Abstract
The present study pertains to the evaluation of urine as a specimen for detection of anti-hepatitis A virus (anti-HAV) antibodies. Immunoglobulin M (IgM), IgG, and IgA capture enzyme-linked immunosorbent assays for hepatitis A were performed on paired serum and urine specimens collected from hepatitis A patients (n = 92), healthy individuals (n = 100), non-A hepatitis patients (n = 70), and patients with nonhepatic diseases (n = 64, including 37 renal disease patients). Hepatitis A patients seropositive for anti-HAV IgM showed 95.65% uropositivity. No false-positive reactions were observed in control groups. The uropositivity of anti-HAV IgM persisted during the convalescent phase of the disease. Anti-HAV IgG uropositivity correlated well with corresponding seropositivity in all groups (P > 0.05 for each). No significant difference between the proportions of serum and urine positivity for anti-HAV IgA was noted (P > 0.05 for each). Using seroreactivity as a “gold standard,” the sensitivity and specificity for anti-HAV IgM, anti-HAV IgG, and anti-HAV IgA tests with urine as a specimen were found to be 95.65 and 100%, 97.76 and 76.47%, and 92.23 and 88.18%, respectively. Urine appears to be comparable to serum for diagnosis of recent and past infection with hepatitis A.
Blood samples are of prime importance in biochemical testing and in seroimmunological diagnosis. Collection of blood specimens, however, is cumbersome on account of the need for sterile equipment and trained staff. In developing countries, the use of disposable syringes, needles, and gloves is not regularly practiced, rendering the subjects at risk for infections. A slippery vein or improper judgment of the location of a vein gives rise to untoward reactions. To circumvent the need for blood samples, the potential of alternative body fluids such as saliva and urine for detection of immunoglobulins against various microbial agents has been investigated (3, 8, 13, 15, 20, 22, 27, 30).
Among the assays employed for detection of salivary or urinary antibodies against infectious agents, antibody class capture assays were preferred to conventional assays (8, 22, 23). The capture assays have been reported to be dependable due to their abilities to capture specific immunoglobulin even at low levels and to establish specificity in the initial stage of the assay. Immunoglobulin M (IgM) and IgG capture radioimmunosorbent assays have been demonstrated to detect urinary and salivary anti-hepatitis A virus (anti-HAV) antibodies (23). An IgG capture enzyme-linked immunosorbent assay (ELISA) has been attempted for detection of antibodies to respiratory syncytial and influenza A/Taiwan (H1N1) viruses in urine (11). However, satisfactory use of IgG capture ELISA for detection of salivary and urinary antibodies against human immunodeficiency virus (HIV) types 1 and 2 has been described (8, 21). This assay appeared to be a promising alternative to conventional tests for use as a new epidemiological tool for surveillance purposes.
We developed an IgM capture ELISA for detection of recent infection with hepatitis A, validated it, and found its sensitivity and specificity to be comparable to those of the commercially available HAVAB-M enzyme immunoassay from Abbott Laboratories, North Chicago, Ill. (5). The assay protocol was further extended for detection of HAV IgG and IgA antibodies in serum. We report here the use of IgM, IgG, and IgA class capture ELISAs for evaluation of urine specimens for the diagnosis of hepatitis A.
MATERIALS AND METHODS
Study subjects and specimens.
Paired serum and urine samples were collected from 100 healthy individuals, 162 hepatitis patients, and 64 patients with nonhepatic diseases. The 100 healthy subjects included children (60 males and 15 females) age 5 to 10 years and adults (9 males and 16 females) age 23 to 58 years. No history of recent illness was reported for these subjects. Most of the subjects belonged to lower-middle socioeconomic classes. The 162 hepatitis patients included patients with sporadic and epidemic cases from Pune in the state of Maharashtra, India. The patients were clinically examined for characteristic symptoms and signs and elevated serum alanine aminotransferase activity and were then referred to the National Institute of Virology, Pune, for serological diagnosis of viral hepatitis. Thus, the samples obtained from patients were after the onset of clinical symptoms with variable postonset periods. This group included 112 males and 50 females age 2 to 55 years. The 64 patients hospitalized on account of nonhepatic diseases included 42 males and 22 females age <1 to 75 years suffering from viral gastritis, bronchitis, anemia, diarrhea, renal disease, or renal failure. Prior to sample collection, informed consent was obtained from healthy adults and patients with nonhepatic diseases. In the case of children, consent was sought from their parents. All serum and urine specimens were stored in 500-μl aliquots at −20 and −70°C, respectively, until processed for ELISAs.
