytosis, cells were stained with the lipophilic dye FM4-64 essentially as described in ref. 26 and examined by using a motorized Zeiss Axioplan II microscope equipped with a Zeiss Axiocam HRc digital camera and Zeiss Plan Apochromat (×100, 1.4 numerical aperture). An invertase assay (27, 28) was used to determine the effect of Gcs1 activity on secretion. ArfGAP activity was determined as described in refs. 13 and 29.
Western Analysis. Recombinant proteins from Escherichia coli were isolated as described in ref. 29. Protein levels were determined by using Bradford reagent with BSA (1 μg/μl) as a standard. To assess protein binding to lipids, Hybond-C membrane was spotted with 500 pmol of each lipid, dried for 1 h at room temperature, and blocked with 3% BSA (fatty acid-free) in TBST (950 ml of H2O/8g of NaCl/0.2 g of KCl/3 g of Tris base, pH 7.4 adjusted with HCl/10 ml of 10% Tris-buffered saline-Tween 20) for 1 h at room temperature. The membrane was then incubated with purified recombinant protein at 15 nM in blocking buffer overnight at 4°C. The membrane was washed with 0.1% TBST six times (5 min each time), incubated with mouse monoclonal anti-(6xHis) antibody (1:2,000, Novagen) for 1.5 h at room temperature, and washed in TBST six times (5 min each time). The secondary antibody (1:5,000, Bio-Rad), goat anti-mouse conjugated to horseradish peroxidase, was incubated with the membrane for 1 h at room temperature. The membrane was then washed 10 times (5 min per wash) in TBST, and the signal was detected by using enhanced chemiluminescence (Amersham Pharmacia Biosciences).
Results
Gcs1-4 Is a Temperature-Sensitive Protein That Is Impaired for Transport from the Trans-Golgi Network. The Gcs1 protein can provide ArfGAP function for distinct stages of vesicular transport. Gcs1 and the ArfGAP Glo3 have overlapping essential function for retrograde transport from the Golgi to the endoplasmic reticulum, whereas Gcs1 and the ArfGAP Age2 have essential overlapping function for trans-Golgi transport. The ArfGAP activity of Gcs1 is required for its function in trans-Golgi transport, because an ArfGAP-dead version of Gcs1 (8) is unable to maintain viability in cells lacking Age2 (unpublished data). To facilitate analyses of ArfGAP function for these two transport stages, we previously generated temperature-sensitive gcs1 mutations and identified the gcs1–28 allele, which encodes a mutant protein that at 37°C is stable but is unable to provide adequate function for retrograde transport in the absence of the Glo3 ArfGAP. Nevertheless, the Gcs1–28 mutant protein at 37°C does provide sufficient ArfGAP function for trans-Golgi transport in the absence of the Age2 ArfGAP. Thus, the gcs1–28 mutation produces a retrograde-specific defect in Gcs1 activity. To create a mutation in the GCS1 gene that only affects Gcs1 activity for trans-Golgi transport, we used a PCR-based mutagenesis approach (11) to identify temperature-sensitive gcs1 alleles that impose a growth defect only in the absence of AGE2.
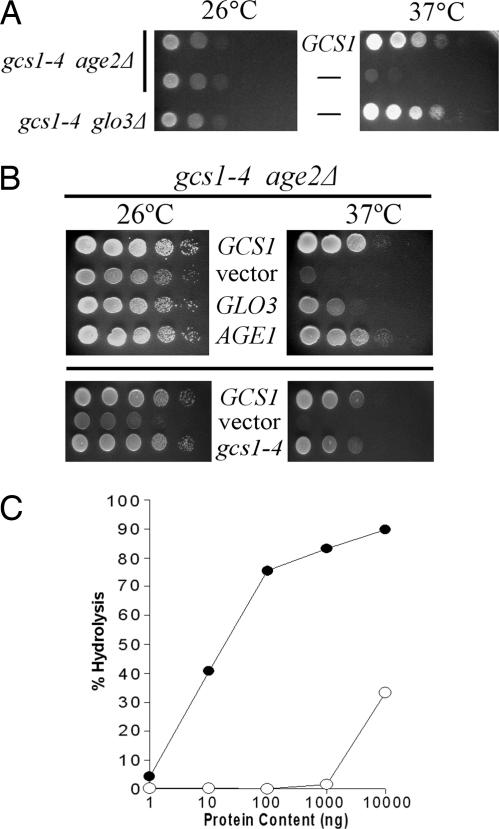
Temperature-sensitive gcs1 mutant genes were analyzed to determine whether the gene encodes a protein that is dysfunctional for retrograde or trans-Golgi transport (or both). Each plasmid-borne gcs1 mutant was transformed into diploid cells heterozygous for gcs1 and age2 deletion mutations (gcs1Δ::LEU2/+ age2Δ::HIS3/+), the transformed diploid cells were induced to undergo meiosis and sporulation, and gcs1Δ age2Δ meiotic segregants carrying gcs1 mutant plasmids were assessed for growth at the restrictive temperature of 37°C. One mutant allele, gcs1-4, encodes a single amino acid substitution (glutamine to aspartate) at position 84, a highly conserved residue that is located at the C-terminal region of the ArfGAP domain. The Gcs1-4 protein provided adequate ArfGAP activity for trans-Golgi transport at the permissive temperature of 26°C but failed to allow growth at 37°C (Fig. 1A). Like the situation for the gcs1–28 mutation after transfer of cells to 37°C, the gcs1-4 mutation encodes a protein that is as stable as WT Gcs1 (data not shown).
