Abstract
Monarch butterflies (Danaus plexippus) undertake one of the most remarkable long-distance insect migrations, travelling thousands of kilometres to overwinter in the central trans-volcanic belt of Mexico. This study explored how monarch butterflies use essential fatty acids (EFA) and nonessential fatty acids (NFA) during overwintering. We collected 150 (male/female) butterflies from the Sierra Chincua wintering colony from the time of arrival (December 2022) to before departure (February 2023) and analysed their lipid content. Our findings revealed that although females have a higher mass fraction of lipids, male and female monarch butterflies depleted their lipids similarly over time, resulting in low abdominal lipid mass fractions by late February. NFA, including oleic and palmitic acid, were predominantly used for energy during overwintering by male and female butterflies. In contrast, the EFA alpha-linolenic and linoleic acids, critical for reproductive success and cellular functions, were conserved in both sexes. Males began the overwintering period with a higher mass fraction of EFA in the polar components of the head and thorax, which may impact the degree of cold acclimation of these tissues during this period. Strategic lipid utilisation, prioritising the preservation of EFA over NFA and optimizing overwintering survival probably enhance readiness for spring remigration and reproduction. This differential fatty acid use underscores the delicate balance monarch butterflies maintain to survive overwintering and highlights the potential impacts of environmental changes on their lipid dynamics and survival.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00359-024-01727-0.
Keywords: Monarch butterfly, Lipids, Wintering, Energetics, Fatty acids
Introduction
The monarch butterfly (Danaus plexippus) is a well-known long-distance insect migrant in North America. In late summer, coincident with changes in food and habitat quality, monarch butterflies migrate up to several thousand kilometres to hibernate at high-altitude overwintering sites in central Mexico (Wassenaar and Hobson 1998; Goehring and Oberhauser, 2002; Chapman et al. 2015). While many other insects become reproductively active soon after completing migration (Southwood 1962; Dingle 1972; Ramenofsky and Wingfield 2007; Chapman et al. 2012, 2015), monarch butterflies undergo a months-long overwintering diapause after their fall migration (Urquhart and Urquhart 1978; Brower 1985; Brower et al. 2006). The Eastern monarch butterflies’ main overwintering sites are located in the central trans-volcanic belt of Mexico (Urquhart and Urquhart 1978). After an energetically costly overwintering period, these butterflies undergo a spring remigration into southern parts of the United States to reproduce (Brower 1985).
The main energy resources for migratory insects, including monarch butterflies, are lipids due to their higher energy density compared to carbohydrates (Stanley-Samuelson et al. 1988; Forte et al. 2002; Brower et al. 2006; Arrese and Soulages 2010). Monarch butterflies rely on lipids they have accumulated and stored during their migratory journey to fuel the overwintering period. Several studies suggested there is no to minimal lipid accumulation during overwintering (Cenedella 1971; Brower 1985; Calvert and Lawton 1993; Brower et al. 2006). Lipids can enhance chill and freeze resilience during high-altitude overwintering (Sinclair and Marshall 2018). The allocation of lipids (synthesized from nectar carbohydrates or directly taken up from larval milkweed) used during these energetically demanding life stages is key to the overall life history success of migrant monarch butterflies.
It is generally assumed that monarch butterflies do not feed during overwintering. Thus, the excessive loss of critically stored energetic lipids to supply the overwintering period can result in premature mortality (Chaplin and Wells 1982; Masters et al. 1988; Williams and Brower, 2015). Overwintering diapause, associated with suppressed metabolic rates (Bale and Hayward 2010; Sinclair and Marshall 2018), reduces the depletion of lipid resources during the overwintering period. However, high temperatures during the day increase the metabolic demands of ectothermic monarch butterflies. Air temperatures > 20 °C may break diapause, triggering premature mating and eliciting premature remigration efforts (Herman et al. 1989; Williams and Brower 2015), all of which threaten the monarch butterflies’ chances of surviving the overwintering period for a successful spring remigration. Moreover, exposure to low night temperatures (< 4 °C) can trigger lipid use as a result of increased heat production or thermogenesis via shivering of thoracic muscles (Masters et al. 1988).
During the overwintering period, monarch butterflies roost in dense colonies in a few high-altitude Oyamel fir (Abies religiosa (Kunth) Schltdl. & Cham.) locations in central Mexico. These fir trees are vital for maintaining stable temperatures (e.g. thermal blanket) for monarch butterflies to survive the entire overwintering period (Anderson and Brower 1996; Brower et al. 2009; Malcolm 2018). Habitat loss due to forest thinning and climatic factors (Brower et al. 2009; Ramirez et al. 2015) reduces the thermal stability of these overwintering sites which can result in increased lipid depletion. Regardless, monarch butterflies do not remain stationary during the overwintering period and are frequently seen seeking drinking water (Calvert and Brower 1981,1986; Brower et al. 2006; Malcom, 2018), an activity which also depletes lipid stores. Monarch butterflies with insufficient total lipid reserve levels are unlikely to survive overwintering and are not able to remigrate and reproduce (Masters et al. 1988; Alonso-Mejia et al. 1997).
Several studies have assessed total lipid loss in monarch butterflies during the overwintering period (e.g., Alonso-Mejia et al. 1997). Still, nothing is known about the use of specific fatty acids (FA). This information is important because while some FA can be synthesised from other macronutrients (nonessential fatty acids, NFA), essential FA (EFA) cannot be de novo synthesised and must come from the diet (Karasov & Martinez del Rio 2007). In nectivorous lepidopterans such as monarch butterflies, NFA can be de novo synthesised from carbohydrates obtained from nectar (Canavoso et al. 2001). Therefore, additional feeding may replenish NFA loss during the overwintering and migratory periods. However, EFA are not commonly found in nectar (Karasov & Martinez del Rio 2007; Nicolson & Thornburg 2007; Krenn 2010; Nicolson 2022). This means the limited pool of EFA largely stems from the larval stage of monarch butterfly development. EFA are vital for reproduction as they play key roles in membrane integrity, regulating reproductive behaviours, navigation, signalling molecule synthesis and brain and eye development (Downer and Matthews 1976; Stanley-Samuelson et al. 1988; Forte et al. 2002; Arrese and Soulages 2010; Malcicka et al. 2018; Pilecky et al. 2021). Understanding patterns of specific FA use during the overwintering period may provide new insight into the delicate balance of lipid resource use in monarch butterflies that facilitates survival during the overwintering period while retaining enough limited EFA to reproduce.
This study aimed to establish patterns of differential use and functional distribution of FA in the key body parts (head, thorax, abdomen) of female and male monarch butterflies (50 per month) during the 2022–2023 overwintering period in Mexico. The FA reported in this study are the following NFA; oleic, palmitic, and stearic acid. These NFA are found in high levels in Lepidoptera, such as monarch butterflies (Subramanyam and Cutkomp 1987; Canavoso et al. 2001; Pilecky et al. 2022). The EFA alpha-linolenic acid, linoleic acid, and eicosapentaenoic acid were also quantified because Lepidopterans cannot de novo synthesise them from nectar carbohydrates, but significant amounts were found in monarch butterflies previously (Pilecky et al. 2022). As the focus of this study was to investigate the differential use of FA in relation to total lipid loss during the overwintering period rather than absolute lipid mass loss, proportional examination of FA (mass fraction of FA/mass fraction of total lipids) was utilized. This method was used to better understand which FA declined similarly to total lipid decline, and which were used to a lesser degree (i.e., conserved) relative to total lipid loss. If certain FA are not readily mobilized as total lipids are lost, this would reflect an increase in the proportion of said FA over time while FA lost at similar rates to the loss of total lipids would reflect a constant or decreasing proportion over time. The changes in proportion can only arise if the FA are being used differently regarding total lipid loss; thus, proportions were used in this study to quantify differential FA use.