Anti-HAV testing.
IgM antibody capture (MAC), IgG antibody capture (GAC), and IgA antibody capture (AAC) ELISAs for HAV antibodies were carried out by the method described earlier (5) Briefly, microwell ELISA plates (Maxisorb; Nunc, Roskilde, Denmark) were separately coated (125 μl/well) at room temperature overnight with rabbit anti-human IgM (μ chain specific, 3.1 g/liter; Dako, Glostrup, Denmark), anti-human IgG (γ chain specific, 5.7 g/liter; Dako), or anti-human IgA (α chain specific, 5.7 g/liter; Dako) antibodies at dilutions of 1:1,000, 1:250, and 1:400, respectively. The test serum samples were added in dilutions of 1:5 for IgM and IgA and 1:2 for IgG, and urine samples were added undiluted, in identified wells. Addition of HAV, anti-HAV IgG-horseradish peroxidase conjugate, and enzyme substrate (o-phenylene diamine [Sigma, St. Louis, Mo.] and urea peroxide [Sigma]) and termination of the reaction with sulfuric acid (Qualigens, Mumbai, India) were performed as described earlier (5). The cutoff value was determined by adding 1/10 of the mean optical density (OD) from duplicate determinations with a known positive control sample to the mean OD from triplicate determinations with a known negative control sample (10). Specimens with absorbance value less than and greater than the cutoff value were considered negative and positive, respectively (5).
Statistical analysis.
McNemar's test (with continuity correction) was used to compare anti-HAV positivity of paired serum and urine samples for IgM, IgG, and IgA markers of all individuals in the study. Student's t test was used to compare mean values between two independent groups, and the paired t test was used to compare mean values between two paired groups for anti-HAV markers. The chi-square test was used to compare uropositivity of anti-HAV IgM at different time intervals during storage. The correlation coefficient between serum and urine sample OD/cutoff OD (S/Co) ratios and its significance was computed using SPSS version 6.0
RESULTS
Reactivities of paired serum and urine samples in anti-HAV MAC ELISA.
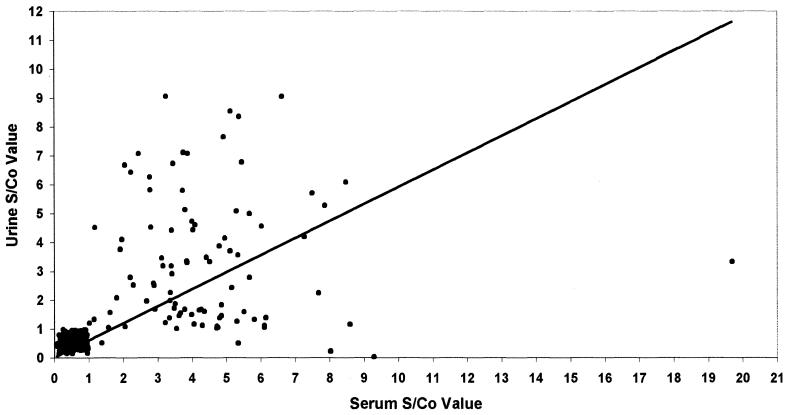
Table 1 shows the comparison between freshly collected paired serum and urine specimens in anti-HAV MAC ELISA. Among anti-HAV IgM-seropositive patients, 95.65% (88 of 92) were uropositive for anti-HAV IgM. There was no statistically significant difference between the percent positivities of serum and urine samples (P > 0.05). All control groups showed sero- and uronegativity for anti-HAV IgM. The mean S/Co ratios obtained for urine specimens from hepatitis A patients were significantly higher than those of control groups (P < 0.05) (Table 1). The correlation between paired serum and urine S/Co ratios was found to be significant (r = 0.59; P < 0.05) (Fig. 1).