Fig. 1.
The Gcs1-4 mutant protein with enfeebled ArfGAP activity is selectively impaired for transport from the trans-Golgi network. (A) Selective impairment for trans-Golgi transport. Ten-fold serial dilutions of cell populations (relevant genotype is indicated on the left) were spotted onto solid enriched medium and incubated at the indicated temperatures. The presence of a plasmid-borne WT GCS1 gene is indicated between the panels. (B) Relief of temperature sensitivity by increased ArfGAP abundance. Ten-fold serial dilutions of gcs1-4 age2Δ cell populations harboring the indicated genes on high-copy plasmids were spotted on solid defined medium for plasmid maintenance and incubated at the indicated temperatures. (C) ArfGAP activities of recombinant Gcs1 (open circles) and Gcs1-4 mutant (filled circles) proteins were assessed by in vitro hydrolysis of radiolabeled Arf-bound GTP.
Gcs1 can also function in retrograde transport in concert with another ArfGAP, Glo3, so we similarly determined whether the gcs1-4 allele is also impaired for this Glo3-mediated transport. The plasmid-borne gcs1-4 allele was transformed into diploid cells heterozygous for chromosomal glo3Δ and gcs1Δ mutations, and individual meiotic products were analyzed. This analysis showed that, in the absence of the chromosomal GCS1 and GLO3 genes, gcs1-4 provides adequate ArfGAP activity regardless of the growth temperature (Fig. 1A). These findings indicate that the gcs1-4 allele specifically affects transport from the trans-Golgi network.
Increased Abundance of Other ArfGAP Proteins Can Mitigate the Consequences of Defective Gcs1 Activity. To determine whether other proteins that are structurally related to Gcs1 can alleviate the effects of the gcs1-4 mutation, we tested the ability of other members of the ArfGAP family to relieve the temperature sensitivity caused by gcs1-4 in the absence of Age2 protein. As shown in Fig. 1B, gcs1-4 age2Δ cells containing high-copy AGE1 or GLO3 plasmids grew at 37°C. Thus, both AGE1 and GLO3 are copy suppressors of gcs1-4 temperature sensitivity.
Copy suppression may reflect the ability of these genes to enhance the effects of an enfeebled Gcs1-4 protein activity or, alternatively, to completely eliminate the need for Gcs1 protein in the absence of Age2. Through standard plasmid-loss procedures, we determined that age2Δ gcs1Δ mutant cells carrying either GLO3 or AGE1 on a high-copy plasmid failed to grow in the absence of the gcs1-4 plasmid, regardless of growth temperature. Thus, suppression of Gcs1-4-mediated temperature sensitivity by Age1 or Glo3 was not through bypass of Gcs1 activity.
Despite the temperature sensitivity in an age2Δ context, the Gcs1-4 mutant protein retains significant activity because, in cells lacking chromosomal copies of GCS1 and GLO3 genes, the Gcs1-4 protein can maintain the essential activities of retrograde transport regardless of growth temperature. We therefore evaluated whether activity of the Gcs1-4 mutant protein is simply insufficient for effective transport from the trans-Golgi network. The gcs1-4 mutant gene was transferred to a high-copy plasmid to allow increased Gcs1-4 expression, and gcs1Δ age2Δ cells harboring this plasmid were assessed for growth at 37°C. Cells harboring the WT GCS1 gene or an empty vector served as positive and negative controls, respectively. As shown in Fig. 1B, overexpression of Gcs1-4 from this high-copy plasmid did indeed relieve the temperature sensitivity, in marked contrast to cells with low-copy gcs1-4. This finding suggests that the Gcs1-4 protein retains some activity for trans-Golgi transport, albeit at a lower efficiency.
To assess the ArfGAP function of the Gcs1-4 protein more directly, we used an in vitro ArfGAP assay (29). Although WT recombinant Gcs1 protein displayed the expected level of ArfGAP activity, we detected only low levels of ArfGAP activity with the Gcs1-4 mutant protein (Fig. 1C), consistent with the low Gcs1-4 activity suggested by our in vivo analysis.