Materials and methods
Overwintering sample collection
Live monarch butterflies were captured at the Sierra Chincua wintering colony in Michoacán, Mexico, in the Monarch Butterfly Biosphere Reserve (permit number: SPARN/DGVS/11879/23). One-hundred-fifty monarch butterflies were collected in the morning (08:00–10:30) using thin mesh nets, sexed, and placed in individual glassine envelopes. Monarch butterflies were immediately transported to UNAM-Campus, Morelia, and freeze-dried, before being transported to the WasserCluster – Biological Station Lunz laboratory for FA quantification and compositional analysis. Monarch butterflies were separated into body segments (head, thorax, and abdomen) due to potential differences in the roles of lipids stored in each body segment. For example, lipids in the abdomen are primarily considered as storage lipids (energy), while lipids in the thorax and head can have varied functional purposes (Brown and Chippendale 1974; Pilecky et al. 2022). Sample collections took place on December 12, 2022, January 16, 2023, and February 28, 2023, covering fall migration arrival up to remigration departure.
Lipid extraction
Monarch butterflies (n = 50 per month; 25m/25f) were separated into three body segments, and the wings were removed. Class separation of lipids was performed for heads and thoraxes due to increased proportions of neutral and polar lipids in these body segments (Pilecky et al. 2022). The abdomen was processed without class separation as most lipids were neutral storage lipids (~ 76%; Pilecky et al. 2022). Lipid extraction was performed using a modified Folch method (Pilecky et al. 2021), and total lipid content as a mass fraction (mg of lipid per g of freeze-dried body segment weight) was determined gravimetrically for each body segment. Briefly, body segments were weighed and placed in test tubes with ice-cold chloroform (VWR Chemicals, Randor, Pennsylvania) (2 mL). Capped vials were then vortexed vigorously (1 min) (Vortex 3, IKA, Staufen, Germany) and left to extract overnight at -80 °C. After that, methanol (VWR Chemicals, Randor, Pennsylvania) (1 mL) and a 0.9% NaCl solution (750 µL) were added, with the resultant solution vortexed. Then, vials were centrifuged at 3,000 g for 5 min (Rotanta 460R, HettichLab, Tuttlingen, Germany). The lower phase was collected using a glass pipette and placed into a new labelled glass vial. Additional chloroform (2 mL) was added to the original vial, which was vortexed and centrifuged to collect the lower lipid phase again; the collected phase was then added to the new vial. For bulk lipid analysis, solvent-extracted total lipids were evaporated to dryness under a gentle flow of N2 (N-Evap, Organomation, Berlin, Massachusetts). For lipid class separation of the head and thorax, solid phase extraction (SPE; Bond Elut LRC–SI, Agilent Technologies, Santa Clara, California) (Pilecky et al. 2023) was performed on solvent-extracted total lipids. The SPE columns were conditioned using hexane (4 mL) and loaded with an aliquot of the total lipid extract (10 µg of lipid at maximum). Neutral lipids were eluted using 4 mL of a chloroform:2-propanol mixture (VWR Chemicals, Randor, Pennsylvania) (2:1 v/v), then collected into a labelled vial. Free FA were separated using 4 mL of 2% acetic acid (VWR Chemicals, Randor, Pennsylvania) in diethyl ether and discarded. Polar lipids were eluted using 4 mL methanol and collected into a labelled vial. Collected neutral and polar lipids were then evaporated to dryness under N2 flow.
FA methyl ester formation
The dried lipid samples were prepared for gas chromatographic (GC) analysis as described by Pilecky et al. (2023, 2024). Following drying, samples were suspended in toluene (1 mL) (VWR Chemicals, Randor, Pennsylvania). Methylation of FA was performed by adding 2 mL of 1% H2SO4 in methanol. The capped solution was then placed in a 70 °C hot water bath and reacted for 2 h. Following the methylation, KHCO3 (2 mL) and hexane (500 µL) were added to the solution, then shaken and allowed to settle. The supernatant was collected and placed into a GC vial (VWR Chemicals, Randor, Pennsylvania), subsequently dried under N2 and re-suspended in hexane (1.5 mL) for GC analysis.
Gas chromatography
FA methyl esters were separated using a gas chromatograph (TRACE GC, ThermoFisher Scientific, Waltham, Massachusetts) and a flame ionisation detector. The injector temperature was 260 °C, with He as the carrier gas (1 mL/min). The TRACE GC housed an Agilent HP-88 column (100 m, 25 mm inner diameter., 0.2 μm film thickness) to separate and quantify FA methyl esters in the sample. The Chromeleon 7 software (ThermoFisher) was used for sample peak integration. The retention times of a polyunsaturated fatty acid (PUFA) standard (37 components; Supelco; Sigma-Aldrich, Bellefonte, Pennsylvania) were averaged to create a library of known FA peaks. The distinct GC peaks in each sample chromatograph were compared to known retention times of standard FA to identify peaks in each sample. The FA mass fractions were quantified as µg of FA per mg of freeze-dried total body segment weight (as with total lipid mass fractions). FA proportions were calculated as a proportion of the total lipid mass fraction (FA mass fraction/total lipid mass fraction) in each sample segment (i.e., head, thorax, abdomen).
Statistics
All statistical tests were performed using R Studio (Version 4.3.2). Two-way ANOVAs and Tukey’s HSD post-hoc analyses were used to evaluate total lipid mass fractions in each body segment and FA proportions in the abdomen. Normality of data was confirmed using Skew (− 2 to + 2) and Kurtosis (− 7 to + 7) analyses (Curran et al. 1996). The collection month (December, January, February) and sex (s; m, f) were independent variables. Three-way ANOVAs and Tukey’s post-hoc analyses were used to evaluate FA proportions in the head and thorax body segments with month collected (M; December, January, February), sex (S; m, f), and lipid class (C; neutral (n), polar (p) as independent variables. Additionally, principal component analysis (PCA) was performed for each body segment with FA mass fractions (combined neutral and polar classes where present) and total lipid mass fractions as variables.
Results
Total lipid mass fractions
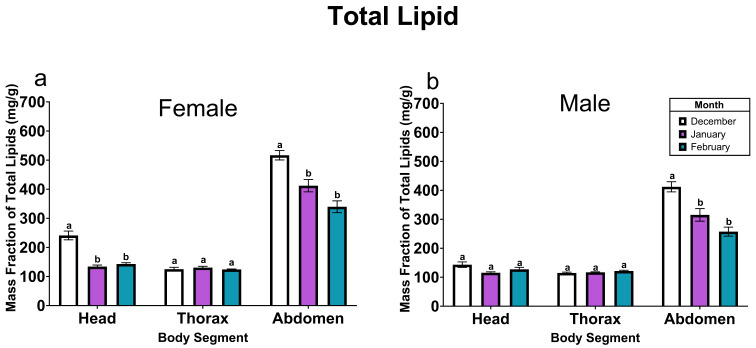
Total lipids were quantified in body segments of the sampled monarch butterflies (Fig. 1). The total lipid mass fraction was affected by month in the heads (Table 1; ~56.49 mg/g loss by February) and abdomens (Table 1; ~166.47 mg/g loss by February) but not in thoraxes (Table 1). Female monarch butterflies had a greater mass fraction of total lipids in the abdomen than males throughout the overwintering period (Table 1; ~94.49 mg/g more; Tukey HSD, pf−m<0.001) while this sex-based difference was only present in the monarch butterfly heads during December (~ 93.01 mg/g more; Tukey HSD, pdecf−decm<0.001). The thorax total lipid mass fraction did not differ between sexes (Table 1). In the PCA, components 1 and 2 described at least 75% of the variation in the data for all body segments. An association between total lipid mass fraction and the mass fractions of all fatty acids was present in each body part. However, NFA (particularly oleic and palmitic acids) displayed a stronger association with total lipid mass fraction than EFA (Online Resource S.4).