TABLE 1.
Comparison of paired serum and urine specimens for anti-HAV IgM
| Group | Serum
|
Urine
|
||
|---|---|---|---|---|
| No. positive/no. tested (% positive) | S/Co (mean ± SD) | No. positive/no. tested (% positive) | S/Co (mean ± SD) | |
| Hepatitis A patients | 92/92 (100) | 4.38 ± 2.38 | 88/92 (95.65) | 3.28 ± 2.23 |
| Healthy controls | 0/98 (0) | 0.58 ± 0.17a | 0/98 (0) | 0.43 ± 0.14 |
| Non-A hepatitis patients | 0/70 (0) | 0.45 ± 0.25a | 0/70 (0) | 0.53 ± 0.21 |
| Patients with nonhepatic diseases | 0/62 (0) | 0.52 ± 0.23a | 0/62 (0) | 0.56 ± 0.20 |
Significant difference compared with value for hepatitis A patients.
FIG. 1.
Scatter diagram displaying anti-HAV IgM activity in simultaneously collected serum and urine specimens from hepatitis A patients (n = 92), healthy controls (n = 98), non-A hepatitis patients (n = 70), and patients with nonhepatic diseases (n = 62).
Of the four patients who were only seropositive, three showed strong reactivity, while one was weakly positive in anti-HAV MAC ELISA. When subjected to total IgM capture ELISA, urine specimens from these patients showed an absence of IgM.
Sequential urine samples were collected from six hepatitis A patients during the postonset period. The S/Co ratios obtained at different days postonset were in the range of 1.02 to 6.55, indicating persistence of anti-HAV IgM for prolonged periods (postonset days were 23, 32, 55, 74, 89, and 130 for the six patients) during the convalescent phase of the disease. A subclinical case of hepatitis A identified in routine surveillance also showed the presence of urinary anti-HAV IgM and its persistence up to day 74 postonset.
Many types of microorganisms multiply rapidly in urine at room temperature. For practical purposes, urine specimens need to be processed rapidly or stored refrigerated during the time before analysis is performed. In order to avoid the problem of bacterial contamination, we preferred to perform tests on fresh samples and store them in aliquots at −70°C. The stability of urine anti-HAV IgM after storage was examined. The percent positivity for anti-HAV IgM in urine specimens declined significantly to 81.25% (26 of 32) (P < 0.05, chi-square test) and 76.08% (35 of 46) (P < 0.01, chi-square test), respectively, after 3 and 6 months, indicating loss of IgM during storage. Six samples were tested after storage under refrigeration (4°C). All six were found to be positive without any loss of anti-HAV IgM activity at 48 to72 h after collection. In order to confirm the stability of anti-HAV IgG and IgA, OD values obtained in GAC and AAC ELISAs were compared using the paired t test. The respective mean ODs ± standard deviations for fresh samples and samples stored for 1 month (n = 10) were 1.07 ± 0.38 and 0.93 ± 0.34 for anti-HAV IgG and 0.45 ± 0.13 and 0.56 ± 0.15 for anti-HAV IgA. No significant difference between the ODs of the two types of samples was noted (P > 0.05).
Anti-HAV IgG.
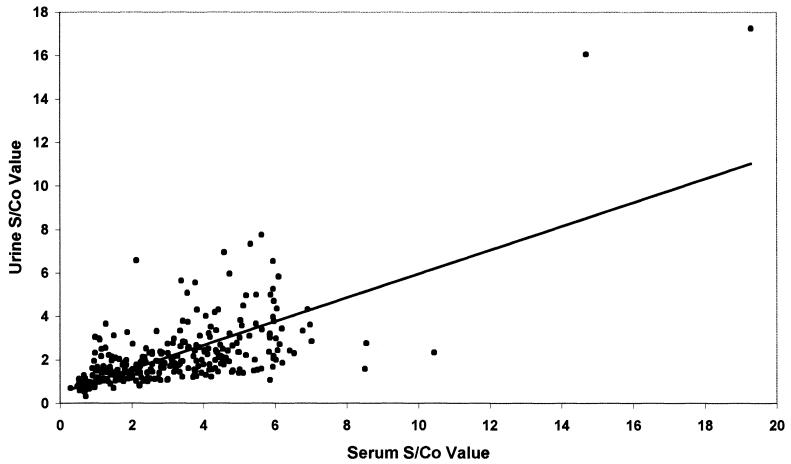
The GAC ELISA test was performed to detect anti-HAV IgG. The proportions of serum and corresponding urine positivity for anti-HAV IgG were in agreement with each other in all groups (McNemar's test, P > 0.05) (Table 2). There were six samples that were only seropositive and eight samples that were only uropositive. However, the correlation between paired serum and urine S/Co ratios was significant (r = 0.7; P < 0.05) (Fig. 2).