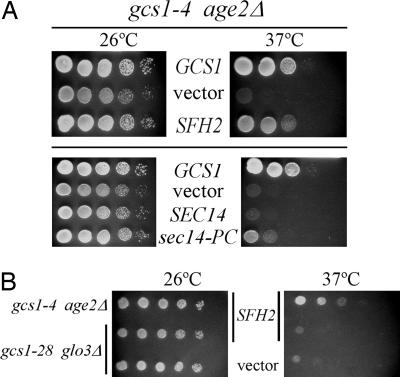
SFH2 Is a Dosage Suppressor of Inadequate Gcs1 Function. To identify additional genes that can relieve gcs1-4 temperature sensitivity, we transformed gcs1-4 age2 mutant cells with a high-copy yeast genomic library, and transformant colonies growing at 26°C were transferred by replica-plating to 37°C for further incubation. Of the ≈12,000 colonies screened in this manner, 50 grew at 37°C. Library plasmids from these temperature-resistant gcs1-4 age2 transformants harbored three types of genomic inserts. As expected, two types of genomic inserts contained either the WT AGE2 gene or the WT GCS1 gene. Two other independent inserts contained the SFH2 gene (Fig. 2A). Sfh2 is not an essential protein and does not display a specific intracellular localization. Although the function of Sfh2 is not well understood, increased expression of Sfh2 can suppress the lethality of cells lacking Sec14 protein (22). Sec14 and other Sfh proteins belong to a family of structurally related proteins that are all characterized as PI transfer proteins. Sec14 differs slightly from other family members in that it can also transfer PC. Each member of the Sfh family was directly tested for suppression of gcs1-4 age2Δ temperature sensitivity by transforming each SFH gene carried on a high-copy plasmid into gcs1-4 age2Δ mutant cells. Testing 10-fold serial dilutions of cultures of these transformants on solid medium showed that, unlike SFH2, SFH1, SFH3, SFH4, and SFH5 were poor suppressors of the gcs1-4 temperature sensitivity (data not shown).
Fig. 2.
Increased SFH2 and sec14-PC-only gene dosage suppresses defective Gcs1 function selectively for trans-Golgi network transport. Populations of gcs1-4 age2Δ (A) and gcs1-4 age2Δ and gcs1–28 glo3Δ (B) cells harboring the indicated genes on high-copy plasmids were treated as described in the legend of Fig. 1 A.
To determine whether increased dosage of the SFH2 gene can bypass the need for the Gcs1 and Age2 ArfGAPs, we determined whether gcs1Δ age2Δ cells (with the gcs1-4 plasmid) harboring the SFH2 plasmid could survive loss of the gcs1-4 plasmid. We were unable to find gcs1Δ age2Δ cells carrying only the SFH2 plasmid (data not shown). Thus, copy suppression by SFH2 requires the presence of the gcs1-4 mutant gene.
To assess whether suppression by increased SFH2 gene dosage was specific for the gcs1-4 age2Δ trans-Golgi transport defect, a high-copy SFH2 plasmid was tested in gcs1–28 glo3Δ mutant cells that display a temperature-sensitive defect only for retrograde transport from the Golgi to the endoplasmic reticulum (11). As shown in Fig. 2B, increased gene dosage of SFH2 did not allow gcs1–28 glo3Δ mutant cells to grow at 37°C, in marked contrast to the SFH2-mediated copy suppression in gcs1-4 age2Δ mutant cells. Thus, the ability of SFH2 to influence vesicular transport may therefore be limited to trans-Golgi transport processes.
Overexpression of a “Sec14-PC-Only” Mutant Protein, but not WT Sec14, Suppresses the Temperature Sensitivity of gcs1-4. Because increased dosage of SFH2 suppresses the lethal effects of a sec14Δ mutation and both Sfh2 and Sec14 can transfer PI in vitro, the two proteins may have similar and perhaps even overlapping functions. Thus, we assessed the ability of increased Sec14 expression to relieve temperature sensitivity caused by gcs1-4. Additionally, we assessed the effects of overexpression of a mutant form of the Sec14 protein that can transfer only PC in vitro. The Sec14-PC-only mutant protein contains two amino acid substitutions (K66A and K239A) that were rationally substituted based on the Sec14 crystal structure to prevent Sec14-PC-only from binding PI (30). Because PI binding is a feature that characterizes Sfh2, we expected that the PC-only form of Sec14 would not be able to provide suppression. Genes encoding WT Sec14 and the Sec14-PC-only mutant were transformed into gcs1-4 age2Δ cells on high-copy plasmids, and despite our expectations, the Sec14-PC-only mutant protein was able to partially suppress the temperature sensitivity of gcs1-4 mutant cells, whereas WT Sec14 had no suppression activity (Fig. 2A). Even after several days of incubation at 37°C, cells containing the high-copy SEC14 plasmid did not grow (data not shown). These observations suggest that increased Sec14 abundance may actually be deleterious under these circumstances, whereas the Sec14-PC-only mutant form is beneficial.