Fig. 1.
Mass fractions of total lipids (mg/g ± SE) in the body segments of overwintering female (a) and male (b) monarch butterflies from December to February. N = 25 per month apart from January female head, December male head, January male thorax, January male abdomen and February female abdomen N = 24. Letters indicate statistically significant differences in lipid mass fraction within each body part for each sex across months (ANOVA, Tukey’s HSD post-hoc test, p < 0.05)
Table 1.
Statistical results of two-way ANOVAs for monarch butterfly head, thorax and abdomen lipid mass fractions with month (M) and sex (S) as independent variables
| Factor | Head | Thorax | Abdomen | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | |
| M | 2, 142 | 23.35 | < 0.001 | 2, 143 | 0.31 | 0.73 | 2, 142 | 39.80 | < 0.001 |
| S | 1, 142 | 23.05 | < 0.001 | 1, 143 | 0.98 | 0.32 | 1, 142 | 37.80 | < 0.001 |
| M × S | 2, 142 | 8.99 | < 0.001 | 2, 143 | 1.30 | 0.28 | 2, 142 | 0.19 | 0.83 |
Monarch butterfly heads
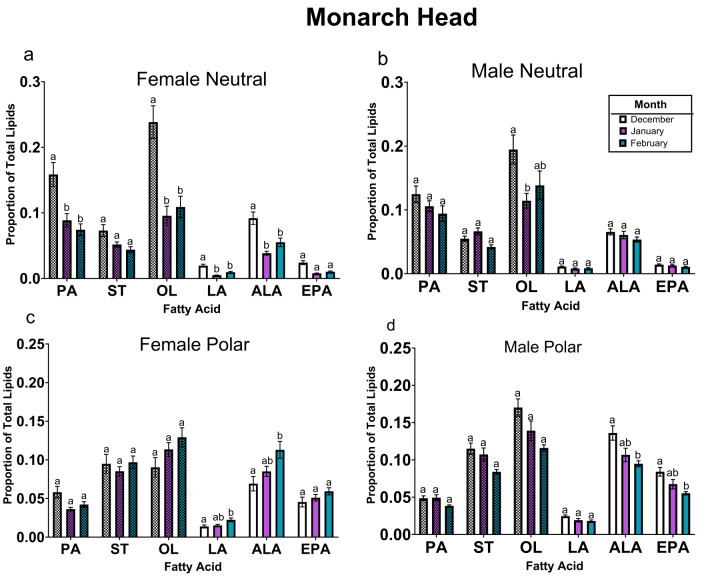
All six FA (palmitic, stearic, oleic, alpha-linolenic, linoleic and eicosapentaenoic acids) were present in the head (Fig. 2). A higher proportion of oleic acid and palmitic acid were found in neutral lipids, while a more substantial proportion of stearic acid was found in the polar lipids (Table 2; oleic: TukeyHSD, pn−p<0.05; palmitic: TukeyHSD, pn−p<0.001; stearic: TukeyHSD, pp−n<0.001). The proportion of palmitic acid declined from December to February in neutral lipids (Table 2; ~0.06 decline; TukeyHSD, pdecn−febn<0.001) but was stable in polar lipids (TukeyHSD, pdecp−febp=0.76). There was no three-way interaction between month, sex, and class for the proportion of palmitic acid (Table 2). For female monarch butterflies, oleic acid proportions declined from December to February in neutral lipids (Table 2; ~0.13 decline; TukeyHSD, pdecfn−febfn<0.001) but were stable in polar lipids (TukeyHSD, pdecfp−febfp=0.87). The decline in oleic acid was absent from December to February in male monarch butterflies (TukeyHSD, pdecmn−febmn=0.35). Males had a higher proportion of oleic acid in polar lipid fraction than females, but only in December (~ 0.08 more; TukeyHSD, pdecmp−decfp<0.05). While there was an interactive effect between month, sex and lipid class for ST, proportions of stearic acid were stable in the neutral lipid fraction of both male and female monarch butterflies during the overwintering period (Table 2; TukeyHSD, pdecmn−febmn=0.98, pdecfn−febfn=0.12), with a similar trend observed in polar fractions (TukeyHSD, pdecmp−febmp=0.09; TukeyHSD, pdecfp−febfp=1.00). All EFA were found in higher proportions in polar lipids (Table 2; linoleic: TukeyHSD, pp−n<0.001; alpha-linolenic: TukeyHSD, pp−n<0.001; eicosapentaenoic: TukeyHSD, pp−n<0.001). A decline in linoleic acid and alpha-linolenic acid proportions was observed during the overwintering period (December to February) in the neutral lipids of female monarch butterflies (Table 2; declines of ~ 0.01 and ~ 0.04, respectively; linoleic: TukeyHSD, pdecfn−febfn<0.01; alpha-linolenic: TukeyHSD, pdecfn−febfn<0.05). This decline was not observed in the polar lipids of male monarch butterflies for linoleic acid proportions but was seen in alpha-linolenic acid proportions (TukeyHSD, linoleic: pdecmp−febmp=0.21; alpha-linolenic: ~0.04 decline, pdecmp−febmp<0.01). However, increases in the proportions of linoleic acid and alpha-linolenic acid were observed in polar lipids of females (~ 0.01 and ~ 0.04 increases, respectively; linolenic: TukeyHSD, pdfebfp−decfp<0.05; alpha-linolenic: pfebfp−decfp<0.01). Proportions of eicosapentaenoic acid were consistent amongst the sexes and lipid classes, apart from polar lipids of males, which declined during the overwintering period (Table 2; ~0.03 decline; TukeyHSD, pdecmp−febmp<0.001). It was also observed that males had greater proportions of polar EFA than females, but only in December (linoleic: TukeyHSD, pdecmp−decfp=0.001; alpha-linolenic: pdecmp−decfp<0.0001; eicosapentaenoic: pdecmp−decfp<0.0001).
Fig. 2.
Monarch butterfly heads: The proportion (± SE) of palmitic (PA), stearic (ST), oleic (OL), linoleic (LA), alpha-linolenic (ALA), and eicosapentaenoic (EPA) acid comprising the total lipid mass fraction in the female neutral (a), male neutral (b), female polar (c), and male polar (d) components from December to February. Letters indicate statistically significant differences in the proportion of each fatty acid within either the neutral or polar lipid class of each sex across months (ANOVA, Tukey’s HSD post-hoc test, p < 0.05). Checkered bars indicate nonessential fatty acids, and solid bars indicate essential fatty acids
Table 2.