TABLE 2.
Comparison of paired serum and urine specimens for anti-HAV IgG
| Group | Serum
|
Urine
|
||
|---|---|---|---|---|
| No. positive/no. tested (% positive) | S/Co (mean ± SD) | No. positive/no. tested (% positive) | S/Co (mean ± SD) | |
| Hepatitis A patientsa | 80/80 (100) | 4.33 ± 2.55 | 80/80 (100)c | 3.07 ± 2.70 |
| Healthy controlsb | 96/100 (96) | 3.77 ± 1.74 | 96/100 (96)c | 2.28 ± 1.03 |
| Non-A hepatitis patientsb | 48/58 (82.75) | 2.11 ± 1.09 | 50/58 (86.21)c | 1.72 ± 0.82 |
| Patients with nonhepatic diseasesb | 44/64 (68.75) | 1.76 ± 1.57 | 44/64 (68.75)c | 1.43 ± 0.70 |
Anti-HAV IgM positive.
Anti-HAV IgM negative.
Marked correlation with corresponding serum value.
FIG. 2.
Scatter diagram displaying anti-HAV IgG activity in simultaneously collected serum and urine specimens from hepatitis A patients (n = 80), healthy controls (n = 100), non-A hepatitis patients (n = 58), and patients with nonhepatic diseases (n = 64).
Anti-HAV IgA.
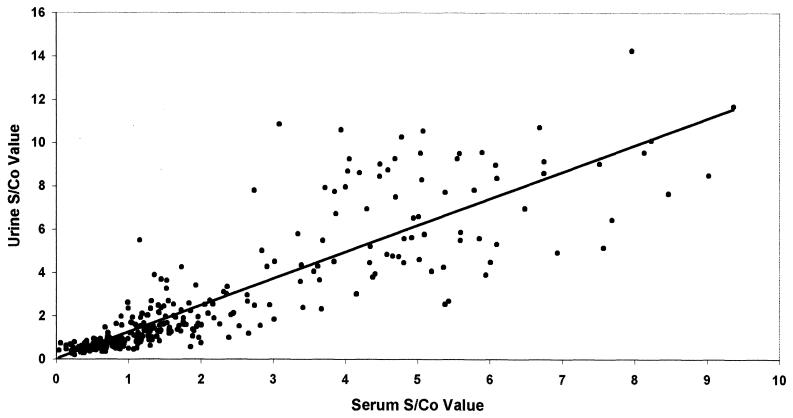
The anti-HAV AAC ELISA indicated that there was no significant difference between the proportions of serum and urine positivity for anti-HAV IgA among all groups studied (McNemar's test, P > 0.05) (Table 3). In comparison to control groups, the mean S/Co ratios obtained for both serum and urine samples from hepatitis A patients were significantly high (P < 0.01 for each comparison). Within the hepatitis A patients, the mean S/Co ratio for urine specimens was significantly higher than that of serum specimens, indicating higher reactivity of urine for anti-HAV IgA (P < 0.01). With this marker, individuals who were only seropositive (n = 16) and who were only uropositive (n = 12) were identified in all groups except the hepatitis A patient group, which included one sample that was only uropositive. The scatter diagram of anti-HAV IgA S/Co ratios showed a correlation coefficient of 0.86 with a P value of <0.05 (Fig. 3).
TABLE 3.