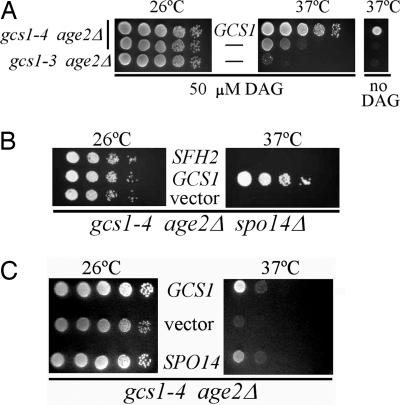
gcs1-4 Temperature Sensitivity Is Relieved by Exogenous Short-Chain Diacylglycerol (DAG). It has been shown (24, 31) that ArfGAP activity is stimulated by the lipid DAG. We tested whether increased DAG levels, which presumably could increase the ArfGAP activity of an enfeebled Gcs1-4 protein, would suppress the temperature sensitivity of gcs1-4 age2Δ cells. Cells were incubated on solid medium supplemented with short-chain di8:0 DAG at 50 μM or 100 μM, concentrations similar to those shown to elevate total intracellular DAG by 20-fold with all of the DAG remaining as di8:0 DAG (32). For comparison, we included gcs1Δ age2Δ mutant cells kept alive at 26°C through expression of the Gcs1-3 mutant protein. This mutant version of Gcs1 is unstable at 37°C; therefore, the temperature sensitivity of these cells was not expected to be suppressed by exogenous DAG. Indeed, DAG did not suppress gcs1-3 temperature sensitivity (Fig. 3A). However, for gcs1-4 mutant cells at 37°C, the temperature sensitivity was relieved by exogenous DAG, and gcs1-4 age2Δ mutant cells grew as well as cells with the WT GCS1 gene. This finding, along with our previous observation that the Gcs1-4 mutant protein is stable at 37°C, provides more evidence that the Gcs1-4 mutant protein retains activity for trans-Golgi vesicular transport that is simply inadequate at 37°C. The data are consistent with a model whereby stimulation of ArfGAP activity by exogenous DAG compensates for the mutationally induced impairment of Gcs1-4 function to restore transport from the trans-Golgi.
Fig. 3.
Gcs1-4-mediated temperature sensitivity is relieved by the presence of exogenous short-chain DAG and increased PLD activity. (A) Ten-fold serial dilutions of cells of indicated genotype (Left), carrying the WT GCS1 gene on a high-copy plasmid where indicated, were spotted on solid enriched selective medium and incubated at the indicated temperature in the presence or absence of 50 μM short-chain DAG. (B) Serial dilutions of gcs1-4 age2Δ spo14Δ triple-mutant cells harboring the indicated high-copy plasmid were treated as described in the legend of Fig. 1 A. (C) Ten-fold serial dilutions of gcs1-4 age2Δ cells harboring the indicated high-copy plasmids were treated as described in the legend of Fig. 1 A.
Inhibition of PLD Activity Abolishes Sfh2 Suppression of gcs1-4. Yeast PLD is encoded by SPO14 and is the sole enzyme in yeast that is responsible for cleaving PC to produce phosphatidic acid and choline. Phosphatidic acid is, in turn, hydrolyzed to form DAG. Because exogenous short-chain DAG relieved the temperature sensitivity associated with the Gcs1-4 protein, we determined the effects of altering PLD activity as another way to affect DAG levels in vivo.
If PLD activity contributes to the ability of SFH2 to suppress gcs1-4 temperature sensitivity, then SFH2 suppression should be diminished if PLD activity is inhibited. This inhibition can be imposed by primary alcohols such as 1-propanol. Normally, water is used as a substrate by PLD enzymes, but many PLDs have a many-fold higher affinity for primary alcohols, resulting in the production of poorly metabolizable phosphatidylalcohol instead of phosphatidic acid. In this way, phosphatidic acid production is substantially reduced, and PLD-mediated DAG production is inhibited. We introduced GCS1, SFH2, SEC14, and SEC14-PC-only into gcs1-4 age2Δ cells and spotted these transformants in 10-fold serial dilutions on solid medium with or without propanol [1% (vol/vol)]. Secondary alcohols are not PLD substrates; thus, isopropanol was used as a control. The gcs1-4 age2Δ transformants containing GCS1 grew well at both 26°C and 37°C on both media. The only growth inhibition on 1% propanol that was seen was for cells containing SFH2 and SEC14-PC-only (data not shown). These differences in growth were subtle but reproducible. This finding suggests that SFH2 and SEC14-PC-only suppress through a mechanism that involves PLD activity. Given that inhibition of PLD activity by primary alcohols is only partial, the involvement of PLD may be underestimated in this assay.
As a more direct assessment of the involvement of PLD activity in SFH2 gene dosage to suppression of gcs1-4, we generated mutant cells lacking the SPO14 gene (encoding PLD). In the resulting gcs1Δ age2Δ spo14Δ cells (carrying the gcs1-4 plasmid), the high-copy SFH2 gene no longer suppressed the temperature sensitivity (Fig. 3B). Thus, Sfh2-mediated suppression of the effects of inadequate Gcs1-4 function requires PLD activity.