Statistical results of three-way ANOVAs for palmitic (PA), stearic (ST), oleic (OL), linoleic (LA), alpha-linolenic (ALA) and eicosapentaenoic (EPA) acid proportions in monarch butterfly heads with month (M), sex (S) and lipid class (C) as independent variables
| Factor | df | PA | ST | OL | LA | ALA | EPA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | ||
| M | 2, 267 | 16.27 | < 0.001 | 6.23 | < 0.01 | 16.81 | < 0.001 | 12.00 | < 0.001 | 6.63 | < 0.01 | 5.73 | < 0.01 |
| S | 1, 267 | 0.01 | 0.91 | 0.97 | 0.33 | 2.54 | 0.11 | 0.39 | 0.53 | 5.93 | < 0.05 | 12.98 | < 0.001 |
| C | 1, 267 | 132.10 | < 0.001 | 111.62 | < 0.001 | 5.55 | < 0.05 | 81.46 | < 0.001 | 87.60 | < 0.001 | 478.29 | < 0.001 |
| M × S | 2, 267 | 4.63 | < 0.05 | 3.58 | < 0.05 | 0.24 | 0.78 | 3.75 | < 0.05 | 5.50 | < 0.01 | 4.57 | < 0.05 |
| M × C | 2, 267 | 6.03 | < 0.01 | 0.55 | 0.58 | 12.54 | < 0.001 | 6.05 | < 0.01 | 3.36 | < 0.05 | 0.21 | 0.81 |
| S × C | 1, 267 | 0.00 | 0.95 | 2.27 | 0.13 | 2.75 | 0.10 | 9.04 | < 0.01 | 9.31 | < 0.01 | 18.94 | < 0.001 |
| M × S × C | 2, 267 | 1.64 | 0.20 | 3.33 | < 0.05 | 7.08 | < 0.01 | 13.10 | < 0.001 | 15.82 | < 0.001 | 13.24 | < 0.001 |
Monarch butterfly thoraxes
All six FA (palmitic, stearic, oleic, alpha-linolenic, linoleic and eicosapentaenoic acids) were present in the thorax (Fig. 3). A greater proportion of both oleic acid and stearic acid were found in polar lipids, while a higher proportion of palmitic acid was found in the neutral lipid fraction (Table 3; oleic: TukeyHSD, pp−n<0.001; stearic: TukeyHSD, pp−n<0.001; palmitic: TukeyHSD, pn−p<0.001). The proportion of palmitic acid declined from December to February in neutral lipids (Table 3; ~0.02 decline; TukeyHSD, pdecn−febn<0.001), but increased in polar lipids (~ 0.02 increase; TukeyHSD, pdecp−febp<0.05). There was no three-way interaction between month, sex, and class for palmitic acid (Table 3). For female monarch butterflies, the proportion of oleic acid declined from December to February in neutral lipids (Table 3; ~0.07 decline; TukeyHSD, pdecfn−febfn<0.05) and increased in polar lipids (~ 0.10 increase; TukeyHSD, pfebfp−decfp<0.001). Male butterflies, however, did not differ in the proportion of oleic acid during the overwintering period in either lipid class (TukeyHSD, pdecmn−febmn=0.67, pdecmp−febmp=0.15). The proportion of stearic acid varied between classes across months (Table 3), wherein it remained stable in neutral lipids (TukeyHSD, pdecfn−febfn=0.37, pdecmn−febmn=0.97), but increased in polar lipids for both sexes (TukeyHSD, pfebfp−decfp<0.001, pfebmp−decmp<0.05). All EFA were found in greater proportions in polar relative to neutral lipids (Table 3; linoleic: TukeyHSD, pp−n<0.001; alpha-linolenic: TukeyHSD, pp−n<0.001; eicosapentaenoic: TukeyHSD, pp−n<0.001). The proportion of linoleic acid varied between classes across months (Table 3), with proportions in neutral lipids remaining stable (TukeyHSD, pdecn−febn=0.99) while increasing in polar lipids from December to February for both males and females (~ 0.02 increase; TukeyHSD, pfebp−decp<0.001). Only female monarch butterflies had increasing proportions of alpha-linolenic acid and eicosapentaenoic acid in polar lipids over the overwintering period (~ 0.09 and ~ 1.0e− 3 increase, respectively) (Table 3; alpha-linolenic: TukeyHSD, pfebfp−decfp<0.001; eicosapentaenoic: TukeyHSD, pfebfp−decfp<0.001). Although female monarch butterflies showed an increase in polar EFA, males had a greater proportion of polar alpha-linolenic acid than females in December (~ 0.05 more; TukeyHSD, pdecmp−decfp<0.001).
Fig. 3.
Monarch butterfly thorax: The proportion (± SE) of palmitic (PA), stearic (ST), oleic (OL), linoleic (LA), alpha-linolenic (ALA), and eicosapentaenoic (EPA) acid comprising the total lipid mass fraction in the female neutral (a), male neutral (b), female polar (c), and male polar (d) components from December to February. N = 25 per month apart from January males where N = 24. Letters indicate statistically significant differences in the proportion of each fatty acid within either the neutral or polar lipid class of each sex across months (ANOVA, Tukey’s HSD post-hoc test, p < 0.05). Checkered bars indicate nonessential fatty acids, and solid bars indicate essential fatty acids
Table 3.
Statistical results of three-way ANOVAs for palmitic (PA), stearic (ST), oleic (OL), linoleic (LA), alpha-linolenic (ALA) and eicosapentaenoic (EPA) acid proportions in monarch butterfly thoraxes with month (M), sex (S) and lipid class (C) as independent variables
| Factor | df | PA | ST | OL | LA | ALA | EPA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | ||
| M | 2, 286 | 0.07 | 0.93 | 18.35 | < 0.001 | 2.93 | 0.06 | 24.42 | < 0.001 | 35.99 | < 0.001 | 23.75 | < 0.001 |
| S | 1, 286 | 0.00 | 0.96 | 0.57 | 0.45 | 3.31 | 0.07 | 1.75 | 0.18 | 5.47 | < 0.05 | 33.71 | < 0.001 |
| C | 1, 286 | 25.43 | < 0.001 | 722.40 | < 0.001 | 38.73 | < 0.001 | 128.37 | < 0.001 | 58.38 | < 0.001 | 740.29 | < 0.001 |
| M × S | 2, 286 | 0.01 | 0.99 | 3.98 | < 0.05 | 0.08 | 0.93 | 0.91 | 0.41 | 8.10 | < 0.001 | 6.87 | < 0.01 |
| M × C | 2, 286 | 11.18 | < 0.001 | 39.09 | < 0.001 | 24.85 | < 0.001 | 13.99 | < 0.001 | 18.87 | < 0.001 | 12.03 | < 0.001 |
| S × C | 1, 286 | 0.15 | 0.70 | 0.40 | 0.53 | 1.83 | 0.17 | 0.97 | 0.33 | 1.29 | 0.26 | 8.28 | < 0.01 |
| M × S × C | 2, 286 | 0.85 | 0.43 | 10.07 | < 0.001 | 3.03 | < 0.05 | 2.37 | 0.10 | 6.29 | < 0.01 | 6.36 | < 0.01 |
Monarch butterfly abdomens
All six FA (palmitic, stearic, oleic, alpha-linolenic, linoleic and eicosapentaenoic acids) were quantified in the abdomen. The highest proportions of NFA were palmitic acid and oleic acid (Fig. 4). Although female monarch butterflies had a higher lipid mass fraction in the abdomen, the proportion of palmitic acid and oleic acid did not differ between males and females nor were there differences between months (Table 4). Additionally, there was no interactive effect of month and sex regarding the proportions of palmitic acid and oleic acid (Table 4). A sex-based difference was observed in stearic acid, with males having a greater proportion than females (Table 4; ~0.01 more; TukeyHSD, pm−f<0.01). The proportion of the EFA linoleic acid and alpha-linolenic acid both increased from December to February (Table 4; ~2.0e− 3 and 0.01 increase, respectively; linoleic: TukeyHSD, pfeb−dec<0.001; alpha-linolenic: TukeyHSD, pfeb−dec<0.001). The proportion of alpha-linolenic acid was similar between males and females (Table 4), but the proportion of linoleic acid was greater in males than in females (Table 4; ~6.0e− 4 more; TukeyHSD, pm−f<0.05). These observations were independent of month for both EFA (Table 4). Unlike other EFA, the proportion of eicosapentaenoic acid was stable across the overwintering period and between sexes (Table 4).
Fig. 4.