Comparison of paired serum and urine specimens for anti-HAV IgA
| Group | Serum
|
Urine
|
||
|---|---|---|---|---|
| No. positive/no. tested (% positive) | S/Co (mean ± SD) | No. positive/no. tested (% positive) | S/Co (mean ± SD) | |
| Hepatitis A patientsa | 83/86 (96.51) | 4.77 ± 1.79 | 84/86 (97.67)c | 6.00 ± 2.56 |
| Healthy controlsb | 67/100 (67) | 1.40 ± 0.71 | 65/100 (65)c | 1.52 ± 0.89 |
| Non-A hepatitis patientsb | 44/66 (66.66) | 1.18 ± 0.51 | 40/66 (60.6)c | 1.21 ± 0.63 |
| Patients with nonhepatic diseasesb | 12/64 (18.75) | 0.69 ± 0.43 | 14/64 (21.87)c | 0.89 ± 0.66 |
Anti-HAV IgM positive.
Anti-HAV IgM negative.
No significant difference compared with corresponding serum value.
FIG. 3.
Scatter diagram displaying anti-HAV IgA activity in simultaneously collected serum and urine specimens from hepatitis A patients (n = 86), healthy controls (n = 100), non-A hepatitis patients (n = 66), patients with nonhepatic diseases (n = 64).
Comparison of anti-HAV antibody contents in serum and urine.
Freshly collected serum and urine specimens from three hepatitis A patients were serially diluted, and the titers of anti-HAV IgM, IgG, and IgA antibodies were determined in capture ELISAs. As reported earlier (6), very high titers (1:32,000 to 1:256,000) were observed in the sera for all three classes of antibodies. Urine anti-HAV antibody titers were, however, significantly lower (undiluted, 1:128).
Sensitivities and specificities of tests using urine as a specimen.
Using seroreactivity as a “gold standard,” the sensitivities and specificities of the assays using urine as a specimen were, respectively, 95.65 and 100% for anti-HAV MAC ELISA, 97.76 and 76.47% for anti-HAV GAC ELISA, and 92.23 and 88.18% for anti-HAV AAC ELISA (Table 4).
TABLE 4.
Concordance between paired serum and urine IgM, IgG, and IgA anti HAV activities
| Serum result | No. of samples with the indicated urine result for anti-HAV:
|
|||||
|---|---|---|---|---|---|---|
| IgM
|
IgG
|
IgA
|
||||
| Positive | Negative | Positive | Negative | Positive | Negative | |
| Positive | 88 | 4 | 262 | 6 | 190 | 16 |
| Negative | 0 | 230 | 8 | 26 | 13 | 97 |
DISCUSSION
The class-specific antibody capture ELISAs described in this study clearly detected urinary HAV antibodies. Use of urine specimens in anti-HAV MAC ELISA correctly identified 95.65% of anti-HAV IgM-seropositive hepatitis A patients and did not produce any false-positive reactions in control groups. The sensitivity of the test using urine may have been equivalent to that of the test used for serum. However, the urine samples classified as negative for anti-HAV IgM lacked total IgM. Thus, the absence of IgM in urine indicated the absence of filtration, local synthesis, or transudation of IgM in urine or its presence below the detection limits of the MAC ELISAs employed. Testing of follow-up urine samples and/or concentration of larger volumes may be useful in such cases. Loss of IgM was observed when urine samples were stored at −70°C, and hence the use of fresh samples is desirable in such tests. Storage period of 3 to 4 days at 4°C may not be detrimental for routine testing. Similar to the persisting seropositivity of anti-HAV IgM reported earlier (5), uropositivity was detected for prolonged times during the convalescent phase.
Hepatitis A is endemic in India. The extent of exposure to HAV is usually very high, resulting in the generation of anti-HAV antibody early in life (1). In our study, sero- and uropositivity displayed 97.76% concordance for anti-HAV IgG, suggesting the utility of urine for detection of past infection. It was also noted that in a group of patients with nonhepatic diseases, anti-HAV IgG positivity in serum and urine was significantly low compared to that in healthy individuals (P < 0.01). The decreased anti-HAV IgG prevalence was mainly in patients with kidney disease. It is unlikely that these patients were not exposed to HAV. However, the reasons for high proportion of negativity could not be traced. Loss of detectable antibody following immunosuppression may occur (2).