PLD Overexpression Suppresses gcs1-4 Temperature Sensitivity. If SFH2 suppression of gcs1-4 is accomplished through increased DAG levels, then increasing PLD activity in gcs1-4 age2Δ cells should lead to a similar suppression of gcs1-4 temperature sensitivity. We increased PLD expression directly by using a high-copy SPO14 plasmid. As shown in Fig. 3C, SPO14 overexpression using this high-copy plasmid alleviated the temperature sensitivity of gcs1-4 age2Δ double-mutant cells. Thus, the suppression observed for SFH2 may indeed work by increasing the rate of DAG formation.
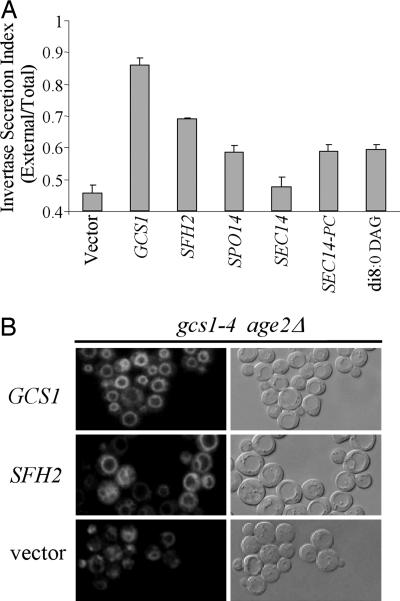
Exogenous DAG and Overexpression of SFH2, SPO14, and SEC14-PC Alleviate Defects in Transport Caused by gcs1-4. Suppression by SFH2 overexpression is indicated by the growth of gcs1-4 age2Δ mutant cells at 37°C. To assess the ability of increased SFH2 gene dosage to improve vesicular transport in these gcs1-4 cells, invertase secretion and endocytosis were assessed.
Invertase is synthesized in the endoplasmic reticulum and transported to the Golgi apparatus, where it undergoes extensive glycosylation and is subsequently transported through the secretory pathway to the periplasmic space. A secretion index comprising the ratio of external invertase activity versus total invertase activity is a reliable indicator of secretory pathway function. To measure the secretion index, cells were grown to the midlogarithmic stage of growth at the permissive temperature of 26°C and shifted to the restrictive temperature of 37°C for a 2-h incubation, at which time invertase activities were determined. The secretion index for gcs1-4 cells was 45%; a secretion index of 85% was found for these cells expressing WT GCS1 (Fig. 4A). This result is consistent with impaired Golgi-derived vesicular transport due to the gcs1-4 mutation. Dosage suppressors of gcs1-4 temperature sensitivity for growth, including SFH2, SPO14, and SEC14-PC, increased secretion indices to 65–70%, whereas the high-copy SEC14 gene, which does not suppress gcs1-4 temperature sensitivity, had no effect on the secretion index. Thus, each of the suppressors of gcs1-4 growth defects also significantly increased the secretion index. The addition of 100 μM di8:0 DAG to the growth medium at the time of shift to 37°C increased the invertase secretion index for gcs1-4 mutant cells to 65%, a value similar to those observed for high-copy suppressors (Fig. 4A). Thus, increased secretion was observed under all conditions that allow growth of gcs1-4 age2Δ cells at the restrictive temperature.
Fig. 4.
Exogenous DAG and overexpression of SFH2, SPO14, and SEC14-PC alleviate transport defects in gcs1-4 age2Δ cells. (A) Ratios of secreted invertase (External) to total invertase (Total) after 2 h at 37°C were determined for gcs1-4 age2Δ cells harboring the indicated genes on high-copy plasmids and also for gcs1-4 age2Δ cells growing in the presence of di8:0 DAG. Results are mean ± SD of three separate experiments, each performed in triplicate. Note that the y axis begins at a value of 0.4. (B) Endocytosis was visualized by uptake and transport of the lipophilic dye FM4-64 in gcs1-4 age2Δ cells harboring plasmids carrying the indicated genes. Samples were taken for analysis after transfer to 37°C for 30 min after the initial loading of the dye at 23°C.
FM4-64 is a lipophilic dye that can be visualized by fluorescence when bound to membranes. Use of this lipophilic dye allows detection of the movement of material from the plasma membrane through the endosomal pathway and to the vacuolar membrane. We found that defective Gcs1 function in gcs1-4 age2Δ mutant cells did not completely block transport of the FM4-64 dye from the plasma membrane because prolonged incubation eventually allowed transport of dye to the vacuole. This delayed transport was in marked contrast to the rapid transport of FM4-64 observed in these mutant cells carrying the WT GCS1 gene on a plasmid (Fig. 4B and data not shown). As shown in Fig. 4B, the delayed transport of FM4-64 in gcs1-4 age2Δ mutant cells was accompanied by a generalized cytoplasmic staining as well as a punctate pattern that likely represented endosomes. The gcs1-4 age2Δ cells with increased SFH2 gene dosage also exhibited efficient FM4-64 transport to the vacuole (Fig. 4B) but with an altered vacuolar morphology characterized by highly fragmented vacuoles. This phenotype was in marked contrast to that of gcs1-4 age2Δ cells with or without the WT GCS1 gene. Although the significance of this morphological change is not clear, it is evident that increased Sfh2 expression restores vesicular-transport function to gcs1-4 age2Δ mutant cells.