Monarch butterfly abdomen: The proportion (± SE) of female nonessential (a), male nonessential (b), female essential (c), and male essential (d) fatty acids comprising the total lipid mass fraction in the abdomens of overwintering monarch butterflies from December to February. N = 25 per month apart from January male and February female where N = 24. Letters indicate statistically significant differences within the proportions each fatty acid of each sex across months (ANOVA, Tukey’s HSD post-hoc test, p < 0.05). Checkered bars indicate nonessential fatty acids, and solid bars indicate essential fatty acids. Nonessential fatty acids: palmitic acid (PA), stearic acid (ST), oleic acid (OL); essential fatty acids: linoleic acid (LA), alpha-linolenic acid (ALA), and eicosapentaenoic acid (EPA)
Table 4.
Statistical results of two-way ANOVAs for palmitic (PA), stearic (ST), oleic (OL), linoleic (LA), alpha-linolenic (ALA) and eicosapentaenoic (EPA) acid proportions in monarch butterfly abdomens with month (M) and sex (S) as independent variables
| Factor | df | PA | ST | OL | LA | ALA | EPA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FF | p | F | p | F | p | F | p | F | p | F | p | ||
| M | 2, 142 | 0.99 | 0.37 | 0.86 | 0.43 | 1.26 | 0.29 | 15.19 | < 0.001 | 11.83 | < 0.001 | 0.77 | 0.47 |
| S | 1, 142 | 1.72 | 0.19 | 8.18 | < 0.01 | 0.55 | 0.46 | 4.35 | < 0.05 | 1.14 | 0.29 | 0.71 | 0.40 |
| M × S | 2, 142 | 0.62 | 0.54 | 1.64 | 0.20 | 0.46 | 0.63 | 0.64 | 0.53 | 1.07 | 0.35 | 0.02 | 0.98 |
Discussion
Most of the lipid mass in monarch butterflies (i.e., mass fractions and total mass of lipid; Online Resource S.1) were in their abdomens (Brown and Chippendale 1974; Pilecky et al. 2022). While the thorax and head had similar mass fractions of total lipids, due to mass differences between the two segments (72.76 mg versus 5.07 mg, respectively; Online Resource S.2, S.8), the head represented a small component in the energetic profile of the monarch butterflies. The thorax, a metabolically active segment in overwintering monarch butterflies (Kammer 1970; Masters et al. 1988), showed no decline in total lipid mass fractions; thus, it can be postulated that FA mobilised and oxidised to supply energy to the thorax were circulated through the haemolymph from the abdomen (Gilby 1965; Beenakkers et al. 1985; Van der Horst et al. 2002; Van der Horst and Rodenburg 2010) wherein the most lipid loss over time was observed in our study. Changes to the thorax FA patterns, particularly unsaturated FA in the polar lipid fraction, may reflect the non-energetic roles of FA in cold acclimation (Hazel and Williams 1990; Danks 2006; Bale and Hayward 2010) in overwintering monarch butterfly flight muscles. Lipid mass fraction loss in the abdomen, expectedly, correlated with total abdominal weight loss (Online Resource S.2). The abdomen was the body segment with greatest relative mass loss across the overwintering period (Online Resource S.2). These FA results provide evidence that monarch butterflies may differentially use NFA and EFA during the overwintering period, increasing the availability of limited EFA for future demands, the most significant of which is reproduction.
In monarch butterfly heads, loss of linoleic acid and alpha-linolenic acid from neutral lipids of females corresponded to proportional increases of these EFA in the polar lipids (collectively ~ 0.05 loss and gain, respectively). This rearrangement of PUFA from neutral to polar lipids may suggest increased investment regarding cognitive traits (Pilecky et al. 2021) by females in late February compared to males, although further research is required regarding the role of PUFA in invertebrate vision (but see Ziegler et al. 2015). Alternatively, the increase in unsaturated FA at the expense of neutral FA may also be a response to maintain membrane fluidity during the overwintering period, as seen in Megaphorura arctica (Purać et al. 2011).
The polar lipids generally had higher proportions of unsaturated FA, such as oleic acid (in thorax), linoleic acid, alpha-linolenic acid, and eicosapentaenoic acid, than neutral lipids. Polar lipids (e.g., phospholipids) are integral to membranes of cells and organelles, and their composition is important for tissues, such as brain and thoracic flight muscle (Chapman 1975; Stanley-Samuelson et al. 1988; Hazel 1995; Karasov & Martinez del Rio 2007). With nightly temperatures occasionally < 0 °C during the coldest months (Calvert and Cohen 1983; Calvert and Brower 1986), higher proportions of unsaturated FA in the functional polar lipid fractions of monarch butterflies could be an adjustment to maintain cells and tissues during the overwintering period (Hazel and Williams 1990; Danks 2006; Bale and Hayward 2010) Increased mobility of unsaturated FA reduces membrane rigidity in lower temperatures, thereby allowing overwintering insects greater tissue functionality and overall survival (Danks 2006; Bale and Hayward 2010; Koštál 2010; Rozsypal et al. 2014). For example, Bennett et al. (1997) found that polar lipid fractions of larval Eurosta solidaginis displayed an increase in the unsaturation ratio in response to declining ambient temperatures. Although not directly tested in this study, we can accordingly speculate that monarch butterflies with higher proportions of unsaturated FA in the total lipids of thoraxes may have greater cold acclimation. However, not all invertebrates display increased accumulation of unsaturated FA in polar lipids (Koštál et al. 2003), so additional research into the differences in cold acclimation and survival in monarch butterflies with varying degrees of FA unsaturation in thorax tissues is warranted.
The unsaturated FA oleic acid and alpha-linolenic acid generally comprised greater portions of thorax lipid content (~ 0.30 and ~ 0.26, respectively) regardless of sex, except for December, when males had a higher proportion of polar thoracic alpha-linolenic acid. In addition to migrant monarch butterflies generally having greater investments into their flight apparatus and body size than non-migrants (Altizer and Davis 2010), there is experimental evidence that males emerged with a greater dry weight of thoracic flight muscle than females (Davis and Holden 2015). An additional variable for male migrants with greater thoracic investment may be the increased allocation of unsaturated FA to the thorax to improve cold acclimation capacity of their tissues and maintain membrane integrity. Females displayed unsaturated FA proportions in polar lipids similar to males by the end of the overwintering period (February), which may come from the rearrangement of thoracic FA from neutral into polar lipids (Bale and Hayward 2010; Çakmak, 2010; Koštál 2010; Rozsypal et al., 2014). For example, a proportional increase of polar oleic acid content in females may occur at the expense of declines in the neutral oleic acid content (~ 0.07 loss compared to a ~ 0.10 gain). However, this pattern was not observed for alpha-linolenic acid, nor were there corresponding declines of alpha-linolenic acid in other body segments to account for this increase. An alternative plausible hypothesis could be a higher survival rate of females with greater unsaturated FA pools during the overwintering period.
Unlike the head and thorax, loss of abdominal total lipid mass fraction, lipid mass (Online Resource S.1), and tissue mass (Online Resource S.2) was observed across the overwintering period in both sexes. Interestingly, the lipid mass fraction loss plateaued in the late overwintering period in both male and female monarch butterflies (also observed in Alonso-Mejia et al. 1997). Previous literature on other overwintering insects has shown an increase in metabolic suppression and subsequent reduction in lipid use in relation to declining temperatures (Hahn and Denlinger 2007, 2011; Sinclair 2015). Monarch butterflies have also shown temperature-related differences in lipid use on the overwintering grounds with butterflies that had access to cooler microclimates having greater amounts of lipid remaining at the end of the overwintering period compared to butterflies that were exposed to warmer microclimates (Brower et al. 2009). While climatic and temperature data were not collected during this study, daily temperature records for the Sierra Chincua site from 2004 to 2023 (co-author MIR, unpublished data) show no significant minimum or maximum trends over the past two decades. However, future research into the direct impacts of ambient overwintering temperature on lipid use in monarch butterflies is warranted. Alternatively, the low but stable lipid mass fractions at the end of the overwintering period may correlate with increases in mating and emigration of monarchs from the overwintering colony as seen in Western overwintering monarch butterfly populations (Chaplin and Wells 1982).