The role of anti-HAV IgA in relation to intestinal or mucosal immunity has been investigated (16, 25, 26). The present study evaluated IgA from urine as a marker of immunity and its possible use in the diagnosis. Uropositivity for anti-HAV IgA was in 95.65% agreement with seropositivity. So far, the seroprevalence of anti-HAV IgA in immune individuals has not been investigated. The conditions required to maintain or boost anti-HAV IgA-type response are also not known. The data obtained in our study on the seroprevalence of anti-HAV IgA in healthy individuals suggest that anti-HAV IgA probably lasts for shorter period than that of IgG (Tables 2 and 3). However, if it persists in serum, its presence can be detected in urine. Similar to the case for anti-HAV IgG, decreased sero- and uroprevalence of anti-HAV IgA was observed mainly in patients with kidney diseases, indicating loss or low concentrations of antibodies not detectable by the test employed.
In the present study, only sero- or uropositivity for anti-HAV IgG or IgA was noted in a small proportion of patients. Such a type of response has been described earlier with HIV antibody screening (17, 28). Only uronegativity or -positivity was counted as false reactivity when serum reactivity was considered the gold standard. Subsequently, when a large number of samples were analyzed, it was demonstrated that neither serum nor urine results alone were as sensitive for HIV type 1 antibody detection as combined results from both samples (29). Mazzoli et al. have suggested the possibility of a compartmentalized immune response to pathogens and protective roles of cell-mediated immunity and mucosal IgA in anti-HIV-negative individuals exposed to HIV (19).
Overall virus-specific IgM, IgG, and IgA synthesis elicited in HAV infection was observed in urine specimens. Such an observation was not made for hepatitis E virus infection which is also transmitted feco-orally with similar clinical and biochemical symptoms (12). Renal involvement and renal failure have been reported in association with fulminant and nonfulminant hepatitis A (24, 32). The exact mechanism involved in affected patients is not known. Immune complex-mediated glomerulonephritis, acute tubular necrosis, and hepatorenal syndrome have been documented in some cases (14, 24, 31). Virus-induced injury has been postulated by Chio and Bakir (4). Monkey kidney cells are known to support in vitro propagation of HAV (7, 9). Glomeruli have also been identified as extrahepatic sites for HAV replication in experimentally infected animals (18). Whether such a renal site exists in vivo in hepatitis A patients, leading to local synthesis of immunoglobulins, needs to be investigated.
Finally, the usefulness of urine as a specimen for diagnosis of hepatitis A, highlighted by our study, could be confirmed in large-scale epidemiological studies. If it stands the test of large sample sizes, this may find several applications in routine surveillance, epidemiological investigations, and hepatitis A vaccination programs.
Acknowledgments
We thank A. R. Bavdekar, Kalpana Kulkarni, and staff of the Department of Paediatrics, KEM Hospital, and Nephro Ward, Sasson General Hospital, Pune, India, for their help in providing clinical samples. We are thankful to V. S. Padbidri and B. L. Rao, National Institute of Virology, for critical evaluation of the manuscript and support. The statistical assistance of A. M. Walimbe and the technical assistance of Prakash Jawalkar and Rajesh Kannalu are gratefully acknowledged.