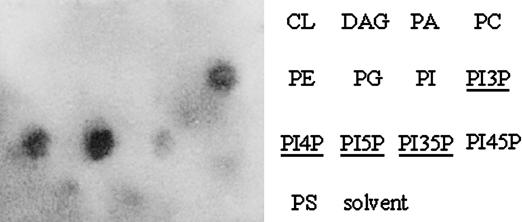
Lipid Binding by Gcs1 in Vitro. The Sfh2 protein binds PI and is thought to be responsible for modifying local lipid environments through an undetermined mechanism (23). In light of the functional relationship demonstrated here between Sfh2 and Gcs1, we assessed the lipid-binding capacity of Gcs1 and Gcs1-4 proteins by using an in vitro lipid-binding assay in which recombinant Gcs1 and Gcs1-4 protein were incubated with a membrane-immobilized lipid as well as a solvent control. As shown in Fig. 5, WT Gcs1 protein bound strongly to monophosphorylated forms of PI. Phosphoinositide binding by Gcs1 is consistent with a previous study that showed that Gcs1 could bind PI-4,5-bisphosphate, but binding to other lipids was not tested (33). Lipid-binding experiments using the Gcs1-4 mutant protein were incubated at 4°C, 23°C, and 37°C. The rationale for the use of different incubation temperatures was that Gcs1-4, as a temperature-sensitive protein, may exhibit temperature-dependent lipid binding. At all temperatures, there was no significant difference in lipid binding between WT and mutant Gcs1 proteins (data not shown).
Fig. 5.
Gcs1 binds to certain phospholipids. Purified recombinant Gcs1 protein (6xHis tagged) was used to probe immobilized lipids.
Neither Gcs1 nor Gcs1-4 gave detectable binding to DAG (Fig. 5 and data not shown), despite the ability of exogenous DAG to relieve gcs1-4 temperature sensitivity. Failure to detect binding of Gcs1 to DAG may reflect the fact that DAG has been demonstrated to activate Gcs1 ArfGAP activity but has not been shown to recruit Gcs1 to membranes. We therefore hypothesize that binding to phosphoinositides may recruit Gcs1 to membranes, where it is subsequently activated by DAG through a mechanism that likely involves a local alteration in membrane curvature or lipid packing to increase Gcs1 ArfGAP activity (31, 34).
Discussion
We show here that the gcs1-4 mutation, in cells lacking the AGE2 gene, causes temperature sensitivity for ArfGAP function, selectively affecting trans-Golgi-specific vesicular transport. The effects of enfeebled Gcs1-4 ArfGAP can be relieved by increased dosage of the SFH2 gene encoding a PI transfer proteins (PITP). The founding member of the yeast PITP protein family is Sec14; Sfh2 protein resembles Sec14 and, like Sec14, can transfer PI in vitro but cannot transfer PC. The Gcs1 and Age2 ArfGAP proteins have been shown previously to be the downstream effectors of the Sec14-mediated activity required for effective vesicular transport from the trans-Golgi. Increased expression of Sec14 did not affect gcs1-4 age2Δ mutant cells, but increased expression of a Sec14 mutant protein able to bind only PC was able to compensate for impaired Gcs1-4 activity in these cells. These findings suggest that regulation of lipid composition may affect the activity of Gcs1 in cells.
Gcs1 ArfGAP activity is known to be affected by phospholipids in vitro, with DAG stimulating and PC (the most abundant phospholipid in yeast membranes) inhibiting its ArfGAP activity (24, 31). Consistent with these findings, we show here that increasing DAG levels either by addition of DAG to the growth medium or by increased PLD expression suppressed the effects of the mutationally enfeebled Gcs1 protein. Despite the stimulatory effect of DAG on ArfGAP function, we did not see direct binding of recombinant Gcs1 to DAG in vitro. Instead, we observed binding to several monophosphorylated PI species, with strongest binding to PI-4-P. Phosphorylated PI species are, at best, only weak activators of Gcs1 ArfGAP activity. PI-4-P is enriched in the Golgi and is thought to localize proteins to the cytosolic face of these membranes (19, 38, 39). Thus, our in vitro findings of phosphorylated PI binding probably reflect the mechanism by which Gcs1 becomes properly localized without direct influence on ArfGAP activity, whereas our observed stimulatory effect of DAG in vivo reflects regulation of ArfGAP activity through alterations in membrane curvature or lipid packing.