As total lipids declined, the proportions of NFA were stable, reflecting the simultaneous loss of NFA and total lipid content. Considering oleic acid and palmitic acid were found in high proportions in the abdomen (~ 0.27 and ~ 0.50, respectively), these NFA are the predominant fuels used by monarch butterflies, a common observation amongst lepidopterans (Wang and Ouyang 1995; Murata and Tojo 2002; Sakamoto et al. 2004; Anparasan 2023). While females may accumulate more lipids to fuel the overwintering period (also observed in Pilecky et al. 2022), NFA proportions, proportional declines and total lipid loss (~ 34% in females and ~ 37% in males) did not differ significantly, suggesting similar use patterns between males and females. In contrast, as total lipid content declined, EFA proportions increased, reflecting the decreased use of EFA during the overwintering period compared to NFA. This study thus provides field evidence for comparatively lower EFA mobilisation relative to NFA to fuel energetic demands during the overwintering period. Selective retention of limited resources (e.g., EFA and essential amino acids) for future life stages has also been shown in other insects (Schneider and Dorn 1994; Wang and Ouyang 1995; Levin et al. 2017; Anparasan et al. 2023). While the proportions of EFA may seem small, considering the large mass fraction of lipids in the abdomen, several hundred µg of EFA were conserved by overwintering monarch butterflies at the end of the sample collection period (538 ± 79.9 µg; Online Resource S.3).
EFA are vital for female monarch butterflies to later produce several hundred eggs (Urquhart 1960; Altizer and Oberhauser 1999) after spring remigration. Interestingly, males also displayed reduced mobilization of EFA in the abdomen. Evidence of EFA donations, particularly of linoleic acid, via the spermatophore to egg production, has been observed in other lepidopterans (Anparasan 2023). Thus, male monarch butterflies may also contribute EFA conserved during the overwintering period, however, the role of male monarch butterflies EFA for reproduction also warrants further research.
While it was evident that NFA was more readily mobilised than EFA during the overwintering period, exercise experiments conducted on monarch butterflies found that long durations (i.e., > 4 h) of forced exercise resulted in a loss of EFA from abdominal stores (Anparasan 2023). A potential contributor to this loss was the lack of nectar provision, which restricted NFA re-accumulation in exercised monarch butterflies. In a similar manner, overwintering EFA conservation may be impacted by the accumulation of required NFA before the start of the overwintering period. Conservation efforts should, therefore, focus on establishing and protecting nectaring locations found in the latter sections of the migratory corridor to Mexico (Brower et al. 2006; Hobson et al. 2023). Preliminary stable isotope analysis (δ2H) has enabled a general prediction of where late-stage nectaring occurs (Hobson et al. 2020). However, considering differences in the sources of EFA and NFA in nectivorous lepidopterans such as monarch butterflies, we recommend the use of compound-specific stable isotopic analysis (Whiteman et al. 2019; Twining et al. 2020) of individual FA with a focus on EFA and de novo synthesised NFA separately to provide greater resolution in predicting late-stage migration nectaring sites.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We mourn the passing of our colleague and co-author Dr. Keith A. Hobson in October 2024. We thank research associates at the UNAM campus for the prompt and efficient preparation and shipment of monarch samples.
Author contributions
LA, LIW and KAH designed the study. LA and MP performed lipid and data analysis. MIR collected the samples. LA, LIW and MP lead the writing of the manuscript. KAH, MJK, and MIR assisted with manuscript editing. All authors critically assessed the manuscript.
Funding
This research was funded by an Austrian Science Foundation grant to LIW (P36013-BBL) and post-doctoral fellowship to LA.
Data availability
The data that supports the findings of this study are available in the supplementary material of this article.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Keith A. Hobson: In Memoria, October 2024
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alonso-Mejia A, Rendon-Salinas E, Montesinos-Patino E, Brower LP (1997) Use of lipid reserves by monarch butterflies overwintering in Mexico: implications for conservation. Ecol Appl 7:934–947. 10.2307/2269444 [Google Scholar]
- Altizer SM, Davis AK (2010) Populations of monarch butterflies with different migratory behaviors show divergence in wing morphology. Evolution 64:1018–1028. 10.1111/j.1558-5646.2009.00946.x [DOI] [PubMed] [Google Scholar]
- Altizer SM, Oberhauser KS (1999) Effects of the protozoan parasite Ophryocystis elektroscirrha on the fitness of monarch butterflies (Danaus plexippus). J Invertebr Pathol 74:76–88. 10.1006/jipa.1999.4853 [DOI] [PubMed] [Google Scholar]
- Anderson JB, Brower LP (1996) Freeze-protection of overwintering monarch butterflies in Mexico: critical role of the forest as a blanket and an umbrella. Ecol Entomol 21:107–116. 10.1111/j.1365-2311.1996.tb01177.x [Google Scholar]
- Anparasan L (2023) Effect of rearing conditions on the allocation of larval and adult acquired essential and nonessential fatty acids to flight in two adult Lepidoptera: Danaus plexippus and Mythimna unipuncta. [PhD Dissertation], University of Western Ontario, London, Ontario, Canada
- Anparasan L, Hobson KA, McNeil JN (2023) Effect of rearing conditions on fatty acid allocation during flight in nectivorous lepidopteran Mythimna unipuncta. Front Ecol Evol 11:1055534. 10.3389/fevo.2023.1055534 [Google Scholar]
- Arrese EL, Soulages JL (2010) Insect fat body: Energy, metabolism, and regulation. Annu Rev Entomol 55:207–225. 10.1146/annurev-ento-112408-085356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale JS, Hayward SAL (2010) Insect overwintering in a changing climate. J Exp Biol 213:980–994. 10.1242/jeb.037911 [DOI] [PubMed] [Google Scholar]
- Beenakkers AMT, Van der Horst DJ, Van Marrewijk WJ (1985) Insect lipids and lipoproteins, and their role in physiological processes. Prog Lipid Res 24:19–67. 10.1016/0163-7827(85)90007-4 [DOI] [PubMed] [Google Scholar]
- Bennett VA, Pruitt NL, Lee Jr RE (1997) Seasonal changes in fatty acid composition associated with cold hardening in third instar larvae of Eurosta solidaginis. J Comp Physiol B 167:249–255. 10.1007/s003600050071 [Google Scholar]
- Brower LP (1985) New perspectives on the migration biology of the monarch butterfly, Danaus plexippus L. In: Rankin MA (ed) Migration: mechanisms and adaptive significance. University of Texas, Austin, Texas, USA, pp 748–785 [Google Scholar]
- Brower LP, Fink LS, Walford P (2006) Fueling the fall migration of the monarch butterfly. Integr Comp Biol 46:1123–1142. 10.1093/icb/icl029 [DOI] [PubMed] [Google Scholar]