REFERENCES
- 1.Arankalle, V. A., M. S., Chadha, S. D. Chitambar, A. M., Walimbe, L. P. Chobe, and S. S. Gandhe. 2001. Changing epidemiology of hepatitis A and E in urban and rural India. J. Viral Hepatitis 8:293-303. [DOI] [PubMed] [Google Scholar]
- 2.Arslan, M., R. H. Wiesner, J. J. Poterucha, J. B. Gross, and N. N. Zein. 1998. Hepatitis A virus antibodies in liver transplant (OLT) recipients: evidence for loss of immunity post transplantation. Hepatology 28:235A. [DOI] [PubMed] [Google Scholar]
- 3.Carli, K. T., H. Batmaz., A. Sen, and A. Minbay. 1993. Comparison of serum, milk and urine as samples in an enzyme immunoassay for bovine leukaemia virus infection. Res. Vet. Sci. 55:394-395. [DOI] [PubMed] [Google Scholar]
- 4.Chio, F., and A. A. Bakir. 1992. Acute renal failure in hepatitis A. Int. J. Artif. Organs 1S:413-416. [PubMed] [Google Scholar]
- 5.Chitambar, S. D., S. Murthy-Grewal, M. Bokil, V. A. Arankalle, M. M. Gore, and K. Banerjee. 1994. Indigenous anti hepatitis A virus IgM capture ELISA for the diagnosis of hepatitis A. Indian J. Med. Res. 99:243-251. [PubMed] [Google Scholar]
- 6.Chitambar, S. D., M. S. Joshi, V. A. Arankalle, and K. Banerjee. 1996. Sensitive ELISA tests for detection of anti hepatitis A virus antibodies. Serodiagn. Immunother. Infect. Dis. 8:63-65. [Google Scholar]
- 7.Chitambar, S. D., S. Murthy Grewal, M. Bokil, M. A. Srinivasan, and K. Banerjee. 1994. Cultivation of buffalo green monkey kidney cells persistently infected with hepatitis A virus. Indian J. Med. Res. 99:115-120. [PubMed] [Google Scholar]
- 8.Connell, J. A., J. V. Parry, P. P. Mortimer, and J. Duncan. 1993. Novel assay for the detection of immunoglobulin G antihuman immunodeficiency virus in untreated saliva and urine. J. Med. Virol. 41:159-164. [DOI] [PubMed] [Google Scholar]
- 9.Daemer, R. J., S. M. Feinstone, I. D. Gust, and R. H. Purcell. 1981. Propagation of human hepatitis A virus in African green monkey kidney cell culture: primary isolation and serial passage. Infect. Immun. 32:388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decker, R. H., S. M. Kosakowski, A. S. Vanderbilt, C. M. Ling, R. Chairez, and L. R. Overby. 1981. Diagnosis of acute hepatitis A by HAVAB-M, a direct radioimmunoassay for IgM anti-HAV. Am. J. Clin. Pathol. 76:140-147. [DOI] [PubMed] [Google Scholar]
- 11.Ireland, D. C., and K. G. Nicholson. 1996. Diagnosis of respiratory virus infections using GACELISA of urinary antibodies. J. Immunol. Methods 195:73-80. [DOI] [PubMed] [Google Scholar]
- 12.Joshi, M. S., A. M. Walimbe, V. A. Arankalle, M. S. Chadha, and S. D. Chitambar. 2002. Hepatitis E antibody profiles in serum and urine. J. Clin. Lab. Anal. 16:137-142. [DOI] [PMC free article] [PubMed]
- 13.Koopmans, M., D. Sanchez-Martinez, J. Patton, and J. Stewart. 1995. Evaluation of antigen and antibody detection in urine specimens from children with congenital human cytomegalovirus infection. J. Med. Virol. 46:321-328. [DOI] [PubMed] [Google Scholar]
- 14.Kramer, M. R., C. Hershko, and I. N. Slotkik. 1986. Acute renal failure associated with non fulminant type A viral hepatitis. Clin. Nephrol. 25:219. [PubMed] [Google Scholar]