The assembly of coat proteins at a membrane site causes deformation of the membrane surface to form a vesicle. ArfGAP activity is required for both formation of transport vesicles and in preparation of transport vesicle fusion with a target membrane (8). The rate of ArfGAP-mediated hydrolysis of Arf-bound GTP has been shown to be responsive to membrane curvature such that lipid compositions that favor curvature, and so mimic a transport vesicle, stimulate ArfGAP function (34). Membrane curvature that accompanies the formation of a spherical vesicle entails altered lipid packing and lipid composition (40, 41). Large head groups (such as phosphorylated PI) favor a more planar bilayer, whereas small head groups (such as DAG) favor the formation of a curved membrane. As the in vitro stimulation of ArfGAP activity by DAG used liposomes, a situation in which DAG levels could affect ArfGAP function through effects on membrane curvature, increased DAG levels may create a lipid environment that stimulates Gcs1 ArfGAP activity.
Previously, we demonstrated that Sec14 generates a lipid environment that favors trans-Golgi transport through stimulation of ArfGAP function (24). Our findings here that Gcs1 responds to DAG levels but does not bind to DAG and instead binds to phosphorylated PI suggest that one role for Sfh proteins is to create the appropriate lipid environment for regulation of vesicular transport, including the stimulation of ArfGAP activity. From our results, it is clear that Sfh2 modulates ArfGAP function through effects on Spo14 activity. Indeed, Sfh proteins are known to be necessary for normal Spo14 activity (22). Stimulation of Spo14 PLD activity will lead to increased levels of DAG, and, as we show here, an increase in DAG levels can compensate for otherwise inadequate Gcs1 activity. We favor a model in which the Gcs1 ArfGAP is localized by binding to phosphorylated PI and is activated by DAG-mediated membrane curvature as the transport vesicle is formed. Thus, ArfGAPs are subject to both temporal and spatial regulation that requires Sfh-mediated modulation of the lipid environment.
Acknowledgments
We thank V. Bankaitis (University of North Carolina, Chapel Hill) and P. Griac (Slovak Academy of Science, Ivanka pri Dunaji, Slovakia) for strains and plasmids and members of the Dalhousie University yeast group for helpful discussions. This work was supported by the Canadian Cancer Society through a grant from the National Cancer Institute of Canada (to G.C.J. and R.A.S.) and grants from the Canadian Institutes of Health Research (to G.C.J., R.A.S., and C.R.M.) and the Canada Research Chairs fund (to C.R.M.).
Author contributions: T.A.W., G.D.F., P.P.P., C.R.M., R.A.S., and G.C.J. designed research; T.A.W., G.D.F., and P.P.P. performed research; M.S. contributed new reagents/analytic tools; T.A.W., G.D.F., P.P.P., and G.C.J. analyzed data; and C.R.M., R.A.S., and G.C.J. wrote the paper.
Abbreviations: Arf, ADP-ribosylation factor; PC, phosphatidylcholine; DAG, diacylglycerol; PI, phosphatidylinositol; GAP, GTPase activating protein; PLD, phospholipase D.
References
- 1.Kirchhausen, T. (2000) Nat. Rev. Mol. Cell. Biol. 1, 187–198. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson, J. G. & Klausner, R. D. (1994) Curr. Opin. Cell Biol. 6, 527–532. [DOI] [PubMed] [Google Scholar]
- 3.Peyroche, A., Paris, S. & Jackson, C. L. (1996) Nature 384, 479–481. [DOI] [PubMed] [Google Scholar]
- 4.Helms, J. B., Palmer, D. J. & Rothman, J. E. (1993) J. Cell Biol. 121, 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cukierman, E., Huber, I., Rotman, M. & Cassel, D. (1995) Science 270, 1999–2002. [DOI] [PubMed] [Google Scholar]
- 6.Tanigawa, G., Orci, L., Amherdt, M., Ravazzola, M., Helms, J. B. & Rothman, J. E. (1993) J. Cell Biol. 123, 1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang, J. S., Lee, S. Y., Gao, M., Bourgoin, S., Randazzo, P. A., Premont, R. T. & Hsu, V. W. (2002) J. Cell Biol. 159, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis, S. M., Poon, P. P., Singer, R. A., Johnston, G. C. & Spang, A. (2004) Mol. Biol. Cell 15, 4064–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Springer, S., Spang, A. & Schekman, R. (1999) Cell 97, 145–148. [DOI] [PubMed] [Google Scholar]