- Brower LP, Williams EH, Slayback DA, Fink LS, Ramirez IM et al (2009) Oyamel fir forest trunks provide thermal advantages for overwintering monarch butterflies in Mexico. Insect Conserv Divers 2:163–175. 10.1111/j.1752-4598.2009.00052.x [Google Scholar]
- Brown JJ, Chippendale GM (1974) Migration of the monarch butterfly, Danaus plexippus: Energy sources. J Insect Physiol 20:1117e1130. 10.1016/0022-1910(74)90218-2 [DOI] [PubMed] [Google Scholar]
- Çakmak O (2010) Seasonal changes in fatty acid composition of Eysarcoris inconspicuous (Herrich-Schaffer, 1844) (Heteroptera: Pentatomidae) adults. Türk. Entomol Derg 34:15–27 [Google Scholar]
- Calvert WH, Brower LP (1981) The importance of forest cover for the survival of overwintering monarch butterflies (Danaus plexippus, Danaidae). J Lepidopt Soc 35:216–225 [Google Scholar]
- Calvert WH, Brower LP (1986) The location of monarch butterfly (Danaus plexippus L.) overwintering colonies in Mexico in relation to topography and climate. J Lepidopt Soc 40:164–187 [Google Scholar]
- Calvert WH, Cohen JA (1983) The adaptive significance of crawling up onto foliage for the survival of grounded overwintering monarch butterflies (Danaus plexippus) in Mexico. Ecol Entomol 8:471474. 10.1111/j.1365-2311.1983.tb00525.x
- Calvert WH, Lawton RO (1993) Comparative phenology of variation in size, weight, and water content of eastern north American monarch butterflies at five overwintering sites in Mexico. In: Malcolm SB, Zalucki MP (eds) Biology and Conservation of the Monarch Butterfly. Natural History Museum of Los Angeles, Los Angeles, California, USA, pp 299–307 [Google Scholar]
- Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA (2001) Fat metabolism in insects. Annu Rev Nutr 21:23–46. 10.1146/annurev.nutr.21.1.23 [DOI] [PubMed] [Google Scholar]
- Cenedella RJ (1971) The lipids of the female monarch butterfly, Danaus plexippus, during fall migration. Insect Biochem 1:244–247. 10.1016/0020-1790(71)90077-1 [Google Scholar]
- Chaplin SB, Wells PH (1982) Energy reserves and metabolic expenditures of monarch butterflies overwintering in Southern California. Ecol Entomol 7:249–256. 10.1111/j.1365-2311.1982.tb00664.x [Google Scholar]
- Chapman D (1975) Phase transitions and fluidity characteristics of lipids and cell membranes. Q Rev Biophys 8:185–235. 10.1017/s0033583500001797 [DOI] [PubMed] [Google Scholar]
- Chapman JW, Bell JR, Burgin LE, Reynolds DR, Pettersson LB et al (2012) Seasonal migration to high latitudes results in major reproductive benefits in an insect. Proc Natl Acad Sci USA 109:14924–14929. 10.1073/pnas.1207255109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JW, Reynolds DR, Wilson K (2015) Long-range seasonal migration in insects: mechanisms, evolutionary drivers, and ecological consequences. Ecol Lett 18:287–302. 10.1111/ele.12407 [DOI] [PubMed] [Google Scholar]
- Curran PJ, West SG, Finch JF (1996) The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychol Methods 1:16–29. 10.1037/1082-989X.1.1.16 [Google Scholar]
- Danks HV (2006) Insect adaptations to cold and changing environments. Can Entomol 138:1–23. 10.4039/n05-802 [Google Scholar]
- Davis AK, Holden MT (2015) Measuring intraspecific variation in flight-related morphology of monarch butterflies (Danaus plexippus): which sex has the best flying gear? J Insects 591705. 10.1155/2015/591705
- Dingle H (1972) Migration strategies of insects. Science 175:1327–1335. 10.1126/science.175.4028.1327 [DOI] [PubMed] [Google Scholar]
- Downer RGH, Matthews JR (1976) Patterns of lipid distribution and utilization in insects. Amer Zool 16:733–745. 10.1093/icb/16.4.733 [Google Scholar]
- Forte SN, Ferrero AA, Alonso TS (2002) Content and composition of phosphoglycerols and neutral lipids at different developmental stages of the eggs of the codling moth, Cydia Pomonella (Lepidoptera: Tortricidae). Arch Insect Biochem Physiol 50:121–130. 10.1002/arch.10036 [DOI] [PubMed] [Google Scholar]
- Gilby AR (1965) Lipids and their metabolism in insects. Annu Rev Entomol 10:141–160. 10.1146/annurev.en.10.010165.001041 [Google Scholar]
- Goehring L, Oberhauser KS (2002) Effects of photoperiod, temperature, and host plant age on induction of reproductive diapause and development time in Danaus plexippus. Ecol. Entomol., 27, 674–685. 10.1046/j.1365-2311.2002.00454.x
- Hahn, D. A. and Denlinger, D. L. (2007) Meeting the energetic demands of insect diapause: nutrient storage and utilization. J Insect Physiol., 53, 760–773. 10.1016/j.jinsphys.2007.03.018 [DOI] [PubMed]
- Hahn DA, Denlinger DL (2011) Energetics of insect diapause. Ann Rev Entomol 56:103–121. 10.1146/annurev-ento-112408-085436 [DOI] [PubMed] [Google Scholar]
- Hazel JR (1995) Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu. Rev Physiol 57:19–42. 10.1146/annurev.ph.57.030195.000315 [DOI] [PubMed] [Google Scholar]
- Hazel JR, Williams EE (1990) The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res 29:167–227. 10.1016/0163-7827(90)90002-3 [DOI] [PubMed] [Google Scholar]
- Herman WS, Brower LP, Calvert WH (1989) Reproductive tract development in monarch butterflies overwintering in California and Mexico. J Lepidopt Soc 43:50–58 [Google Scholar]
- Hobson KA, García-Rubio OR, Carrera-Treviño R, Anparasan L, Kardynal KJ et al (2020) Isotopic (δ2H) analysis of stored lipids in migratory and overwintering monarch butterflies (Danaus plexippus): evidence for Southern critical late-stage nectaring sites? Front. Ecol Evol 8:572140. 10.3389/fevo.2020.572140 [Google Scholar]
- Hobson KA, Taylor O, Ramírez MI, Carrera-Treviño R, Pleasants J et al (2023) Dynamics of stored lipids in fall migratory monarch butterflies (Danaus plexippus): Nectaring in northern Mexico allows recovery from droughts at higher latitudes. Conserv Physiol 11:coad087. 10.1093/conphys/coad087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer AE (1970) Thoracic temperature, shivering, and flight in the monarch butterfly, Danaus plexippus (L). Z Vergl Physiol 68:334–344. 10.1007/BF00298260 [Google Scholar]
- Karasov WH, Martinez del Rio C (2007) Physiological ecology: how animals process energy, nutrients, and toxins. Princeton University Press, New Jersey, USA. 10.2307/j.ctvzsmfh4 [Google Scholar]
- Koštál V (2010) Cell structural modifications in insects at low temperatures. In: Denlinger DL, Lee RE Jr (eds) Low Temperature Biology of insects. Cambridge University Press, Cambridge, UK, pp 116–140 [Google Scholar]
- Koštál V, Berkova P, Simek P (2003) Remodelling of membrane phospholipids during transition to diapause and cold-acclimation in the larvae of Chymomyza costata (Drosophilidae). Comp Biochem Physiol B 135:407–419. 10.1016/S1096-4959Ž03.00117-9 [DOI] [PubMed]