- 15.Lerner, M., J. S. Remington, and M. Finland. 1962. Neutralizing antibody to poliovirus in normal human urine. J. Clin. Investig. 41:805-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locarnini, S. A., A. G. Coulepis, J. Kaldor, and I. D. Gust. 1980. Coproantibodies in hepatitis A: detection by enzyme linked immunosorbent assay and immune electron microscopy. J. Clin. Microbiol. 11:71-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez, P., R. Ortiz de Lejarazu, J. M. Eiros, J. De Benito, Rodriguez, and A Torres. 1996. Urine samples as a possible alternative to serum for human immunodeficiency virus antibody screening. Eur. J. Clin. Microbiol. Infect. Dis. 15:810-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathiesen, L. R., Feinstone, S. M., R. H Purcell, and J. A. Wagner. 1977. Detection of hepatitis A antigen by immunofluorescence. Infect. Immun. 18:524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzoli, S., D. Trabattoni, C. S. Lo, S. Piconi, C. Ble, F. Meacci, S. Ruzzante, A. Salvi, F. Semplici, R. Longhi, M. L. Fusi, N. Tofani, M. Biasin, M. L. Villa, F. Mazzotta, and M. Clerici. 1997. HIV specific mucosal and cellular immunity in HIV seronegative partners of HIV seropositive individuals. Nat. Med. 3:1250-1257. [DOI] [PubMed] [Google Scholar]
- 20.Miwa, H., M. Hirose, S. Kikuchi, T. Terai, R. Iwazaki, O. Kobayashi, Y. Takei, T. Ogihara, and N. Sato. 1999. How useful is the detection kit for antibody to Helicobactor pylori in urine (Urine ELISA) in clinical practice? Am. J. Gastroenterol. 94:3460-3463. [DOI] [PubMed] [Google Scholar]
- 21.Mortimer, P., and J. V. Parry. 1991. Non invasive virological diagnosis: are saliva and urine specimens adequate substitutes for blood? J. Med. Virol. 1:73-78. [Google Scholar]
- 22.Ochnio, J. J., D. W. Scheifele, H. O. Margaret, and L. A. Mitchell. 1997. New ultrasensitive enzyme immunoassay for detecting vaccine- and disease-induced hepatitis A virus-specific immunoglobulin G in saliva. J. Clin. Microbiol. 35:98-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry, K. R., J. V. Parry, E. M. Vandervelde, and P. P. Mortimer. 1992. The detection in urine specimens of IgG and IgM antibodies to hepatitis A and hepatitis B core antigens. J. Med. Virol. 38:265-270. [DOI] [PubMed] [Google Scholar]
- 24.Philips, A. O., D. M. Thomas, and G. A. Coles. 1993. Acute renal failure associated with nonfulminating hepatitis A. Clin. Nephrol. 39:156-157. [PubMed] [Google Scholar]
- 25.Stapleton, J. T., D. K. Lange, J. W. LeDuc, L. N. Binn, R. W. Jansen, and S. M. Lemon. 1991. The role of secretory immunity in hepatitis A virus infection. J. Infect. Dis. 163:7-11. [DOI] [PubMed] [Google Scholar]
- 26.Suga, M., Y. Akahonai, M. Arashi, T. Sasanami, H. Yoshizaki, H. Fujita, M. Ikebe, and A. Yachi. 1982. Studies on IgA type antibody in patients with hepatitis A. Acta Hepatol. Jpn. 23:9-14. [Google Scholar]
- 27.Terda, K., T. Niizuma, N. Kataoka, and Y. Niitani. 2000. Testing for rubella specific IgG antibody in urine. Pediatr. Infect. Dis. J. 19:104-108. [DOI] [PubMed] [Google Scholar]
- 28.Urnovitz, H. B., M. Clerici, G. M. Shearer, T. D. Gottfried, D. J. Robinson, L. I. Lutwick, L. Montagnier, and D. V. Landers. 1993. HIV-1 antibody serum negativity with urine positivity. Lancet 342:1458-1459. [DOI] [PubMed] [Google Scholar]
- 29.Urnovitz, H. B., J. C. Sturge, and T. D. Gottfried. 1997. Increased sensitivity of HIV-1 antibody detection. Nat. Med. 3:1258. [DOI] [PubMed] [Google Scholar]
- 30.Verta, L. A., T. D. Elisova, and G. M. Voronkova. 1993. The detection of antibodies to the hantan virus in the urine of patients with hemorrhagic fever with renal syndrome. Vopr. Virusol. 38:18-21. [PubMed] [Google Scholar]
- 31.Watanabe, S., H. Nomoto, and M. Matsuda. 1986. A case of acute renal failure associated with type A acute hepatitis responds dramatically to plasmapheresis. Tokii J. Exp. Clin. Med. 11:1-4. [PubMed] [Google Scholar]
- 32.Wilkinson, S. P., M. J. Weston, V. Parsons, and R. Williams. 1977. Dialysis in the treatment of renal failure in patients with liver disease. Clin. Nephrol. 8:287-292. [PubMed] [Google Scholar]