- 10.Poon, P. P., Wang, X. M., Rotman, M., Huber, I., Cukierman, E., Cassel, D., Singer, R. A. & Johnston, G. C. (1996) Proc. Natl. Acad. Sci. USA 93, 10074–10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poon, P. P., Cassel, D., Spang, A., Rotman, M., Pick, E., Singer, R. A. & Johnston, G. C. (1999) EMBO J. 18, 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang, C. J., Cavenagh, M. M. & Kahn, R. A. (1998) J. Biol. Chem. 273, 19792–19796. [DOI] [PubMed] [Google Scholar]
- 13.Poon, P. P., Nothwehr, S., Singer, R. A. & Johnston, G. C. (2001) J. Cell Biol. 155, 1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Meer, G. & Sprong, H. (2004) Curr. Opin. Cell Biol. 16, 373–378. [DOI] [PubMed] [Google Scholar]
- 15.Itoh, T. & De Camilli, P. (2004) Nature 429, 141–143. [DOI] [PubMed] [Google Scholar]
- 16.De Matteis, M. A. & Godi, A. (2004) Biochim. Biophys. Acta 1666, 264–274. [DOI] [PubMed] [Google Scholar]
- 17.Godi, A., Di Campli, A., Konstantakopoulos, A., Di Tullio, G., Alessi, D. R., Kular, G. S., Daniele, T., Marra, P., Lucocq, J. M. & De Matteis, M. A. (2004) Nat. Cell Biol. 6, 393–404. [DOI] [PubMed] [Google Scholar]
- 18.Bankaitis, V. A., Aitken, J. R., Cleves, A. E. & Dowhan, W. (1990) Nature 347, 561–562. [DOI] [PubMed] [Google Scholar]
- 19.Hama, H., Schnieders, E. A., Thorner, J., Takemoto, J. Y. & DeWald, D. B. (1999) J. Biol. Chem. 274, 34294–34300. [DOI] [PubMed] [Google Scholar]
- 20.Cleves, A. E., McGee, T. P., Whitters, E. A., Champion, K. M., Aitken, J. R., Dowhan, W., Goebl, M. & Bankaitis, V. A. (1991) Cell 64, 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, X., Xie, Z. & Bankaitis, V. A. (2000) Biochim. Biophys. Acta 1486, 55–71. [DOI] [PubMed] [Google Scholar]
- 22.Li, X., Routt, S. M., Xie, Z., Cui, X., Fang, M., Kearns, M. A., Bard, M., Kirsch, D. R. & Bankaitis, V. A. (2000) Mol. Biol. Cell 11, 1989–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Routt, S. M. & Bankaitis, V. A. (2004) Biochem. Cell Biol. 82, 254–262. [DOI] [PubMed] [Google Scholar]
- 24.Yanagisawa, L. L., Marchena, J., Xie, Z., Li, X., Poon, P. P., Singer, R. A., Johnston, G. C., Randazzo, P. A. & Bankaitis, V. A. (2002) Mol. Biol. Cell 13, 2193–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guthrie, C. & Fink, G. R. (1991) Methods in Enzymology (Academic, New York), Vol. 194.
- 26.Vida, T. A. & Emr, S. D. (1995) J. Cell Biol. 128, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bankaitis, V. A., Malehorn, D. E., Emr, S. D. & Greene, R. (1989) J. Cell Biol. 108, 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein, A. & Lampen, J. O. (1975) Methods Enzymol. 42, 504–511. [DOI] [PubMed] [Google Scholar]
- 29.Huber, I., Rotman, M., Pick, E., Makler, V., Rothem, L., Cukierman, E. & Cassel, D. (2001) Methods Enzymol. 329, 307–316. [DOI] [PubMed] [Google Scholar]
- 30.Phillips, S. E., Sha, B., Topalof, L., Xie, Z., Alb, J. G., Klenchin, V. A., Swigart, P., Cockcroft, S., Martin, T. F., Luo, M. & Bankaitis, V. A. (1999) Mol. Cell 4, 187–197. [DOI] [PubMed] [Google Scholar]
- 31.Antonny, B., Huber, I., Paris, S., Chabre, M. & Cassel, D. (1997) J. Biol. Chem. 272, 30848–30851. [DOI] [PubMed] [Google Scholar]
- 32.Henneberry, A. L., Lagace, T. A., Ridgway, N. D. & McMaster, C. R. (2001) Mol. Biol. Cell 12, 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blader, I. J., Cope, M., Jackson, T. R., Profit, A. A., Greenwood, A. F., Drubin, D. G., Prestwich, G. D. & Theibert, A. B. (1999) Mol. Biol. Cell 10, 581–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bigay, J., Gounon, P., Robineau, S. & Antonny, B. (2003) Nature 426, 563–566. [DOI] [PubMed] [Google Scholar]
- 35.Schneiter, R., Brugger, B., Sandhoff, R., Zellnig, G., Leber, A., Lampl, M., Athenstaedt, K., Hrastnik, C., Eder, S., Daum, G., et al. (1999) J. Cell Biol. 146, 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zinser, E., Sperka-Gottlieb, C. D., Fasch, E. V., Kohlwein, S. D., Paltauf, F. & Daum, G. (1991) J. Bacteriol. 173, 2026–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raetz, C. R. (1986) Annu. Rev. Genet. 20, 253–295. [DOI] [PubMed] [Google Scholar]
- 38.Walch-Solimena, C. & Novick, P. (1999) Nat. Cell Biol. 1, 523–525. [DOI] [PubMed] [Google Scholar]
- 39.Audhya, A., Foti, M. & Emr, S. D. (2000) Mol. Biol. Cell 11, 2673–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, Q., Guo, Y., Li, L. & Hui, S. W. (1997) Biophys. J. 73, 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lafleur, M., Bloom, M., Eikenberry, E. F., Gruner, S. M., Han, Y. & Cullis, P. R. (1996) Biophys. J. 70, 2747–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]