- Krenn HW (2010) Feeding mechanisms of adult lepidoptera: structure, function, and evolution of the mouthparts. Annu Rev Entomol 55:307–327. 10.1146/annurev-ento-112408-085338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E, McCue MD, Davidowitz G (2017) More than just sugar: allocation of nectar amino acids and fatty acids in a lepidopteran. Proc R Soc B 284:2016–2126. 10.1098/rspb.2016.2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcicka M, Visser B, Ellers J (2018) An evolutionary perspective on linoleic acid synthesis in animals. Evol Biol 45:15–26. 10.1007/s11692-017-9436-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm SB (2018) Anthropogenic impacts on mortality and population viability of the monarch butterfly. Annu Rev Entomol 63:277–302. 10.1146/annurev-ento-020117-043241 [DOI] [PubMed] [Google Scholar]
- Masters AR, Malcolm SB, Brower LP (1988) Monarch butterfly (Danaus plexippus) thermoregulatory behavior and adaptations for overwintering in Mexico. Ecology 69:458–467. 10.2307/1940444 [Google Scholar]
- Murata M, Tojo S (2002) Utilization of lipid for flight and reproduction in Spodoptera litura (Lepidoptera: Noctuidae). Eur J Entomol 99:221–224. 10.14411/eje.2002.031 [Google Scholar]
- Nicolson SW (2022) Sweet solutions: nectar chemistry and quality. Phil Trans R Soc B 377:20210163. 10.1098/rstb.2021.0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson SW, Thornburg RW (2007) Nectar chemistry. In: Nicolson S, Nepi M, Pacini E (eds) Nectaries and nectar. Springer, Dordrecht, The Netherlands, pp 215–264. 10.1007/978-1-4020-5937-7_5 [Google Scholar]
- Pilecky M, Závorka L, Arts MT, Kainz MJ (2021) Omega-3 PUFA profoundly affect neural, physiological, and behavioural competences– implications for systemic changes in trophic interactions. Biol Rev 96:2127–2145. 10.1111/brv.12747 [DOI] [PubMed] [Google Scholar]
- Pilecky M, Wassenaar LI, Kainz MJ, Anparasan L, Ramirez IM et al (2022) Isotopic (δ2H and δ13C) tracing the provenance and fate of individual FAs fueling migrating animals: a case study of the monarch butterfly (Danaus plexippus). Front Ecol Evol 10:1051782. 10.3389/fevo.2022.1051782 [Google Scholar]
- Pilecky M, Wassenaar LI, Taipale S, Kainz MJ (2023) Protocols for sample preparation and compound-specific stable-isotope analyses (δ2H, δ13C) of fatty acids in biological and environmental samples. MethodsX 11:102283. 10.1016/j.mex.2023.102283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilecky M, Kainz MJ, Wassenaar LI (2024) Evaluation of lipid extraction methods for fatty acid quantification and compound-specific δ13C and δ2H analyses. Anal Biochem 687:115455. 10.1016/j.ab.2023.115455 [DOI] [PubMed] [Google Scholar]
- Purać J, Pond DW, Grubor-Lajsić G, Kojić D, Blagojević DP et al (2011) Cold hardening induces transfer of fatty acids between polar and nonpolar lipid pools in the Arctic collembolan Megaphorura arctica. Physiol Entomol 36:135–140. 10.1111/j.1365-3032.2010.00772.x [Google Scholar]
- Ramenofsky M, Wingfield JC (2007) Regulation of migration. Bioscience 57:135–143. 10.1641/B570208 [Google Scholar]
- Ramirez IM, Saenz-Romero C, Rehfeldt G, Salas-Canela L (2015) Threats to the availability of overwintering habitat in the monarch butterfly biosphere reserve: land use and climate change. In: Oberhauser KS, Nail KR, Altizer SM (eds) Monarchs in a changing world: biology and conservation of an iconic insect. Cornell University Press, Ithaca, New York, USA, pp 157–168 [Google Scholar]
- Rozsypal J, Koštál V, Berková P, Zahradníčková H, Šimek P (2014) Seasonal changes in the composition of storage and membrane lipids in overwintering larvae of the codling moth, Cydia Pomonella. J Therm Biol 54:20–22. 10.1016/j.jtherbio.2014.08.011 [DOI] [PubMed] [Google Scholar]
- Sakamoto R, Murata M, Tojo S (2004) Effects of larval diets on flight capacity and flight fuel in adults of the common cutworm, Spodoptera litura (Lepidoptera: Noctuidae). Appl Entomol Zool 39:133–138. 10.1303/aez.2004.133 [Google Scholar]
- Schneider M, Dorn A (1994) Lipid storage and mobilization by flight in relation to phase and age of Schistocerca Gregaria females. Insect Biochem Mol Biol 24:883–889. 10.1016/0965-1748(94)90017-5 [Google Scholar]
- Sinclair BJ (2015) Linking energetics and overwintering in temperate insects. J Therm Biol 54:5–11. 10.1016/j.jtherbio.2014.07.007 [DOI] [PubMed] [Google Scholar]
- Sinclair BJ, Marshall KE (2018) The many roles of fats in overwintering insects. J Exp Biol 221:jeb161836. 10.1242/jeb.161836 [DOI] [PubMed] [Google Scholar]
- Southwood TRE (1962) Migration of terrestrial arthropods in relation to habitat. Biol Rev 37:171–214. 10.1111/j.1469-185X.1962.tb01609.x [Google Scholar]
- Stanley-Samuelson DW, Jurenka RA, Cripps C, Blomquist GJ, Renobales M (1988) Fatty acids in insects: composition, metabolism, and biological significance. Arch Insect Biochem Physiol 9:l–33. 10.1002/arch.940090102 [Google Scholar]
- Subramanyam B, Cutkomp LK (1987) Total lipid and fatty acid composition in male and female larvae of indian-meal moth and almond moth (Lepidoptera: Pyralidae). Gt Lakes Entomol 20:10. 10.22543/0090-0222.1604 [Google Scholar]
- Twining CW, Taipale SJ, Ruess L, Bec A, Martin-Creuzburg D et al (2020) Stable isotopes of FAs: current and future perspectives for advancing trophic ecology. Philos Trans R Soc Lond B Biol Sci 375:20190641. 10.1098/rstb.2019.0641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart FA (1960) The Monarch Butterfly. University of Toronto, Toronto, ON [Google Scholar]
- Urquhart FA, Urquhart NR (1978) Autumnal migration routes of the eastern population of the monarch butterfly (Danaus plexippus L.; Danaidae: Lepidoptera) in North America to the overwintering site in the neovolcanic plateau of Mexico. Can J Zool 56:1754–1764. 10.1139/z78-240 [Google Scholar]
- Van der Horst DJ, Rodenburg KW (2010) Locust flight activity as a model for hormonal regulation of lipid mobilization and transport. J Insect Physiol 56:844–853. 10.1016/j.jinsphys.2010.02.015 [DOI] [PubMed] [Google Scholar]
- Van der Horst DJ, van Hoof D, van Marrewijk WJA, Rodenburg KW (2002) Alternative lipid mobilization: the insect shuttle system. Mol Cell Biochem 239:113–119 [PubMed] [Google Scholar]
- Wang Z, Ouyang Y (1995) Flight activity and fatty acid utilization in Mythimna Separata (Walker) moths. Insect Sci 2:370–376. 10.1111/j.1744-7917.1995.tb00061.x [Google Scholar]
- Wassenaar LI, Hobson KA (1998) Natal origins of migratory monarch butterflies at wintering colonies in Mexico: New isotopic evidence. P Natl Acad Sci USA 95:15436–15439. 10.1073/pnas.95.26.15436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman JP, Elliott-Smith EA, Besser AC, Newsome SD (2019) A guide to using compound-specific stable isotope analysis to study the fates of molecules in organisms and ecosystems. Diversity 118. 10.3390/d11010008
- Williams EH, Brower LP (2015) Microclimate protection of overwintering monarchs provided by Mexico’s high-elevation oyamel fir forests. In: Oberhauser KS, Nail KR, Altizer S (eds) Monarchs in a changing World: Biology and Conservation of an iconic Butterfly. Cornell University Press, Ithaca, New York, USA, pp 109–116 [Google Scholar]
- Ziegler AB, Ménagé C, Grégoire S, Garcia T, Ferveur J et al (2015) Lack of Dietary Polyunsaturated fatty acids causes synapse dysfunction in the Drosophila Visual System. PLoS ONE 10:e0135353. 10.1371/journal.pone.0135353 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.